Abstract
Germin-like proteins (GLPs) are defined by their sequence homology to germins from barley and are present ubiquitously in plants. Analyses of corresponding genes have revealed diverse functions of GLPs in plant development and biotic and abiotic stresses. This study describes the identification of a family of 14 germin-like genes from Brassica napus (BnGLP) designated BnGLP1–BnGLP14 and investigated potential functions of BnGLPs in plant defense against the necrotrophic fungus Sclerotinia sclerotiorum. Sequence alignment and phylogenetic analyses classify the 14 BnGLPs into four groups, which were clearly distinguished from known germin oxalic acid oxidases. Transcriptional responses of the BnGLP genes to S. sclerotiorum infection was determined by comparing cultivars of susceptible B. napus ‘Falcon’ and partially resistant B. napus ‘Zhongshuang 9’. Of the 14 BnGLP genes tested, BnGLP3 was transcriptionally upregulated in both B. napus cultivars at 6h after S. sclerotiorum infection, while upregulation of BnGLP12 was restricted to resistant B. napus ‘Zhongshuang 9’. Biochemical analysis of five representative BnGLP members identified a H2O2-generating superoxide dismutase activity only for higher molecular weight complexes of BnGLP3 and BnGLP12. By analogy, H2O2 formation at infected leaf sites increased after 6h, with even higher H2O2 production in B. napus ‘Zhongshuang 9’ compared with B. napus ‘Falcon’. Conversely, exogenous application of H2O2 significantly reduced the susceptibility of B. napus ‘Falcon’. These data suggest that early induction of BnGLP3 and BnGLP12 participates in an oxidative burst that may play a pivotal role in defence of B. napus against S. sclerotiorum.
Key words: Brassica napus, germin-like proteins (GLPs), oxidative burst, plant disease resistance, Sclerotinia sclerotiorum, superoxide dismutase (SOD)
Introduction
Proteins with sequence homology to germins from wheat and barley have been identified in mosses and mono- and dicotyledonous plants and thus have been named germin-like proteins (GLPs). Initially, germins were found to accumulate in germinating wheat embryos (Lane et al., 1992), and later Lane et al. (1993) demonstrated that this germin degraded oxalic acid to H2O2 and CO2 by an oxalate oxidase (OXO) activity. So far, only germins from barley have been proven to share this OXO activity (Dumas et al., 1993; Lane et al., 1993; Whittaker and Whittaker, 2002), and this distinguishes these proteins from GLPs for which no OXO activity has yet been found. Instead, some GLPs have been shown to possess superoxide dismutase activity, which converts superoxide to H2O2 and O2 (Christensen et al., 2004; Gucciardo et al., 2007), while others remain elusive in terms of enzymatic activity, or function as an auxin receptor (Woo et al., 2002; Yin et al., 2009), reflecting the high functional diversity among GLPs.
Genome and transcriptome analysis of rice (Manosalva et al., 2009), barley (Zimmermann et al., 2006), wheat (Schweizer et al., 1999), maize (Breen and Bellgard, 2010), Physcomitrella patens (Nakata et al., 2004), Arabidopsis thaliana (Carter et al., 1998), and peanut and soybean (Chen et al., 2011) have revealed that GLPs are encoded by gene families with multiple gene members. For example, the A. thaliana genome contains 32 sequences annotated as ‘germin-like’ genes (www.uniprot.org). Expression of germin-like genes is not restricted to germinating seeds, as initially ascribed to wheat germin, but is found in leaves (Membré et al., 2000; Fan et al., 2005; Banerjee and Maiti, 2010), stems (Minic et al., 2009; Banerjee and Maiti, 2010), flowers (Fernández et al., 2003; Yang et al., 2006) and roots (Zimmermann et al., 2006; Gucciardo et al., 2007). The exact function of these proteins during plant development is unclear, but their apoplastic localization in combination with H2O2 generating superoxide dismutase (SOD) activity offers a role in cell-wall fortification through the cross-linkage of proteins and carbohydrates (Schopfer, 1996; Barceló, 1998; Banerjee and Maiti, 2010). Additionally, some GLPs bind to the plant hormone auxin and mediate auxin-induced physiological responses during plant development (Inohara et al., 1989; Robert et al., 2010; Effendi et al., 2011). Gene expression analysis in different species has revealed that many germin-like genes are regulated following abiotic and biotic stresses. Ke et al. (2009) identified a GLP among ten drought-induced proteins in rice, and GLPs from wheat, barley and Barbula unguiculata are regulated under salt or metal stress conditions (Hurkman et al., 1991; Berna and Bernier, 1999; Nakata et al., 2002; Caliskan, 2009).
Pathogen infection is one of the major triggers inducing germin-like gene expression. Corresponding sequences have been identified after pathogen challenge in barley (Wei et al., 1998; Hückelhoven et al., 2001), wheat (Berna and Bernier, 1999), rice (Manosalva et al., 2009; Banerjee and Maiti, 2010), pepper (Park et al., 2004), A. thaliana (Collins et al., 2010), sugar beet (Knecht et al., 2010), and grape (Ficke et al., 2004; Godfrey et al., 2007). Accordingly, germin and GLP activities significantly contribute to plant defence reactions against different pathogens. Heterologous expression of barley or wheat germin was found to lead to increased resistance against Sclerotinia sp. fungus in rape (Dong et al., 2008), peanut (Livingstone, 2005), sunflower (Hu, 2003), and tomato (Walz et al., 2008), as well as in transgenic poplar leaves against Septoria musiva (Liang et al., 2001). However, OXO activity is not necessarily essential for the defence function. Expression of mutated germin gf-2.8 and germin-like TaGLP2a, which both lack OXO activity, from wheat, conferred like native gf-2.8 increased resistance in wheat leaves against Blumeria graminis (Schweizer et al., 1999). Knecht et al. (2010) enhanced the resistance in A. thaliana plants against the fungal pathogens Verticillium longisporum and Rhizoctonia solani through transgenic expression of germin-like BvGLP-1 from sugar beet, and silencing of GLP genes in rice intensified the development of fungal rice blast and sheath blight diseases (Manosalva et al., 2009). Furthermore, tobacco plants silenced for a germin-like gene (NaGLP) showed increased susceptibility against two insect herbivores (Lou and Baldwin, 2006), indicating a basal function of GLPs beyond microbial pathogens.
