Abstract
The SE7 somaclonal line of finger millet (Eleusine coracana) achieved increased grain yield in field trials that apparently resulted from a higher number of inflorescences and seeds per plant, compared with the wild type. Levels of endogenous cytokinins, especially those of highly physiologically active iso-pentenyl adenine, were increased during early inflorescence development in SE7 plants. Transcript levels of cytokinin-degrading enzymes but not of a cytokinin-synthesizing enzyme were also decreased in young leaves, seedlings, and initiating inflorescences of SE7. These data suggest that attenuated degradation of cytokinins in SE7 inflorescences leads to higher cytokinin levels that stimulate meristem activity and result in production of more inflorescences. Gene expression was compared between SE7 and wild-type young inflorescences using the barley 12K cDNA array. The largest fraction of up-regulated genes in SE7 was related to transcription, translation, and cell proliferation, cell wall assembly/biosynthesis, and to growth regulation of young and meristematic tissues including floral formation. Other up-regulated genes were associated with protein and lipid degradation and mitochondrial energy production. Down-regulated genes were related to pathogen defence and stress response, primary metabolism, glycolysis, and the C:N balance. The results indicate a prolonged proliferation phase in SE7 young inflorescences characterized by up-regulated protein synthesis, cytokinesis, floral formation, and energy production. In contrast, wild-type inflorescences are similar to a more differentiated status characterized by regulated protein degradation, cell elongation, and defence/stress responses. It is concluded that attenuated degradation of cytokinins in SE7 inflorescences leads to higher cytokinin levels, which stimulate meristem activity, inflorescence formation, and seed set.
Key words: Cytokinin, cytokinin metabolism, finger millet, gene expression, inflorescence development, somaclonal variation
Introduction
Grain yield of crop plants largely depends on grain number per plant, which is correlated with the number of inflorescences formed in the meristems. To understand the mechanisms, which determine and control meristem differentiation is economically relevant, and enhanced inflorescence formation is important to maximize yield potential.
The balance between meristem maintenance and differentiation is mediated by auxin to cytokinin (CK) ratios (Barazesh and McSteen, 2008). CK deficiency diminishes the activity of vegetative and floral shoot apical meristems, indicating an absolute CK requirement for stimulation of cell division (Werner et al., 2003). CK levels depend on de novo synthesis, conjugation, and degradation, as well as on local and long-distance transport. Thereby, a precise CK homeostasis is maintained within a certain organ (Kudo et al., 2010). The CK biosynthesis pathway and the participating key genes have been identified (Kamada-Nobusada and Sakakibara, 2009; Frebort et al., 2011). Adenosine phosphate-iso-pentenyltransferase (IPT) catalyses the initial step of N 6-(Δ2-isopentenyl) adenine (iP) and trans-zeatin (tZ) biosynthesis utilizing dimethylallyl diphosphate (DMAPP) and ATP/ADP to generate iP-ribotides (Sakamoto et al., 2006). iP-ribotides are hydroxylated to tZ-ribotides by cytokinin trans-hydroxylase (CYP735A; Takei et al., 2004). tRNA isopentenyltransferase (tRNA-IPT) synthesizes cis-zeatin (cZ; Miyawaki et al., 2006). Conversion of iP-, tZ-, and cZ-ribotide 5′-monophosphate to active forms may occur by two-step activation. In this pathway, ribotides are dephosphorylated to ribosides and converted to free-base CKs (Kudo et al., 2010). The genes involved, however, have not been identified. In the direct activation pathway (Kuroha et al., 2009; Tokunaga et al., 2012), CK ribotide 5′-monophosphates are converted to free-base CKs by cytokinin nucleoside 5′-monophosphate phosphoribohydrolase, also called LONELY GUY (LOG; Kurakawa et al., 2007). CK is degraded by cytokinin oxidase/dehydrogenase (CKX; Galuszka et al., 2001; Schmülling et al., 2003), which is important for regulation of CK activity. Plants perceive and respond to CKs through two-component systems (Werner and Schmülling, 2009; Perilli et al., 2010; Müller, 2011) consisting of CK receptors and response regulators. Enzymes involved in CK biosynthesis, perception, and degradation are generally encoded by gene families (Müller, 2011).
Local CK biosynthesis within the meristem in rice is essential, as shown for log mutants encoding a CK biosynthesis gene. LOG loss-of-function mutants exhibit reduced CK levels and panicle size, branching, and numbers of flowers and stamens (Kurakawa et al., 2007). On the other hand, CKX loss of function results in increased CK levels and seed set in cereals (Ashikari et al., 2005; Zalewski et al., 2010) and Arabidopsis (Bartrina et al., 2011). CKs can affect different metabolic pathways stimulating assimilate transporters for nitrate, ammonium, sulphate, phosphate, and iron (Sakakibara, 2006; Séguéla et al., 2008; Werner et al., 2010). In barley, CKs participate in regulation of grain size, possibly by influencing both the accumulation and the duration of the filling period (Mechael and Seiler-Kelbitsch, 1972).
Finger millet [Eleusine coracana (L.) Gaerth.] is an ancient crop plant cultivated mainly as a cereal in the arid areas of Africa and Asia. Eleusine coracana is originally native to the Ethiopian Highlands and was introduced into India ~4000 years ago. It is very adaptable to higher elevations and is grown in the Himalaya up to 2300 m in elevation. It is estimated that finger millet is grown on ~38000 km2 of land (http://en.wikipedia.org/wiki/Eleusine_coracana). Due its ability to grow in semi-arid regions, tolerance to severe diseases, its nutritional values (especially a high methionine content), and good storage properties of grains, E. coracana can become an attractive crop in sustainable agriculture of developing countries contributing to a secure food resource. However, genetic characterization of finger millet is just beginning. Construction of genetic maps has been initiated (Dida et al., 2007) and comparative analysis revealed high levels of co-linearity between finger millet and rice genomes (Srinivasachary et al., 2007). Recently, a transformation protocol has been published for finger millet (Ceasar and Ignacimuthu, 2011).
In this work, the somaclonal line SE7 of finger millet, which exhibits decreased plant height and considerably increased grain yield compared with the wild type, is reported. Increased CK levels were found during early flower development together with decreased amounts of CKX transcripts. This suggests attenuated degradation of CKs in SE7 inflorescences resulting in higher levels of CKs, which stimulate meristem activity, inflorescence formation, and seed set.
