Abstract
Bacterial surface structures such as capsules and adhesins are generally regarded as important virulence factors. Here we demonstrate that capsules block the function of the self-recognizing protein antigen 43 through physical shielding. The phenomenon is not restricted to Escherichia coli but can occur in other gram-negative bacteria. Likewise, we show that other short adhesins exemplified by the AIDA-I protein are blocked by the presence of a capsule. The results support the notion that capsule polysaccharides sterically prevent receptor-target recognition of short bacterial adhesins. This negative interference has important biological consequences, such as affecting the ability of bacteria to form biofilms.
All members of the Enterobacteriaceae are able to elaborate a layer of surface-associated polysaccharides called the capsule. The composition of these capsular polysaccharides is very much strain dependent. In Escherichia coli it may be one of the 80 distinct polysaccharides (designated the K antigens) or a polymer derived from the 170 different O antigens. In effect, whereas all polysaccharide K antigens form a capsule structure, the inverse is not always true: not all capsules are composed of K antigens (53).
Capsule polysaccharides provide protection against desiccation and attack from phages, however, they are first and foremost recognized virulence determinants. It has been known for years that some capsular antigens, notably K1 and certain types of O antigen, are strongly associated with extraintestinal infections such as septicemia, meningitis, and urinary tract infection (UTI) (19, 25, 28, 32, 46). E. coli strains of serotype K1 represent 80% of all E. coli strains isolated from cases of newborn meningitis and sepsis in humans. Also, K1 strains are often implicated in UTI. The K1 capsule is a linear homopolymer of sialic acid residues. The capsule is identical to the polysialic acid found on certain human cells and is poorly immunogenic due to molecular mimicry of host structures (49). Strains causing UTI, meningitis, and sepsis are generally resistant to the bactericidal action of human serum (reviewed in references 34, 53). Furthermore, K1 capsules are antiphagocytic, arguably a trait that would be helpful to a bacterium for survival in blood and tissue.
Another group of virulence factors is constituted by the bacterial adhesins, protein structures that recognize a wide range of molecular motifs and provide targeting of the bacteria to specific tissue surfaces in the host (for a recent review, see reference 24). While most adhesins recognize nonself targets present in the environment (e.g., a specific saccharide), some mediate self-recognition.
Antigen 43 (Ag43) is exceptional in being such a self-recognizing adhesin (15, 21). Ag43 is a surface protein that confers bacterial cell-to-cell aggregation, a trait that can be visualized macroscopically as flocculation and settling of cells from static liquid suspensions; hence, the gene name flu was originally coined for the corresponding genetic locus (12). In an independent study, a major E. coli outer membrane protein antigen was investigated by virtue of its aggregative properties and termed Ag43 (29). Only later was Ag43 identified as the product of the flu gene (15, 18). In line with the gene product name Ag43, the term agn43 is also frequently used for the corresponding gene instead of flu. Ag43 expression confers a characteristic frizzy colony morphology (16). Expression is phase variable with switching rates of ∼10−3 per cell per generation under normal growth conditions due to the concerted action of the Dam methylase (positive regulation) and the OxyR redox sensor (negative regulation) (18, 38, 41, 52). Ag43 is an autotransporter protein, and all information required for traverse of the bacterial membrane system and final routing to the surface resides in the protein itself. This is also supported by the fact that Ag43 can be expressed in a wide range of gram-negative bacteria (20, 21). In E. coli, Ag43 is present in ∼50,000 copies per cell (30). It is processed into two subunits, α and β, each constituting about half of the protein. The β subunit is an outer membrane component that presumably forms a pore through which Ag43α gains access to the surface. The α subunit remains attached to the cell surface via interaction with the β subunit but can be detached by brief heat treatment (18).
Expression of Ag43 dramatically enhances biofilm formation in bacteria (9, 21, 22, 23), and Ag43 expression in E. coli is specifically correlated with the biofilm mode of growth (39). Conversely, lesions in the flu gene causing abolishment of Ag43 expression in many cases result in cells with a very limited ability to form a biofilm (9, 22, 39). Biofilm formation is often correlated with bacterial virulence (8, 37). Expression of Ag43 was recently reported to be correlated with biofilm formation by uropathogenic E. coli during infection of bladder cells (1). Furthermore, Ag43-mediated cell aggregation was found to protect bacteria against killing agents like hydrogen peroxide (38). Ag43 is widespread in E. coli, and it is expressed in many pathogenic strains. A survey of enteropathogenic and UTI strains showed that 77 and 60%, respectively, of these were capable of Ag43 expression (31). Also, many strains possess duplex or multiple copies of the gene, as seems to be the case with enteropathogenic and enterohemorrhagic subtypes (23, 35, 48). Ag43 exhibits significant homology to several other members of the autotransporter protein family; for example, the primary structure of the AIDA-I adhesin, involved in diffuse adherence of enteropathogenic E. coli strains, shows ∼31% identity to Ag43 (5).