This study reports on the identification and characterization of germin-like genes in the rapeseed (Brassica napus) genome and evaluated their potential in defence against the fungal pathogen S. sclerotiorum, the causal agent of Sclerotinia stem rot disease. S. sclerotiorum is a necrotrophic pathogen that thrives on more than 400 plant species (Bolton et al., 2006) and poses a considerable threat to rape farming. Efforts to increase rape resistance against S. sclerotiorum to the generation of varieties with increased tolerance (Wang et al., 2004; Liu et al., 2005;Li et al., 2009) and intensive research is ongoing. In the available sequence databases, we identified a germin-like gene family in B. napus composed of 14 members designated BnGLP1–BnGLP14. To identify BnGLP members with a putative function in defence against S. sclerotiorum, we measured the corresponding transcripts by quantitative real-time PCR (qPCR) in rape after infection and compared the susceptible B. napus ‘Falcon’ variety with the more tolerant B. napus ‘Zhongshuang 9’ cultivar. Biochemical characterization of five selected BnGLP proteins revealed a H2O2-generating SOD activity for two members, while none showed OXO activity. by analogy, S. sclerotiorum evoked an oxidative burst in the plant at 6h after infection, and H2O2 itself was shown to augment plant tolerance against S. sclerotiorum. Taken together, these results describe for the first time the family of germin-like genes in B. napus and demonstrate that early upregulation of SOD-active BnGLPs correlates with H2O2 formation in plants and may play a pivotal role in resistance of B. napus to S. sclerotiorum infection.
Materials and methods
Plant material and growth conditions
The commercial rape variety B. napus ‘Falcon’ was provided by the Norddeutsche Pflanzenzucht Hans-Georg Lembke KG (Hohenlieth, Germany) and B. napus ‘Zhongshuang 9’ was obtained from Professor Wang Hanzhong (Oil Crops Research Institute, Wuhan, China). Plants of A. thaliana and B. napus were cultivated in growth cabinets with 10h light d–1 at 300 µmoles m–2 s–1 and the temperature set to 22 and 20 °C during day and night periods, respectively.
Infection with S. sclerotiorum
The S. sclerotiorum isolate used throughout this work was obtained from Professor W. Qian (Mei et al., 2011). S. sclerotiorum mycelium was grown on potato dextrose agar (PDA) plates (26.5g l–1 potato dextrose, 15g l–1 agar, pH 5.6) for 2 d at 22 °C. Infection of fully expanded leaves from 5–6-week-old plants was performed as described by Zhao and Meng (2003). In brief, scissored leaves were arranged in moistened trays and inoculated with S. sclerotiorum-grown PDA plugs cut with a 0.6cm cork borer from the margin of the expanding mycelium. Trays were sealed with plastic foil and incubated at 22 °C. Developing lesions were measured with a caliper at the indicated time points. To measure lesion formation following water or H2O2 infiltration, leaves were not cut from the plant but were wrapped with plastic foil after inoculation to prevent drying of the agar plugs. Infiltration of ~20 µl solution was applied from the bottom side of the leaf at the site of plug infection. In the case of plant sampling for gene transcript analysis, leaves were also not cut from the plant.
Infection of A. thaliana was performed with S. sclerotiorum grown in 70ml liquid Czapek Dox medium (33.4g l–1 Czapek Dox, 2g l–1 yeast extract, 2g l–1 malt extract, pH 5.5) for 2 d at 22 °C. The mycelium was homogenized for 3 s using an Ultra-Turrax, centrifuged at 6000 g for 10min, and the sedimented mycelium resuspended in 10mM MgCl2 to a concentration of 0.7g in 50ml. A. thaliana plants were grown in 9×9cm pots filled with peat soil and four plants per pot. Fully expanded leaves of 5–6-week-old soil-grown plants were infected with 30 µl drops of the mycelium suspension and covered with a plastic lid to maintain a high humidity. At 3 d after infection, lesion development was divided in three classes: 1, necrotic spot, poor fungal expansion; 2, round wet lesion; and 3, macerated leaf. Based on each class, a disease index was calculated with the formula: (0.5×class 1/total number of drops+class 2/total number of drops+2×class 3/total number of drops)×100. Two independent experiments were performed to validate the results.
For S. sclerotiorum growth inhibition assays, 20 µl of H2O2 solution was applied to Whatman paper discs (0.8cm diameter) in the centre of a PDA plate before inoculation and incubated for 2 d.