Materials and methods
Plant materials
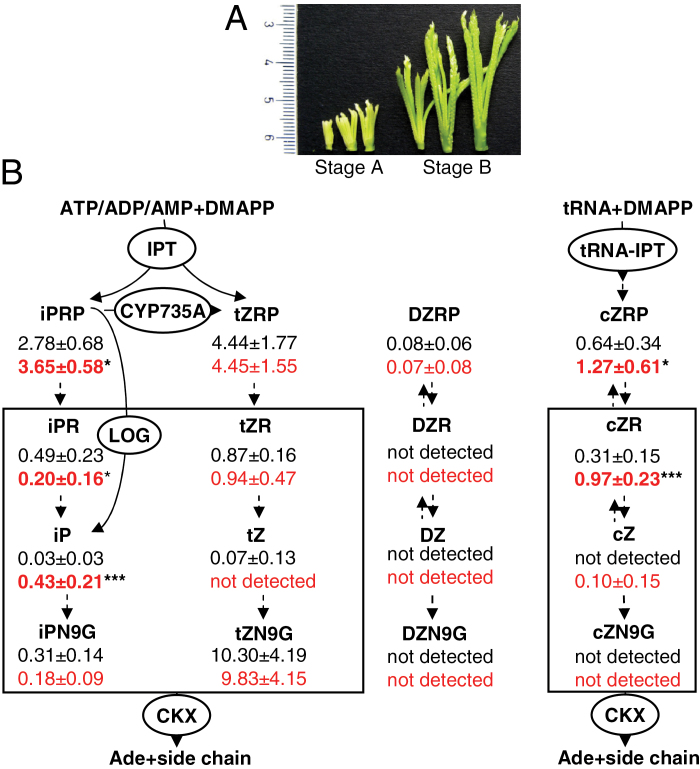
Field evaluation of the phenotype was done using ~100 wild-type (variety Tropikanka) and SE7 finger millet plants, grown for 2 weeks in a greenhouse and then transplanted into the field in Gatersleben, Germany in May 2011 with 20 cm×20cm distances between single plants. Plant height and yield from 25 plants were measured in late September 2011. For metabolite and array analyses, whole developing inflorescences were collected (Fig. 3A). For quantitative reverse transcription-PCR (qRT-PCR) analyses of different tissues, total RNA was isolated form seedlings at 5 d after imbibition, young leaves at the tillering stage, and old leaves from maturating plants. All samples were collected at least in triplicate from biologically independent plant material.
Fig. 3.
(A) General view of developing finger millet inflorescences used for cytokinin measurements as well as for molecular biological analyses. (B) Levels of cytokinins measured in young inflorescences (stage A) of SE7 and wild-type finger millet. Wild-type cytokinin contents are depicted in black; and those of the SE7 mutant in red. Data represent mean values (in pmol g–1 fresh weight) ±SD. Values representing significant differences between SE7 and the wild type are shown in bold (*P < 0.05; ***P < 0.001, calculated by Student’s t-test). Key enzymes involved in cytokinin biosynthesis and degradation are shown in circles. Cytokinin derivatives shown in a box are potential targets for the CKX enzyme (Frébort et al., 2011). CKX, cytokinin oxidase/dehydrogenase; cZ, cis-zeatin; cZNG, cis-zeatin 9-glucoside; cZR, cis-zeatin riboside; cZRP, cis-zeatin ribotide-phosphate; DZ, dihydro-zeatin; DZNG, dihydro-zeatin 9-glucoside; DZR, dihydro-zeatin riboside; DZRP, dihydro-zeatin ribotide-phosphate; iP, N 6-(Δ2-isopentenyl) adenine; iPR, iP riboside; iPNG, iP 9-glucoside; iPRP, iP ribotide-phosphate; IPT, adenosine phosphate-isopentenyltransferase; LOG, LONELY GUY; tRNA-IPT, tRNA isopentenyltransferase; tZ, trans-zeatin; tZNG, trans-zeatin 9-glucoside; tZR; trans-zeatin riboside; tZRP, trans-zeatin ribotide-phosphate.
Cloning of the cDNA for genes involved in cytokinin metabolism
Total RNA was extracted from different tissues of the wild type and the SE7 mutant of finger millet using Trizol reagent (Invitrogen). For this, 100mg of tissue was ground in liquid nitrogen, mixed with 1ml of pre-heated Trizol (60 °C) for 5min, and centrifuged at 13000rpm for 10min at 4 °C. The supernatant was transferred into a new tube, mixed with 0.2ml of chloroform for 2min, and centrifuged at 13000rpm for 10min at 4 °C. The aqueous phase was transferred into a new tube and mixed with 0.6vol. of iso-propanol, left at room temperature for 10min, and then centrifuged at 13 000rpm for 10min at 4 °C. The pellet was rinsed once with 70% cold ethanol and dissolved in 100 µl of distilled water. The isolated RNA was treated with RNase-free DNase (Qiagen), purified using an RNeasy plant mini kit (Qiagen), and used for the synthesis of cDNA, quantitative RT-PCRs, and cDNA array.
cDNA fragments of the CKX, LOG, and CYTOKININ RESPONSE 1 (CRE1) genes were amplified from wild-type inflorescences of finger millet by RT-PCRs using gene-specific primers selected from conserved regions of the corresponding rice genes. Primers used for RT-PCRs are listed in Supplementary Table S1 available at JXB online. All synthesized cDNAs were cloned into the pGEM-T easy vector (Promega) and sequenced. Sequence analysis and alignment were performed using DNAstar. The phylogenetic tree construction was drawn with the ClustalW tool.
Cytokinin extraction and purification
CKs were extracted from 500 µg of corresponding tissue (fresh weight) and purified according to Dobrev and Kaminek (2002). For analyses, 50 pmol of each of the following 17 deuterium-labelled standards were added: [2H5]Z, [2H5]Z9R, [2H5]Z7G, [2H5]Z9G, [2H5]ZOG, [2H5]Z9ROG, [2H6]iP, [2H6]iP9R, [2H6]iP7G, [2H6]iP9G, [2H3]DHZ, [2H3]DHZ9R, [2H3]DHZ9G, [2H7]DHZOG, [2H5]Z9RP, [2H6]iP9RP, and [2H3]DHZ9R (Apex Organics, Honiton, UK). Derivatives of cZ were determined from the retention time and the mass spectra of unlabelled standards and the response ratio of their tZ counterparts. Briefly, after homogenization in liquid nitrogen, samples were mixed with modified Bieleski solution [MeOH:water:COOH pH 2.5 (15:4:1, v/v/v), –20 °C]. The internal standards were added immediately. After overnight extraction at –20 °C, samples were purified using reverse phase chromatography. The nucleotide fraction was separated from the second fraction containing CK bases, ribosides, and glucosides by ion exchange chromatography (Oasis MCX extraction columns, 6 cc/150 mg, Waters). The nucleotide fraction was treated with alkaline phosphatase and then analysed in the same way as above.
High-performance liquid chromatography/mass spectrometry
High-performance liquid chromatography/mass spectrometry (HPLC/MS) analysis was performed as described by Dobrev et al. (2002) using an HPLC/MS system consisting of an HTS-Pal auto-sampler with a cooled sample stack (CTC Analytics, Zwingen, Switzerland), a quaternary HPLC pump Rheos 2200 (Flux Instruments, Basel, Switzerland), a Delta Chrom CTC 100 Column oven (Watrex, Praha, Czech Republic), and a TSQ Quantum Ultra AM triple-quad high resolution mass spectrometer (Thermo Electron, San Jose, CA, USA). Ternary gradient elution (water/acetonitrile/acetic acid) was used. The mass spectrometer was operated in the positive MS/MS mode (SRM; single reaction monitoring) with monitoring of 2–4 transitions for each compound. The most intensive ion was used for quantification and the remainder for identity confirmation. Multilevel calibration graphs with 2H-labelled CK internal standards were used for quantification. Detection limits of different CKs varied from 0.05 pmol to 0.1 pmol per sample. Each sample was injected at least twice.
