Abstract
Proanthocyanidins (PAs) are products of the flavonoid pathway, which also leads to the production of anthocyanins and flavonols. Many flavonoids have antioxidant properties and may have beneficial effects for human health. PAs are found in the seeds and fruits of many plants. In apple fruit (Malus × domestica Borkh.), the flavonoid biosynthetic pathway is most active in the skin, with the flavan-3-ols, catechin, and epicatechin acting as the initiating units for the synthesis of PA polymers. This study examined the genes involved in the production of PAs in three apple cultivars: two heritage apple cultivars, Hetlina and Devonshire Quarrenden, and a commercial cultivar, Royal Gala. HPLC analysis shows that tree-ripe fruit from Hetlina and Devonshire Quarrenden had a higher phenolic content than Royal Gala. Epicatechin and catechin biosynthesis is under the control of the biosynthetic enzymes anthocyanidin reductase (ANR) and leucoanthocyanidin reductase (LAR1), respectively. Counter-intuitively, real-time quantitative PCR analysis showed that the expression levels of Royal Gala LAR1 and ANR were significantly higher than those of both Devonshire Quarrenden and Hetlina. This suggests that a compensatory feedback mechanism may be active, whereby low concentrations of PAs may induce higher expression of gene transcripts. Further investigation is required into the regulation of these key enzymes in apple.
Abbreviations:
- ANOVA
analysis of variance
- ANR
anthocyanidin reductase
- DAD
diode array detector
- DAFB
days after full bloom
- DFR
dihydroflavonol reductase
- LAR
leucoanthocyanidin reductase
- LC-MS
liquid chromatography/mass spectrometry
- PA
proanthocyanidin
- qPCR
real-time quantitative PCR
Key words: anthocyanidin reductase, apple, catechin, epicatechin, flavonoid, leucoanthocyanidin reductase, proanthocyanidin biosynthesis, regulation, tannin
Introduction
Proanthocyanidins (PAs) are products of the flavonoid pathway, which also leads to the production of anthocyanins and flavonols. These three classes of secondary metabolites are called flavonoids and are synthesized from common precursor compounds. PAs (also known as condensed tannins) are produced from the condensation of flavan-3-ol units that accumulate in the vacuoles of plants (Dixon et al., 2005; Lepiniec et al., 2006). They are found in the bark of trees, leaves of tea and forage plants, and the seeds and fruits of many plants including grapes, apples, kiwifruit, cranberries, and persimmon. Anthocyanins, PAs, and flavonols are all antioxidants and may have beneficial effects for human health when incorporated into the diet (Wolfe et al., 2003; Tsao et al., 2005; Stevenson and Hurst, 2007; Aron and Kennedy, 2008). PAs also impart astringency to fresh fruits, fruit juices, and wine, oxidize to form brown pigments in seeds and other tissues, and may act as feeding deterrents in reproductive tissues and developing fruits (Wrangham et al., 1998; Forkner et al., 2004). Apples provide a diet high in polyphenolics and flavonoids, and therefore represent a major source of dietary antioxidants (Vinson et al., 2001; Lee et al., 2003). Apples contain a large number of different types of polyphenolics, and the concentrations of these compounds within the apple vary among cultivars, growing conditions, and location within the tree (van der Sluis et al., 2001; McGhie et al., 2005; Tsao et al., 2005).
PA biosynthesis results from a pathway controlled by genes encoding enzymes involved in the formation of the biochemical structure of the compound and by regulatory genes that control gene expression of these enzymes (Lepiniec et al., 2006). The biochemical functions of these enzymes have been well established in some plant species (Holton and Cornish, 1995), and significant progress has been made in this area (Bogs et al., 2005; Takos et al., 2006c; Almeida et al., 2007; Ikegami et al., 2007). For many years, PA biosynthesis was assumed to branch from the anthocyanin pathway at the level of leucoanthocyanidin (Fig. 1). Most models of PA biosynthesis maintain that the extension units arise by condensation of molecular units derived from leucoanthocyanidin. However, this model fails in one important aspect: the stereochemistry of leucoanthocyanidin is 2,3-trans when formed by the proposed pathway, whereas in most cases the major extension unit is 2,3-cis (Xie and Dixon, 2005).
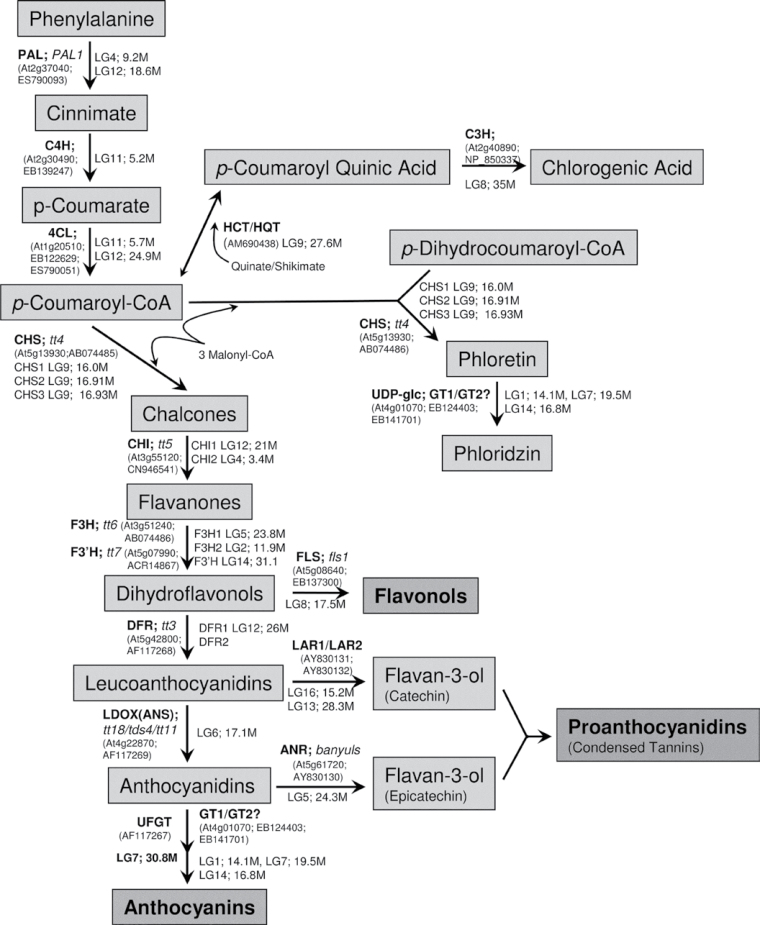
Fig. 1.
Scheme of the apple polyphenolic pathway, showing structural genes involved in flavonoid biosynthesis in Arabidopsis thalianaseed and Malus × domestica fruit, annotated in bold with the TAIR locus and GenBank accession numbers, and their predicted linkage group in the apple reference genome, with approximate position (to the nearest 100kb). PAL, phenylalanine ammonia lyase;C4H, cinnamate-4-hydroxymate; 4CL, 4-coumarate:coenzyme A ligase; CHS, chalcone synthase; CHI, chalcone isomerase; F3H, flavanone 3-hydroxylase, F3'H, flavanone 3'-hydroxylase; DFR, dihydroflavonol 4-reductase; ANS, anthocyanidin synthase; UFGT, UDP-glucose flavonoid 3-O-glucosyl transferase; FLS, flavonol synthase; LAR1/2, leucoanthocyandin reductase; ANR, anthocyanidin reductase; GT1/2, glycosyltransferases; HQT/HCT, quinate hydroxycinnamoyl/hydrxycinnamoyl CoA shikimate; C3H, p-coumarate 3-hydroxylase.
A possible solution to this stereochemical paradox was the discovery of the BANYULS (BAN) gene in Arabidopsis thaliana. Mutations in BAN result in a transparent testa associated with a lack of PAs in the seed coat (Devic et al., 1999). Based on the phenotype and the protein sequence similarity of BAN to dihydroflavonol reductase (DFR; catalyses production of leucoanthocyanidins from dihydroflavanols), it was suggested that BAN encodes leucoanthocyanidin reductase (LAR) and therefore converts flavan-3,4-diols to the corresponding 2,3-trans-flavan-3-ols. However, expression of recombinant BAN proteins from A. thaliana and Medicago truncatula in Escherichia coli failed to produce in vitro LAR activity. Instead, BAN genes were shown to encode a new enzyme, anthocyanidin reductase (ANR), which converts anthocyanidins to 2,3-cis-flavan-3-ols (Xie et al., 2003).