RNA extraction and qPCR
Plant samples were excised with a 0.8cm cork borer at the sites ofS. sclerotiorum agar plug infection and immediately frozen in liquid nitrogen. Agar plugs without mycelium were used as controls. Total RNA was extracted with TRIzol Reagent (Invitrogen; www.invitrogen.com/) from ground tissue of three leaf discs per sample and three samples were processed for each treatment. Reverse transcription of mRNA into cDNA was performed from 2 µg of total RNA using SuperScriptIII (Invitrogen) following the manufacturer’s instructions. qPCR was conducted with a 7300 Real Time PCR System (Applied Biosystems; www.appliedbiosystems.com) using MAXIMA®SYBR Green Master Mix (Fermentas; www.fermentas.de) for gene amplification. The primer combinations to amplify BnGLP1–BnGLP 14 and β-tubulin as the reference gene are given in Supplementary Table S1 at JXB online. All PCR products were sequenced to verify specificity for the respective gene, and invariant expression of the β-tubulin reference gene in response to S. sclerotiorum infection was assured beforehand. Data were analysed using 7300 System SDS software by the comparative ΔΔC t method (Supplementary Fig. S1 at JXB online).
Gene cloning and transient expression in Nicotiana benthamiana
Full-length sequences of BnGLP3, BnGLP7, BnGLP8, BnGLP10 and BnGLP12 were amplified from genomic DNA of B. napus ‘Zhongshuang 9’ using Pfu polymerase (Fermentas). As control genes, GFP was amplified from pGWB5 (Nakagawa et al., 2007) and wheat germin gf-2.8 template DNA was kindly provided by Dr Bornemann (Gucciardo et al., 2007). The respective primers contained attB sites at their 5' ends to allow cloning of the PCR products via the Gateway®BP recombination reaction (Invitrogen) into the pDONR201 Entry vector. The primer sequences are listed in Supplementary Table S2 at JXB online. For each BnGLP gene, at least six independent clones were sequenced to confirm consistent sequence identity. In the cases of BnGLP3 and BnGLP12, two copies of these family members were amplified with nucleotide polymorphisms. Subsequently, genes were transferred into the Gateway compatible binary pGWB414 vector (Nakagawa et al., 2007) in frame with a C-terminal triple haemagglutinin (HA)-tag coding sequence. For transient plant transformation, the binary vector constructs were transformed into Agrobacterium tumefaciens strain GV3101::pMP90RK and infiltrated with a 1ml syringe without a needle into fully expanded leaves of N. benthamiana, as described by Witte et al. (2004). Tissue for protein extraction was harvested at 6–8 d post-infiltration and frozen at –80°C until further processing.
Generation of transgenic A. thaliana
Transformation of A. thaliana ‘Columbia-0’ was conducted according to Clough and Bent (1998). Transgenic plants (T1) were selected on half-strength Murashige and Skoog medium (Duchefa; www.duchefa.com) supplemented with 50g l–1 kanamycin and later transferred to soil for seed setting. Infection experiments were conducted with T2 plants selected for kanamycin resistance as above.
Enzyme analysis and immunodetection of proteins
Unless otherwise noted, protein extraction from ~10mg of ground tissue was performed in 110 µl extraction buffer (20mM Tris/HCl, pH 7.5, 0.5% SDS, 5mM DTT), except for BnGLP3.1 and BnGLP3.2, which were extracted with 20mM Tris/HCl (pH 7.5), 2% SDS, 50mM DTT. Subsequently, protein samples were adjusted to 2% SDS, 50mM DTT, 10% glycerol and 0.01% bromophenol blue and loaded on an SDS-polyacrylamide gel (10% acrylamide) without boiling (semi-native) for separation. An in-gel SOD activity assay was performed following the protocol of Beauchamp and Fridovich (1971). OXO activity was tested after transferring the proteins to nitrocellulose membrane (Roth; www.carlroth.com) as described by Lane et al. (1993). For immunodetection, proteins were blotted on a PVDF membrane (Roche; www.roche.de) and visualized with anti-HA antibody (Roche) in combination with a Lumi-LightPLUS Western Blotting Kit (Roche) following the manufacturer’s instructions.
Measurement of H2O2 in rape leaves
Measurement of H2O2 from leaf tissue was performed using the FOX reagent as described by Cheeseman (2006) with slight modifications. B. napus leaf discs were collected with a 0.6cm cork borer at sites of S. sclerotiorum infection or from control treatment plants and immediately ground in liquid nitrogen. To extract the H2O2, 400 µl of 25mM HCl was added and the sample thawed on ice with shaking. After centrifugation for 5min at 17 000 g at 4 °C, 100 µl of the supernatant was added to 900 µl of FOX reagent [250 µM ammonium iron (II) sulfate, 100 µM sorbitol, 100 µM xylenol orange, 25mM H2SO4, 1% ethanol] and incubated for 15min in the dark. Complex formation of FeIII+ with xylenol orange in the presence of H2O2 was measured with a photometer at 560nm.