Quantitative RT-PCR analyses
For qRT-PCR, 5 µg of the total RNA isolated as described above were used for reverse transcription by SuperScript III reverse transcriptase (Invitrogen) with an oligo(dT) primer. The resulting cDNAs were used as template for qRT-PCR analyses which were performed as described earlier (Radchuk et al., 2011). The efficiencies of PCRs were estimated using the LinRegPCR software (Ramakers et al., 2003). Primer sets for each gene have been selected based on the recommendations by Udvardi et al. (2008) and are listed in Supplementary Table S1 at JXB online. All samples were run in biological triplicates for each experiment. Dissociation curves confirmed the presence of a single amplicon in each PCR. The Ct of each gene of interest (GOI) from each sample was normalized against the endogenous reference gene actin from finger millet by using the formula ΔCt=CtGOI–Ctactin and calculated as an arithmetic mean of the replicates. In order to highlight the relative gene expression levels in SE7 versus the wild type, the fold changes of gene expression values were presented as 2–ΔΔCt according to Livak and Schmittgen (2001), where ΔΔCt is the difference between ΔCtWT and ΔCtSE7.
cDNA array and data analysis
For cDNA array analysis, total RNA was extracted as described above from very early developing inflorescences (stage A, see Fig. 3A) of wild-type and SE7 finger millet and used for the synthesis of [33P]dCTP-labelled probes. Probe preparation, hybridization, and processing of the 12K barley seed cDNA array was done essentially as described (Sreenivasulu et al., 2006) except for the hybridization temperature. In order to increase cross-hybridization between finger millet probes and the barley array, the hybridization temperature was set to 60 °C. Images of hybridized nylon membranes were subjected to automatic spot detection using the MATLAB program. Signal intensities of 11 787 genes were scored from the double spots, enabling the assessment of two replications. Additionally, two biological repetitions were performed using RNA from independently grown plants. Quantile normalization (Bolstad et al., 2003) was carried out on the complete data set. Fold changes between wild-type and mutant probes were calculated from the replicates. P-values were calculated based on the moderated t-test to detect false positives. Genes that showed statistically significant differences in expression in SE7 in comparison with wild-type inflorescences at the level of ≥1.5-fold were selected for further analyses. The detailed set of the normalized values, fold difference, and P-values of differentially expressed genes are provided in Supplementary Table S2 at JXB online.
Accession numbers
Sequence data from this article can be found in the GenBank/EMBL databases under the following accession numbers: HE800184 (EcCKX1), HE800185 (EcCKX2), HE800186 (EcLOG1), HE800187 (EcCRE1), and HE800188 (Ec actin).
Results
Phenotypic analysis of somaclonal variant SE7 of finger millet
The somaclonal line SE7 of finger millet [E. coracana (L.) Gaertn.] was selected after in vitro regeneration of var. Tropikanka (Yemets et al., 2003) due to its higher seed yield and more rapid germination at low temperature compared with the initial variety (Baer et al., 2007). These agricultural traits were stably inherited over >5 generations.
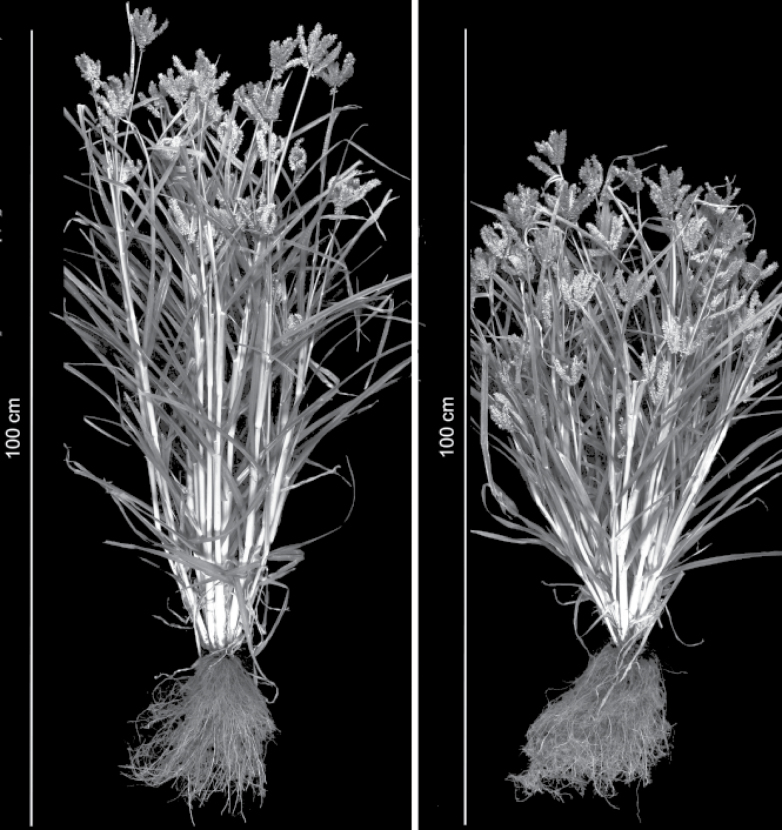
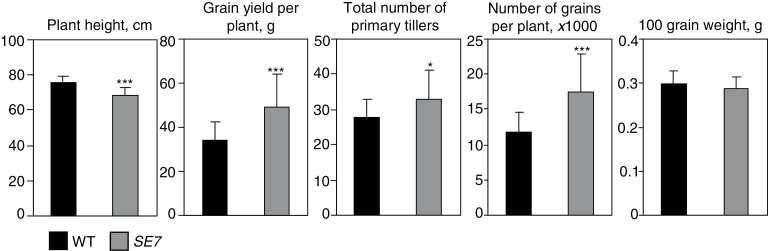
Phenotypic analyses of the somaclonal line SE7 under field conditions revealed lower plant height and increased number of tillers per plant compared with the wild type (Fig. 1). To analyse yield-related parameters, field trials were performed in plots of 100 plants planted 20cm apart. In the plots, the plant height of SE7 was 10% lower compared with the wild type. Grain yield per plant was increased by 40% and total tiller number was 17% higher. The number of grains per plant was also increased by 40%, while the 100 grain weight was unchanged (Fig. 2). The results show that the SE7 line under field conditions has achieved considerably higher grain yield, which was probably caused by a higher seed number due to an increased number of tillers per plant.
Fig. 1.
Lower plant height and increased number of inflorescences in the somaclonal line SE7 (right) compared with the wild-type finger millet (left).
Fig. 2.
Phenotypic analysis of field-grown SE7 plants compared with wild-type finger millet. Each bar represents the mean values ±SD of a trait. Significant differences between the mutant and wild type, calculated by Student’s t-test are: *P < 0.05; and ***P < 0.001).
Cytokinin contents in developing inflorescences of SE7 and wild-type finger millet
The higher number of inflorescences formed in the SE7 line indicates that more generative/floral spikes were produced in the meristems. Such an increased meristematic activity is often caused by modified levels of phytohormones, especially of CKs (Ashikari et al., 2005; Bartrina et al., 2011). Therefore, endogenous levels of 16 CK derivatives were measured in the inflorescences of SE7 and the wild type at two developmental stages: flower initiation with total inflorescence lengths <1cm and flower development with inflorescence length between 1cm and 3cm (Fig. 3A). Significantly different levels were measured only at the stage of flower initiation (Fig. 3B) but not during flower development (Supplementary Fig. S1 at JXB online).
The active CK pool included predominantly iP (Fig. 3B). In SE7 inflorescences, iP levels were as much as 14-fold higher compared with the wild type. The amounts of tZ and dihydrozeatin (DZ) were almost not detectable. Levels of iP riboside-phosphate (iPRP) were 30% higher in SE7, whereas that of the nucleoside form, iP riboside (iPR), was 60% lower. Levels of the iP deactivation product, iP 9-glucoside (iPN9G), were also decreased in the SE7 line, although not significantly. Amounts of tZ were barely detectable in early inflorescences, whereas levels of tZ precursors and the riboside forms, tZRP and tZR, did not differ between SE7 and the wild type. Levels of cZ were barely detectable, whereas those of cZRP and cZR were increased by 2- and 3-fold (Fig. 3B).