Both Ag43 and capsules seem to play important roles in bacterial survival and pathogenicity. However, while Ag43 protrudes ∼10 nm beyond the outer membrane (23), the capsule may extend 0.2 to 1.0 μm from the bacterial surface, depending on its type and composition. On one hand, the capsule constitutes a somewhat ephemeral structure that might not interfere with the close cellular contact required for intercellular Ag43 contact. On the other hand, it could be that the presence of a capsule would sterically shield Ag43 and abolish Ag43-Ag43-mediated cell aggregation. To resolve this apparent paradox, we investigated possible interference between Ag43 and capsule.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The strains and plasmids used in this study are listed in Table 1. Cells were grown at 37°C on solid or in liquid Luria-Bertani (LB) medium supplemented with the appropriate antibiotics unless otherwise stated.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| E. coli strains | ||
| 1177 | UTI isolate (O1:K1:H7) | 7 |
| CN1016 | 1177 fim::kan | 7 |
| MG1655 | K-12 reference strain | 2 |
| MS427 | MG1655 flu | 33 |
| MS428 | MG1655 fim | 22 |
| MS528 | MG1655 flu fim | 22 |
| MS641 | MG1655 oxyR::kan | This study |
| DD14 | MS427 containing pKKJ128 and pBR322 | This study |
| DD19 | MS641 containing pBR322 | This study |
| DD26 | MS641 containing pKT274 | This study |
| DD27 | MS641 containing pIR100 | This study |
| DD34 | MS427 containing pACYC184 and pKT274 | This study |
| DD35 | MS427 containing pACYC184 and pIR100 | This study |
| DD36 | MS427 containing pACYC184 and pBR322 | This study |
| DD37 | MS427 containing pKKJ128 and pKT274 | This study |
| DD38 | MS427 containing pKKJ128 and pIR100 | This study |
| MS718 | MS428 containing pFCH2235 | This study |
| PK1081 | CN1016 containing pFCH2235 | This study |
| K. pneumoniae strains | ||
| C105 | K35 capsule | 45 |
| C105 NCV | Spontaneous C105 NCV | 45 |
| LH71 | C105 containing pIB264 | This study |
| LH72 | C105 NCV containing pIB264 | This study |
| PK1013 | C105 containing pHHA147 | This study |
| PK1014 | C105 NCV containing pHHA147 | This study |
| PK1016 | C105 containing pBR322 | This study |
| PK1021 | C105 NCV containing pBR322 | This study |
| Plasmids | ||
| pFHC2235 | IPTG-inducible dam gene in pUC19a | Gift from F. G. Hansen |
| pHHA147 | flu gene from MG1655 in pBR322 | 38 |
| pIR100 | E. coli K5 capsule gene operon in pBR322(cos4) | Gift from I. Roberts |
| pKKJ128 | flu gene from MG1655 in pACYC184 | 21 |
| pKT274 | E. coli K1 capsule gene operon in pHC79 | 13 |
| pKKJ101 | flu gene in pUCP22 | 21 |
| pIB264 | AIDA-I autotransporter and glycosylation genes in pBR322 | 4 |
IPTG, isopropyl-β-d-thiogalactopyranoside.
Construction of an oxyR::kan mutant.
An E. coli MG1655 oxyR::kan mutant was constructed by using the λ Red recombinase gene replacement system (10). Briefly, the kanamycin gene from plasmid pKD4 was amplified by primers containing 40-nucleotide oxyR homology extensions (288, 5′-GGCTGAACACCGCCATTTTCGGCGTGCGGCAGATTCCTGCGTGTAGGCTGGAGCTGCTTC, and 289, 5′-CGCGGATGGCCTCTGCCAGCTGCTCATAGCGGCTGCGCAGCATATGAATATCCTCCTTAG). This product was digested with DpnI and transformed into MG1655(pKD46), and kanamycin-resistant colonies were selected. The correct double-crossover and recombination event was confirmed by PCR and Southern blotting. The λ Red helper plasmid pKD46 was cured by growth at 37°C, and the subsequent strain was designated MS641.
DNA manipulations.
Isolation of plasmid DNA was carried out with the QIAprep Spin miniprep kit (Qiagen). Restriction endonucleases were used according to the manufacturer's specifications (Biolabs). PCRs were made as previously described (44). Amplified products were sequenced to ensure fidelity of the PCR by using the ABI PRISM BigDye Terminator cycle sequencing ready reaction kit (PE Applied Biosystems). Samples were run on an ABI PRISM 310 genetic analyzer (PE Applied Biosystems) as described in the manufacturer's specifications.
Immunofluorescence microscopy.
Surface presentation of Ag43 was assessed by immunofluorescence microscopy employing a polyclonal rabbit antiserum raised against the α subunit of Ag43. A fluorescein isothiocyanate-labeled anti-rabbit serum was used as a secondary antibody (Sigma). Cell fixation, immunolabeling, and microscopy were carried out as previously described (16).
Colony morphology.
Colony morphology was assayed by employing a Carl Zeiss Axioplan epifluorescence microscope, and digital images were captured with a 12-bit cooled slow-scan charge-coupled device camera (KAF 1400 chip; Photometrics, Tucson, Ariz.) controlled by the PMIS software (Photometrics).
Biofilm assay.
Biofilm formation on plastic surfaces was monitored in 96-well polystyrene microtiter plates. Cells were grown statically for 24 h in LB medium at 37°C, washed to remove unbound cells, and stained with crystal violet as previously reported (40). Quantification of bound cells was performed by addition of acetone-ethanol (20:80) and measurement of the dissolved crystal violet at an optical density of 600 nm.
Cell adhesion assay.
Adherence of bacteria to mammalian HT29 colon cancer cells was performed as follows. A monolayer of cells was grown to 95% confluence in standard tissue culture medium. Exponentially growing bacterial cells were harvested, washed, and resuspended in phosphate-buffered saline (containing 0.5% methyl-α-d-mannopyranoside to rule out any contribution from type 1 fimbriae) at a concentration of 108 cells per ml. Bacteria were incubated together with the HT29 cells for 2 h at 37°C, and afterwards, nonadhered bacteria were removed by extensive washing with phosphate-buffered saline. The cells were then fixed with 70% ice-cold methanol for 15 min and examined by phase-contrast microscopy.
RESULTS
Ag43 function is inhibited in a wild-type encapsulated uropathogenic E. coli strain.