Results
The germin-like gene family in B. napus
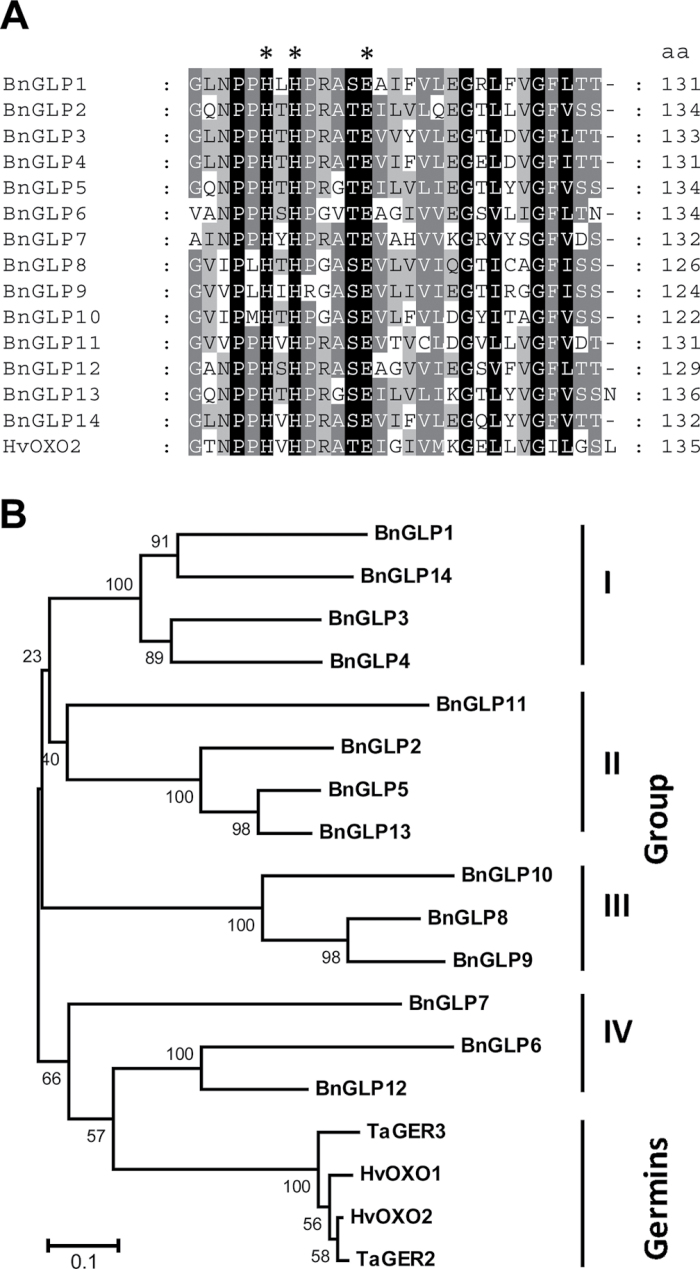
To identify germin-like genes in B. napus (BnGLP), we searched genomic and expressed sequence tag (EST) databases from the NCBI, TGI (http://compbio.dfci.harvard.edu/tgi/plant.html) and the Shanghai Rapeseed database (http://rapeseed.plantsignal.cn) with an original germin sequence from barley using tblastn. ESTs provide an alternative source for gene identification in plants whose genome sequences are not fully available (Rudd, 2003), as is the case for B. napus. Gene candidates from genomic database were verified by EST fragments to exclude non-transcribed pseudo-genes. Putative full-length sequences matching an E-value of a maximum of 10–3 were selected and sequences were only considered that contained the two conserved histidines essential for binding the manganese co-factor (Fig. 1A; Woo et al., 2000), giving 307 candidate sequences in total. The average sequence length of the BnGLPs was around 220 aa, matching the length of the original germins including the signal peptide, and amino acid identity ranged from 30 to 48% between the BnGLPs and HvOXO2 germin from barley. Polyploidity of B. napus and independent sequence donations from various B. napus accessions to the databases can result in redundancy of gene family members. We thus performed a protein sequence alignment and subsequent phylogenetic analysis (http://bioweb2.pasteur.fr; Felsenstein, 1989; Thompson et al., 1994) to distinguish classes of highly similar sequences. For each class, a representative BnGLP sequence was chosen that showed no more than 65% amino acid identity to any protein of the other aligned clusters. This produced a GLP family in B. napus comprising 14 members, which could be clustered into four groups (Fig. 1A, 1B). Comparison of the BnGLP family with the proven germin OXOs TaGER2, TaGER3, HvOXO1 and HvOXO2 did not reveal high homology and thus these were separated from the germins in the phylogenetic analysis (Fig. 1B).
Fig. 1.
Primary structure analysis of GLPs from B. napus (BnGLP). (A) Partial amino acid alignment of the 14 BnGLP members with HvOXO2 germin from barley. Asterisks denote conserved amino acids involved in manganese ion binding of germins (Woo et al., 2000). (B) Phylogenetic tree of GLPs from B. napus and germins from Hordeum vulgare (HvOXO1 and HvOXO2) and Triticum aestivum (TaGER2 and TaGER3). Analysis was conducted using MEGA5 software (Tamura et al., 2011) with the neighbour-joining method. The sum of branch length is 4.9. Numbers next to the branches indicate percentage of replicate trees in which the sequences clustered together in a bootstrap test (100 replicates). Bar, 0.1 amino acid substitutions per site.