This discovery of the BAN gene highlighted two possible branch pathways for the formation of PAs in plant cells: (i) LAR synthesizing 2,3-trans-flavan-3-ol from leucoanthocyanidins (Tanner et al., 2003); and (ii) ANR, encoded by the BANYULS gene (Devic et al., 1999; Xie et al., 2003), synthesizing 2,3-cis-flavan-3-ol from anthocyanidins (Fig. 1). PA biosynthesis in Arabidopsis is exclusively epicatechin (2,3-cis-flavan-3-ol)-based and limited to the seed coat, while many other plants produce both epicatechin and catechin (2,3-trans-flavan-3-ol)-based PAs of various amounts and compositions and in a range of different tissues (Dixon et al., 2005). Arabidopsis lacks a functional LAR gene and it is not known what regulates LAR expression in other plants (Bogs et al., 2007). In apple fruit (Malus × domestica Borkh.), the flavonoid biosynthetic pathway is most active in the skin (Lister et al., 1994) with the trans- and cis-flavan-3-ols being catechin and epicatechin, respectively, acting as the initiating units for the synthesis of PA polymers. In apple (Takos et al., 2006c), the MdLAR1, MdLAR2, and MdANR genes were cloned from the Cripps Red variety and real-time PCR carried out to correlate steady-state transcript levels with flavonoid accumulation (Takos et al., 2006c). The results from this study indicated that transcription of these PA synthesis genes in apple skin was differentially regulated from transcription of other flavonoid pathway genes.
Recent studies have elucidated the transcriptional regulation of anthocyanin accumulation in apple skin and flesh (Takos et al., 2006a; Ban et al., 2007; Espley et al., 2007, 2009). Several of the flavonoid biosynthesis pathway genes involved in anthocyanin synthesis and accumulation are also required for the synthesis of PAs and flavonols (Fig. 1). However, the exact processes involved are not fully understood. The regulation of PA biosynthesis and accumulation has also been described in persimmon (Ikegami et al., 2007; Akagi et al., 2009a,b; Ikegami et al., 2009) and grape (Czemmel et al., 2009; Gagne et al., 2009; Terrier et al., 2009) fruit. Recently an apple orthologue of Arabidopsis TRANSPARENT TESTA GLABRA1 (TTG1), involved in production of seed coat PA-based colour, was shown to activate the AtBAN promoter (Brueggemann et al., 2010). While these studies contribute to a global picture of flavonoid regulation, the specific transcriptional regulation of PAs in apple fruit is still not well understood.
In this study, we compared three apple cultivars: two heritage cultivars, Hetlina and Devonshire Quarrenden with high PA concentrations, and a commercial cultivar, Royal Gala, with a low PA concentration. To understand better the genes involved in the regulation of PAs in the flesh and skin of these cultivars, we isolated cDNA over a developmental period and compared the expression of flavonoid biosynthesis pathway structural genes by real-time quantitative PCR (qPCR). HPLC analysis provided an insight into the accumulation of PAs and other polyphenolics in these different apple cultivars. The results suggested that the regulation of gene expression of the apple PA pathway in fruit is well controlled, in a tissue- and developmentally specific fashion. However, comparisons of transcript levels between cultivars do not explain differences in final PA concentration or the magnitude of differences between tissues. Future study is required on the possible mechanism controlling final PA concentration in apple.
Material and methods
Tissue collection
Developing apple fruit from three cultivars were collected at five times throughout the 2007–2008 growing season. Because of differing flowering dates and maturity rates, each cultivar was sampled at five stages based on known developmental indices: 37, 65, 72, 100, and 128 days after full bloom (DAFB, tree ripe—as assessed by starch index and internal ethylene levels) for Hetlina; 30, 37, 65, 72, and 114 DAFB (tree ripe) for Devonshire Quarrenden; and 37, 65, 100, 128, and 135 DAFB (tree ripe) for Royal Gala. These fruit were sampled from trees at the Plant & Food Research orchard in Havelock North, Hawke’s Bay, New Zealand. Twelve fruit were collected for each cultivar, from two different trees (Royal Gala and Hetlina) or one tree (Devonshire Quarrenden). For each apple, the skin, excised from the cortex, and the skin-free cortex were stored separately, frozen immediately in liquid nitrogen and stored at –80 °C. Confirmatory samples of stage 5 (tree ripe) apple fruit from the three cultivars were also taken during the 2009–2010 growing season.
Real-time qPCR expression analysis
RNA was isolated from the tissue samples collected (see above) using a method adapted from that previously described by Chang et al. (1993). The RNA was treated with DNAse I using an Ambion DNA-free™ kit. After DNAse I treatment, cDNA synthesis was carried out on 2 µg of each RNA sample using anchored-oligo(dT)18 primers and random hexamer primers following the protocol set out in the Roche Transcriptor First Strand cDNA synthesis kit.
Genes encoding apple flavonoid biosynthesis pathway enzymes and branch points were identified by best BLAST homology in the Plant & Food Research EST database (BioPipe and BioView – automated cDNA sequence annotation pipeline and viewer system © Dr Ross Crowhurst and The New Zealand Institute for Plant & Food Research Ltd) and in the apple genome (Velasco et al., 2010). Where a location in the genome was apparent, with no contradiction between haplotypes, linkage group, and approximate position (to the nearest 100kb), this is indicated on Fig. 1. Where putative gene family members existed, candidates were selected by abundance in fruit library tissues. Gene-specific primers corresponding to these genes were designed using Vector NTI version 9.0.0 (http://www.invitrogen.com) to a stringent set of criteria, enabling application of universal reaction conditions. The sequences of each primer pair and the relevant accession numbers are shown in Supplementary Table S1 at JXB online.
qPCR DNA amplification and analysis was carried out using a LightCycler® 480 Real-Time PCR System (Roche Diagnostics). All reactions were performed using the LightCycler® 480 SYBR Green I Master Mix (Roche Diagnostics) according to the procedure described by the manufacturer. Reactions were performed four times using 2.5 µl Master Mix, 0.25 µl each primer (10 µM), 1.25 µl diluted cDNA (1:50) and nuclease-free water (Roche Diagnostics) to a final volume of 5 µl. A negative water control was included in each run. Fluorescence was measured at the end of each annealing step. Amplification was followed by melting curve analysis with continual fluorescence data acquisition during the 65–95 °C melt. For each gene, a standard curve was generated using a cDNA serial dilution, and the resultant PCR efficiency calculations (ranging between 1.443 and 2.00) were imported into the relative expression data analysis. Relative expression levels were quantified using a developed quantification method (Andre et al., 2009). Analysis of the raw data for this study used a single reference gene and gene-specific amplification efficiencies. Malus × domestica Actin (MdActin, GenBank accession number CN938023) was selected as the reference gene because of its consistent transcript level throughout fruit tissues and leaves. For each gene, a standard curve was generated using a cDNA serial dilution, and the resultant PCR efficiency (E) values (ranging between 1.443 and 2.00) were imported into the relative expression data analysis. For each biological sample, the relative quantity (RQ) of each target gene was determined by calculating the difference in quantification cycle value (Cq) between the average Cp value for each sample (from four technical replicates) and a calibrator value (i.e. a fixed Cp value was used for all samples; the minimum Cp value over the entire experiment).
RQ = EΔCq
where △Cq = Cqcalibrator – Cqtarget
The RQs of the target genes were normalized to the relative quantities of the reference gene MdActin to give normalized RQs (NRQs):
NRQ = RQtarget/RQactin
NRQ values were further rescaled to the sample with the lowest RQ over the entire experiment:
Rescaled NRQ = NRQtarget/NRQlowest
Error bars shown in the qPCR data are technical replicates, representing the standard deviation (SD) of four replicate qPCRs. These rescaled normalized relative quantities were used to compare the expression levels of the structural genes in the flavonoid biosynthetic pathway among the three apple cultivars over a developmental series.
Extraction and Identification of polypropanoids
Tissue samples from the 12 fruit collected for each cultivar, Hetlina, Devonshire Quarrenden, and Royal Gala, were used for extraction and identification of polypropanoids by HPLC.
For the total phenolics and flavan-3-ols developmental series analysis (Fig. 2), frozen samples of the skin and cortex from each cultivar at each developmental time from the 2007/2008 growing season were freeze dried, ground to powder under low light conditions, and the polyphenols extracted using absolute ethanol:water:formic acid (80:20:1, v/v/v) extraction buffer at a 5:1 buffer:sample ratio. The extraction mixture was homogenized using a vortex for 30 s and then incubated at 4 °C for 24h. After centrifugation at 3000 g for 10min, the supernatant was collected and stored at –20°C. These extractions represented triplicate technical replicates of each tissue sample.
