Abstract
Aims
To determine whether specific IgE and skin prick test correlate better in predicting reaction severity during a double-blinded placebo controlled food challenge (DBPCFC) for egg, milk, and multiple tree nut allergens.
Study design
Prospective study.
Place and Duration of Study
Department of Pediatrics, Stanford University School of Medicine, August 2009 and ongoing.
Methodology
We examined the reaction severity of twenty-four subjects to nine possible food allergens: milk, egg, almond, cashew, hazelnut, peanut, sesame, pecan and walnut. Specific IgE and SPT were performed before each DBPCFC. DBPCFC results were classified into mild (1), moderate (2), or severe (3) reactions using a modified Bock’s criteria.
Results
Twenty four subjects underwent a total of 80 DBPCFC. Eighty percent of all DBPCFCs resulted in a positive reaction. A majority, 71%, were classified as mild. No reactions occurred with a SPT of zero mm while three reactions occurred with a negative specific IgE. All reactions were reversible with medication.
Conclusion
These data suggest that SPT and specific IgE levels are not associated with reaction severity (p<0.64 and 0.27, respectively). We also found that combining specific IgE and SPT improved specificity but did not help to achieve clinically useful sensitivity. For instance, an SPT > 5mm had a sensitivity of 91% and specificity of 50%. Combining SPT > 5mm and IgE > 7 resulted in a reduced sensitivity of 64%. Unexpectedly, a history of anaphylaxis 70% (n=17) was not predictive of anaphylaxis on challenge 4% (n=2).
Keywords: Double-blinded placebo controlled food challenge, food allergy, desensitization, milk allergy, treenut allergy, peanut allergy, skin prick test, specific IgE
1. INTRODUCTION
More than 3 million Americans have a peanut or tree nut allergy (Sicherer, 2010). According to the Centers for Disease Control and Prevention, food allergy prevalence has increased by 18% and the prevalence of peanut allergy has tripled from 0.4% to 1.4% in American children younger than 18 years within the past two decades (Branum, 2009). Not all allergies are outgrown. Whereas 85% of children allergic to foods such as cow’s milk, egg, wheat and soy develop tolerance to their allergy, only 15% to 20% of children allergic to peanut, tree nuts, fish, and shellfish show eventual tolerance (Nowak-Wegrzyn, 2011). With food allergies on the rise, there is great concern for potential anaphylaxis from accidental ingestion particularly from incomplete or misleading labels on packaged foods.
Peanuts are one of the most researched food allergen, and some speculate that peanut allergy is higher in America because consumption is higher. However, per capita consumption of peanuts in China is similar to that of the United States but peanut allergy is rare (Hatahet et al., 1994). Unlike China, the peanuts in the United States are dry-roasted whereas peanuts in China are typically boiled. This leads to the speculation that higher temperatures required for dry-roasting potentially increases the allergenicity of peanut proteins more than the lower temperatures used for boiling (Hatahet et al., 1994; Sporik et al., 2000).
It is difficult for clinicians to predict the degree of reaction severity upon exposure to food allergens given the limitations of specific IgE and skin prick testing (SPT). SPT has a high negative predictive value but an overall positive predictive value (PPV) of only 50% (Sampson 1997; Skolnick, 2001). Not all studies concur with these values. Others have shown that a SPT > 8 mm or a specific IgE ≥ 15 kU/L has a PPV of 95%. Given the discordance between the accuracy of SPT and specific IgE levels, oral food challenges remain the gold standard for diagnosing food allergy, and a history of a severe reaction is the best predictor of reaction severity (Hourihane, 2005). A literature search shows weak associations between specific IgE, SPT and the severity of allergic reaction (Perry, 2004a; Hourihane, 2005; Flinterman, 2006).
To confirm this observation, a prospective study of subjects considered to have severe food allergies underwent food challenges and allergy testing. We determined whether combining specific IgE and SPT improved the prediction of reaction severity for DBPCFC.
2. MATERIALS AND METHODS
2.1 Study Design
We examined the reaction severity of twenty-four subjects to nine possible food allergens: milk, egg, almond, cashew, hazelnut, peanut, sesame, pecan and walnut. Subjects were given detailed written and verbal advice on nut avoidance of all nut types. Family members and subjects were trained in the use of emergency medication, with trainer pens and a written treatment plan. A physician was available 24 hours daily for questions. The study was approved by a Stanford ethics committee IRB 16882 and the Human Subjects Institutional Review Board at Stanford University.
The study population included subjects aged 3–55 years. Inclusion criteria included a history of allergic reaction within one to two hours after ingestion, and either a positive SPT or a specific IgE ≥ 7 kU/L to the allergen. Exclusion criteria included a history of severe anaphylaxis to nut requiring intubation or admission to an ICU, frequent allergic or non-allergic urticaria, and a history consistent with poorly controlled persistent asthma. A baseline visit, history, physical exam, serum basophil, SPT and specific IgE was collected. Participants underwent DBPCFC of up to six of the nine possible allergens plus placebo.
Subjects were randomized to the order they received their DBPCFCs. The primary investigator, subject, and families remained blinded with the exception of a clinical research registered dietitian who prepared all food challenge doses and food mixtures. Subjects were unblinded after completion of all DBPCFCs. All the allergen mixture underwent spectrometer analysis. During the DBPCFC the allergen was mixed with a food vehicle to the subjects’ liking to improve palatability. Because a recent study from Jones and Burks et al. demonstrated that the risk of an allergic reaction was associated with dosing on empty stomach and concurrent febrile illness (Buchanan, 2007), all subjects were required to eat before their DBPCFC. DBPCFC for each allergen were completed within the same day. If subjects participated in multiple DBPCFCs, subsequent visits were scheduled. A physician or registered nurse administered all challenges.
2.2 SPT
Within 6 months of the first DBPCFC, SPTs were performed on the back with a 1 mm point plastic lancet after application of a drop of allergen extract. Single-lancet technique was performed with allergen extracts from Greer Allergy Immunotherapy (Lenoir, NC, USA). Histamine (1 mg/mL) and a negative control solution were used for internal validation. Antihistamine medication was withheld for at least 72 hours prior to the test. Wheals were measured at 15 minutes. The wheal measurement was recorded by taking the average of the two perpendicular diameters (mm). Subjects also had specific IgE levels drawn within two years of the initial DBPCFC. A specific IgE level of 0.35 kU/L was considered negative.
2.3 DBPCFC
DBPCFC were performed in a clinical research setting equipped with vital sign monitors and resuscitation equipment. DBPCFC doses contained the allergen in flour form mixed in a food vehicle of apple sauce, pudding, ice cream, or yogurt. Patients were started at a low dose which increased incrementally every 30 minutes until a maximum dose of 2500 mg (cumulative 4,000 mg) was reached within the same day. Before every dose a small amount was placed on the lower vermillion to observe for reaction. If no objective signs developed, the full dose was given. Vital signs including blood pressure, pulse, respiratory rate, percutaneous oxygen saturation and a focused clinical exam were taken every 15 minutes. Subjects were continually monitored two hours after ingestion of the last dose.
If at anytime during the DBPCFC objective symptom arose, the test was terminated. The highest tolerated dose was recorded, and the DBPCFC was considered reactive. Objective symptoms are outlined in Table 1 (Nowak-Wegrzyn et al., 2009). Symptoms were treated as medically indicated. Epinephrine, albuterol nebulizer, cetirizine, benadryl, famotidine, prednisolone, and intubation kit were available. Reactions to DBPCFC were categorized as mild(1), moderate(2), or severe(3) per modified Bock’s criteria, Table 2 (Nowak-Wegrzyn et al., 2009). Subjects were considered nonreactive if they completed the DBPCFC without a reaction.
Table 1.