Transcript profiling of BnGLP genes in response toS. sclerotiorum infection
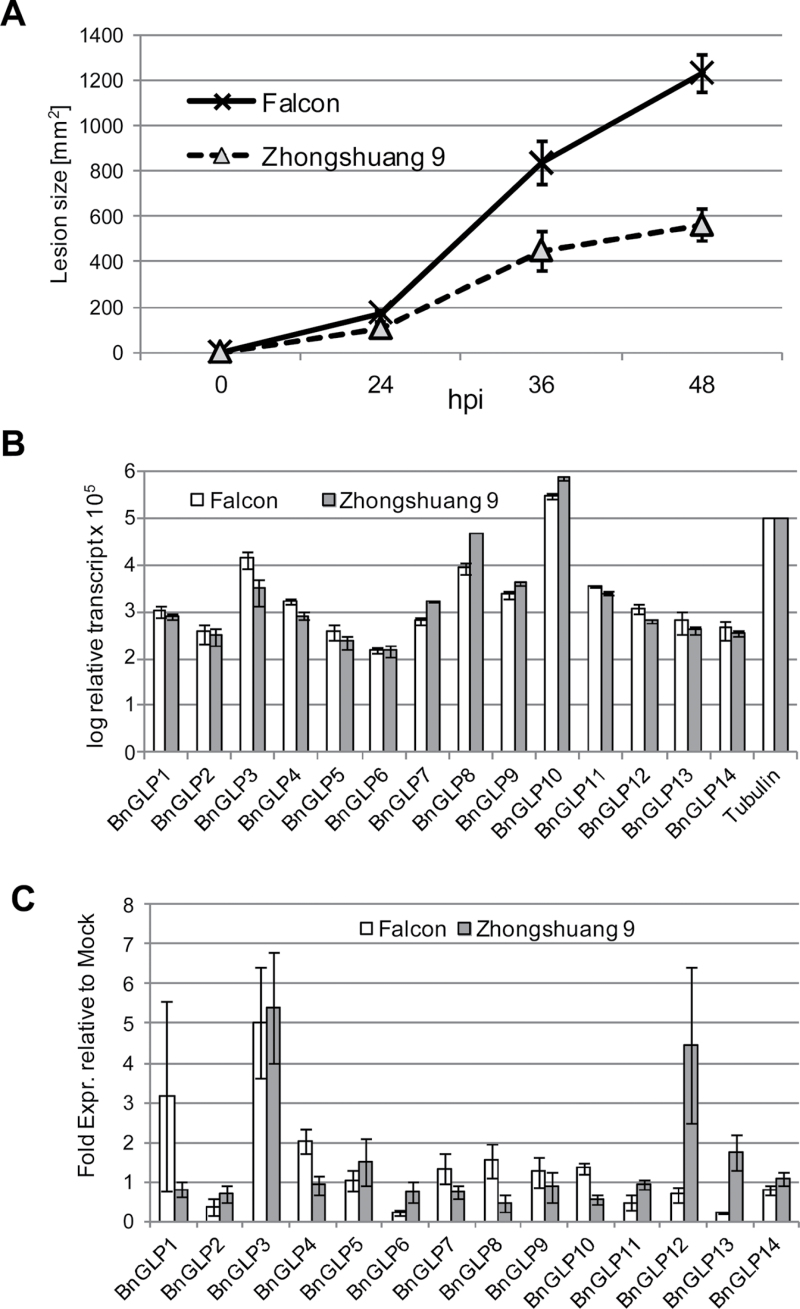
B. napus is a highly susceptible host for S. sclerotiorum and only a few varieties exist with partial resistance, such as B. napus ‘Zhongshuang 9’ derived from native Chinese cultivars (Wang et al., 2004). In order to find two B. napus cultivars with differential susceptibility to Sclerotinia disease, we compared B. napus ‘Zhongshuang 9’ with the commercial cultivar B. napus ‘Falcon’ that was previously shown to have a high susceptibility against V. longisporum (Eynck et al., 2009). Detached leaves of both varieties were infected with S. sclerotiorum grown on PDA and incubated at 20–22 °C. Around the infection sites, circular lesions developed that were twice the size on B. napus ‘Falcon’ leaves at 36 and 48h after infection compared with those on B. napus ‘Zhongshuang 9’ leaves (Fig. 2A), revealing significant differences in susceptibility to S. sclerotiorum infection. To verify the expression of BnGLP genes in leaf tissue, we first extracted RNA from non-treated leaves and measured the transcript abundance by qPCR using primers specifically amplifying fragments of each of the 14 BnGLP genes (Fig. 2B). Gene transcripts were detected for all BnGLP genes, although the relative amounts varied by three orders of magnitudes, with BnGLP6 and BnGLP10 being the least and most abundant transcripts, respectively. Significant differences in BnGLP gene expression between B. napus ‘Falcon’ and ‘Zhongshuang 9’ were not detected. Furthermore, we used these cultivars to test whether members of the BnGLP family were transcriptionally regulated in response to infection with S. sclerotiorum and whether differences in regulation existed between the susceptible B. napus ‘Falcon’ and the partially resistant B. napus ‘Zhongshuang 9’ lines. We chose a 6h time point for transcript analysis as an early stage of infection, assuming that molecular effects decisive for the success of plant defence appear in the beginning and to limit secondary effects derived from massive cell degradation at later time points. In the susceptible B. napus ‘Falcon’ background, BnGLP3 was upregulated at 6h post-infection, while in B. napus ‘Zhongshuang 9’, in addition to BnGLP3, BnGLP12 also became transcriptionally induced. None of the other BnGLP genes showed a significant response in gene expression, although BnGLP8 and BnGLP10 showed a tendency in multiple experiments to become suppressed in B. napus ‘Zhongshuang 9’ following S. sclerotiorum infection. Together, these data demonstrated that B. napus ‘Zhongshuang 9’ responded to the S. sclerotiorum infection differently in the regulation of BnGLP genes compared with B. napus ‘Falcon’.
Fig. 2.
B. napus infection with S. sclerotiorum and transcript analysis of BnGLP genes. (A) Infection of B. napus ‘Falcon’and ‘Zhongshuang 9’ with S. sclerotiorum in a detached-leaf assay. Lesion sizes were measured at the indicated hours post-infection (hpi) and results are shown as means ± standard error (SE) (n=8). (B) Transcript abundance of the 14 BnGLP genes relative to that of β-tubulin in leaves of B. napus ‘Falcon’ and ‘Zhongshuang 9’. Transcripts were measured by qPCR and values calculated following the equation 2CtTubulin/2CtBnGLP×105. Results are shown as means ±SE (n=3) and independent experiments showed the same trend.(C) qPCR analysis of the 14 BnGLP genes relative to mock treatment at 6h after infection with S. sclerotiorum.