For mature fruit analysis (Fig. 3), frozen samples of the skin (0.5g) and cortex (2g) from each cultivar at the final tree-ripe stage 5 time point, from both the 2007/2008 and the 2009/2010 growing seasons, were ground under liquid nitrogen and the polyphenols extracted using 10ml absolute ethanol:water (80:20, v/v) extraction buffer. The extraction mixture was homogenized using a vortex for 30 s and then shaken for 2h at room temperature. After centrifugation at 6000 g for 15min, the supernatant was collected and dried by evaporation using a Centrivap. The resulting pellet containing polyphenols was resuspended in 1ml water:absolute ethanol (50:50, v/v), filtered through a 0.45 µm filter, and stored at –20°C. These extractions represented triplicate technical replicates of the samples analysed.
HPLC analysis
For total phenolics and flavan-3-ols developmental series HPLC (Fig. 2), the HPLC system used to measure polyphenolics in sample extracts was a Waters Alliance 2690 with a Waters 996 photodiode array and Waters 474 fluorescence detectors (Waters, Milford, MA). The analytical column was a Zorbax SB-C18 (150×4.6mm, 1.8 µm; Agilent, Melbourne, Australia) maintained at 40 °C. The injection volume was 5 µl. A gradient elution was performed with solvent A (5% formic acid in water) and solvent B (acetonitrile) at a flow rate of 0.8ml min–1. The solvent programme was as follows: 0–9min, linear gradient from 0 to 20% solvent B; 10–18min, linear gradient from 20 to 80% solvent B; 19–20min, 80% solvent B isocratic; 21–22min, linear gradient from 80 to 0% solvent B in order to return to the initial conditions before injecting another sample at 24min. Spectral data were collected for the entire run, and the polyphenolic components were quantified by extracting chromatograms at 280, 370, and 530nm. Quercetin, phloridzin, and phloridzin-xyloside were quantified at 280nm, quercetin glycosides and chlorogenic acid at 370nm, and cyanidin glycosides at 530nm. Catechin, epicatechin, and procyanidins were quantified using fluorescence detection with excitation at 276nm and emission at 316nm. Chromatographic data were collected and manipulated using the Chromeleon® Chromatography Management System version 6.8. The polyphenolic standards quercetin 3-O-rutinoside, quercetin 3-O-galactoside, quercetin 3-O-glucoside, quercetin 3-O-rhamnoside, cyanidin 3-O-glucoside, and procyanidin B2 were purchased from Extrasynthese (Genay, France). Epicatechin, catechin, chlorogenic acid, phloridzin, and quercetin were purchased from Sigma (Sydney, Australia). Total phenolics values were calculated by totalling individual detection data for catechin, epicatechin, procyanidin B2, chlorogenic acid, anthocyanin, quercetin derivatives (flavonols), and phloridzin. Error bars shown are technical replicates, representing the standard error (SE) of three replicate HPLC extractions.
Mature fruit HPLC identification of the polyphenolic compounds (Fig. 3) was performed using a Ultimate 3000 system (Dionex, Sunnyvale, CA) equipped with a diode array detector (DAD). A 5 µl aliquot was injected onto a Dionex C18 Acclaim PolarAdvantage II column (150×2.1mm internal diameter, 3 µm particle size). The mobile phases were water with 0.1% formic acid (A) and acetonitrile with 0.1% formic acid (B). The flow rate was 0.35ml min–1, and the column temperature was 35 °C. The 42min gradient was as follows: 0–5min, 0–8% B; 6–10min, 8–15% B; 11–20min, 15–20% B; 21–27min, 20% B linear; 28–34min, 27–100% B; 35–36min, 100% B linear; 37–42min, 0% B, re-equilibration time. Simultaneous monitoring was set at 254, 280, and 320nm, and at 520nm for quantification. Polyphenol compounds were identified by their retention time and spectral data compared with standards and were quantified using five-point calibration curves. High-UV-absorbing peaks were first putatively identified by liquid chromatography/mass spectrometry (LC-MS) from their mass spectra and from data in previous literature (Alonso-Salces et al., 2004; Volz and McGhie, 2011). These assignments were confirmed by HPLC-DAD comparison of their UV spectra and retention times with those of authentic standards when available. Flavonols, hydroxycinnamic acids, and anthocyanins were quantified as rutin, chlorogenic acid, and cyanidin-3-galactoside equivalents, respectively. Oligomeric (up to hexamer) and polymeric procyanidins were determined according to their mass spectra and data from previous studies (Guyot et al., 1998; Hamauzu et al., 2005) and were quantified as catechin equivalents. Error bars shown in the HPLC data are technical replicates, representing the SE of three replicate HPLC extractions.
Mass spectrometry
Identification of the main phenolic compounds was performed by LC-MS using a LCQ Deca ion trap mass spectrometer fitted with an electrospray ionization interface (ThermoQuest, Finnigan, San Jose, CA) and coupled to a Surveyor™ HPLC instrument. Column and elution conditions were as described above for the HPLC-DAD analysis. Spectra were recorded in positive ion mode between 100 and 2000 atomic mass units.
Statistical analysis of polyphenolic content
The statistical significance of variations in total polyphenolic compound content between cultivars was determined by one-way analysis of variance (ANOVA) of data (significant at P = 0.00001), followed by multiple comparisons using Tukey’s test for least-squared-difference (LSD at P = 0.05).
Phylogeny and sequence alignment
Protein consensus sequences from the three apple cultivars (Hetlina, Devonshire Quarrenden and Royal Gala) were aligned with published protein sequences of reductases and a phylogenetic tree was generated using Geneious Pro 4.8.5 Tree Builder (Drummond et al., 2010). The tree alignment options were: cost matrix = Blosum62, gap opening penalty = 10, gap extension penalty = 0.1, and alignment type = global alignment with free end gaps. The tree builder options were: genetic distance model = Jules–Cantor, tree build method = neighbour joining, and no outgroups.
Protein consensus sequences from the three apple cultivars were determined from the coding sequences of ANR, LAR1, and LAR2 from each cultivar and aligned with translated apple genome reference sequences (Velasco et al., 2010) and published protein sequences of ANR, LAR1, and LAR2 (Takos et al., 2006c) using Geneious Pro 4.8.5 Alignment (Drummond et al., 2010). The alignment options were: cost matrix = Blosum62, gap opening penalty = 10, gap extension penalty = 0.1, and alignment type = global alignment with free end gaps.
Results
HPLC analysis reveals polyphenolic diversity between the three apple cultivars
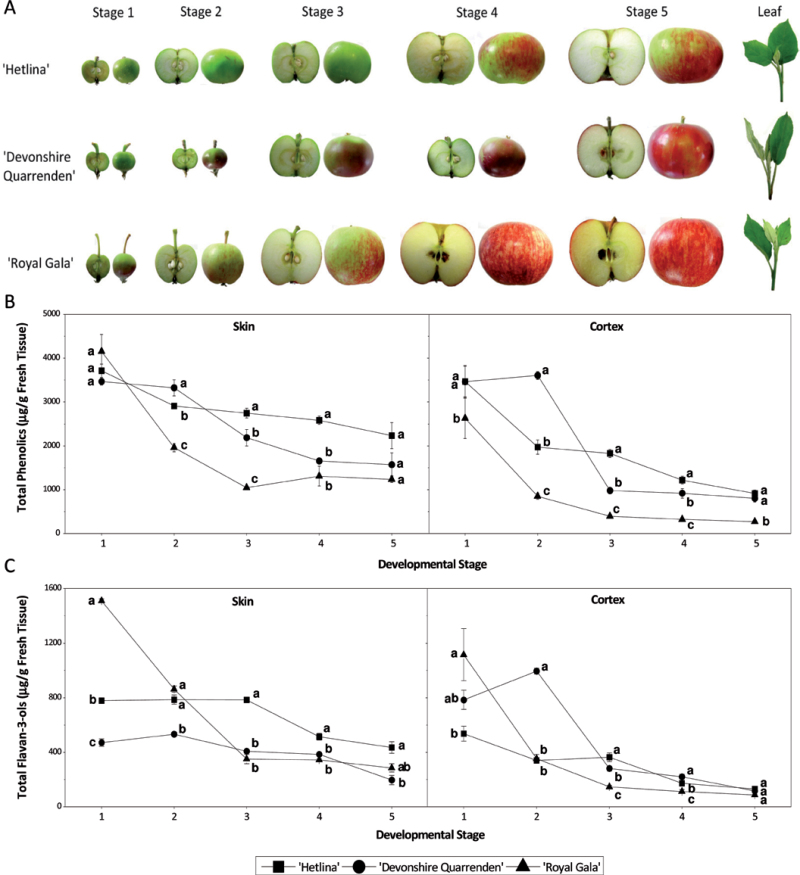
Three apple cultivars were chosen for this study based on polyphenolic compound concentrations identified in heritage and commercially grown apples (Volz and McGhie, 2011). The two heritage apple cultivars, Hetlina and Devonshire Quarrenden, have high polyphenolic concentrations, while the commercial cultivar Royal Gala has low polyphenolic concentrations (Volz and McGhie, 2011). A full developmental series of fruit tissue was collected for the three cultivars over the 2007/2008 growing season (Fig. 2A). As flavonoid concentrations are affected by environmental cues (Lin-Wang et al., 2011), the results were validated in a second season (2009/2010). Stages 1–5 represent five time points within the apple growing season that allowed full coverage of the season. The full developmental series was used for extraction for HPLC analysis of phenolic compound composition. When total phenolic composition was examined per gram of fresh weight, it was apparent that apple skin consistently contained higher amounts of polyphenolic compounds than apple cortex across the whole growing season (Fig. 2B). When only catechin and epicatechin (total flavan-3-ols) were examined (Fig. 2C) a similar trend was observed as for total phenolics (Fig. 2B) with a decline in the total amount over the growing season. When the proportions of each compounds’ contribution to the total phenolic profile were examined for both cortex and skin (Supplementary Fig. S1 in JXB online), the cortex was shown to contain predominantly chlorogenic acid with varying amounts of catechin, epicatechin, and procyanidin B2 across all three cultivars studied. Royal Gala showed the highest proportions of catechin, epicatechin, and procyanidin B2 across the growing season with Hetlina and Devonshire Quarrenden showing proportions similar to each other. The skin composition showed a significant decrease in the proportion of chlorogenic acid and an increase in flavonols and phloridzin. Royal Gala showed the highest catechin, epicatechin, and procyanidin B2 proportions of the three cultivars. Leaf tissue proportions were also calculated for Hetlina, Devonshire Quarrenden, and Royal Gala showing 85, 86, and 91% phloridzin content and 14, 13 and 8% flavonol content, respectively, with the other compounds studied making up the remainder (data not shown).