Objective Allergic Signs*
| System | Signs |
|---|---|
| Skin | Urticaria, angioedema |
| Upper respiratory | Rhinoconjunctivitis, sneezing bursts, periocular swelling, cough |
| Lower respiratory | Wheeze |
| Gastrointestinal | Emesis, diarrhea |
| Cardiovascular | Hypotension, mental status change, collapse |
(Nowak-Wegrzy, 2009)
Table 2.
Classification of reaction severity
| Severity | Reactions |
|---|---|
| Mild | Skin and/or oral symptoms |
| Moderate | Upper respiratory and/or gastrointestinal or any 3 systems above |
| Severe | Lower respiratory and/or cardiovascular symptoms or any 4 systems above |
2.4 Statistical Analyses
Statistical analyses were conducted using SAS software (SAS Institute Inc., Cary, NC, USA). Spearman coefficients were used to assess the correlation between reaction severity and IgE or SPT values, in order to account for non-normality in the distribution of these variables. The correlations were re-calculated after excluding DBPCFCs in which severity was 0.
3. RESULTS
3.1 Study Population
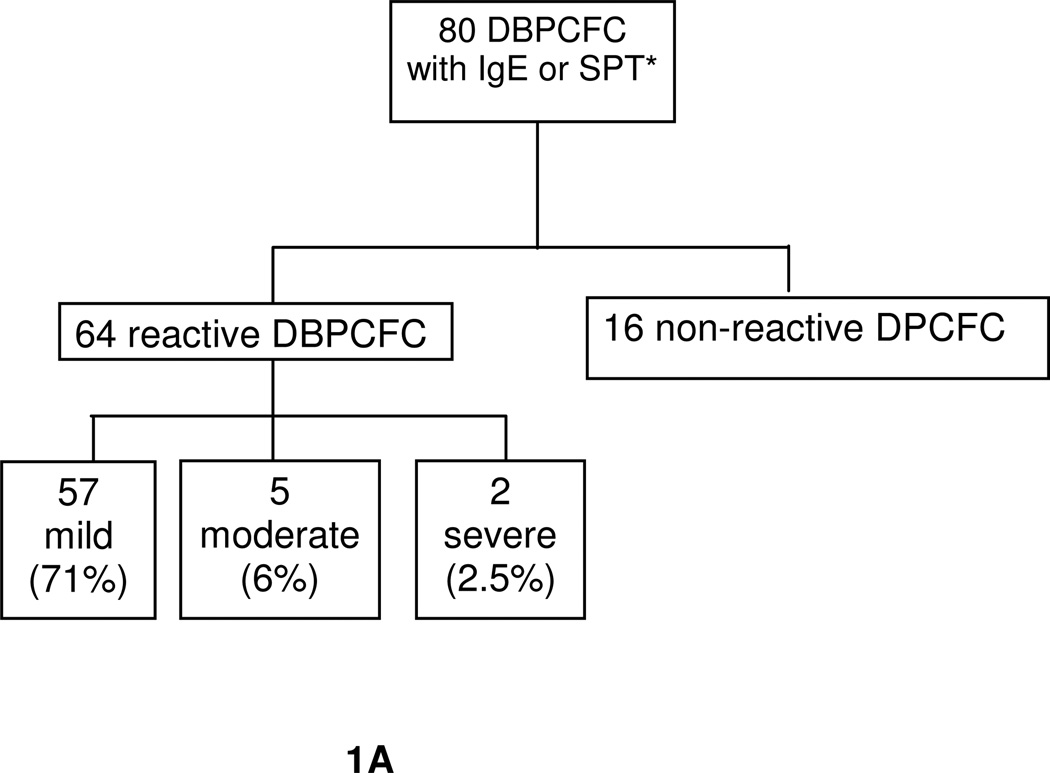
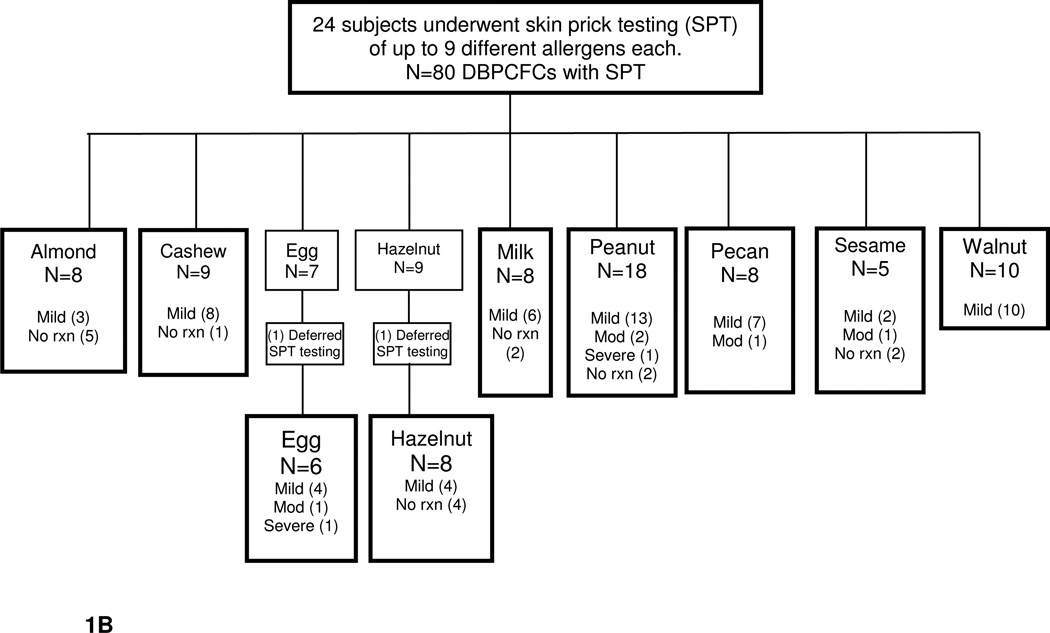
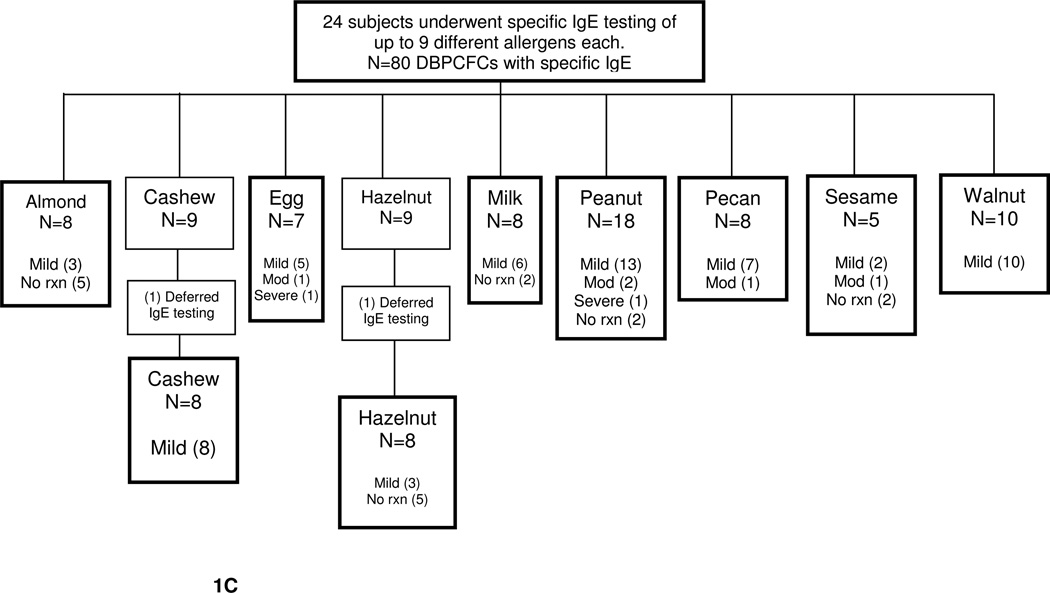
At the time of interim analysis, 82 DBPCFCs were performed on 24 subjects but only 80 DBPCFCs were accounted for due to lack of IgE or SPT levels provided by subjects, Fig. 1B, C. The study population mean age was 11 years. The most common atopic history, documented in table 3, was atopic dermatitis, asthma, and anaphylaxis. Of the 24 subjects, all developed some degree of reaction with the exception of one subject. Of the reactions observed in the study, the majority were classified as mild, 71%, Fig. 1A, B, C.