Cloning and biochemical characterization of BnGLP3, BnGLP7, BnGLP8, BnGLP10 and BnGLP12
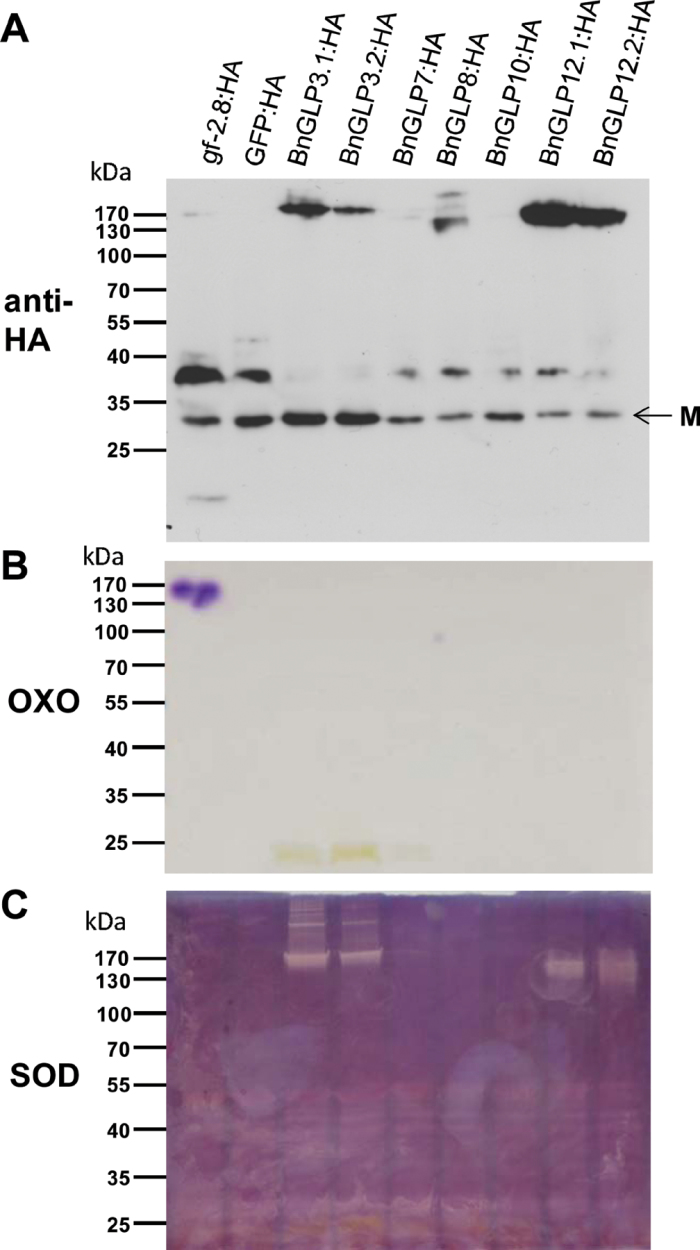
As GLPs from B. napus have not been characterized to date, we cloned five representative members of the BnGLP gene family with regard to their transcriptional regulation after S. sclerotiorum infection (Fig. 2B). These comprised the inducible BnGLP3 and BnGLP12 and the non-induced BnGLP8, BnGLP10 and BnGLP7 genes. The full-length open reading frames were cloned behind a 35S promoter and fused to the coding sequence of a triple HA tag (3×HA) to allow later immunodetection of the expressed proteins. Six to ten clones of each gene were sequenced to verify sequence consistency and to identify gene copies in the Brassica genome. While BnGLP7, BnGLP8 and BnGLP10 were cloned as single genes, amplification of BnGLP3 and BnGLP12 each yielded two homologues coding for proteins with 95% identities and were named BnGLP3.1 and BnGLP3.2, and BnGLP12.1 and BnGLP12.2, respectively. The homologous genes were included in the further biochemical analysis to test for alterations resulting from the amino acid differences. Additionally, we generated 3×HA-tagged fusion proteins of wheat germin gf-2.8 (Lane et al., 1993) and GFP to serve as positive and negative controls, respectively. The recombinant proteins were transiently expressed in N. benthamiana, and total protein extracts were separated by semi-native SDS-PAGE omitting a reducing agent in the loading buffer and without boiling before loading the sample (Fig. 3A). The calculated molecular weight of monomeric germin and all BnGLPs fused to the 3×HA tag was ~29 kDs and that of GFP:HA was ~32.8kDa, matching the band sizes seen at the bottom of the HA-specific immunoblot. The larger proteins between 35 to 40kDa and around 170kDa were not observed under full denaturating conditions (data not shown) and thus probably reflect different oligomerizations of the proteins. The active gf-2.8 enzyme is a hexameric complex and has been shown to migrate at ~125kDa by semi-native SDS-PAGE (Lane et al., 1993; Walz et al., 2008). In Fig. 3A, the majority of gf-2.8:HA protein migrated at between 25 and 40kDa and a minor amount also formed a complex at ~170kDa. Taking the mass of the 3×HA tag into account, the latter probably represents the hexameric gf-2.8:HA complex. By analogy, of the seven investigated BnGLPs, both homologues of BnGLP3:HA and BnGLP12:HA, and BnGLP8:HA migrated as monomers and also formed higher-molecular-weight complexes. In contrast, BnGLP7:HA and BnGLP10:HA were expressed as monomeric proteins only and did not form higher-molecular-weight complexes.
Fig. 3.
Biochemical characterization of the fusion proteins BnGLP3.1:HA, BnGLP3.2:HA, BnGLP7:HA, BnGLP8:HA, BnGLP10:HA, BnGLP12.1:HA, BnGLP12.2:HA, gf-2.8:HA and GFP:HA transiently expressed in N. benthamiana. (A) Immunodetection of recombinant proteins using HA-specific antibody in total protein extracts. Samples were loaded without DTT in the loading buffer and without prior boiling (semi-native).M indicates the monomer. (B) Protein extracts separated as in(A) were blotted on nitrocellulose membrane and assayed for OXO activity. (C) Protein extracts separated as in (A) were assayed for SOD activity.