Fig. 2.
(A) Apple fruit developmental series. Developing Hetlina, Devonshire Quarrenden, and Royal Gala apple fruit were collected at five time points throughout the 2007/2008 growing season. (B) HPLC data of apple skin and cortex for total polyphenolic composition for Hetlina, Devonshire Quarrenden, and Royal Gala over a developmental series. (C) HPLC data of apple skin and cortex for total flavan-3-ols (catechin and epicatechin) for Hetlina, Devonshire Quarrenden, and Royal Gala over a developmental series. Error bars show SE. Data points with the same letter are not significantly different at P = 0.05, using one-way ANOVA analysis, followed by a multiple-comparison t-test.
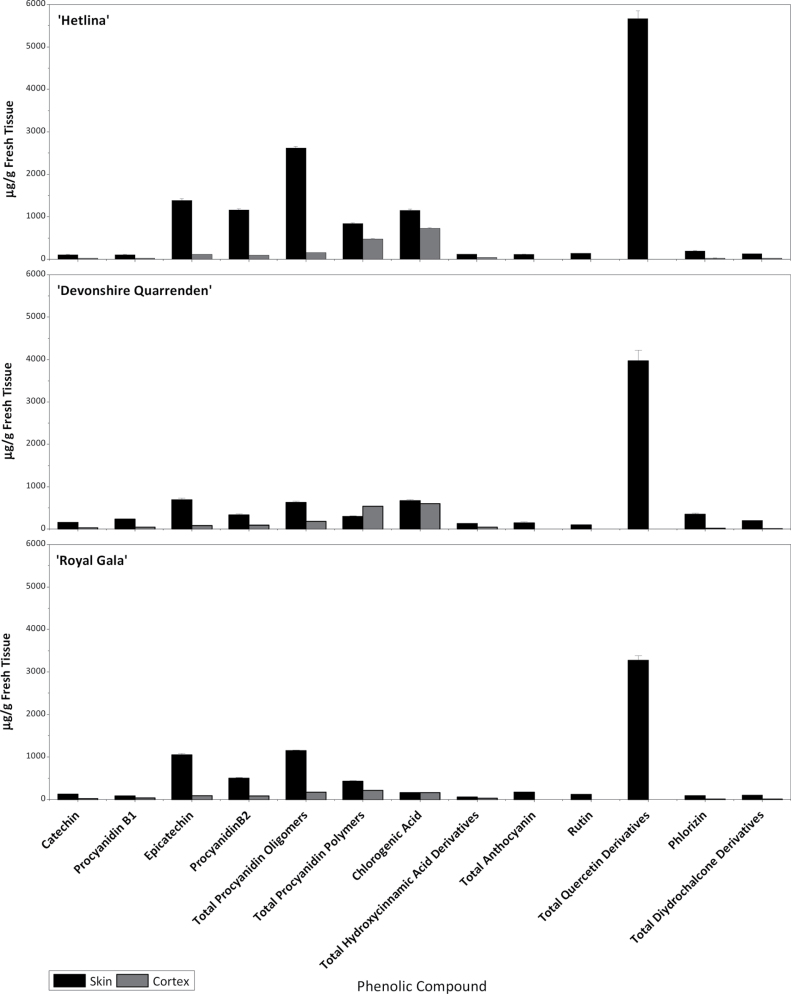
The phenolic composition of mature (stage 5) apples from the three cultivars was examined using an alternative HPLC protocol (Fig. 3 and Supplementary Table S2 at JXB online). For every compound, the phenolic amounts were higher in the apple skin than in the cortex, on a per gram of fresh weight basis. Apples make both catechin and epicatechin as primary subunits of procyanidin dimers and resulting oligomers and polymers. Catechin and procyanidin B1 (catechin dimer) were seen in all three cultivars, with the highest amounts in Devonshire Quarrenden. Amounts of epicatechin and procyanidin B2 (epicatechin dimmer) were highest in Hetlina skin. The procyanidin B2 concentrations were approximately half that of epicatechin in Devonshire Quarrenden and Royal Gala but not in Hetlina, where they were similar in concentration. This could be an indication of a ‘block’ in the pathway leading to dimerization for the Devonshire Quarrenden and Royal Gala cultivars. Hetlina also had significantly higher total procyanidin oligomer and polymer concentrations than Devonshire Quarrenden and Royal Gala. Total quercetin derivatives (Fig. 1, flavonol branch) showed very high levels in the skin, with Hetlina the highest and Royal Gala the lowest, with no detectable amounts in the cortex in all three cultivars. Anthocyanins showed a similar pattern to flavonols, found in the skin of all three cultivars and not detected in the cortex, with Royal Gala the highest and Hetlina the lowest.
Fig. 3.
HPLC analysis for identification of the polyphenolic compounds in tree-ripe (stage 5) apple skin and cortex from Hetlina, Devonshire Quarrenden, and Royal Gala cultivars. Error bars show SE.
RNA expression profile of the phenylpropanoid pathway reveals tissue and cultivar differences
With the recent publication of the apple genome sequence (Velasco et al., 2010), we were able to use the best BLAST match to known Arabidopsis genes to add several potential new steps to the phenylpropanoid pathway (Fig. 1). For example, good matches to gene family members for CHS and CHI were identified. In addition, linkage group information is shown in Fig. 1, which agrees with recent mapping and reverse genetics of apple phenylpropanoid quantitative trait loci (Chagne et al., 2012).