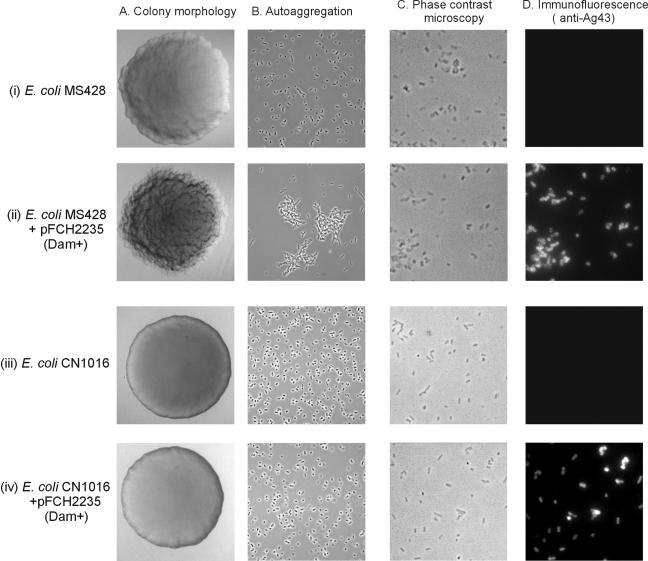
Expression of Ag43 is positively regulated by Dam methyltransferase. We have previously found that the presence of type 1 fimbriae sterically blocks intercellular Ag43-Ag43 interaction (15) and also affects its expression (41, 42). Accordingly, when the E. coli K-12 strain MS428, a fim derivative of the K-12 reference strain MG1655, was transformed with the high-copy-number plasmid pFHC2235 (encoding the dam methyltransferase gene) we expected it to produce high levels of Ag43 by virtue of the enhanced level of the Dam protein. In line with this tenet, the MS428(pFHC2235) transformant produced colonies with a frizzy morphology and aggregating cells that settled from static liquid solutions, i.e., traits that were indicative of high levels of Ag43 production (Fig. 1).
FIG. 1.
Colony morphology (A), cell-cell autoaggregation (B), phase-contrast microscopy (C), and immunofluorescence microscopy with anti-Ag43 serum (D) of (i) E. coli MS428 (MG1655 fim), (ii) E. coli MS428 harboring pFCH2235 (induced with isopropyl-β-d-thiogalactopyranoside [IPTG] for Dam overexpression), (iii) E. coli CN1016 (1177 fim), and (iv) E. coli CN1016 harboring pFCH2235 (induced with IPTG for Dam overexpression). The frizzy colony morphology and cell-cell aggregation imparted by Dam-induced expression of Ag43 are not observed in the K1 capsulated UTI fim mutant strain CN1016.
The uropathogenic E. coli strain 1177 of serotype O1:K1:H7 was previously studied, and a derivative, CN1016, deficient in expression of type 1 fimbriae was constructed (7). Strain 1177 has at least one copy of the flu gene on the chromosome (our unpublished data). When the pFHC2235 plasmid was introduced into these strains, we expected it to induce production of high levels of Ag43 in line with the K-12 control. However, the frizzy colony morphology, aggregation, and cell settling profiles we expected (at least in the case of the Fim-negative CN1016[pFHC2235] strain) were not observed (Fig. 1). We therefore speculated whether a surface feature other than type 1 fimbriae could interfere with the expression or function of Ag43. Although this strain is also able to produce P-fimbriae, overexpression of the Dam protein prevents transition from the phase on to the phase off state (6), and we observed that only a small proportion (less than 5%) of the colonies produced these organelles. A good candidate therefore seemed to be the K1 capsule of 1177 and derivatives.
Ag43 function is blocked upon capsule expression in E. coli K-12.
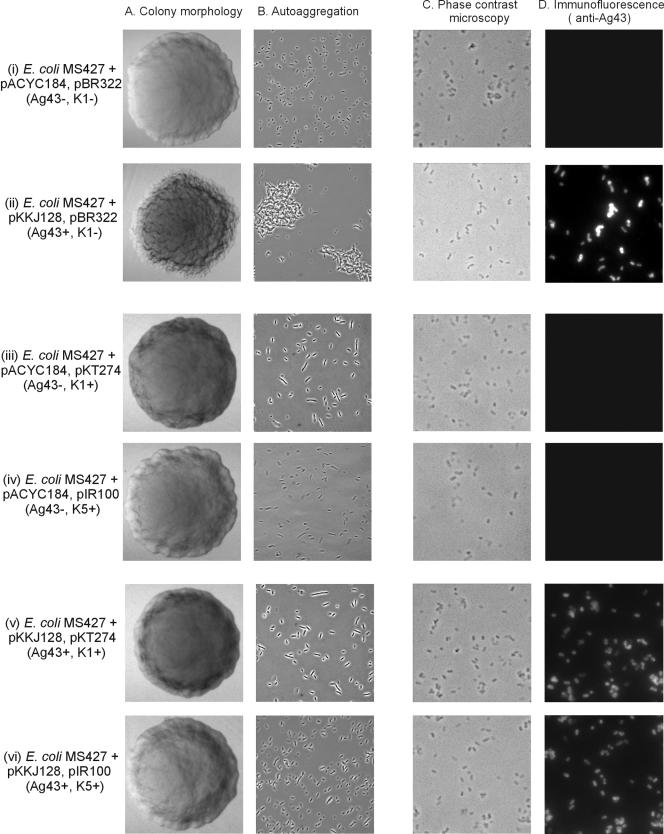
Unlike many wild-type strains, E. coli K-12 strains are normally incapable of producing extended surface-associated polysaccharides and produce rough colonies; the polysaccharide has a complete core but no O antigen due to the insertion of an IS5 element in the rfb gene cluster controlling O antigen biosynthesis (27). This makes K-12 a perfect test bed for monitoring potential interference of capsular polysaccharides with Ag43. When plasmids carrying the gene cluster required for either K1 or K5 capsule production were introduced in strain MG1655, colonies with a characteristic smooth appearance resulted (Fig. 1). It should be noted that we also used a version of MG1655 (i.e., strain MS427) where the flu gene was deleted from the chromosome (33). Such cells do not aggregate or settle from liquid suspension. Meanwhile, expression of Ag43 in MS427 resulted in cells which formed frizzy colonies, aggregated, and settled from static liquid suspensions (Fig. 2). When cells were forced to concomitantly express both surface factors, i.e., capsule and Ag43, the capsule phenotype was dominant and the cells were unable to aggregate. This could either be due to sterical shielding of Ag43 by the capsule or capsule production somehow interfering with synthesis or transport of Ag43 to the cell surface. To differentiate between these possibilities, immunofluorescence microscopy was employed (Fig. 2). This demonstrated that the cells did express Ag43 on the surface and that the signal strengths in capsulated and noncapsulated pairs of strains were similar, indicative of similar levels of Ag43 on the surface (Fig. 2). Western blotting of whole-cell lysates also indicated that similar amounts of Ag43 were present irrespective of encapsulation (data not shown). When settling assays were performed with these cells, they were incapable of aggregation (data not shown). Taken together, the results suggest that a capsule blocks the close cell contact needed for intercellular Ag43-Ag43 interaction.