Fig. 1.
A. Outcome of DBPCFC
All DBPCFC had specific IgE levels. *Subject that declined SPT or specific IgE were excluded. Mild: skin and/or oral symptoms. Moderate: upper respiratory and/or gastrointestinal symptoms or any 3 systems above. Severe: lower respiratory and/or cardiovascular symptoms or any 4 above systems (Perry, 2004).
B. DBPCFC with SPT severity reaction breakdown into mild, moderate, and severe
C. DBPCFC with IgE severity reaction breakdown into mild, moderate, and severe
Table 3.
Demographics of 24 subjects
| Characteristic | N (%) |
|---|---|
| Female | 10 (42) |
| Asthma | 16 (67) |
| Atopic Dermatitis | 13 (54) |
| Allergic Rhinitis | 16 (67) |
| Urticaria | 11 (46) |
| Anaphylaxis | 16 (67) |
3.2 Specific IgE
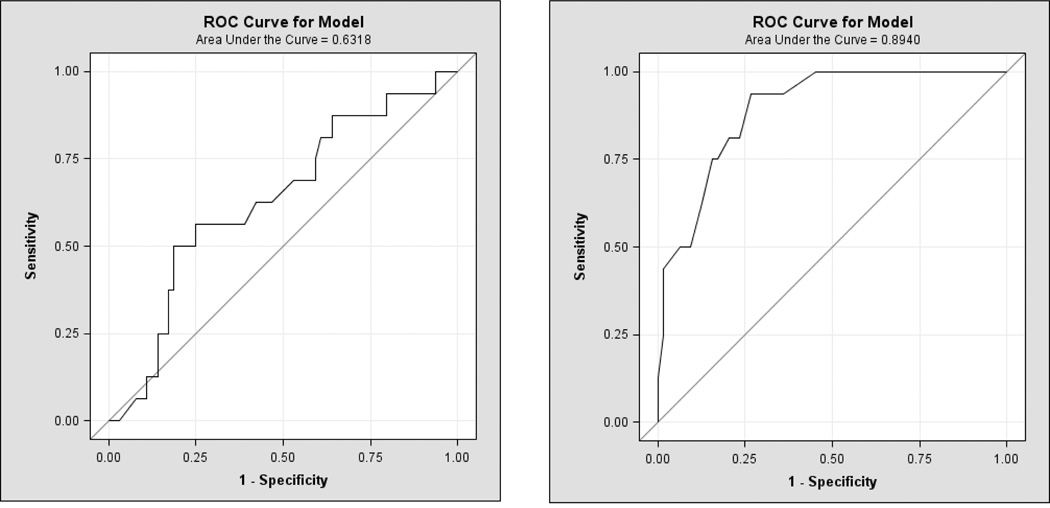
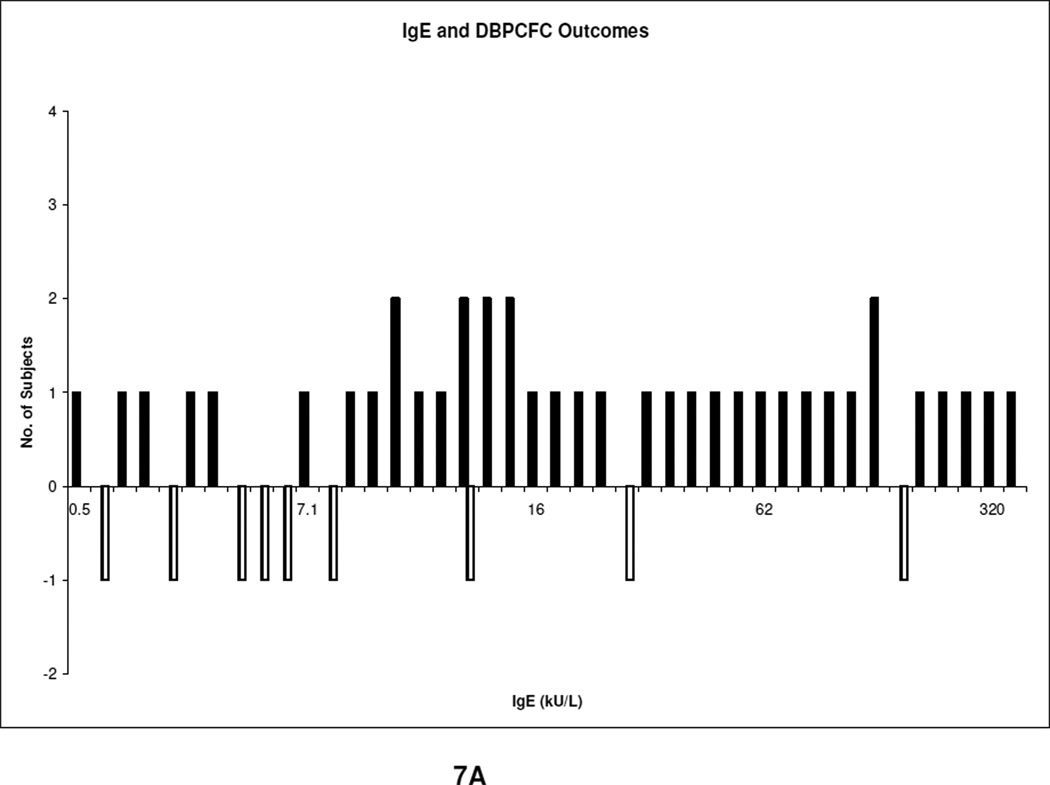
Of the 80 DBPCFCs, 64 (80%) reacted, Fig 6. Of these 64, the most common types of reactions were skin reactions 53%, Table 4. No cardiac symptoms were noted. The baseline mean specific IgE levels in the reactive and non-reactive groups were 54 kU/L (range, 0.35– 531 kU/L) and 22 kU/L (range, 0.35–144 kU/L) respectively. Table 5 shows the specific IgE mean, median, and range of all the food allergens. The sensitivity for clinical allergy of a specific IgE ≥ 15 mm was 69% with a sensitivity of 49%. The cutoff was then lowered from 15 to 7 mm to observe if the sensitivity improved, Table 6, of which it did (49% vs 81%); but the specificity reduced from 69 to 50%. If subjects with a specific IgE ≤ 1 kU/L were assumed to be non reactors, 7.5% would have been wrongly assumed non reactive. Number of reactors per corresponding specific IgE levels are demonstrated in Fig. 7A.
Fig. 6. A, B. Receiver operating characteristic (ROC) curves.
ROC for skin prick test and specific IgE in predicting a reactive DBPCFC.
Table 4.
Reactions for all DBPCFC (N = 99)
| DBPCFC Outcome | N (%) |
|---|---|
| Skin | 52 (53) |
| Upper respiratory | 20 (20) |
| Lower respiratory | 3 (3) |
| Gastrointestinal | 24 (24) |
| Cardiovascular | 0 (0) |
Table 5.