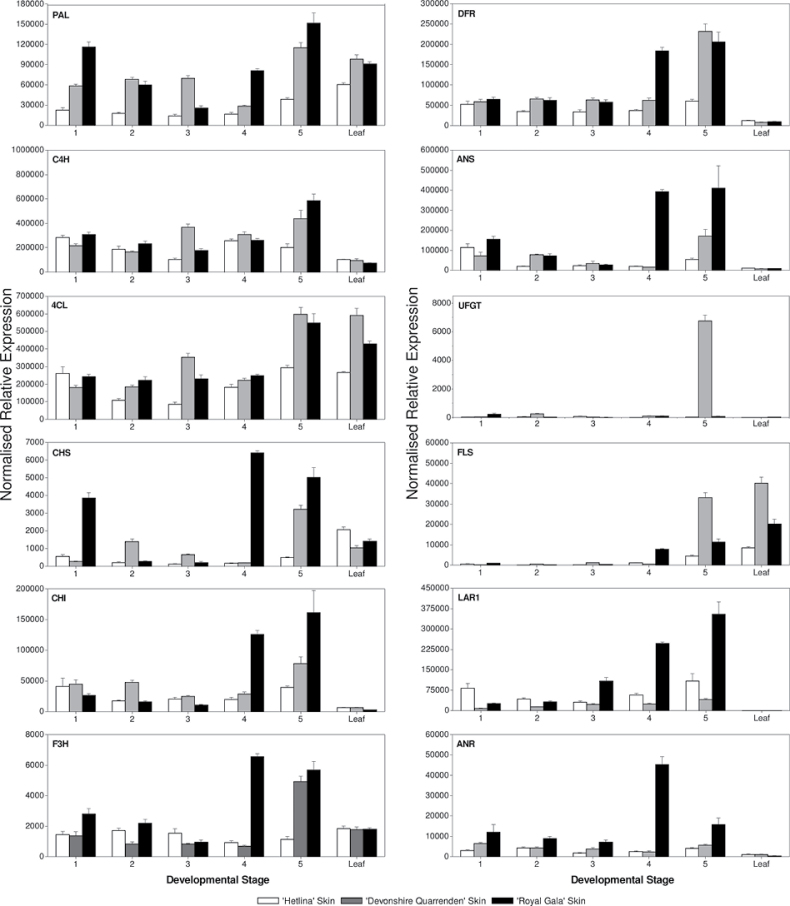
Gene expression analysis of apple skin (Fig. 4) revealed some surprising observations. Royal Gala showed the highest expression of the core phenylpropanoid biosynthetic genes PAL, C4H, CHS, CHI, F3H, DFR, and ANS. Genes encoding the synthesis of chlorogenic acid and phloretin/phloridzin have not yet fully been characterized. However, expression levels of the published LAR1 and ANR genes (Takos et al., 2006c) were also highest in Royal Gala skin. FLS expression was highest in Devonshire Quarrenden tree-ripe fruit but was still not as high as in leaf. A lower expression level of FLS in Hetlina could direct more substrate down the phenylpropanoid pathway towards anthocyanin and PAs. However, Hetlina also had the highest concentration of quercetin derivatives (Fig. 3). Expression analysis revealed that certain genes were expressed in a highly fruit-specific manner. All the assayed genes showed high expression values (normalized to MdActin) with good fold induction during fruit development. In general, transcript levels increased in the skin with fruit development, even though total skin phenolics declined on a fresh weight basis (Fig. 2B).
Fig. 4.
Real-time qPCR expression analysis for all major steps of the polyphenolic biosynthetic pathway in apple skin from Hetlina, Devonshire Quarrenden, and Royal Gala cultivars. MdActin used as the reference gene. Error bars show SD.
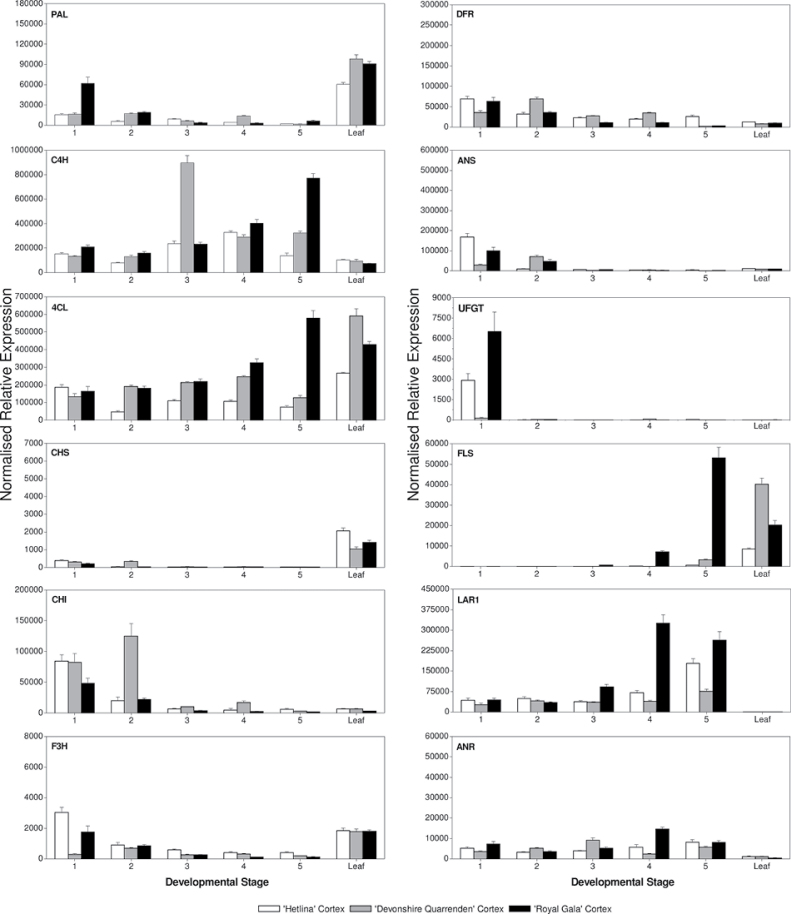
As apple flesh is by far the bulk of the tissue eaten by the consumer, the importance of phenolics in this tissue and the genes that control compound synthesis were examined separately (Fig. 5). In the cortex, there were generally much lower expression levels for PAL, CHS, CHI, F3H, DFR, and ANS compared with those in the skin, for all cultivars (Fig. 5). In contrast, cortical expression of C4H and 4CL was similar to skin expression levels (Figs 4 and 5). In the apple cortex, expression of the reductases LAR1 and ANR was significant, even though the flesh had lower catechin, epicatechin, procyanidin B1, and procyanidin B2 concentrations in the cortex (Fig. 3). LAR1 expression was similar in the cortex and skin (Figs 4 and 5). ANR expression levels were significantly lower in Royal Gala flesh than in skin. However, in Hetlina and Devonshire Quarrenden, ANR expression levels were similar in skin and flesh.
Fig. 5.
Real-time qPCR expression analysis for all major steps of the polyphenolic biosynthetic pathway in apple cortex from three apple cultivars, Hetlina, Devonshire Quarrenden, and Royal Gala. MdActin used as the reference gene. Error bars show SD.
Another striking example of mRNA expression not corresponding to compound concentrations occurred with flavonol synthesis. Quercetin derivatives were very high in the skin but not detected in the cortex using HPLC (Supplementary Table S1). However, in Royal Gala, FLS showed cortical expression that was higher than skin expression at maturity (relative to reference genes). Expression of FLS in Hetlina was lower but still significant. As a proportion, flavonols made up almost 50% of skin total polyphenolics in all three cultivars, and nothing in the flesh (Supplementary Fig. S1). Therefore, other processes must be occurring to explain the absence of flavonols in the flesh.
Phylogenetic analysis of apple phenolic reductases
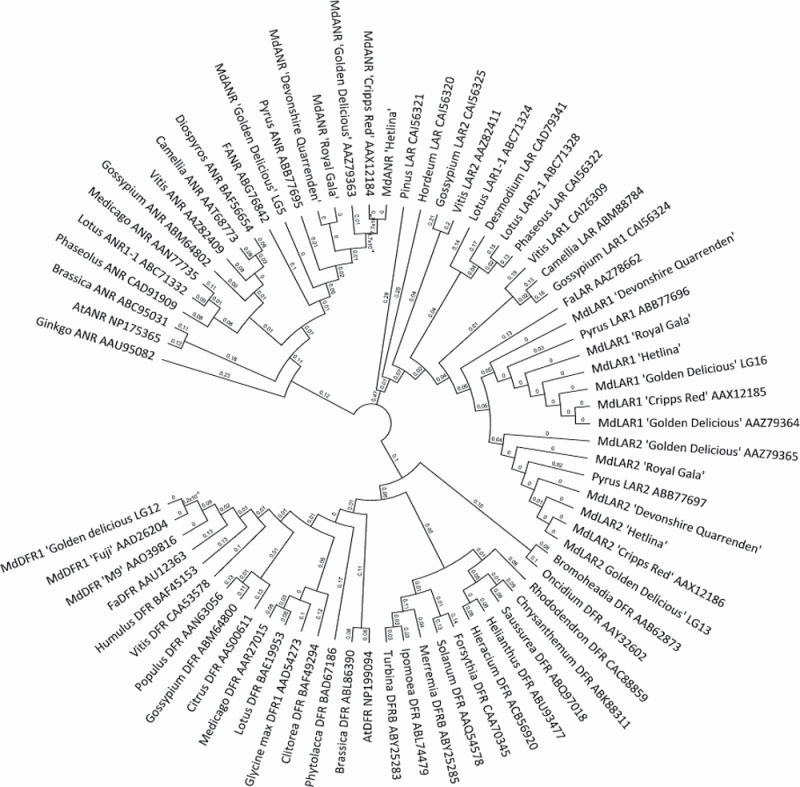
Because of the lack of correlation between gene expression and polyphenolic concentrations, we sequenced the full-length genes of the reductase family members (LAR1, LAR2, and ANR) from all three cultivars in our study and compared these with sequences from the recently published Golden Delicious (Velasco et al., 2010) and Cripps Red (Takos et al., 2006c) reductases. The deduced protein sequences showed high sequence homology among the three cultivars studied here and the additional cultivars. All Malus × domestica ANR protein sequences clustered together, with the addition of the Pyrus ANR sequence, as did LAR1, LAR2, and DFR1 (Fig. 6).