The CK measurement revealed increased levels of several derivatives, especially of the highly physiologically active iP in initiating inflorescences of the SE7 mutant finger millet. Such changes could be affected by altered expression of genes related to CK metabolism.
Cloning of genes involved in cytokinin metabolism and perception
To elucidate whether altered endogenous CK levels in the SE7 mutant were caused by differential expression of genes involved in CK metabolism and/or perception, the corresponding cDNAs were cloned from finger millet. As little sequence information is available for finger millet, cDNA fragments were PCR amplified from early developing inflorescences using primers from conserved regions of known rice and barley homologues. For CKX, 10 barley genomic and corresponding cDNA sequences (Matsumoto et al., 2011; Mameaux et al., 2012) and 11 rice (Ashikara et al., 2005) cDNAs were examined. Sequences for barley and rice LOG have not been described and were therefore selected from available full-length cDNA and expressed sequence tag (EST) collections according to homology (Zhang et al., 2004; Kuroha et al., 2009; Matsumoto et al., 2011). Five full-length barley LOG cDNAs were identified. The rice genome contains all four counterparts of Arabidopsis hybrid kinases (HKs) (Supplementary Fig. S2 at JXB online). In addition, one full-length HvHK3 and one partial HvCRE1 cDNA of barley were identified.
Differences in CK levels between the wild type and SE7 were measured at initiation (stage A) of inflorescence development (Fig. 3A). Therefore, total RNA from this stage was used to amplify cDNA fragments by RT-PCR. The 1411bp EcCKX1 fragment is 61.8% identical to Arabidopsis AtCKX6, 86.6% identical to barley HvCKX4, and 84.6% identical to rice OsCKX4 at the amino acid level. The 1758bp EcCKX2 fragment is 83.8% identical to OsCKX3, 77.7% identical to HvCKX3, and 44.6% identical to AtCKX1 (Supplementary Fig. 2A at JXB online). The 957bp EcLOG1 fragment is 78.7% identical to HvLOG4 and 78.4% identical to OsLOG4 (Supplementary Fig. 2B). The EcCRE1 fragment of 706bp contains conserved receptor-like (RLD) and receiver domains with highly conserved aspartate residues and is 68.8% identical to OsCRE1, 61.1% identical to HvCRE1, and 42.7% identical to CRE1/WOL1/AHK4 of Arabidopsis (Supplementary Fig. 2C). Further members of CKX, LOG, or HK gene families could not been cloned, possibly due to absent transcripts in early inflorescences or to unsuitable primers selected for amplification. Differential temporal and spatial expression patterns for the particular members of these gene families have been described previously (Werner et al., 2003; Zalewski et al., 2010; Tokunaga et al., 2012).
Corresponding fragments of EcCKX1, EcCKX2, EcLOG1, and EcCRE1 cDNAs were also amplified from inflorescences of the line SE7. The sequences in the wild type and SE7 were not different. This indicates that changed expression rather than the sequence structure of CK-related genes may alter CK levels in the SE7 line.
Genes involved in cytokinin degradation are down-regulated in SE7 finger millet
Expression of EcCKX1, EcCKX2, EcLOG1, and EcCRE1 genes was analysed in different tissues of SE7 and the wild type by qRT-PCR. Remarkably, gene expression of EcCKX2 was 8.2-fold lower in young leaves of SE7 compared with the wild type. The mRNA levels of EcCKX1 were 3.9-fold lower in inflorescences of SE7 at stage A but unchanged at later stages. Transcript levels of EcCKX1 and EcCKX2 genes were lower by 3.2- and 3.1-fold in seedlings but unchanged in mature leaves. The mRNA levels of EcLOG1, encoding a CK-synthesizing enzyme, were undetectable in leaves and unchanged in seedlings. EcCRE1 transcript levels were 3.9-fold lower in young leaves but unchanged in older leaves and inflorescences (Fig. 4).
Fig. 4.
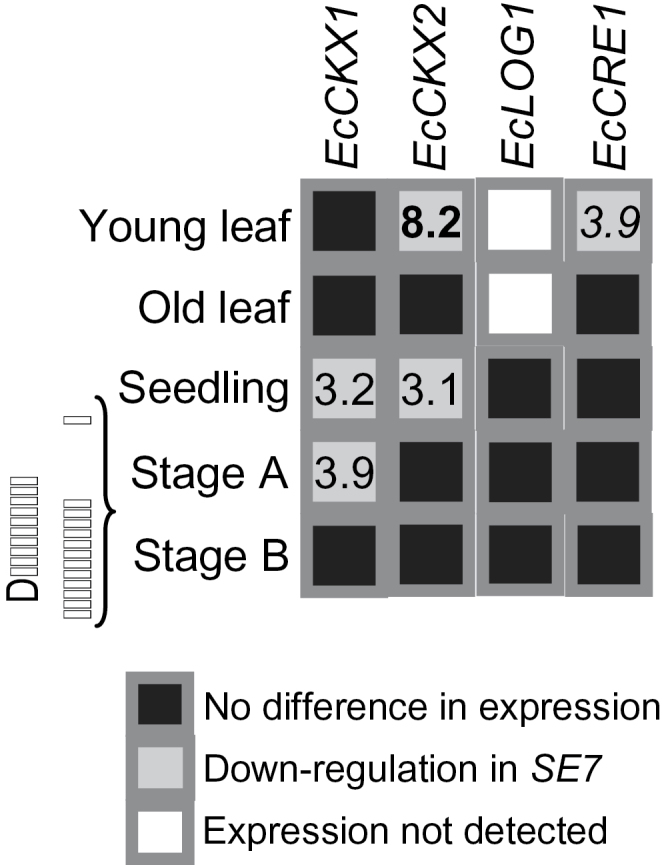
Differences in expression of EcCKX1, EcCKX2, EcLOG1, and EcCRE1 genes in different tissues of wild-type and SE7 finger millet plants as analysed by qRT-PCR. Significant expression differences between SE7 and the wild type are shown by normal (t-test, P < 0.05), italic (t-test, P < 0.01), and bold (t-test, P < 0.001) numerals.
In summary, transcript levels of CK-degrading enzymes but not of the CK-synthesizing enzyme were decreased in young and actively growing tissues such as young leaves, seedlings, and initiating inflorescences of SE7 compared with the wild type. This suggests attenuated degradation of CKs in SE7 inflorescences relative to the wild type, which is correlated with higher CK levels.
Transcript profiling of early inflorescences of the SE7 mutant finger millet
Comparison of gene expression patterns between developing inflorescences of SE7 and the wild type (Supplementary Table S2 at JXB online) was performed using the barley seed-specific 12K cDNA array (Sreenivasulu et al., 2006). Samples from finger millet inflorescences were hybridized on barley seed cDNA arrays, which could explain the low number of differentially expressed genes detected. In early inflorescences of SE7 and the wild type, 126 genes were differentially expressed at the threshold of ≥1.5-fold (four replicates, P < 0.05). Of these, 69 genes were up-regulated and 57 were down-regulated. A total of 17 up- and 23 down-regulated genes could not be annotated (Supplementary Table S2).