Originally, germins from wheat and barley were found to oxidize oxalic acid to CO2 and H2O2 (Dumas et al., 1993; Lane et al., 1993), defining them as true germins. This OXO activity has so far not been shown for any of the GLPs present in mono- and dicotyledonous plant species and mosses. Instead, some GLPs are SODs, reducing superoxide to H2O2, while the enzyme activity of other GLPs remains elusive (Yamahara et al., 1999; Christensen et al., 2004; Nakata et al., 2004; Zimmermann et al., 2006; Gucciardo et al., 2007; Banerjee and Maiti, 2010). We thus tested for OXO and SOD activities of the transient expressed proteins (Fig. 3B, 3C). In the OXO activity assay, the gf-2.8:HA protein gave a clear signal at the size of the higher-molecular-weight complex of ~170kDa, confirming the OXO activity for recombinant gf-2.8:HA protein (Fig. 3B). Under the same conditions, none of the BnGLPs or GFP:HA displayed an OXO activity at any protein complex size. However, when we tested for SOD activity, the protein complexes of BnGLP3:HA and BnGLP12:HA and their respective homologues showed a clear activity at 170kDa or higher (Fig. 3C), but none of the other tested BnGLPs or gf-2.8:HA and GFP:HA appeared to possess SOD activity under the experimental conditions used. Notably, despite higher-molecular-weight complex formation, BnGLP8:HA did not show any OXO or SOD activity. We also expressed BnGLP10 as native protein to test for potential interference of the 3×HA tag with enzyme function but again did not observe SOD activity (data not shown).
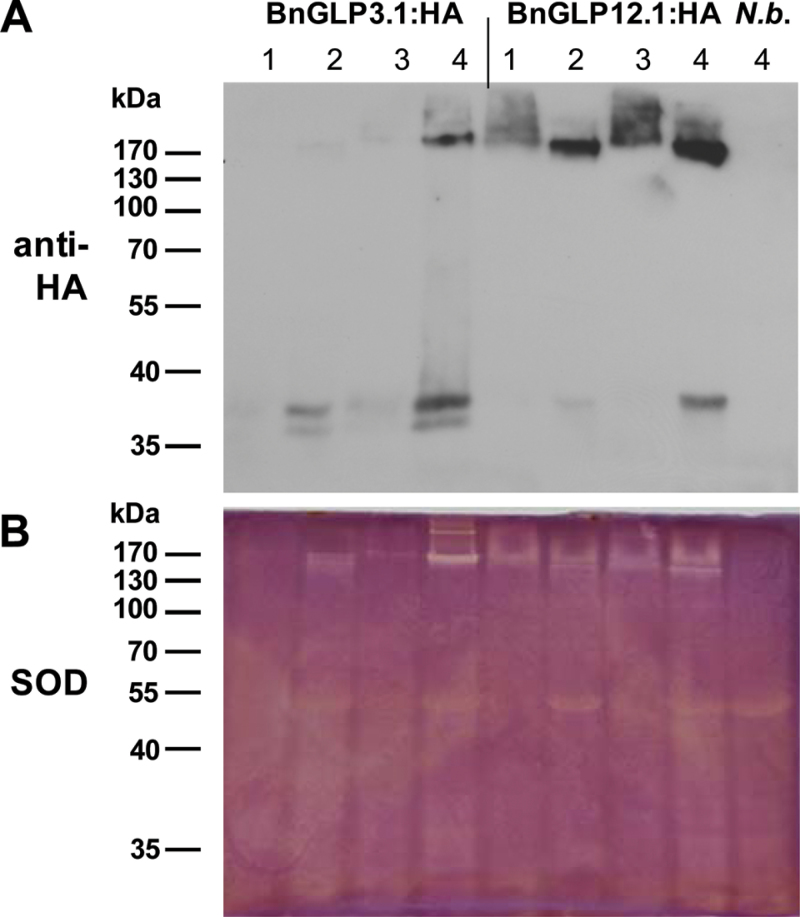
In order to evaluate whether BnGLP3 and BnGLP12 represent SODs with redundant functions in the plant, we investigated their solubility under different extraction conditions (Fig. 4). Both proteins exhibited maximum solubility when extracted with 50mM Tris/HCl (pH 7.5), 2% SDS and 50mM DTT. In contrast, 50mM Tris/HCl (pH 7.5) without detergent and reducing agent extracted BnGLP12.1:HA but not BnGLP3.1:HA, as shown by anti-HA immunodetection and SOD activity assay (Fig. 4, lane 1). The addition of either 50mM DTT or 2% SDS (Fig. 4, lanes 2 and 3) slightly increased the solubility of both proteins, although the majority of BnGLP3.1:HA was extracted with the combination of both. Together, these results are in line with earlier findings that disproved an OXO activity for GLPs, and instead we have demonstrated SOD activity for two members of five tested GLPs from B. napus. Interestingly, it was BnGLP3 and BnGLP12, which were transcriptionally induced in response to S. sclerotiorum infection, that displayed SOD activity.
Fig. 4.
Total proteins from leaves of N. benthamiana expressing BnGLP3.1:HA, BnGLP12.1:HA or no (N.b.) recombinant protein. Extraction was performed as follows: lane 1, 50mM Tris/HCl(pH 7.5); lane 2, 50mM Tris/HCl (pH 7.5), 50mM DTT; lane 3, 50mM Tris/HCl (pH 7.5), 2% SDS; lane 4, 50mM Tris/HCl (pH 7.5), 50mM DTT, 2% SDS. Prior to SDS-PAGE loading, all samples were adjusted to the same concentration of DTT and SDS but not boiled. (A) Immunodetection of transblotted proteins with HA-specific antibody. (B) In-gel SOD activity assay.