Specific IgEs for reacted and non reacted DBPCFCs
| Reacted | Non reacted | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Allergen | DBPCFC (n) |
Mean IgE (kU/L) |
Median IgE (kU/L) |
Range | DBPCFC (n) |
Mean IgE (kU/L) |
Median IgE (kU/L) |
Range | P value |
| Almond | 3 | 5.8 | 3.7 | 0.5–13 | 5 | 4.2 | 3.1 | 0.35–11 | 0.7 |
| Cashew | 8 | 25 | 16 | 0.35–69 | 0 | na | na | na | * |
| Egg | 7 | 25 | 9.5 | 0.8–124 | 0 | na | na | na | * |
| Hazelnut | 3 | 25 | 22 | 13–39 | 5 | 30 | 6.5 | 0.7–144 | 0.8 |
| Milk | 6 | 33 | 18 | 0.6–92 | 2 | 13 | 13 | 4.6–22 | 0.5 |
| Peanut | 16 | 147 | 100 | 4.2–531 | 2 | 19 | 19 | 3.8–35 | 0.01 |
| Pecan | 8 | 23 | 10.9 | 0.35–63 | 0 | na | na | na | * |
| Sesame | 2 | 26 | 12 | 7.1–60 | 2 | 45 | 45 | 13–77 | 0.6 |
| Walnut | 10 | 21 | 11 | 0.35–82 | 0 | na | Na | na | * |
| Total | 64 | 54 | 13 | 0.35–531 | 16 | 22 | 6.5 | 0.35–144 | 0.105 |
No calculated P value due to zero sample size of non reactants
Table 6.
Sensitivity, specificity, NPV and PPV for specific cutoff levels
| Sensitivity % | Specificity % | Odds Ratio | 95% CI | PPV | NPV | |
|---|---|---|---|---|---|---|
| SPT > 5mm | 91 | 50 | 9.7 | 2.3–43.6 | 88 | 57 |
| SPT > 8mm | 77 | 81 | 14.2 | 3.1–73 | 94 | 46 |
| IgE > 7 kU/L | 81 | 50 | 4.3 | 1.2–16.3 | 82 | 40 |
| IgE > 15 kU/L | 49 | 69 | 2.1 | 0.6–7.8 | 86 | 25 |
| Combination 1 | 64 | 80 | 7.1 | 1.6–35.9 | 93 | 34 |
| Combination 2 | 62 | 88 | 11.4 | 2.2–79.8 | 95 | 37 |
Combination 1: SPT > 5 mm and IgE >7; Combination 2: SPT > 8 mm and IgE >7
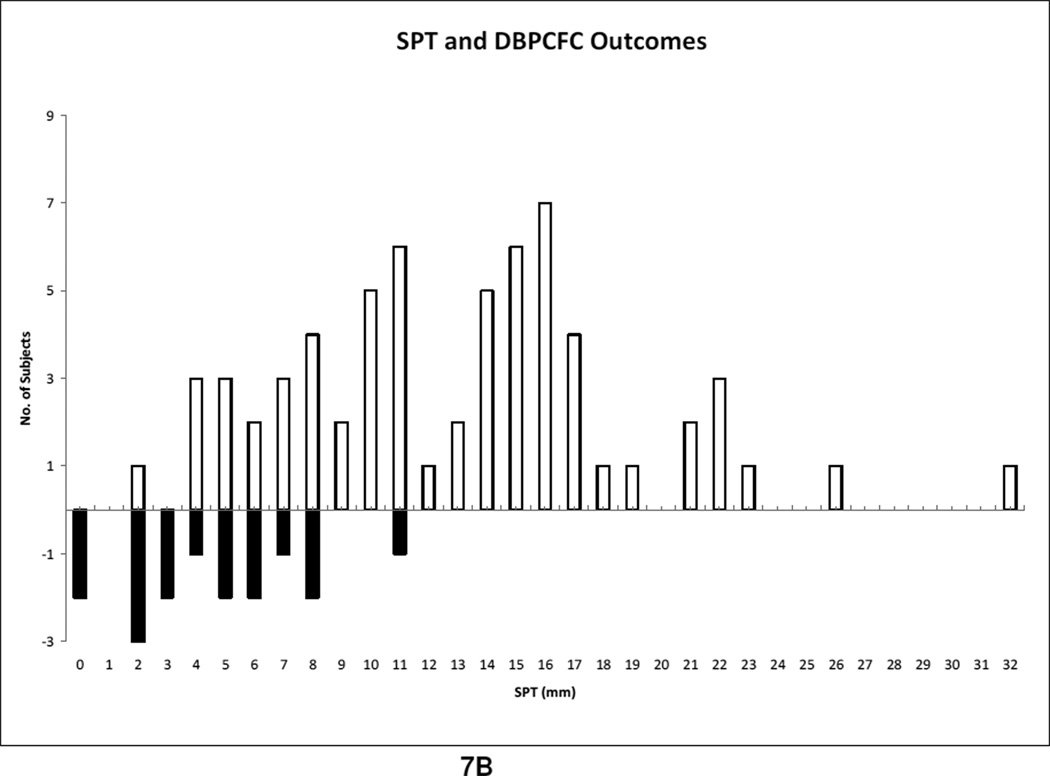
Fig. 7.
A. IgE outcome
The outcome of IgE (kU/L) and DBPCFC. The solid shade represents subjects with no reactions.
B. SPT outcome
The outcome of skin prick test (mm) and DBPCFC. The solid shade represents subjects with no reactions.
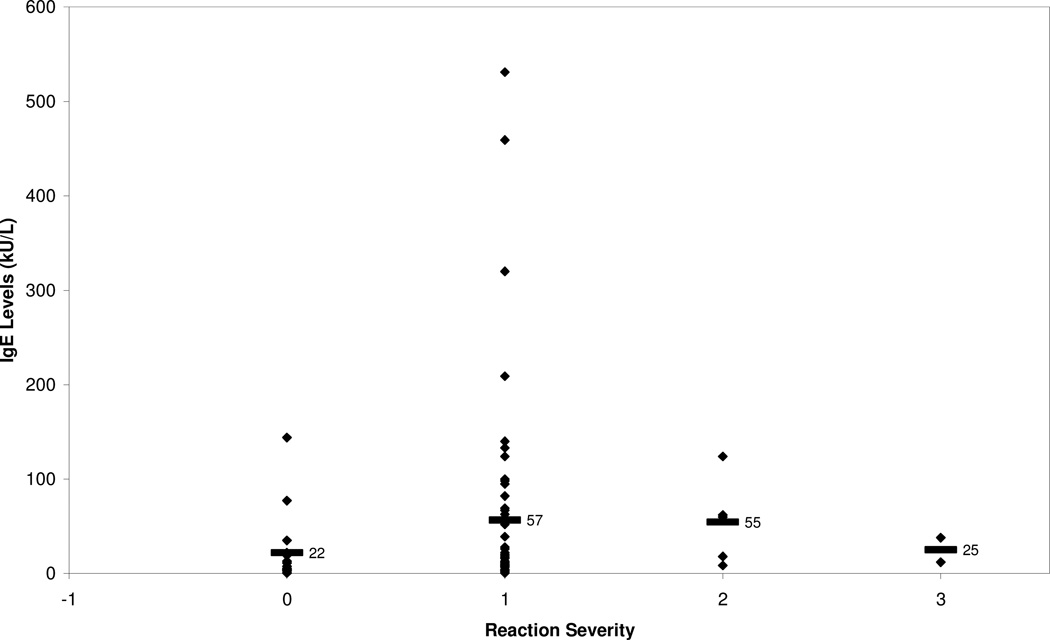
Reaction severity was not found to be associated with an increasing specific IgE means. This is contrary to the results of Perry et al. in which increasing IgE levels for peanut were associated with a statically significant increased reaction severity, p<0.05 (Perry, 2004b). Specific IgE averages for the mild, moderate, and severe groups were 57, 55, and 25 kU/L respectively, Fig. 2. Of the two subjects that developed a severe reaction, specific IgE was less than 38 kU/L and the reacted dose was less than 400 mg, which is roughly equivalent to a peanut. We also looked at specific IgE levels and reaction severity correlations within individuals but saw no significant trend. The ROC curve for IgE and reaction status indicates that it is not possible to produce concurrent high specificity and sensitivity, Fig 6. The maximum achievable area under the curve is one, yet the area under the curve attained in this study was 0.63.