FIG. 2.
Colony morphology (A), cell-cell autoaggregation (B), phase-contrast microscopy (C), and immunofluorescence microscopy with anti-Ag43 serum (D) of E. coli MS427 (MG1655 Δflu) harboring (i) pACYC184 and pBR322 (control), (ii) pKKJ128 and pBR322 (Ag43+), (iii) pACYC184 and pKT274 (K1+), (iv) pACYC184 and pIR100 (K5+), (v) pKKJ128 and pKT274 (Ag43+ K1+), or (vi) pKKJ128 and pIR100 (Ag43+ K5+). The frizzy colony morphology and cell-cell aggregation imparted by Ag43 are prevented by the concomitant surface expression of either a K1 or K5 capsule.
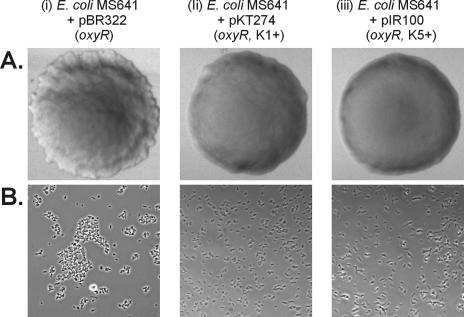
We also performed the same experiments with an MG1655 oxyR::kan host. In this host, transcription of the flu gene is driven from its natural promoter and the absence of OxyR causes constitutive Ag43 expression. The results indicated that Ag43 was produced but that its function was blocked by the capsule (Fig. 3). Arguably, unless OxyR is involved, capsule production does not seem to influence transcription of the flu gene and transport of Ag43 to the cell surface. The data support the notion that a capsule sterically shields Ag43-Ag43 interaction.
FIG. 3.
Colony morphology (A) and phase-contrast microscopy (B) of E. coli MS641 (MG1655 oxyR::kan) harboring (i) pBR322 (control), (ii) pKT274 (K1+), or (iii) pIR100 (K5+). Derepression of Ag43 expression in the absence of OxyR results in a frizzy colony morphology and clumping phenotype that is prevented by the concomitant surface expression of a capsule.
Ag43 function can be restored by capsule loss in Klebsiella pneumonia.
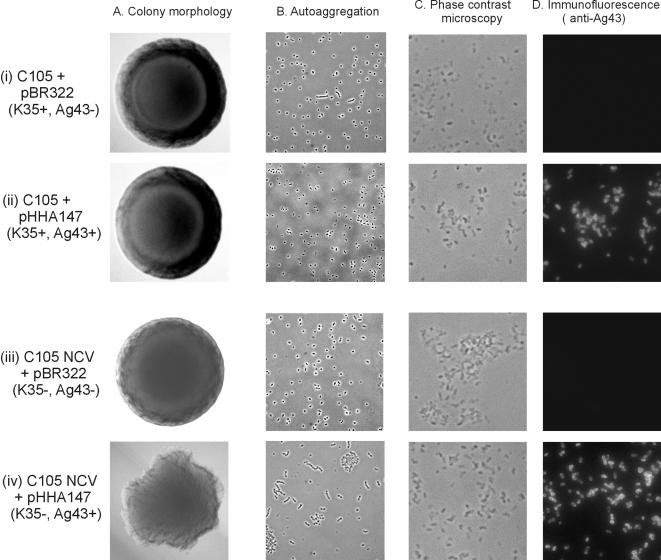
The vast majority of K. pneumoniae strains express a large polysaccharide capsule. However, noncapsulated variants (NCVs) arise spontaneously. An isogenic pair of K. pneumoniae strain C105 clones, differing only in their ability to express K35 capsule antigen, was recently characterized (45). When this C105/C105NCV pair was transformed with plasmid pHHA147, harboring a constitutively expressed flu gene, a characteristic frizzy colony morphology resulted in the case of strain C105NCV(pHHA147), whereas little or no difference in colony morphology was seen in strain C105(pHHA147) (Fig. 4). Microscopic observation of C105NCV(pHHA147) revealed the presence of cell aggregates, whereas no aggregates were observed with C105(pHHA147) cells (Fig. 4). Additionally, we introduced Ag43-expressing plasmids into a number of other gram-negative bacteria from the institute strain collection with a known ability to produce capsules, viz., Enterobacter cloacae, Serratia liquefaciens, Burkholderia cepacia, and Pseudomonas aeruginosa. Although Ag43 was expressed on the cell surface, none of the transformed strains were capable of aggregating or settling from standing overnight cultures (data not shown). These observations further bolster the notion of negative interference between capsule and Ag43; they also take it from being a phenomenon restricted to E. coli to being a general phenomenon valid in a range of gram-negative bacteria.