The largest set of 30 genes (40% of total) up-regulated in SE7 inflorescences was related to transcription and translation and contained genes encoding 10 histones, nine ribosomal proteins, and several elongation and initiation factors. In contrast, only 12 genes from this category were down-regulated (Supplementary Table S2 at JXB online). In SE7, 16 up-regulated genes were related to cell proliferation, different aspects of cell wall assembly/biosynthesis, and to regulation of growth of young and meristematic tissues including floral formation (Table 1). Dynamin and α-tubulin are involved in cytokinesis and microtubule formation. Uridine monophosphate kinase is engaged in pyrimidine biosynthesis. γ-Glutamyl hydrolase sustains folate homeostasis and one-carbon metabolism necessary for nucleotide biosynthesis and methylation reactions occurring during cell wall formation (Loizeau et al., 2008). Extensins and glycine-rich proteins represent common structural cell wall proteins. Two peroxidases can potentially polymerize cell wall lignin (Fagerstedt et al., 2010). ADP-ribosylation factors are involved in vesicle and metabolite transport towards the cell wall. Nodulin 21 (MtM21) represents a potential tonoplast metabolite exporter. The respective mutants show defective cell wall formation and elongation (Ranocha et al., 2010). Choline kinase is involved in phospholipid biosynthesis. Purple acid phosphatase mediates phosphate acquisition and redistribution, which is important for efficient growth. Its overexpression in Arabidopsis results in higher seed yield (Sun et al., 2012). Heterotrimeric G-protein modulates cell proliferation, which is reduced in the respective null mutants (Ullah et al., 2001). In contrast, from this category, only an actin-related protein was down-regulated in SE7. Two up-regulated genes in SE7 participate in floral formation. Argonaute 1 regulates the temporal programme of floral stem cells (Ji et al., 2011). ALWAYS EARLY (ALY2/Lin-9) contains Myb domains, is nuclear localized, and regulates germ-like specific gene expression in Drosophila with presumed similar functions in plants (Bhatt et al., 2004; Doggett et al., 2011). Three up-regulated genes in SE7 are associated with protein or lipid degradation and another two with mitochondrial energy production.
Table 1.
Partial list of the genes differentially expressed in the finger millet SE7 inflorescences compared with the wild type
| Clone IDa | Gene identification | Blast score | Closest homologue in rice | Fold differenceb |
| Genes up-regulated in the SE7 inflorescences | ||||
| HY07H07 | Peroxidase, class III | 842 | LOC_Os03g02920 | 6.86 |
| HF04C06 | Elongation factor 1β | 529 | LOC_Os07g42300 | 4.89 |
| HY05A19 | Uridylate monophosphate kinase | 577 | LOC_Os04g33300 | 4.09 |
| HA10K05 | Histone H2B-1 | 1540 | LOC_Os01g05610 | 3.38 |
| HA12L08 | Poly(A)-binding protein | 825 | LOC_Os09g02700 | 3.36 |
| HA03D02 | Peroxidase II | 264 | LOC_Os04g59150 | 3.36 |
| HA05J13 | 40S ribosomal protein S15A | 637 | LOC_Os07g10720 | 3.03 |
| HB17F08 | Extensin | 1011 | LOC_Os05g43280 | 3.03 |
| HA29L06 | Eukaryotic translation initiation factor 3-1α | 688 | LOC_Os02g02990 | 2.89 |
| HB21M05 | Chloroplast proteinase cnd41 | 2085 | LOC_Os02g48900 | 2.82 |
| HA01F14 | Histone H4-2 | 1478 | LOC_Os05g38740 | 2.81 |
| HA11D18 | Glycine-rich protein | 198 | LOC_Os01g15310 | 2.80 |
| HY10A12 | 60S ribosomal protein L3 | 770 | LOC_Os11g06750 | 2.72 |
| HY07G20 | Nodulin | 649 | LOC_Os05g01580 | 2.58 |
| HB19G05 | Argonaute AGO1 | 570 | LOC_Os01g16870 | 2.37 |
| HA08C04 | 60S ribosomal protein L11-1 | 989 | LOC_Os04g51630 | 2.34 |
| HY08K24 | 40S ribosomal protein S20 | 633 | LOC_Os06g04290 | 2.12 |
| HA23C20 | ATP synthase γ subunit, mitochondrial | 748 | LOC_Os05g45740 | 2.10 |
| HY10P24 | MYB TF Always Early (ALY/Lin-9) | 432 | LOC_Os03g43800 | 2.04 |
| HB13O10 | Outer envelope membrane protein OEP75 | 284 | LOC_Os02g10260 | 1.99 |
| HZ61A07 | Acidic ribosomal protein P2 | 903 | LOC_Os01g09510 | 1.94 |
| HY10K07 | Heterotrimeric G protein, α subunit | 287 | LOC_Os05g27520 | 1.88 |
| HB11P08 | Dynamin | 517 | LOC_Os03g50520 | 1.84 |
| HY10L09 | Choline kinase | 815 | LOC_Os01g08760 | 1.78 |
| HA29K04 | Nucleolar protein 5A | 187 | LOC_Os07g46720 | 1.72 |
| HB03J03 | DEAD-Box RNA helicase | 302 | LOC_Os07g05050 | 1.70 |
| HA14I09 | α-Tubulin | 1576 | LOC_Os11g14220 | 1.68 |
| HB14O18 | Elongation factor 2 | 2087 | LOC_Os02g32030 | 1.68 |
| HF06L15 | γ-Glutamyl hydrolase | 424 | LOC_Os05g44130 | 1.68 |
| HB11K04 | ATP synthase β subunit, mitochondrial | 1504 | LOC_Os05g47980 | 1.62 |
| HA13H05 | Seryl-tRNA synthetase | 276 | LOC_Os11g39670 | 1.62 |
| HB05B24 | Purple acid phosphatase 1 | 1105 | LOC_Os03g13540 | 1.61 |
| HY04C14 | Glycosyl hydrolase 5, cellulase | 1138 | LOC_Os02g28040 | 1.58 |
| HB28B23 | Lipase class 3 | 1402 | LOC_Os04g40510 | 1.57 |
| HA31P02 | ADP-ribosylation factor | 673 | LOC_Os05g41060 | 1.55 |
| Genes down-regulated in SE7 inflorescences | ||||
| HA22P12 | Microsomal signal peptidase | 263 | LOC_Os09g38370 | –12.72 |
| HY10P05 | Heat shock protein HSP80 | 1144 | LOC_Os08g39140 | –4.10 |
| HZ57F19 | Endoplasmic reticulum ATPase | 335 | LOC_Os10g30580 | –3.29 |
| HY10H24 | DnaK-type molecular chaperone HSP70 | 1142 | LOC_Os11g47760 | –2.92 |
| HZ65I21 | Histone H2A | 890 | LOC_Os03g17100 | –2.87 |
| HF15K21 | Peptide chain release factor subunit 1–3 | 513 | LOC_Os03g49580 | –2.78 |
| HA06A05 | Alcohol dehydrogenase 1 | 389 | LOC_Os08g01760 | –2.77 |
| HA28N18 | Chaperone DnaJ-like protein | 585 | LOC_Os03g57340 | –2.57 |
| HZ59A18 | Ubiquitin-associated (UBA) protein | 819 | LOC_Os02g38050 | –2.34 |
| HB04C08 | Cap-binding protein nCBP | 320 | LOC_Os03g15590 | –2.32 |
| HZ48I16 | Phytochelatin synthetase | 809 | LOC_Os05g32110 | –2.23 |
| HY10N19 | Elongation factor 1α | 3735 | LOC_Os03g08010 | –2.12 |
| HF05E04 | SUMO-activating enzyme 2 (SAE2) | 1394 | LOC_Os05g32110 | –2.06 |
| HY06G19 | Ubiquitin | 2693 | LOC_Os07g39780 | –2.05 |
| HY09G12 | DNA repair helicase XPB1 | 238 | LOC_Os01g49680 | –2.04 |
| HY10G03 | Enolase | 857 | LOC_Os06g46770 | –1.95 |
| HZ57O13 | Ubiquitin-activating enzyme E1 | 704 | LOC_Os01g42850 | –1.89 |
| HB17M09 | BRI1-interacting protein | 309 | LOC_Os03g58480 | –1.82 |
| HB06H23 | Actin-related protein 2/3 | 858 | LOC_Os01g46580 | –1.78 |
| HB30D08 | Zinc carboxypeptidase | 643 | LOC_Os02g02710 | –1.61 |
| HB31J16 | Quinone reductase | 888 | LOC_Os05g24880 | –1.60 |
| HF21C12 | GAPDH, cytosolic | 614 | LOC_Os08g03290 | –1.59 |
| Genes up-regulated in the SE7 inflorescences | ||||
| HF02G08 | Glutamate decarboxylase GAD1 | 809 | LOC_Os08g36320 | –1.57 |
| HB02N21 | Brassinosteroid-insensitive 1 (BRI1) | 83 | LOC_Os11g39370 | –1.55 |
a Clone ID is taken from http://pgrc.ipk-gatersleben.de/est/index.php. The full list of differentially regulated genes is given in Supplementary Table S1 at JXB online.