Fig. 6.
Phylogenetic relationships for the deduced amino acid sequences of the ANR, LAR1, LAR2, and DFR biosynthetic genes from Malus × domestica and other species. All ANR protein sequences clustered together, as did LAR1, LAR2, and DFR.
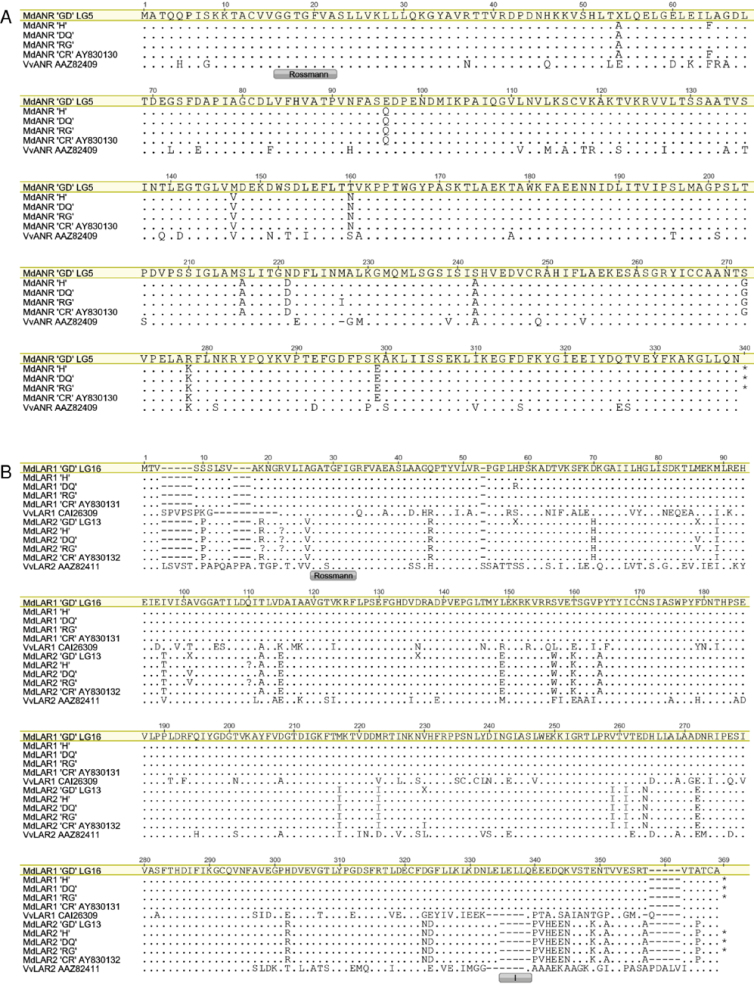
The LAR1 protein sequences were almost identical among the five cultivars (Fig. 7B). Only a single amino acid residue appeared to differ in Devonshire Quarrenden at position 59. As this alteration did not occur in Hetlina, it is unlikely to be responsible for a difference in compound concentrations among cultivars. LAR1 and LAR2 also shared high sequence homology among cultivars and with one another, and were on homologous linkage groups (LG16 and LG13, respectively). They are therefore likely to represent a genome-wide duplication that occurred in apple evolution (Velasco et al., 2010). All LAR1 sequences contained the motif LELLQEEEDQK located between residues 335 and 345, while LAR2 sequences contained PVHEEN in this region at the C-terminal end (without the protein sequence marked I in Fig. 7B). LAR1 and LAR2 also both contained sequence motifs common to the reductase–epimerase–dehydrogenase family of proteins. These included the Rossmann dinucleotide-binding domain (sequence motif GXXGXXG) starting at Gly19 and three residues, Ser129, Tyr148, and Lys151, critical for the catalytic site (Takos et al., 2006c).
Fig. 7.
Protein sequence alignment of various apple cultivars for (A) ANR protein and (B) LAR1 and LAR2 protein. The conserved reductase Rossmann dinucleotide-binding domain (Bottoms et al., 2002) is shown for ANR, LAR1, and LAR2. An additional motif at the C-terminal end differing between LAR1 and LAR2 is marked I (Takos et al., 2006c).
Alignment of the ANR protein sequences also showed high sequence homology with very little variation among cultivars or in comparison with the grape ANR protein sequence. The ANR protein sequences for all the cultivars studied contained the Rossmann dinucleotide-binding domain (motif GXGXXA) starting at Gly17 (Takos et al., 2006c). There were 11 amino acid differences between the Golden Delicious sequence and the other cultivars (Fig. 7A). However, none of these changes correlated with differences between high and low polyphenolic apples. Most changes were within the Golden Delicious reference, so may represent sequencing errors from the high-throughput approach used to generate the genome sequence (Velasco et al., 2010). At position 65, there was an amino acid change in Hetlina and Cripps Red, while at position 228 Royal Gala had a unique amino acid change. These may be relevant for enzymatic activity.
Discussion
Apples are a major source of dietary polyphenolics (Sampson et al., 2002). These compounds have beneficial effects for human health, as well as implications for apple taste and appearance. We sought to characterize the accumulation of PAs, anthocyanins, and flavonols in diverse genotypes of apple. Apples contain high concentrations of these compounds, and there are a diversity of different types, including flavonols (quercetin glycosides), cinnamic acids (chlorogenic and caffeic acids), flavanols (catechin, epicatechin, and polymeric PAs), dihydrochalcones (phloridzin), and anthocyanins (cyanidin glycosides) (McGhie et al., 2005). The profile of these types is affected by cultivar (McGhie et al., 2005; Wojdylo et al., 2008), environment (McGhie et al., 2005; Lin-Wang et al., 2011), and growth conditions (Takos et al., 2006b). Our results confirmed previous reports of restriction of some polyphenolic components to the skin, for example, anthocyanins and flavonols (quercetin glycosides) (Takos et al., 2006a,b,c; Ban et al., 2007; Espley et al., 2007). Other compounds such as chlorogenic acid, phloridzin, epicatechin, and condensed tannins are present in both skin and flesh (McGhie et al., 2005; Volz and McGhie, 2011).
Our HPLC analysis showed that tree-ripe fruit from the heritage cultivar Hetlina contained higher amounts of phenolic compounds than the commercial cultivar Royal Gala or the heritage cultivar Devonshire Quarrenden. PAs and condensed tannins are the predominant apple phenolic compounds, making up 80% of the total (Wojdylo et al., 2008). We have compared the genes involved in the production of PAs in three apple cultivars: two heritage apple cultivars, Hetlina and Devonshire Quarrenden, with high polyphenolic amounts, and a commercial cultivar, Royal Gala, with low polyphenolic amounts. Epicatechin and catechin biosynthesis is under the control of biosynthetic enzymes ANR and LAR1, respectively. Counter-intuitively, real-time qPCR analysis showed that expression levels of Royal Gala LAR1 and ANR were significantly higher than those of both Devonshire Quarrenden and Hetlina. However, although Royal Gala was lowest in total polyphenolics and flavan-3-ols, the proportion of flesh and skin polyphenolics that were in the PA fraction was highest in Royal Gala.
The expression of apple flavonoid pathway genes and PA concentration has been measured previously (Takos et al., 2006b; Szankowski et al., 2009). In the study carried out by Takos et al. (2006b), fruit bagging of red skinned Cripps’ Red resulted in downregulation of the entire apple anthocyanin and flavonol pathway. Transcripts of ANR and LAR1 were less affected by bagging. For example, there was a 40- and 70-fold decrease in UFGT and FLS transcript levels, respectively, without light, while ANR and LAR1 declined only two- to four-fold (Takos et al., 2006b). Apple skin PAs were little affected by this treatment (100 d in bags on the tree), while anthocyanins disappeared completely and flavonols declined by 40% (Takos et al., 2006b). Further investigation is required into the regulation of these key enzymes in apple.