FIG. 4.
Colony morphology (A), cell-cell autoaggregation (B), phase-contrast microscopy (C), and immunofluorescence microscopy with anti-Ag43 serum (D) of the capsule-producing K. pneumoniae strain C105 containing either (i) pBR322 (control) or (ii) pHHA147 (Ag43+) and the capsule-negative strain C105 NCV containing either (iii) pBR322 (control) or (iv) pHHA147 (Ag43+). The frizzy colony morphology and cell-cell aggregation imparted by Ag43 is prevented by the concomitant surface expression of the K. pneumoniae K35 capsule.
Biofilm aspects.
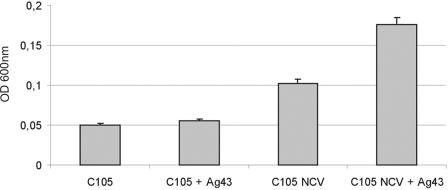
We and others have demonstrated that Ag43 expression confers excellent biofilm forming properties upon a variety of bacteria (9, 21, 22, 23, 38, 39). Since we have demonstrated here that the presence of a capsule virtually abolishes Ag43 functionality, we surmised that encapsulated cells expressing both capsule and Ag43 would be impaired in biofilm formation compared to noncapsulated cells. To test this hypothesis, a set of strains differing in their ability to produce capsule and Ag43 were investigated for their ability to form biofilms on an abiotic surface (in this case polystyrene microtiter plates). Our results revealed that in capsulated cells expression of Ag43 did not improve biofilm formation, whereas in a noncapsulated strain a significant improvement was observed when Ag43 was expressed (Fig. 5). It should also be noted that when the capsulated and noncapsulated pair of strains were compared, the noncapsulated variant appeared to be a slightly better biofilm former. This is probably due to capsule shielding of other (non-Ag43) factors that improve biofilm formation.
FIG. 5.
Biofilm formation by the capsule-producing K. pneumoniae strain C105 containing either (i) pBR322 (control) or (ii) pHHA147 (Ag43+) and the capsule-negative strain C105 NCV containing either (iii) pBR322 (control) or (iv) pHHA147 (Ag43+). Surface expression of Ag43 promoted an enhanced biofilm formation phenotype only in the absence of any capsular material. Strains were grown in LB media on polystyrene microtiter plates. Adhered cells were stained with 0.1% crystal violet, and the absorbance was measured at 600 nm. Shown are the averages of readings from 3 experiments (± standard deviation).
The action of other autotransporter adhesins similar to Ag43 is abolished by capsule shielding.
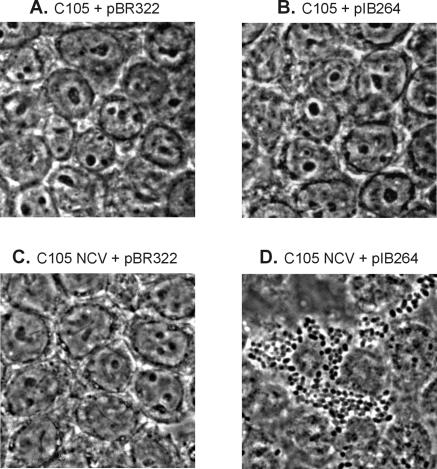
The AIDA-I autotransporter is a potent adhesin involved in the adherence of enteropathogenic E. coli strains to various mammalian cells (4, 5). The AIDA-I adhesin and Ag43 are predicted to have the same overall tertiary structure. We speculated that, since capsulation obstructs the activity of Ag43, it might also interfere with the activity of similar-sized proteins such as AIDA-I. To test this, we introduced a plasmid, pIB264, encoding the AIDA-I locus (4) into a pair of host cells differing in their ability to produce a capsule, viz., C105 and C105 NCV. The ability of these strains to adhere to human intestinal cell line cells (HT29 cells) was assayed. Bacteria producing both AIDA-I and capsule were unable to adhere, whereas bacteria producing AIDA-I, but not capsule, bound in large numbers (Fig. 6).
FIG. 6.
Adherence of the capsule-producing K. pneumoniae strain C105 containing either pBR322 (control) (A) or pIB264 (AIDA-I) (B) and the capsule-negative strain C105 NCV containing either pBR322 (control) (C) or pIB264 (AIDA-I) (D) to HT29 colon cancer cells. Adherence mediated by the AIDA-I autotransporter protein was blocked by the concomitant expression of a surface capsule layer.
DISCUSSION
Bacteria express a number of surface structures that enable them to interact with the environment, e.g., flagella for swimming and adhesins for attachment. These surface structures have a highly diverse size spectrum, and it must be implicit that sometimes they must interfere physically with each other in such a way that the function of some will be obstructed by the presence of other (more extended) structures. As an example, it was previously demonstrated that fimbriae, which extend ∼1 μm from the bacterial surface, physically block the action of the much shorter Ag43 in bacteria (15). Based on this observation, we predict that fimbriae will also block the action of other adhesins similar in size to Ag43. Such surface structure interference must have important consequences for the interplay of bacteria with the environment. Here, we have studied the interference between nonfimbrial adhesins and capsule.