b A positive value indicates gene up-regulation in SE7 inflorescences, and a negative value indicates gene down-regulation.
From the genes down-regulated in SE7 inflorescences, eight are related to pathogen defence and stress response (Table 1). Among them are three heat shock proteins, alcohol dehydrogenase, and phytochelatin synthase. Five down-regulated genes are involved in regulated protein degradation via the ubiquitin pathway. Brassinosteroid insensitive 1 (BRI1) together with a BRI1-interacting protein is also transcriptionally down-regulated. The former encodes a leucine-rich repeat receptor-like kinase recognizing brassinosteroids, which are engaged in cell elongation. Interestingly, attenuating brassinosteroid signalling in Arabidopsis enhances FLC expression and the late flowering autonomous pathway (Domagalska et al., 2007), indicating relationships between brassinosteroids and flowering. Three down-regulated genes are related to primary metabolism such as glycolysis (enolase, GAPDH) and C:N balance (glutamate decarboxylase).
In summary, differential gene expression suggests that SE7 young inflorescences possess a prolonged proliferation phase characterized by up-regulated transcription and translation, cell division, floral formation, and energy production. In contrast, wild-type inflorescences are similar to a more differentiated status characterized by regulated protein degradation, brassinosteroid-mediated cell elongation, and defence/stress responses.
Discussion
Plants regenerated in vitro from undifferentiated cells often exhibit some level of variation called somaclonal variation. Somaclonal variation in plants is caused by increased mutation rates mainly due to nucleotide substitutions and small indels during the in vitro regeneration process and could be provoked by exogenous growth stimulators (Jiang et al., 2011; Sato et al., 2011). Thereby, genetic modifications lead to somaclonal variations in regenerated plantlets. Here the somaclonal line SE7 of finger millet was analysed. Compared with the wild type, this line exhibited lower plant height as well as increased numbers of inflorescences and flowers over several generations, resulting in considerably higher seed yield per plant by up to 40% in field trials. Phenotypic features of SE7 plants, decreased CK degradation and elevated active CK content, correlate well with CK function in stimulation of meristem activity, leading to enhanced inflorescence formation and seed set.
Measurements of CK metabolites revealed iP as the major form of active CKs in developing inflorescences of finger millet, whereas iP together with tZ are major forms in Arabidopsis (Tokunaga et al., 2012). iP levels and that of the precursor iPRP are strongly increased in SE7 inflorescences at the initiation of differentiation, but are similar to the wild type later on (Fig. 3; Supplementary Fig. S1 at JXB online). Increased iP levels are probably caused by attenuated degradation, as evidenced by lower gene expression of the EcCKX1 gene in young inflorescences. Such a shift in CK homeostasis, brought about by either decreased CK degradation or enhanced biosynthesis, can excite plant productivity. Accordingly, down-regulated CKX gene expression lowered CK-degrading activity and increased CK levels in rice and barley and enhanced seed numbers per plant and thousand grain weight (Ashikari et al., 2005; Zalewski et al., 2010). Overexpression of AtLOG genes (AtLOG2, AtLOG4, and AtLOG7) also increased CK biosynthesis and led to more axillary stems and larger seeds (Kuroha et al., 2009).
It was also found that finger millet inflorescences accumulated more tZ than cZ derivatives. It was reported earlier that several species from Arundinoideae, Panicoideae, and Pooideae subfamilies of the Poaceae family accumulate predominantly cZ derivatives in their leaves (Gajdosova et al., 2011). Eleusine coracana belongs to the Chloridoideae subfamily (Glemin and Bataillon, 2009), which is phylogenically separate from subfamilies analysed by Gajdosova et al. (2011), which may explain this discrepancy. Besides, the ratio between cZ and tZ derivatives may change dramatically throughout the life span of a single plant (Gajdosova et al., 2011; Tarkowská et al., 2012) with cZ derivatives prevalent in the developing stages associated with limited growth. Therefore, a high level of tZ derivatives might be specific for actively grown and developing finger millet inflorescences. Interestingly, the levels of cZ precursors, cis-zeatin riboside-phosphate and cis-zeatin riboside, were also increased in the SE7 line, additionally supporting the idea that cZ can play a role in a fine-tuning regulation of reproductive development (Tarkowská et al., 2012).
The SE7 plants exhibited decreased plant height and increased seed yield mainly due to an increased number of inflorescences and grains per plant. Levels of EcCKX1 and/or EcCKX2 transcripts but not those of EcLOG1 were decreased in seedlings, young developing inflorescences, and in younger but not in older leaves (Fig. 4). It is concluded that the phenotypic changes observed in the SE7 plants are caused by decreased expression of EcCKX1 and EcCKX2 genes, which resulted in elevation of iP levels in actively growing tissues and as a consequence promoted maintenance and activity of vegetative and generative meristems. This assumption is further supported by enhanced expression in the SE7 young inflorescences of genes related to transcription, translation, and cell proliferation, to different aspects of cell wall assembly/biosynthesis, and to regulation of growth of young and meristematic tissues including floral formation. Such changes indicate enhanced cell proliferation and metabolic activity in the SE7 meristems. Accordingly, on the transcriptional level, lipid degradation and ATP production were stimulated presumably to supply energy to the metabolically more active SE7 meristems. The expression data support the hypothesis that enhanced levels of active CKs stimulate cell proliferation in SE7 inflorescences. Increased meristem activity would then increase flower and seed formation in SE7 mutant plants.
Rice plants with reduced expression of OsCKX2 also exhibit lower plant height, but this feature is caused by mutated gibberellin-20 oxidase (Ashikari et al., 2005). Overexpression of a LOG isoform in Arabidopsis generates semi-dwarf phenotypes (Kuroha et al., 2009), indicating reduced shoot apical dominance. It is unclear why plant height is reduced together with lower expression of CKX in SE7 plants. Possibly, differentiation and growth of more inflorescences as highly energy-demanding process may result in suppression of the final plant height, due to the limited energy sources. SE7 mutant finger millet seeds germinated faster and produced longer roots (Baer et al., 2007), a feature also found for barley with decreased CKX activity (Zalewski et al., 2010).