Fig. 2. Specific IgE and Reaction Severity.
Reaction severity classified according to modified Bock’s criteria. 0: no reaction (n=16), 1: mild (n=57), 2: moderate (n=5), 3: severe (n=2). Means indicated with black bar.
3.3 SPT
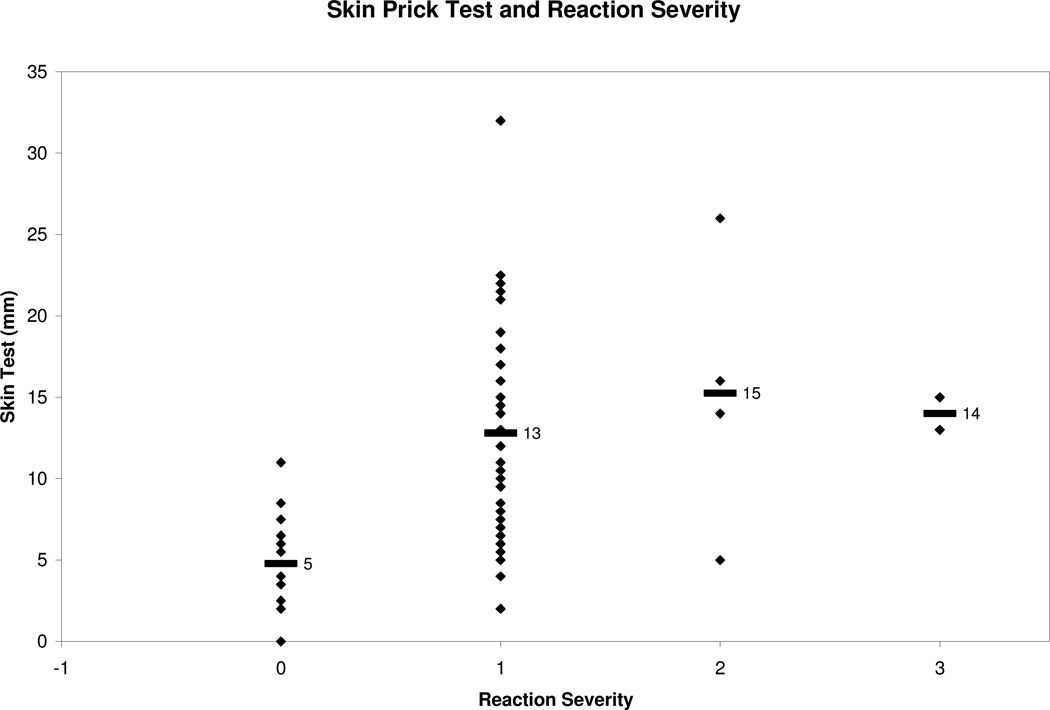
We compared the mean of all reactive SPT to the non reactive SPT and found that the reactive group had a larger mean SPT wheal of 12.9 mm compared to 4.8 mm in the non reactive group, Table 7. There was no correlation between SPT and severity of reaction. To analyze the sensitivity and specificity of a SPT value, we used a cutoff of 8 mm because Sporik et al. reported a SPT wheal of greater than 8 mm to be 100% specific in predicting reactive challenges in peanut allergic subjects (Roberts 2005). We did not duplicate similar results in our study. At SPT ≥ 8 mm, the specificity was 81% and the sensitivity was 77%. When the diameter cutoff was lowered to 5 mm, the sensitivity increased but the specificity decreased as expected. Figure 6 is a receiving operator curve (ROC) used to depict the trade off between sensitivity and specificity among different specific IgE and SPT cutoffs. We sought to determine if combing specific IgE ≥ 7 kU/L and SPT ≥ 8 mm increased the predictive value of a reaction but this only lowered the sensitivity to 62%, with a specificity of 88%, Table 6. Specific IgE and SPT showed a low correlation coefficient of 0.27. We hypothesized that as SPT size increases, severity would also raise. However, when we examined the subset of positive DBPCFCs to see if SPT was associated with greater reaction severity, we observed a low correlation coefficient (r = 0.05, p<0.64). Number of reactors per corresponding SPT levels are demonstrated in Fig. 7B.
Table 7.
SPT for reacted and non reacted DBPCFCs
| Reacted | Non reacted | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Allergen | DBPCFC (n) |
Mean SPT (mm) |
Median SPT (mm) |
Range | DBPCFC (n) |
Mean SPT (mm) |
Median SPT (mm) |
Range | P value |
| Almond | 3 | 11 | 13 | 5.5–16 | 5 | 3.2 | 3.5 | 0–6.5 | 0.1 |
| Cashew | 8 | 14 | 16 | 8–19 | 1 | 8.5 | 8.5 | 8.5 | * |
| Egg | 6 | 10 | 10 | 5–14 | 0 | NA | NA | NA | * |
| Hazelnut | 4 | 9.5 | 7.8 | 2–21 | 4 | 3 | 3 | 0–6 | 0.2 |
| Milk | 6 | 12 | 14 | 6–16 | 2 | 8 | 8 | 7.5–8 | 0.3 |
| Peanut | 16 | 17 | 15 | 4–32 | 2 | 3.8 | 3.8 | 2–5.5 | 0.008 |
| Pecan | 8 | 11 | 10 | 4–21 | 0 | NA | NA | NA | * |
| Sesame | 2 | 15 | 14 | 11–22 | 2 | 8.3 | 8.3 | 5.5–11 | 0.19 |
| Walnut | 10 | 9.8 | 10 | 4–15 | 0 | NA | NA | NA | * |
| Total | 64 | 12.9 | 13.5 | 2–32 | 16 | 4.8 | 4.7 | 0–11 | 0.0001 |
No calculated P value due to zero sample size of non reactants
3.4 DBPCFC
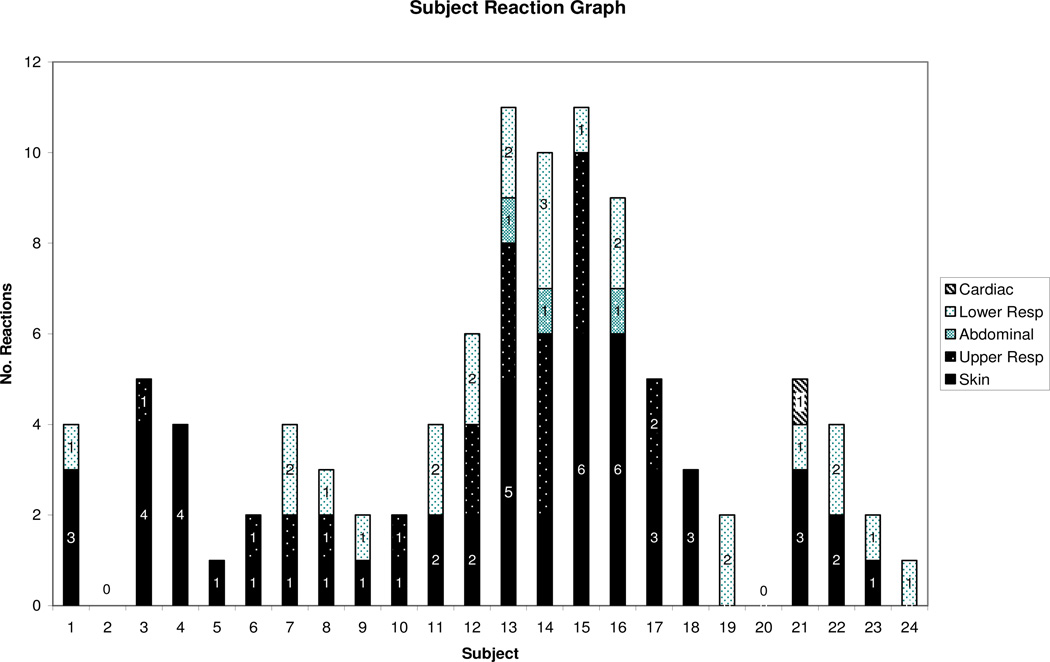
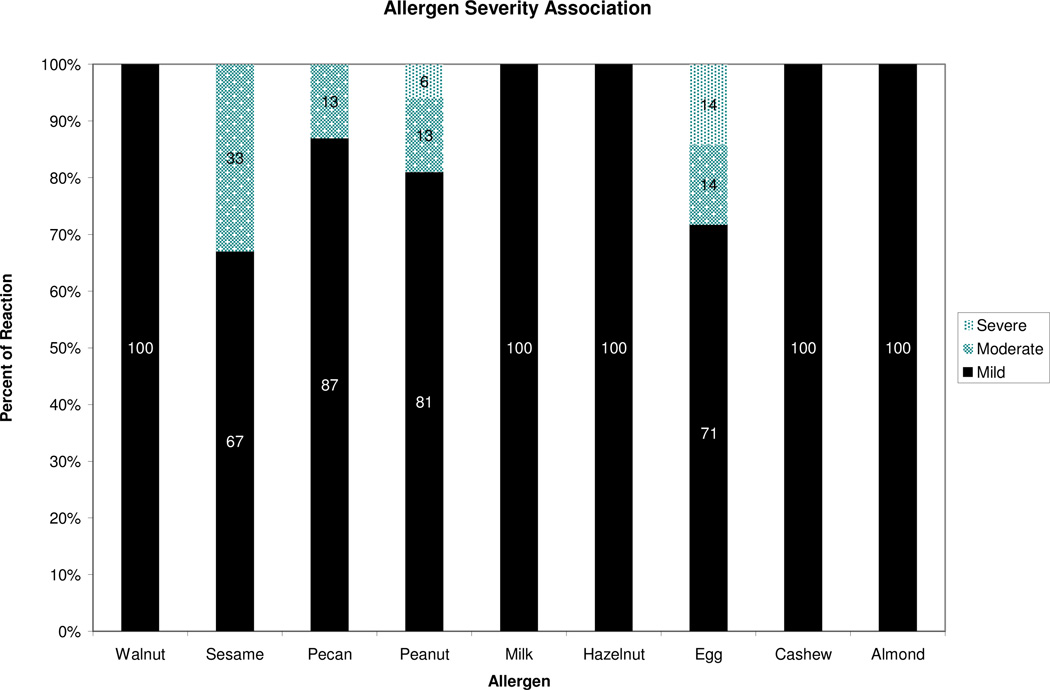
According to Table 4, skin was the most common site of reaction. One subject developed only abdominal symptoms while others had multiple symptoms. We also found no trends between subjects and their reaction presentation, Fig. 4. Of the multiple allergens in this study, egg and peanut DBPCFCs were associated with more severe reactions compared to the other allergens in this study, Fig 5. Treatment data revealed that cetirizine was given with the most frequency, Table 8. All reactions were treated with medication and the mean time to resolution was 30 min (figure not shown). The types of reactions these subjects developed are shown in Table 4. Three subjects had a reaction to the placebo. There were no reports of subject who had a non reactive DBPCFC at two hours developing symptoms of a delayed reaction in hours or days. There were no hospitalizations or deaths.
Fig. 4. Reactions observed per subject.
Skin: urticaria, angioedema. Upper respiratory: rhinoconjunctivitis, sneezing bursts, periocular swelling, and cough. Lower respiratory: wheeze. Gastrointestional: emesis and diarrhea. Cardiovascular: hypotension, collapse and mental status change.
Fig. 5. Food allergen and reactions observed.