Several lines of evidence indicated that Ag43 function was sterically blocked by extended polysaccharides. In a uropathogenic E. coli strain of serotype K1, Ag43 could be expressed but it was inactive. In E. coli K-12 strains expressing Ag43, aggregation was abolished when these strains were made to express either K1 or K5 capsules. Furthermore, in a well-characterized strain set of K. pneumoniae, differing only in capsulation, Ag43 activity was only observed in the NCV. In line with this, Ag43 activity was blocked in a range of bacterial strains, arguably due to capsule interference. All phenotypic traits associated with Ag43 were affected: cell aggregation, settling from static liquid suspension, biofilm formation, and in most cases, colony morphology. The interference between capsule and Ag43 was physical in nature rather than by genetic cross talk. Negative interference between capsule and Ag43 seemed to be a general phenomenon in a range of gram-negative bacteria and was not isolated to E. coli. Bacterial capsules might provide various degrees of blockage depending on their thickness and quantity and perhaps also on their charge.
An important aspect of this study was the finding that capsulation not only interfered with the function of Ag43 but also shielded and blocked the action of another autotransporter adhesin, namely AIDA-I. Both adhesins have been predicted to protrude ∼10 nm from the bacterial surface. The implications of these findings are far-ranging because it means that an entire class of short nonorganelle bacterial adhesins and invasins are nonfunctional in the presence of a capsule. This class encompasses not only the autotransporter adhesins and invasins such as TibA, AIDA-I, and Ag43 from enterics but also autotransporters from a wide spectrum of bacteria, including pertactin P.69 and TcfA from Bordetella, Hsf from Haemophilus influenzae, and UspA1 from Moraxella catarrhalis (for a review, see reference 17). Additionally, there are numerous other nonfimbrial adhesins and invasins exemplified by Afa-I and intimin (reviewed in references 24 and 43), which would be candidates for capsule shielding. In line with our data, several other studies have actually reported that capsulated bacteria adhere poorly to epithelial cells and exhibit severe reductions in their abilities to invade compared to their capsulated counterparts (14, 36). In Neisseria meningitidis, the adhesive Opc protein was reported to be active only in acapsulated strains (51). A study of E. coli P-fimbrial adhesins expressed in a nonorganelle context revealed their activity could be blocked by extensive O antigens (50). Finally, the synthesis of lipopolysaccharide by enterohemorrhagic E. coli O157:H7 was shown to interfere with adherence to HeLa cells in vitro (47).
The capsule shielding concept leaves the bacteria with an obvious dilemma. They cannot adhere or invade without the assistance of adhesin proteins, but at the same time, the capsule provides protection against many of the countermeasures at the disposal of a mammalian host such as phagocytosis or complement, etc. Arguably, bacteria that use short adhesins and invasins would have to coordinate their expression with that of capsules. An alternative solution would be to express adhesins that can penetrate and reach beyond the capsule, viz., fimbriae.
At present, it is hard to say whether bacteria actually coordinate production of capsules and nonorganelle adhesins, and to our best knowledge, no reports to this end are available from the literature. The regulation of capsule gene expression is complex, with overlapping regulatory circuits. Capsule expression has in many cases been reported to be phase variable, for example, in Bacteroides fragilis (26), N. meningitidis (11), and Campylobacter (3). In K. pneumoniae, some types of capsule production were reported to be influenced by environmental conditions (14). Arguably, differential expression of capsules will intermittently result in a noncapsulated state where nonorganelle adhesins like Ag43 and AIDA-I would be exposed.
Ag43 promotes bacterial biofilm formation and aggregation, both of which are traits closely associated with bacterial virulence (1, 8). Capsulation protects bacteria that live in close association with a mammalian host against several defense mechanisms. The future challenge is to decipher how the bacteria orchestrate the expression of these two important types of surface structures to make use of their full survival and virulence capabilities.
Acknowledgments
We thank Birthe Jul Jorgensen and Louise Hjerrild for technical assistance, Ian Roberts, University of Manchester, for providing plasmids pKT274 and pIR100, Alexander Schmidt, Westfälische Wilhelms-Universität, for providing plasmid pIB264, and Karen Krogfelt, Statens Serum Institut Denmark, for providing Klebsiella strains C105 and C105 NCV.
This work was supported by the Danish Natural Sciences (grants 21-01-0296 and 51-00-0291) and the Danish Technical (26-02-0183) Research Councils.
REFERENCES
- 1.Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. [DOI] [PubMed] [Google Scholar]
- 2.Bachmann, B. 1996. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12, p. 2460-2488. In F. C. Neidhardt et al. (ed.), Escherichia coli and Salmonella: cellular and molecular biology, 2nd ed. ASM Press, Washington, D.C.