A study by Szankowski et al. (2009) investigated the consequences of blocking anthocyanin biosynthesis in apple by silencing ANS in transgenic plants of a red-leaved apple cultivar. As expected, anthocyanin biosynthesis was strongly reduced. What was not expected was an increase in epicatechin biosynthesis in the ANS-silenced plants, as well as a strong decrease in the epicatechin derivatives procyanidin B2, B5, and E-B5. There was a decrease in ANR transcript levels, which is counter-intuitive to the increase in epicatechin biosynthesis. Szankowski et al. (2009) suggested a second biosynthetic pathway to epicatechin as an alternative explanation for the increase in epicatechin amounts in the ANS-silenced apples. They suggested several alternative hypotheses for the increase in epicatechin, such as epimerization of catechin to epicatechin, depolymerization in a non-stereospecific manner from oligomeric epicatechin derivatives, or redundant secondary ANS enzyme activity of another dioxygenase of the flavonoid pathway. Royal Gala, a commercial variety not known for its astringency, displayed significantly higher gene expression levels for key procyanidin biosynthesis enzymes such as LAR1 and ANR than the heritage cultivars Hetlina and Devonshire Quarrenden. There appear to be other events occurring in each cultivar that contribute to the final polyphenolic profile.
A study into apple flesh polyphenolic content suggested that polyphenols accumulated in the first 30–50 d of fruit development (Renard et al., 2007), followed by a marked decrease in concentration between 30 and 60 d for both cider and table apple cultivars. After that point, polyphenol concentration would evolve mostly by dilution, which they postulated could also explain the dramatic decrease in total phenolics towards maturity of the fruit. Renard et al. (2007) suggest a possible link between fruit growth phases and polyphenol accumulation via modifications of enzyme activities, as well as a correlation between the end of active biosynthesis and the beginning of cell enlargement. They concluded that the possible mechanisms are still unknown but that the metabolic shift between cell proliferation and cell expansion clearly appears to include the polyphenol biosynthesis pathway. Our observation that the transcripts of ANR and LAR1 increased towards maturity further suggests that enzymatic activities are vital to PA and condensed tannin concentrations as fruit mature.
Our study showed that procyanidin concentration in apple is only partially regulated at the transcriptional level. As can be seen in Fig. 2, total phenolics decreased according to the developmental stage, probably associated with a decrease in astringency, whereas the relative gene expression data followed the opposite trend. For example, apples at stages 4 and 5 generally had higher levels of gene expression for most enzymes and in all cultivars. This suggests the presence of degradation processes or polymerization to an extent that the compounds are no longer extracted, which increases with maturity. Alternatively, polyphenolic content per gram of ry weight may be relatively constant, suggesting a dilution effect as fruit grows towards maturity.
The discrepancies between metabolite concentrations and gene expression could be explained by the relative importance of some of the following factors: (i) spontaneous chemical degradation of the compounds due to variations in the conditions in planta (such as pH); (ii) active degradation of the compounds by enzymes such as oxidases and peroxidises; (iii) variation of biosynthetic enzymatic activities by post-translational modification; (iv) an absence of substrate supply; and (v) the presence of an additional reductase or redundant secondary ANS enzyme activity of another dioxygenase of the flavonoid pathway.
Conclusions
In this study, we have shown strong evidence that the procyanidin concentration observed in both heritage and commercial apple cultivars is only partially regulated at the transcriptional level, with a combination of events occurring in each cultivar to contribute to a final polyphenolic profile. In order to determine the regulatory events that are occurring in apple fruit, both transcriptional and post-translational modifications of the biosynthetic steps need investigation. The ratio of catechin:epicatechin in the PA polymers determined via thiolysis of the condensed tannins will also highlight a point at which epicathechin and catechin amounts are determined.
Supplementary Material
Supplementary data
Fig. S1. Pie charts showing proportions of individual polyphenolic compounds that constitute the total polyphenolic amounts displayed in Fig. 1B for (a) skin and (b) cortex for each of the cultivars Hetlina, Devonshire Quarrenden and Royal Gala.
Table S1. Forward and reverse primers for the apple genes used in qPCR analysis.
Table S2. HPLC analysis for identification of the polyphenolic compounds in tree-ripe (stage 5) apple skin and cortex from Hetlina, Devonshire Quarrenden and Royal Gala cultivars.
Acknowledgements
This research was funded by the New Zealand Foundation for Research Science and Technology (Horticultural Genomics C06X0812). Thanks to Richard Volz and Claire Whitworth (Plant & Food Research, Hawke’s Bay, New Zealand) for providing developmental series fruit and Linda Boyd for her help with the statistical analysis. We also thank William Laing and Anne Gunson for their comments on the manuscript.
References
- Akagi T, Ikegami A, Suzuki Y, Yoshida J, Yamada M, Sato A, Yonemori K. 2009a. Expression balances of structural genes in shikimate and flavonoid biosynthesis cause a difference in proanthocyanidin accumulation in persimmon (Diospyros kaki Thunb.) fruit Planta 230 899–915 [DOI] [PubMed] [Google Scholar]
- Akagi T, Ikegami A, Tsujimoto T, Kobayashi S, Sato A, Kono A, Yonemori K. 2009b. DkMyb4 is a Myb transcription factor involved in proanthocyanidin biosynthesis in persimmon fruit Plant Physiology 151 2028–2045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida JRM, D’Amico E, Preuss A, et al. 2007. Characterization of major enzymes and genes involved in flavonoid and proanthocyanidin biosynthesis during fruit development in strawberry (Fragaria3ananassa) Archives of Biochemistry and Biophysics 465 61–71 [DOI] [PubMed] [Google Scholar]
- Alonso-Salces RM, Ndjoko K, Queiroz EF, Ioset JR, Hostettmann K, Berrueta LA, Gallo B, Vicente F. 2004. On-line characterisation of apple polyphenols by liquid chromatography coupled with mass spectrometry and ultraviolet absorbance detection Journal of Chromatography A 1046 89–100 [PubMed] [Google Scholar]
- Andre CM, Schafleitner R, Legay S, Lefevre I, Aliaga CAA, Nomberto G, Hoffmann L, Hausman JF, Larondelle Y, Evers D. 2009. Gene expression changes related to the production of phenolic compounds in potato tubers grown under drought stress Phytochemistry 70 1107–1116 [DOI] [PubMed] [Google Scholar]
- Aron PM, Kennedy JA. 2008. Flavan-3-ols: nature, occurrence and biological activity Molecular Nutrition & Food Research 52 79–104 [DOI] [PubMed] [Google Scholar]
- Ban Y, Honda C, Hatsuyama Y, Igarashi M, Bessho H, Moriguchi T. 2007. Isolation and functional analysis of a MYB transcription factor gene that is a key regulator for the development of red coloration in apple skin Plant and Cell Physiology 48 958–970 [DOI] [PubMed] [Google Scholar]
- Bogs J, Downey MO, Harvey JS, Ashton AR, Tanner GJ, Robinson SP. 2005. Proanthocyanidin synthesis and expression of genes encoding leucoanthocyanidin reductase and anthocyanidin reductase in developing grape berries and grapevine leaves Plant Physiology 139 652–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogs J, Jaffe FW, Takos AM, Walker AR, Robinson SP. 2007. The grapevine transcription factor VvMYBPA1 regulates proanthocyanidin synthesis during fruit development Plant Physiology 143 1347–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottoms CA, Smith PE, Tanner JJ. 2002. A structurally conserved water molecule in Rossmann dinucleotide-binding domains Protein Science 11 2125–2137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemann J, Weisshaar B, Sagasser M. 2010. A WD40-repeat gene from Malus3domestica is a functional homologue of Arabidopsis thaliana TRANSPARENT TESTA GLABRA1 Plant Cell Reports 29 285–294 [DOI] [PubMed] [Google Scholar]
- Chagne D, Krieger C, Rassam M, et al. QTL and candidate gene mapping for polyphenolic composition in apple fruit. BMC Plant Biology. 2012;12:12. doi: 10.1186/1471-2229-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S, Puryear J, Cairney J. 1993. A simple and efficient method for isolating RNA from pine trees Plant Molecular Biology Reporter 11 113–116 [Google Scholar]