Reaction severity classified according to modified Bock’s criteria. Skin: urticaria and angioedema. Upper respiratory: rhinoconjunctivitis, sneezing bursts, periocular swelling, and cough. Lower respiratory: wheeze. Gastrointestional: emesis and diarrhea. Cardiovascular: hypotension, collapse and mental status change
Table 8.
Medications given during DBPCFC n = 88
| Treatment | n (%) |
|---|---|
| Cetirizine | 48 (54) |
| Famotidine | 15 (17) |
| Loratidine | 2 (0.03) |
| Benedryl | 14 (17) |
| Albuterol Nebulizer | 5 (0.01) |
| Triamcinolone Cream | 2 (0.040 |
| Epinephrine | 1 (0.02) |
| Oral Prednisone | 1 (0.02) |
4. DISCUSSION
The aim of this prospective study was to determine whether specific IgE and skin prick test correlated to the severity of reaction during a DBPCFC for egg, milk, peanut and multiple tree nut allergens. Furthermore we aimed to evaluate the specificity and sensitivity of specific IgE ≥ 7 kU/L and SPT ≥ 8mm. Several studies such as that done by Sampson and Sicherer reported no correlation between the severity of a reaction to specific IgE level or SPT (Sicherer, 2000; Sampson, 1997; Rance, 2002; Clark, 2003). On the other hand, Perry et al. found a statistically significant trend between specific IgE levels and severity of reaction for peanuts (Perry, 2004b). Our data (Fig 2) showed a correlation. We conclude that subjects with elevated specific IgE have a reaction (p < 0.06), but not necessarily a more severe reaction.
4.1 Specific IgE
IgE cutoff levels found in this study are consistent with those reported by Sampson and Ho. Sampson and Ho noted that a specific IgE level ≥ 15 kU/L was found to have >95% PPV for a positive challenge in a study of subjects with atopic dermatitis and in our study we found a PPV 86%. Although only 60% of our subjects had atopic dermatitis compared to 100% in Sampson’s study, we considered our subjects to be highly allergic given that 67% had a history of anaphylaxis and allergic rhinitis, Table 3. The trend for increasing reactions with increasing peanut-specific IgE level was statistically significant (p < .0001), with 100% passing with a level between 0.35 and 2, and 6% with levels between 2–4.9 kU/L. We had three DBPCFCs react that were associated with IgE <0.35, which is considered a negative test. Our finding differs from Perry and et al. who had 77%, 44%, 40%, and 0% passing with a level of less than 0.35 kU/L, 0.36–2 kU/L, 2–4.9 kU/L, and greater than 5 kU/L respectively (Perry, 2004a). Given that our subjects are considered highly allergic, we had 11% passing with IgE ≥ 5 compared to Perry’s study which had 0%. This emphasizes that our study did not exclude the more sensitive patients and therefore our results could be confounded by the more prevalent atopic history making comparisons with prior studies skewed. In this study, a history of either any previous reaction to a food allergen was not associated with the development of anaphylaxis during the DBPCFC. Potentially subjects that would have developed anaphylaxis did not because a food challenge ended once any allergic objective sign was noted and medication was given immediately.
We did not reproduce the results seen by Nicalou et al. Their study also used a specific IgE cutoff of 15 kU/L but had 96% of subjects at greater than this level being correctly classified as allergic while we only demonstrated a correct diagnosis of 48% (31 with IgE > 15 kU/L of 64 that had a reaction) (Nicalou, 2011). 9% with specific IgE ≤ 2 kU/L had a reaction. This suggests that food-specific IgE levels have quantitative value and can provide clinically useful information, even at low levels for many foods. From our data we inferred that using higher cutoffs for specific IgE levels ≥ 15 mm or SPT ≥ 8 mm resulted in relatively poor sensitivity and higher specificity. We also found that combining specific IgE and SPT did not help to achieve clinically useful sensitivity and specificity. This would suggest that combinations of tests are not more useful than many individual tests, a finding supported by other studies (Roberts, 2005).