- 3.Bacon, D. J., C. M. Szymanski, D. H. Burr, R. P. Silver, R. A. Alm, and P. Guerry. 2001. A phase variable capsule is involved in virulence of Campylobacter jejuni 81-176. Mol. Microbiol. 40:769-777. [DOI] [PubMed] [Google Scholar]
- 4.Benz, I., and M. A. Schmidt. 1989. Cloning and expression of an adhesin (AIDA-I) involved in diffuse adherence of enteropathogenic Escherichia coli. Infect. Immun. 57:1506-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benz, I., and M. A. Schmidt. 1992. AIDA-I, the adhesin involved in diffuse adherence of the diarrhoeagenic Escherichia coli strain 2787 (O126:H27), is synthesized via a precursor molecule. Mol. Microbiol. 6:1539-1546. [DOI] [PubMed] [Google Scholar]
- 6.Blyn, L. B., B. A. Braaten, and D. A. Low. 1990. Regulation of pap pilin phase variation by a mechanism involving differential dam methylation states. EMBO J. 9:4045-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connell, H., W. Agace, P. Klemm, M. Schembri, S. Mårild, and C. Svanborg. 1996. Type 1 fimbrial expression enhances Escherichia coli virulence for the urinary tract. Proc. Natl. Acad. Sci. USA 93:9827-9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costerson, J. W., P. S. Stewart, and E. P. Greenberg. 1999. Bacterial biofilms: a common cause of persistent infections. Science 284:1318-1322. [DOI] [PubMed] [Google Scholar]
- 9.Danese, P. N., I. A. Pratt, S. Dove, and R. Kolter. 2000. The outer-membrane protein, Ag43, mediates cell-to-cell interactions within E. coli biofilms. Mol. Microbiol. 37:424-432. [DOI] [PubMed] [Google Scholar]
- 10.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 97:6640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Vries, F. P., A. van der Ende, J. P. van Putten, and J. Dankert. 1996. Invasion of primary nasopharyngeal epithelial cells by Neisseria meningitidis is controlled by phase variation of multiple surface antigens. Infect. Immun. 64:2998-3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Diderichsen, B. 1980. flu, a metastable gene controlling surface properties of Escherichia coli. J. Bacteriol. 141:858-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Echarti, C., B. Hirschel, G. J. Boulnois, J. M. Varley, F. Waldvogel, and K. N. Timmis. 1983. Cloning and analysis of the K1 capsule biosynthesis genes of Escherichia coli: lack of homology with Neisseria meningitidis group B DNA sequences. Infect. Immun. 41:54-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Favre-Bonte, S., T. R. Licht, C. Forestier, and K. A. Krogfelt. 1999. Klebsiella pneumoniae capsule expression is necessary for colonization of large intestines of streptomycin-treated mice. Infect. Immun. 67:6152-6156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hasman, H., T. Chakraborty, and P. Klemm. 1999. Antigen-43-mediated autoaggregation of Escherichia coli is blocked by fimbriation. J. Bacteriol. 181:4834-4841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hasman, H., M. A. Schembri, and P. Klemm. 2000. Antigen 43 and type 1 fimbriae determine colony morphology of Escherichia coli K-12. J. Bacteriol. 182:1089-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Henderson, I. R., and J. P. Nataro. 2001. Virulence functions of autotransporter proteins. Infect. Immun. 69:1231-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henderson, I. R., and P. Owen. 1999. The major phase-variable outer membrane protein of Escherichia coli structurally resembles the immunoglobulin A1 protease class of exported protein and is regulated by a novel mechanism involving Dam and OxyR. J. Bacteriol. 181:2132-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jann, B., and K. Jann. 1997. Capsules of Escherichia coli, p. 113-143. In M. Sussman (ed.), Mechanisms of virulence. Cambridge University Press, Cambridge, England.
- 20.Kjaergaard, K., H. Hasman, M. A. Schembri, and P. Klemm. 2002. Antigen 43-mediated autotransporter display, a versatile bacterial cell surface presentation system. J. Bacteriol. 184:4197-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kjaergaard, K., M. A. Schembri, H. Hasman, and P. Klemm. 2000. Antigen 43 from Escherichia coli induces inter- and intraspecies cell aggregation and changes in colony morphology of Pseudomonas fluorescens. J. Bacteriol. 182:4789-4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kjaergaard, K., M. A. Schembri, C. Ramos, S. Molin, and P. Klemm. 2000. Antigen 43 facilitates formation of multispecies biofilms. Environ. Microbiol. 2:695-702. [DOI] [PubMed] [Google Scholar]
- 23.Klemm, P., L. Hjerrild, M. Gjermansen, and M. A. Schembri. 2004. Structure-function analysis of the self-recognizing antigen 43 autotransporter protein from Escherichia coli. Mol. Microbiol. 51:283-296. [DOI] [PubMed]
- 24.Klemm, P., and M. A. Schembri. 2000. Bacterial adhesins: function and structure. Int. J. Med. Microbiol. 290:27-35. [DOI] [PubMed] [Google Scholar]
- 25.Korhonen, T. K., M. V. Valtonen, J. Parkkinen, V. Väisanen-Rhen, J. Finne, F. Ørskov, I. Ørskov, S. B. Svenson, and P. H. Mäkelä. 1985. Serotypes, hemolysin production, and receptor recognition of Escherichia coli strains associated with neonatal sepsis and meningitis. Infect. Immun. 48:486-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krinos, C. M., M. J. Coyne, K. G. Weinacht, A. O. Tzianabos, D. L. Kasper, and L. E. Comstock. 2001. Extensive surface diversity of a commensal microorganism by multiple DNA inversions. Nature 414:555-558. [DOI] [PubMed] [Google Scholar]
- 27.Liu, D., and P. R. Reeves. 1994. Escherichia coli K12 regains its O antigen. Microbiology 140:49-57. [DOI] [PubMed] [Google Scholar]
- 28.Ott, M., L. Bender, G. Blum, M. Schmittroth, M. Achtman, H. Tschärpe, and J. Hacker. 1991. Virulence patterns and long-range genetic mapping of extraintestinal Escherichia coli K1, K5, K100 isolates: use of pulse-field electrophoresis. Infect. Immun. 59:2664-2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Owen, P. 1983. Antigens of the Escherichia coli cell envelope, p. 347-373. In O. J. Bjerrum (ed.), Electroimmunochemical analysis of membrane proteins. Elsevier Science Publishing, Inc., Amsterdam, The Netherlands.