EcCKX1 and EcCKX2 cDNA sequences do not differ between the wild type and SE7 mutant, indicating that down-regulation of EcCKX1 and EcCKX2 genes in SE7 occurs at the transcriptional level. CK activity is controlled by balancing its biosynthesis, catabolism, and signalling, which generates specific homeostasis for a given tissue (Sakakibara, 2006; Werner and Schmülling, 2009; Perilli et al., 2010; Frebort et al., 2011). Presumably, mutations of genes which are responsible for the transcriptional regulation, such as transcription factors, might cause down-regulation of expression of CK-related genes in SE7, although direct evidence is lacking. However, down-regulation of EcCRE1 gene expression together with that of CKX in young leaves implicates complex networks regulating CK signalling at the transcriptional level. Altogether, it seems that a mechanism leading to the increased level of cytokinins in SE7 inflorescences most probably differs from that described by Ashikari et al. (2005).
This work demonstrates a good example where somaclonal rearrangements can be advantageous for agronomic application. In vitro plant regeneration was successfully employed to produce finger millet plants with altered hormonal status and useful agronomic properties. The somaclonal line SE7 is now registered as a variety, Yaroslav-8, in Ukraine. Since genetic characterization of finger millet is just beginning, the genetic resource analysed here can be of great importance, especially to finger millet breeding programmes in developing countries.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Levels of cytokinins measured in young inflorescences of the SE7 mutant and the wild type of finger millet at the stage of flower development (stage B).
Figure S2. Dendrograms of cytokinin oxidase/dehydrogenase (CKX; A) and LONELY GUY (LOG; B) genes from barley, rice, and finger millet, and genes encoding hybrid kinases (C) implicated in cytokinin signalling.
Table S1. Primers used in cloning experiments and for qRT-PCR analyses.
Table S2. Complete list of differentially expressed genes in the SE7 finger millet inflorescences at stage A compared with the wild type, together with gene annotation, normalized gene expression values, and their statistical significance.
Acknowledgements
We are grateful to Angela Stegmann and Elsa Fessel for excellent technical assistance. This work was supported by Deutsche Forschungsgemeinschaft, grants WE 1608/4-1 to VK, WE1608/7-1 to VR, WE 1608/9-1 to YP, and the Czech Science Foundation, project no. 522/09/2058.
Glossary
Abbreviations
- CK
cytokinin
- CKX
cytokinin oxidase/dehydrogenase
- CRE1
cytokinin RESPONSE 1
- IPT
adenosine phosphate-iso-pentenyltransferase
- iP
N6-(Δ2-isopentenyl)-adenine
- LOG
LONELY GUY.
References
- Ashikari M, Sakakibara H, Lin S, Yamamoto T, Takashi T, Nishimura A, Angeles ER, Qian Q, Kitano H, Matsuoka M. 2005. CK oxidase regulates rice grain production Science 309 741–745 [DOI] [PubMed] [Google Scholar]
- Bayer GY, Yemets AI, Stadnichuk NA, Rakhmetov DB, Blume YB. 2007. Somaclonal variability as a source for creation of new varieties of finger millet (Eleusine coracana (L.) Gaertn.) Cytology and Genetics 41 204–208 [PubMed] [Google Scholar]
- Barazesh S, McSteen P. 2008. Hormonal control of grass inflorescence development Trends in Plant Sciences 13 656–662 [DOI] [PubMed] [Google Scholar]
- Bartrina I, Otto E, Strnad M, Werner T, Schmülling T. 2011. CK regulates the activity of reproductive meristems, flower organ size, ovule formation, and, thus, seed yield in Arabidopsis thaliana The Plant Cell 23 69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt AM, Zhang Q, Harris SA, White-Cooper H, Dickinson H. 2004. Gene structure and molecular analysis of Arabidopsis thaliana ALWAYS EARLY homologs Gene 336 219–229 [DOI] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. 2003. A comparison of normalization methods for high density oligonucleotide array data based on bias and variance Bioinformatics 19 185–193 [DOI] [PubMed] [Google Scholar]
- Ceasar SA, Ignacimuthu S. 2011. Agrobacterium-mediated transformation of finger millet (Eleusine coracana (L.) Gaertn.) using shoot apex explants Plant Cell Reports 30 1759–1770 [DOI] [PubMed] [Google Scholar]
- Dida MM, Srinivasachary, Ramakrishnan S, Bennetzen JL, Gale MD, Devos KM. 2007. The genetic map of finger millet, Eleusine coracana Theoretical and Applied Genetics 114 321–332 [DOI] [PubMed] [Google Scholar]
- Dobrev PI, Kaminek M. 2002. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction Journal of Chromatography A 950 21–29 [DOI] [PubMed] [Google Scholar]
- Dobrev P, Motyka V, Gaudinova A, Malbeck J, Travnickova A, Kaminek M, Vankova R. 2002. Transient accumulation of cis- and trans-zeatin type cytokinins and its relation to cytokinin oxidase activity during cell cycle of synchronized tobacco BY-2 cells Plant Physiology and Biochemistry 40 333–337 [Google Scholar]
- Doggett K, Jiang J, Aleti G, White-Cooper H. 2011. Wake-up-call, a lin-52 paralogue, and Always early, a lin-9 homologue physically interact, but have opposing functions in regulating testis-specific gene expression Developmental Biology 355 381–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domagalska MA, Schomburg FM, Amasino RM, Vierstra RD, Nagy F, Davis SJ. 2007. Attenuation of brassinosteroid signaling enhances FLC expression and delays flowering Development 134 2841–2850 [DOI] [PubMed] [Google Scholar]
- Fagerstedt KV, Kukkola EM, Koistinen VV, Takahashi J, Marjamaa K. 2010. Cell wall lignin is polymerised by class III secretable plant peroxidases in Norway spruce Journal of Integrative Plant Biology 52 186–194 [DOI] [PubMed] [Google Scholar]
- Frébort I, Kowalska M, Hluska T, Frébortová J, Galuszka P. 2011. Evolution of cytokinin biosynthesis and degradation Journal of Experimental Botany 62 2431–2452 [DOI] [PubMed] [Google Scholar]
- Galuszka P, Frebort I, Sebela M, Sauer P, Jacobsen S, Pec P. 2001. Cytokinin oxidase or dehydrogenase? Mechanism of cytokinin degradation in cereals . European Journal of Biochemistry 268 450–461 [DOI] [PubMed] [Google Scholar]
- Glemin S, Bataillon T. 2009. A comparative view of the evolution of grasses under domestication New Phytologist 183 273–290 [DOI] [PubMed] [Google Scholar]
- Ji L, Liu X, Yan J, et al. ARGONAUTE10 and ARGONAUTE1 regulate the termination of floral stem cells through two microRNAs in Arabidopsis. PLoS Genetics. 2011;7:e1001358. doi: 10.1371/journal.pgen.1001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C, Mithani A, Gan X, Belfield EJ, Klingler JP, Zhu JK, Ragoussis J, Mott R, Harberd NP. 2011. Regenerant Arabidopsis lineages display a distinct genome-wide spectrum of mutations conferring variant phenotypes Current Biology 21 1385–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada-Nobusada T, Sakakibara H. 2009. Molecular basis for cytokinin biosynthesis Phytochemistry 70 444–449 [DOI] [PubMed] [Google Scholar]
- Kudo T, Kiba T, Sakakibara H. 2010. Metabolism and long-distance translocation of cytokinins Journal of Integrative Plant Biology 52 53–60 [DOI] [PubMed] [Google Scholar]