4.2 SPT
In regards to SPT, there is wide variation in SPT cutoff to achieve 100% specificity levels from one study to another. Rance et al. found a SPT of >16 mm to be 100% specific with peanut extract in non- allergic peanut subjects while Wainstein found that a SPT ≥ 15 mm had a specificity of 100% (Rance, 2002; Wainstein, 2007). Kagan et al. had a similar specificity finding but with a lower SPT cutoff of ≥ 13 mm in subjects with a positive peanut SPT but no prior peanut ingestion (Kagan, 2003). On the other hand, a recent study by Roberts et al. reported a specificity of 98.5% for a SPT of > 8 mm as did Sporik et al. (Roberts, 2005). Our results did not achieve similar specificity. When we set our SPT cutoff to > 8mm, specificity was 81%, but when we lowered the SPT cutoff to 5 mm the specificity dropped drastically to 50%, Table 6. In comparison to the traditional clinical cutoff of 3 mm, it appears that higher SPT provides more specificity but the sensitivity of a positive test becomes compromised, as expected. The wide variation noted in multiple studies may be due to factors such as sample size, use of different skin test devices, techniques and non standardized sources of allergen. Historically SPT wheal diameter correlated with a likelihood of a positive food challenge and some studies such as Clark noted a correlation to reaction severity as well (Clark, 2003). We however saw no correlation between SPT and reaction severity.
4.3 DBPCFC
Of the failed challenges, skin was the most common system involved (52%). Of those that developed a severe reaction, the allergens were egg and peanut. The tree nuts sesame, pecan, and peanut appear to be the most associated with moderate and severe reactions, although these trends were not statically significant. Three subjects reacted to the placebo. Their reactions included acute onset of copious rhinorrhea, persistent abdominal pain, and oral pruritus. All three subjects had negative SPT to rice, the placebo ingredient. Factors that may explain these reactions are numerous, including environmental cross contamination. We also wanted to determine if our subjects that developed severe reactions were false positives because they received higher doses. Wensing and Wood found the opposite effect in that subjects with severe reactions did so at lower doses than those with mild symptoms, p < 0.05 (Hourihane, 2005). We concur with Wensing and Wood as our data shows that those who had a severe reaction reacted at lower doses 50 and 400 mg, in comparison to the maximum 2,500 mg. Participants were not likely to have more severe reactions when larger doses of the challenge food were ingested.
5. CONCLUSION
The limitations in this study include the time interval at which SPT and specific IgE were drawn. These tests were typically collected within 6 months and 2 years, respectively, prior to DBPCFC, but although it is possible that results may differ if the test were performed closer to the challenge, it seems unlikely that our results would have differed significantly. Also, aborting the DBPCFC at the first development of any objective signs avoided many cases of anaphylaxis, therefore not taking into account the more severe reactions. There is also considerable discordance between the occurrence of severe reaction and the use of epinephrine (n=1) 0.02% in which the medication was given due to a parent’s request.
In summary, we found no association between SPT, specific IgE and reaction severity. Combining specific IgE and SPT improved specificity but did not help to achieve clinically useful sensitivity.
Fig. 3. Skin prick test (SPT) and Reaction Severity.
Reaction severity classified according to modified Bock’s criteria. 0: no reaction (n=16), 1: mild (n=57), 2: moderate (n=5), 3: severe (n=2). Means indicated with black bar.
ACKNOWLEDGEMENTS
We would like to thank the Fund for Food Allergy at Stanford, the Food Allergy Initiative, the Stanford Immunity, Transplantation and Infectious Disease Institute, and the Lucile Packard Foundation. We also acknowledge the help of our Data Safety Monitoring Board and the Stanford Alliance for Food Allergy Research clinical research team and the staff of the Clinical Trial Research Unit.
ABBREVIATIONS
- DBPCFC
Double blinded, placebo controlled food challenge
- SPT
Skin prick test
- PPV
Positive predictive value
- NPV
Negative predictive value
- CI
Confidence interval
- IgE
Immunoglobulin E
- kU/L
Kilounits of allergen specific IgE per liter
- ROC
Receiving operator curve
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution License (http://creativecommons.org/licenses/by/3.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
REFERENCES
- 1.Bock SA, Munoz-Furlong A, Sampson HA. Fatalities due to anaphylactic reactions to foods. J Allergy Clin Immunol. 2001;107:191–193. doi: 10.1067/mai.2001.112031. [DOI] [PubMed] [Google Scholar]
- 2.Boyano-Martı´nez T, Garcı´a-Ara C, Dı´az-Pena JM, Mun˜oz FM, Sa´nchez GG, Esteban MM. Validity of specific IgE antibodies in children with egg allergy. Clin Exp Allergy. 2001;31:1464–1469. doi: 10.1046/j.1365-2222.2001.01175.x. [DOI] [PubMed] [Google Scholar]
- 3.Branum AM, Lukacs SL. Food allergy among U. S. children: trends and hospitalization National Center for Health Statistics, Centers for disease control and prevention No. 10. 2009. [Google Scholar]
- 4.Buchanan AD, Green TD, Jones SM, Scurlock AM, Christie L, Althage KA, et al. Egg oral immunotherapy in nonanaphylactic children with egg allergy. J Allergy Clin Immunol. 2007;119:199–205. doi: 10.1016/j.jaci.2006.09.016. [DOI] [PubMed] [Google Scholar]
- 5.Clark AT, Ewan PW. Interpretation of tests for nut allergy in one thousand patients, in relation to allergy or tolerance. Clin Exp Allergy. 2003;33:1041–1045. doi: 10.1046/j.1365-2745.2003.01624.x. [DOI] [PubMed] [Google Scholar]
- 6.Fleiss J. Statistical Methods for Rates and Proportions 2nd Ed Section 5.6. New York: Publisher John Wiley & Sons; 1981. [Google Scholar]
- 7.Flinterman AE, Pasmans SG, Hoekstra MO, et al. Determination of no-observed- adverse-effect levels and eliciting doses in a representative group of peanut-sensitized children. J Allergy Clin Immunol. 2006;117:448–454. doi: 10.1016/j.jaci.2005.11.035. [DOI] [PubMed] [Google Scholar]
- 8.Garcı´a-Ara C, Boyano-Martı´nez T, Dı´az-Pena JM, Martı´n-Mun˜oz F, Reche-Frutos M, Martı´n-Esteban M. Specific IgE levels in the diagnosis of immediate hypersensitivity to cow’s milk protein in the infant. J Allergy Clin Immunol. 2001;107:185–190. doi: 10.1067/mai.2001.111592. [DOI] [PubMed] [Google Scholar]
- 9.Garcı´a-Ara C, Martı´nez TB, Dı´az-Pena JM, Martin-Mu~oz F, Reche-Frutos M, Esteban MM. Specific IgE levels in the diagnosis of immediate hypersensitivity to cow’s milk protein in the infant. J Allergy Clin Immunol. 2001;107:185–190. doi: 10.1067/mai.2001.111592. [DOI] [PubMed] [Google Scholar]
- 10.Hatahet R, Kirch F, Kanny G, Moneret-Vautrin DA. Sensibilisation auxallergènes d’arachide chez les nourrissons de moins de quatre mois: à propos de 125 observations. Rev Fr Allergol Immunol Clin. 1994;34:377–381. [Google Scholar]
- 11.Hill DJ, Hosking CS, Reyes-Benito LV. Reducing the need for food allergen challenges in young children: a comparison of in vitro and in vivo tests. Clin Exp Allergy. 2001;31:1031–1035. doi: 10.1046/j.1365-2222.2001.01113.x. [DOI] [PubMed] [Google Scholar]