- 30.Owen, P. 1992. The gram-negative outer membrane: structure, biochemistry and vaccine potential. Biochem. Soc. Trans. 20:1-6. [DOI] [PubMed] [Google Scholar]
- 31.Owen, P., M. Meehan, H. de Loughry-Doherty, and I. Henderson. 1996. Phase-variable outer membrane proteins in Escherichia coli. FEMS Immunol. Med. Microbiol. 16:63-76. [DOI] [PubMed] [Google Scholar]
- 32.Podshun, R., D. Sievers, A. Fischer, and U. Ullmann. 1993. Serotypes, hemagglutinins, siderophore synthesis, and serum resistance of Klebsiella isolates causing human urinary tract infections. J. Infect. Dis. 168:1415-1421. [DOI] [PubMed] [Google Scholar]
- 33.Reisner, A., J. A. J. Haagensen, M. A. Schembri, E. L. Zechner, and S. Molin. 2003. Development and maturation of Escherichia coli K-12 biofilms. Mol. Microbiol. 48:933-946. [DOI] [PubMed] [Google Scholar]
- 34.Roberts, I. S. 1995. Bacterial polysaccharides in sickness and in health. Microbiology 141:2023-2031. [DOI] [PubMed] [Google Scholar]
- 35.Roche, A. J., J. P. McFadden, and P. Owen. 2001. Antigen 43, the major phase-variable protein of the Escherichia coli outer membrane, can exist as a family of proteins encoded by multiple alleles. Microbiology 147:161-169. [DOI] [PubMed] [Google Scholar]
- 36.Sahly, H., R. Podschun, T. Oelschlaeger, M. Greiwe, H. Parlois, D. L. Hasty, J. Kekow, U. Ullman, I. Ofek, and S. Sela. 2000. Capsule impedes adhesion to and invasion of epithelial cells by Klebsiella pneumoniae. Infect. Immun. 68:6744-6749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schembri, M. A., M. Givskov, and P. Klemm. 2002. An attractive surface: Gram negative bacterial biofilms. Sci. STKE 132:RE6. [DOI] [PubMed] [Google Scholar]
- 38.Schembri, M. A., L. Hjerrild, M. Gjermansen, and P. Klemm. 2003. Differential expression of the Escherichia coli autoaggregation factor antigen 43. J. Bacteriol. 185:2236-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schembri, M. A., K. Kjærgaard, and P. Klemm. 2003. Global gene expression in Escherichia coli biofilms. Mol. Microbiol. 48:253-257. [DOI] [PubMed] [Google Scholar]
- 40.Schembri, M. A., and P. Klemm. 2001. Biofilm formation in a hydrodynamic environment by novel FimH variants and ramifications for virulence. Infect. Immun. 69:1322-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schembri, M. A., and P. Klemm. 2001. Coordinate gene regulation by fimbriae-induced signal transduction. EMBO J. 20:3074-3081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schembri, M. A., D. W. Ussery, C. Workman, H. Hasman, and P. Klemm. 2002. DNA microarray analysis of fim mutations in Escherichia coli. Mol. Gen. Genet. 267:721-729. [DOI] [PubMed] [Google Scholar]
- 43.Schmidt, M. A. 1994. Nonfimbrial adhesins of Escherichia coli, p. 85-96. In P. Klemm (ed.), Fimbriae adhesin, genetics, biogenesis and vaccines. CRC Press, Boca Raton, Fla.
- 44.Stentebjerg-Olesen, B., L. Pallesen, L. B. Jensen, G. Christiansen, and P. Klemm. 1997. Authentic display of a cholera toxin epitope by chimeric type 1 fimbriae: effects of insert position and host background. Microbiology 143:2027-2038. [DOI] [PubMed] [Google Scholar]
- 45.Struve, C., and K. A. Krogfelt. 2003. Role of capsule in Klebsiella pneumoniae virulence: lack of correlation between in vitro and in vivo studies. FEMS Microbiol. Lett. 218:149-154. [DOI] [PubMed] [Google Scholar]
- 46.Tarkanen, A. M., B. L. Allen, P. H. Williams, M. Kauppi, K. Haahtela, A. Siitonen, I. Orskov, F. Orskov, S. Clegg, and T. K. Korhonen. 1992. Fimbriation, capsulation, and iron-scavenging systems of Klebsiella strains associated with human urinary tract infection. Infect. Immun. 60:1187-1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Torres, A. G., and J. B. Kaper. 2003. Multiple elements controlling adherence of enterohemorrhagic Escherichia coli O157:H7 to HeLa cells. Infect. Immun. 71:4985-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Torres, A. G., N. T. Perna, V. Burland, A. Ruknudin, F. R. Blattner, and J. B. Kaper. 2002. Characterization of Cah, a calcium-binding and heatextractable autotransporter of enterohemorrhagic Escherichia coli. Mol. Microbiol. 45:951-966. [DOI] [PubMed] [Google Scholar]
- 49.Troy, F. A. 1992. Polysialylation: from bacteria to brains. Glycobiology 2:5-23. [DOI] [PubMed] [Google Scholar]
- 50.van Die, I., E. Zuidweg, W. Hoekstra, and H. Bergmans. 1986. The role of fimbriae of uropathogenic Escherichia coli as carrier of the adhesin involved in mannose-resistant hemagglutination. Microb. Pathog. 1:51-56. [DOI] [PubMed] [Google Scholar]
- 51.Virji, M., K. Makepeace, I. R. A. Peak, D. J. P. Ferguson, M. P. Jennings, and E. R. Moxon. 1995. Opc- and pilus-dependent interactions of meningococci with human endothelial cells: molecular mechanisms and modulation by surface polysaccharides. Mol. Microbiol. 18:741-754. [DOI] [PubMed] [Google Scholar]
- 52.Waldron, D. E., P. Owen, and C. J. Dorman. 2002. Competitive interaction of the OxyR DNA-binding protein and the Dam methylase at the antigen 43 gene regulatory region in Escherichia coli. Mol. Microbiol. 44:509-520. [DOI] [PubMed] [Google Scholar]
- 53.Whitfield, C., and I. S. Roberts. 1999. Structure, assembly and regulation of expression of capsules in Escherichia coli. Mol. Microbiol. 31:1307-1319. [DOI] [PubMed] [Google Scholar]