- Kurakawa T, Ueda N, Maekawa M, Kobayashi K, Kojima M, Nagato Y, Sakakibara H, Kyozuka J. 2007. Direct control of shoot meristem activity by a cytokinin-activating enzyme Nature 445 652–655 [DOI] [PubMed] [Google Scholar]
- Kuroha T, Tokunaga H, Kojima M, Ueda N, Ishida T, Nagawa S, Fukuda H, Sugimoto K, Sakakibara H. 2009. Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis The Plant Cell 21 3152–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T) (–Delta Delta C) method Methods 25 402–408 [DOI] [PubMed] [Google Scholar]
- Loizeau K, De Brouwer V, Gambonnet B, Yu A, Renou JP, Van Der Straeten D, Lambert WE, Rébeillé F, Ravanel S. 2008. A genome-wide and metabolic analysis determined the adaptive response of Arabidopsis cells to folate depletion induced by methotrexate Plant Physiology 148 2083–2095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Tanaka T, Sakai H, et al. 2011. Comprehensive sequence analysis of 24,783 barley full-length cDNAs derived from 12 clone libraries Plant Physiology 156 20–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechael G, Seiler-Kelbitsch H. 1972. Cytokinin content and kernel size of barley grain as affected by environmental and genetic factors Crop Science 12 162–165 [Google Scholar]
- Miyawaki K, Tarkowski P, Matsumoto-Kitano M, Kato T, Sato S, Tarkowska D, Tabata S, Sandberg G, Kakimoto T. 2006. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis Proceedings of the National Academy of Sciences, USA 103 16598–16603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller B. 2011. Generic signal-specific responses: cytokinin and context-dependent cellular responses Journal of Experimental Botany 62 3273–3288 [DOI] [PubMed] [Google Scholar]
- Perilli S, Moubayidin L, Sabatini S. 2010. The molecular basis of cytokinin function Current Opinion in Plant Biology 13 21–26 [DOI] [PubMed] [Google Scholar]
- Radchuk V, Weier D, Radchuk R, Weschke W, Weber H. 2011. Development of maternal seed tissue in barley is mediated by regulated cell expansion and cell disintegration and coordinated with endosperm growth Journal of Experimental Botany 62 1217–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramakers C, Ruijter JM, Deprez RHL, Moorman AFM. 2003. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data Neuroscience Letters 339 62–66 [DOI] [PubMed] [Google Scholar]
- Ranocha P, Denancé N, Vanholme R, et al. 2010. Walls are thin 1 (WAT1), an Arabidopsis homolog of Medicago truncatula NODULIN21, is a tonoplast-localized protein required for secondary wall formation in fibers The Plant Journal 63 469–483 [DOI] [PubMed] [Google Scholar]
- Sakakibara H. 2006. Cytokinins: activity, biosynthesis, and translocation Annual Review of Plant Biology 57 431–449 [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Sakakibara H, Kojima M, Nagasaki H, Yamamoto Y, Inukai Y, Sato Y, Matsuoka M. 2006. Ectopic expression of KNOTTED1-like homeobox protein induces expression of cytokinin biosynthesis genes in rice Plant Physiology 142 54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Hosokawa M, Doi M. Somaclonal variation is induced de novo via the tissue culture process: a study quantifying mutated cells in Saintpaulia . PLoS One. 2011;6:e23541. doi: 10.1371/journal.pone.0023541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmülling T, Werner T, Riefler M, Krupková E, Manns IB. 2003. Structure and function of cytokinin oxidase/dehydrogenase genes of maize, rice, Arabidopsis and other species Journal of Plant Research 116 241–252 [DOI] [PubMed] [Google Scholar]
- Séguéla M, Briat JF, Vert G, Curie C. 2008. Cytokinins negatively regulate the root iron uptake machinery in Arabidopsis through a growth-dependent pathway The Plant Journal 55 289–300 [DOI] [PubMed] [Google Scholar]
- Sreenivasulu N, Radchuk V, Strickert M, Miersch O, Weschke W, Wobus U. 2006. Gene expression patterns reveal tissue-specific signaling networks controlling programmed cell death and ABA-regulated maturation in developing barley seeds The Plant Journal 47 310–327 [DOI] [PubMed] [Google Scholar]
- Srinivasachary, Dida MM, Gale MD, Devos KM. 2007. Comparative analyses reveal high levels of conserved colinearity between the finger millet and rice genomes Theoretical and Applied Genetics 115 489–499 [DOI] [PubMed] [Google Scholar]
- Sun F, Suen PK, Zhang Y, Liang C, Carrie C, Whelan J, Ward JL, Hawkins ND, Jiang L, Lim BL. 2012. A dual-targeted purple acid phosphatase in Arabidopsis thaliana moderates carbon metabolism and its over-expression leads to faster plant growth and higher seed yield New Phytologist 194 206–219 [DOI] [PubMed] [Google Scholar]
- Takei K, Yamaya T, Sakakibara H. 2004. Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans-zeatin Journal of Biological Chemistry 279 41866–41872 [DOI] [PubMed] [Google Scholar]
- Tarkowská D, Filek M, Biesaga-Kościelniak J, Marcińska I, Macháčková I, Krekule J, Strnad M. 2012. Cytokinins in shoot apices of Brassica napus plants during vernalization Plant Science 187 105–112 [DOI] [PubMed] [Google Scholar]
- Tokunaga H, Kojima M, Kuroha T, Ishida T, Sugimoto K, Kiba T, Sakakibara H. 2012. Arabidopsis lonely guy (LOG) multiple mutants reveal a central role of the LOG-dependent pathway in CK activation The Plant Journal 69 355–365 [DOI] [PubMed] [Google Scholar]
- Udvardi MK, Czechowski T, Scheible WR. 2008. Eleven golden rules of quantitative RT-PCR The Plant Cell 20 1736–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah H, Chen JG, Young JC, Im KH, Sussman MR, Jones AM. 2001. Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis Science 292 2066–2069 [DOI] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T. 2003. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity The Plant Cell 15 2532–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Nehnevajova E, Kollmer I, Novak O, Strnad M, Kramer U, Schmülling T. 2010. Root-specific reduction of cytokinin causes enhanced root growth, drought tolerance, and leaf mineral enrichment in Arabidopsis and tobacco The Plant Cell 22 3905–3920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Schmülling T. 2009. Cytokinin action in plant development Current Opinion in Plant Biology 12 527–538 [DOI] [PubMed] [Google Scholar]
- Yemets AI, Bayer GY, Klimkina LA, Stadnichuk NA, Abramov AA. 2003. Cultivation and regeneration in vitro of finger millet plants Eleusine coracana (L.) Gaertn. var. Tropikanka Physiology and Biochemistry of Cultivated Plants 35 152–159 (in Russian) [Google Scholar]
- Zalewski W, Galuszka P, Gasparis S, Orczyk W, Nadolska-Orczyk A. 2010. Silencing of the HvCKX1 gene decreases the cytokinin oxidase/dehydrogenase level in barley and leads to higher plant productivity Journal of Experimental Botany 61 1839–1851 [DOI] [PubMed] [Google Scholar]
- Zhang H, Sreenivasulu N, Weschke W, et al. 2004. Large-scale analysis of the barley transcriptome based on expressed sequence tags The Plant Journal 40 276–290 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.