- Czemmel S, Stracke R, Weisshaar B, Cordon N, Harris NN, Walker AR, Robinson SP, Bogs J. 2009. The grapevine R2R3-MYB transcription factor VvMYBF1 regulates flavonol synthesis in developing grape berries Plant Physiology 151 1513–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devic M, Guilleminot J, Debeaujon I, Bechtold N, Bensaude E, Koornneef M, Pelletier G, Delseny M. 1999. The BANYULS gene encodes a DFR-like protein and is a marker of early seed coat development The Plant Journal 19 387–398 [DOI] [PubMed] [Google Scholar]
- Dixon RA, Xie DY, Sharma SB. 2005. Proanthocyanidins – a final frontier in flavonoid research? New Phytologist 165 9–28 [DOI] [PubMed] [Google Scholar]
- Drummond AJ, Ashton B, Buxton S, et al. 2010. Geneious v5.5: http://www.geneious.com/web/geneious/
- Espley RV, Brendolise C, Chagne D, et al. 2009. Multiple repeats of a promoter segment causes transcription factor autoregulation in red apples Plant Cell 21 168–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espley RV, Hellens RP, Putterill J, Stevenson DE, Kutty-Amma S, Allan AC. 2007. Red colouration in apple fruit is due to the activity of the MYB transcription factor, MdMYB10. The Plant Journal 49 414–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forkner RE, Marquis RJ, Lill JT. 2004. Feeny revisited: condensed tannins as anti-herbivore defences in leaf-chewing herbivore communities of Quercus Ecological Entomology 29 174–187 [Google Scholar]
- Gagne S, Lacampagne S, Claisse O, Geny L. 2009. Leucoanthocyanidin reductase and anthocyanidin reductase gene expression and activity in flowers, young berries and skins of Vitis vinifera L. cv. Cabernet-Sauvignon during development Plant Physiology and Biochemistry 47 282–290 [DOI] [PubMed] [Google Scholar]
- Guyot S, Marnet N, Laraba D, Sanoner P, Drilleau JF. 1998. Reversed-phase HPLC following thiolysis for quantitative estimation and characterization of the four main classes of phenolic compounds in different tissue zones of a French cider apple variety (Malus domestica var. Kermerrien) Journal of Agricultural and Food Chemistry 46 1698–1705 [Google Scholar]
- Hamauzu Y, Yasui H, Inno T, Kume C, Omanyuda M. 2005. Phenolic profile, antioxidant property, and anti-influenza viral activity of Chinese quince (Pseudocydonia sinensis Schneid.), quince (Cydonia oblonga Mill.), and apple (Malus domestica Mill.) fruits Journal of Agricultural and Food Chemistry 53 928–934 [DOI] [PubMed] [Google Scholar]
- Holton TA, Cornish EC. 1995. Genetics and biochemistry of anthocyanin biosynthesis Plant Cell 7 1071–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami A, Akagi T, Potter D, Yamada M, Sato A, Yonemori K, Kitajima A, Inoue K. 2009. Molecular identification of 1-cys peroxiredoxin and anthocyanidin/flavonol 3-O-galactosyltransferase from proanthocyanidin-rich young fruits of persimmon (Diospyros kaki Thunb.) Planta 230 841–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami A, Eguchi S, Kitajima A, Inoue K, Yonemori K. 2007. Identification of genes involved in proanthocyanidinbiosynthesis of persimmon (Diospyros kaki) fruit Plant Science 172 1037–1047 [Google Scholar]
- Lee KW, Kim YJ, Kim DO, Lee HJ, Lee CY. 2003. Major phenolics in apple and their contribution to the total antioxidant capacity Journal of Agricultural and Food Chemistry 51 6516–6520 [DOI] [PubMed] [Google Scholar]
- Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, Caboche M. 2006. Genetics and biochemistry of seed flavonoids Annual Review of Plant Biology 57 405–430 [DOI] [PubMed] [Google Scholar]
- Lin-Wang K, Micheletti D, Palmer J, et al. 2011. High temperature reduces apple fruit colour via modulation of the anthocyanin regulatory complex Plant, Cell & Environment 34 1176–1190 [DOI] [PubMed] [Google Scholar]
- Lister CE, Lancaster JE, Sutton KH, Walker JRL. 1994. Developmental changes in the concentration and composition of flavonoids in skin of a red and a green apple cultivar Journal of the Science of Food and Agriculture 64 155–161 [Google Scholar]
- McGhie TK, Hunt M, Barnett LE. 2005. Cultivar and growing region determine the antioxidant polyphenolic concentration and composition of apples grown in New Zealand Journal of Agricultural and Food Chemistry 53 3065–3070 [DOI] [PubMed] [Google Scholar]
- Renard CM, Dupont N, Guillermin P. 2007. Concentrations and characteristics of procyanidins and other phenolics in apples during fruit growth Phytochemistry 68 1128–1138 [DOI] [PubMed] [Google Scholar]
- Sampson L, Rimm E, Hollman PC, de Vries JH, Katan MB. 2002. Flavonol and flavone intakes in US health professionals Journal of the American Dietetic Association 102 1414–1420 [DOI] [PubMed] [Google Scholar]
- Stevenson DE, Hurst RD. 2007. Polyphenolic phytochemicals – just antioxidants or much more? Cellular and Molecular Life Sciences 64 2900–2916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szankowski I, Flachowsky H, Li H, Halbwirth H, Treutter D, Regos I, Hanke MV, Stich K, Fischer TC. 2009. Shift in polyphenol profile and sublethal phenotype caused by silencing of anthocyanidin synthase in apple (Malus sp.) Planta 229 681–692 [DOI] [PubMed] [Google Scholar]
- Takos AM, Jaffe FW, Jacob SR, Bogs J, Robinson SP, Walker AR. 2006a. Light-induced expression of a MYB gene regulates anthocyanin biosynthesis in red apples Plant Physiol 142 1216–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takos AM, Robinson SP, Walker AR. 2006b. Transcriptional regulation of the flavonoid pathway in the skin of dark-grown ‘Cripps’ Red’ apples in response to sunlight Journal of Horticultural Science & Biotechnology 81 735–744 [Google Scholar]
- Takos AM, Ubi BE, Robinson SP, Walker AR. 2006c. Condensed tannin biosynthesis genes are regulated separately from other flavonoid biosynthesis genes in apple fruit skin Plant Science 170 487–499 [Google Scholar]
- Tanner GJ, Francki KT, Abrahams S, Watson JM, Larkin PJ, Ashton AR. 2003. Proanthocyanidin biosynthesis in plants: purification of legume leucoanthocyanidin reductase and molecular cloning of its cDNA Journal of Biological Chemistry 278 31647–31656 [DOI] [PubMed] [Google Scholar]
- Terrier N, Torregrosa L, Ageorges A, Vialet S, Verries C, Cheynier V, Romieu C. 2009. Ectopic expression of VvMybPA2 promotes proanthocyanidin biosynthesis in grapevine andsuggests additional targets in the pathway Plant Physiology 149 1028–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao R, Yang R, Xie S, Sockovie E, Khanizadeh S. 2005. Which polyphenolic compounds contribute to the total antioxidant activities of apple? Journal of Agricultural and Food Chemistry 53 4989–4995 [DOI] [PubMed] [Google Scholar]
- van der Sluis AA, Dekker M, de Jager A, Jongen WM. 2001. Activity and concentration of polyphenolic antioxidants in apple: effect of cultivar, harvest year, and storage conditions Journal of Agricultural and Food Chemistry 49 3606–3613 [DOI] [PubMed] [Google Scholar]
- Velasco R, Zharkikh A, Affourtit J, et al. 2010. The genome of the domesticated apple (Malus3domestica Borkh.) Nat Genet 42 833–839 [DOI] [PubMed] [Google Scholar]
- Vinson JA, Su XH, Zubik L, Bose P. 2001. Phenol antioxidant quantity and quality in foods: fruits Journal of Agricultural and Food Chemistry 49 5315–5321 [DOI] [PubMed] [Google Scholar]
- Volz RK, McGhie TK. 2011. Genetic variability in apple fruit polyphenol composition in Malus3domestica and Malus sieversii germplasm grown in New Zealand Journal of Agricultural and Food Chemistry 59 11509–11521 [DOI] [PubMed] [Google Scholar]
- Wojdylo A, Oszmianski J, Laskowski P. 2008. Polyphenolic compounds and antioxidant activity of new and old apple varieties Journal of Agricultural and Food Chemistry 56 6520–6530 [DOI] [PubMed] [Google Scholar]
- Wolfe K, Wu XZ, Liu RH. 2003. Antioxidant activity of apple peels Journal of Agricultural and Food Chemistry 51 609–614 [DOI] [PubMed] [Google Scholar]
- Wrangham RW, Conklin-Brittain NL, Hunt KD. 1998. Dietary response of chimpanzees and cercopithecines to seasonal variation in fruit abundance. I. Antifeedants International Journal of Primatology 19 949–970 [Google Scholar]
- Xie DY, Dixon RA. 2005. Proanthocyanidin biosynthesis – still more questions than answers? Phytochemistry 66 2127–2144 [DOI] [PubMed] [Google Scholar]
- Xie DY, Sharma SB, Paiva NL, Ferreira D, Dixon RA. 2003. Role of anthocyanidin reductase, encoded by BANYULS in plant flavonoid biosynthesis Science 299 396–399 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.