- 12.Hourihane JO, Crimshaw K, Briggs RA, Trewin JB, Kilburn SA, Warner JO. Does Severity of low-dose, double-blinded, placebo-controlled food challenges reflect severity of allergic reactions to peanut in the community? Clin Exp Allergy. 2005;35:1227–1233. doi: 10.1111/j.1365-2222.2005.02312.x. [DOI] [PubMed] [Google Scholar]
- 13.Kagan R, Hayami D, Joseph L, St Pierre Y, Clarke AE. The predictive value of a positive prick skin test to peanut in atopic, peanut-naive children. Ann Allergy Asthma Immunol. 2003;90:640–645. doi: 10.1016/S1081-1206(10)61869-8. [DOI] [PubMed] [Google Scholar]
- 14.Kulis M, Li Y, Lane H, Pons L, Burks W. Single tree nut immunotherapy attenuates allergic reactions in mice with hypersensitivity to multiple tree nuts. J Allergy Clin Immunol. 2011;127:81–88. doi: 10.1016/j.jaci.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 15.Longo G, Barbi E, Berti I, Meneghetti R, Pittalis A, Ronfani L, et al. Specific oral tolerance induction in children with very severe cow’s milk-induced reactions. J Allergy Clin Immunol. 2008;121:343–347. doi: 10.1016/j.jaci.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 16.Martı´nez TB, Garcı´a-Ara C, Dı´az-Pena JM, Mu~oz FM, Sa´nchez GG, Esteban MM. Validity of specific IgE antibodies in children with egg allergy. Clin Exp Allergy. 2001;31:1464–1469. doi: 10.1046/j.1365-2222.2001.01175.x. [DOI] [PubMed] [Google Scholar]
- 17.Martinez, Garcia-Ara, Osterballe M, Bindslev-Jensen C, et al. Threshold levels in food challenge and specific IgE in patients with egg allergy: is there a relationship? J Allergy Clin Immunol. 2003;112:196–201. doi: 10.1067/mai.2003.1603. [DOI] [PubMed] [Google Scholar]
- 18.Nicalou N, Murray C, Belgrave D, Poorafshar M, Simpson A, Custovic A. Quantification of specific IgE to whole peanut extract and peanut components in prediction of peanut allergy. J Allergy Clin Immunol. 2011;127:684–685. doi: 10.1016/j.jaci.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 19.Nicolaou N, Poorafshar M, Murray C, Simpson A, Winell H, Kerry G, et al. Allergy or tolerance in children sensitized to peanut: prevalence and differentiation using component-resolved diagnostics. J Allergy Clin Immunol. 2010;125:191–197. e1–e13. doi: 10.1016/j.jaci.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Nowak-Wegrzyn A, Assaad A, Bahna S, Bock A, Sicherer S, Teuber S. J Allergy Clin Immunol. 2009;123:S365–S383. doi: 10.1016/j.jaci.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 21.Nowak-Wegrzyn A, Sampson HA. Future therapies for food allergies. J Allergy Clin Immunol. 2011;127:558–573. doi: 10.1016/j.jaci.2010.12.1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patriarca G, Nucera E, Pollastrini E, Roncallo C, Pasquale De. T, Lombardo C, et al. Oral specific desensitization in food-allergic children. Dig Dis Sci. 2007;52:1662–1672. doi: 10.1007/s10620-006-9245-7. [DOI] [PubMed] [Google Scholar]
- 23.Perry TT, Matsui EC, Conover-Walker MK, Wood RA. The relationship of allergen-specific IgE levels and oral food challenge outcomes. J Allergy Clin Immunol. 2004a;114:144–149. doi: 10.1016/j.jaci.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 24.Perry TT, Matsui EC, Conover-Walker MK, Wood RA. Risk of oral food challenges. J Allergy Clin Immunol. 2004b;114:1164–1168. doi: 10.1016/j.jaci.2004.07.063. [DOI] [PubMed] [Google Scholar]
- 25.Press W, et al. Numerical Recipes in C 2nd Ed. Section 15.6. Cambridge UK: Publisher Cambridge University Press; 1992. [Google Scholar]
- 26.Pucar F, Kagan R, Lim H, Clarke AE. Peanut challenge: a retrospective study of 140 patients. Clin Exp Allergy. 2001;31 doi: 10.1046/j.1365-2222.2001.00962.x. 40-6.10. [DOI] [PubMed] [Google Scholar]
- 27.Rance F, Abbal M, Lauwers-Cances V. Improved screening for peanut allergy by the combined use of skin prick tests and specific IgE assays. J Allergy Clin Immunol. 2002;109:1027–1033. doi: 10.1067/mai.2002.124775. [DOI] [PubMed] [Google Scholar]
- 28.Roberts G, Lack G. Diagnosing peanut allergy with skin prick and specific IgE testing. J Allergy Clin Immunol. 2005;115:1291–1296. doi: 10.1016/j.jaci.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 29.Roux KH, Teuber SS, Sathe SK. Tree nut allergens. Int Arch Allergy Immunol. 2003;131 doi: 10.1159/000072135. 234-44.4. [DOI] [PubMed] [Google Scholar]
- 30.Sampson HA, Ho DG. Relationship between food specific IgE concentrations and the risk of positive food challenges in children and adolescents. J Allergy Clin Immunol. 1997;100:444–451. doi: 10.1016/s0091-6749(97)70133-7. [DOI] [PubMed] [Google Scholar]
- 31.Sampson HA. Utility of food-specific IgE concentrations in predicting symptomatic food allergy. J Allergy Clin Immunol. 2001;107:891–896. doi: 10.1067/mai.2001.114708. [DOI] [PubMed] [Google Scholar]
- 32.Sampson HA. Update on food allergy. J Allergy Clin Immunol. 2004;113:805–819. doi: 10.1016/j.jaci.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Sampson HA. Peanut allergy. N Engl J Med. 2002;346:1294–1299. doi: 10.1056/NEJMcp012667. [DOI] [PubMed] [Google Scholar]
- 34.Sicherer SH, Morrow EH, Sampson HA. Dose-response in double-blind, placebo controlled oral food challenges in children with atopic dermatitis. J Allergy Clin Immunol. 2000;105:582–586. doi: 10.1067/mai.2000.104941. [DOI] [PubMed] [Google Scholar]
- 35.Sicherer SH, Sampson HA. Peanut allergy: emerging concepts and approaches for an apparent epidemic. J Allergy Clin Immunol. 2007;120:491–503. doi: 10.1016/j.jaci.2007.07.015. [DOI] [PubMed] [Google Scholar]
- 36.Sicherer SH, Mu~noz-Furlong A, Godbold JH, Sampson HA. US prevalence of self-reported peanut, tree nut, sesame allergy: 11-year follow-up. J Allergy Clin Immunol. 2010;125:1322–1326. doi: 10.1016/j.jaci.2010.03.029. [DOI] [PubMed] [Google Scholar]
- 37.Skolnick HS, Conover-Walker MK, Koemer CB, Sampson HA, Burks AW, Wood RA. The natural history of peanut allergy. J Allergy Clin Immunol. 2001;107:367–374. doi: 10.1067/mai.2001.112129. [DOI] [PubMed] [Google Scholar]
- 38.Sporik R, Hill DJ, Hosking CS. Specificity of allergen skin testing in predicting open food challenges to milk, egg, peanut in children. Clin Exp Allergy. 2000;30:1540–1546. doi: 10.1046/j.1365-2222.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- 39.Staden U, Rolinck-Werninghaus C, Brewe F, Wahn U, Niggemann B, Beyer K. Specific oral tolerance induction in food allergy in children: Efficacy and clinical patterns of reaction. Allergy. 2007;62:1261–1269. doi: 10.1111/j.1398-9995.2007.01501.x. [DOI] [PubMed] [Google Scholar]
- 40.Wainstein BK, Yee A, Jelley D, Ziegler M, Ziegler JB. Combining skin prick, immediate skin application and specific-IgE testing in the diagnosis of peanut allergy in children. Pediatr Allergy Immunol. 2007;18:231–239. doi: 10.1111/j.1399-3038.2007.00517.x. [DOI] [PubMed] [Google Scholar]
- 41.Wensing M, Penninks AH, Hefle SL, Kopelman SJ, Bruijnzeel-Koomen CAFM, Knulst AC. The distribution of individual threshold doses eliciting allergic reactions in a population with peanut allergy. J Allergy Clin Immunol. 2002;110:915–920. doi: 10.1067/mai.2002.129235. [DOI] [PubMed] [Google Scholar]