Impact of H2O2 on defence of B. napus againstS. sclerotiorum
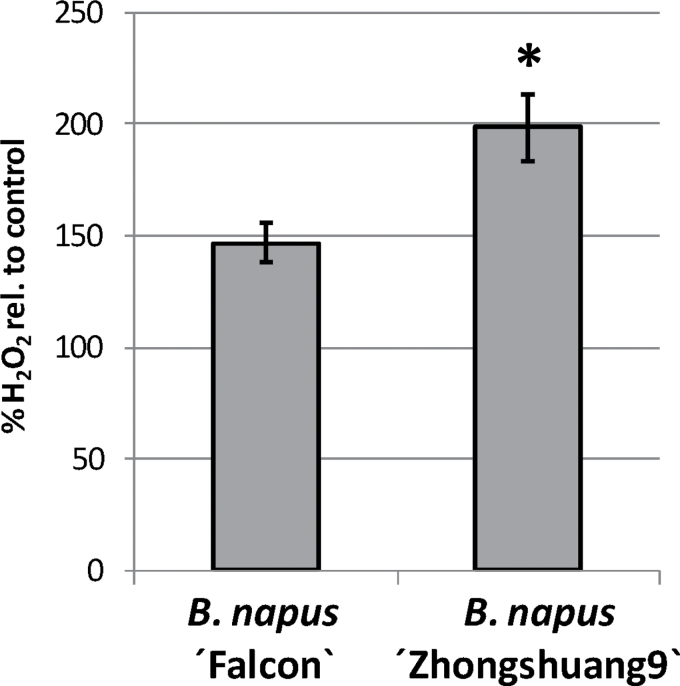
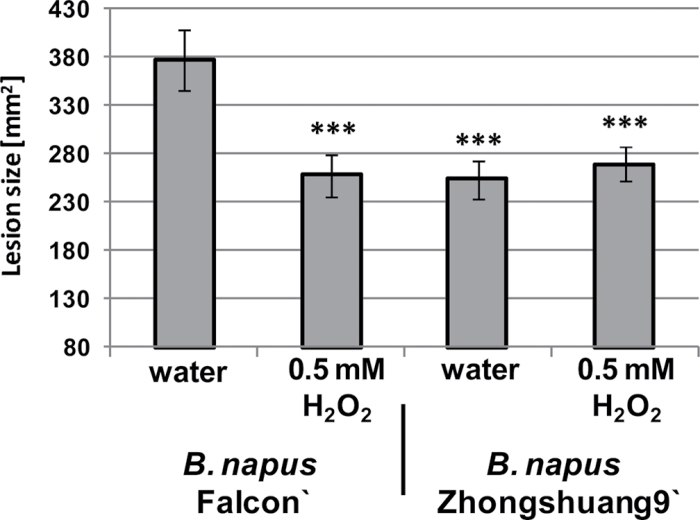
The correlation between BnGLP3 and BnGLP12 gene transcript activation in response to S. sclerotiorum infection and the SOD activity of the corresponding proteins prompted us to further investigate the role of H2O2 as one reaction product of SOD activity during the defence response of B. napus to S. sclerotiorum infection. The BnGLP transcript analyses shown in Fig. 2C revealed gene induction of SOD BnGLP3 and BnGLP12 at 6h post-infection. We thus measured H2O2 production in the leaves of B. napus ‘Falcon’ and B. napus ‘Zhongshuang 9’ at 6h after infection with S. sclerotiorum infection. Figure 5 shows the change in H2O2 formation relative to the control treatment. Both B. napus varieties generated more H2O2 at 6h post-infection, but the increase in the partially resistant B. napus ‘Zhongshuang 9’ variety was significantly higher compared with B. napus ‘Falcon’. Thus, H2O2 production is part of the early plant defence against S. sclerotiorum infection and its magnitude may contribute to the resistance phenotype of B. napus ‘Zhongshuang 9’. To strengthen this idea further, we infiltrated leaves of B. napus ‘Falcon’ and ‘Zhongshuang 9’ from the bottom side with 0.5mM H2O2 or water as a control and infected the infiltration sites from the top with S. sclerotiorum grown on PDA plugs. At 29h post-infection, we measured the lesion sizes of the spreading S. sclerotiorum fungus to evaluate whether artificial supply with H2O2 could influence the progress of S. sclerotiorum infection (Fig. 6). Lesion sizes were significantly smaller on B. napus ‘Zhongshuang 9’ compared with B. napus ‘Falcon’ when only water was infiltrated, in line with the detached-leaf assay shown in Fig. 2A. In contrast, infiltration of H2O2 resulted in significantly smaller lesion formation on B. napus ‘Falcon’, and was approximately equal to that observed on the more tolerant ‘Zhongshuang 9’ genotype. Lesion formation was not significantly different on B. napus ‘Zhongshuang 9’ between H2O and H2O2 infiltration. Thus, an external supply of H2O2 can increase the resistance of B. napus ‘Falcon’ to S. sclerotiorum infection and this observation was not caused by a direct toxic effect of H2O2 on S. sclerotiorum growth, as H2O2 did not induce a further lesion size reduction on B. napus ‘Zhongshuang 9’ and did not affect S. sclerotiorum growth in vitro (data not shown).
Fig. 5.
H2O2 formation at 6h after S. sclerotiorum infection on leaves of B. napus ‘Falcon’ and ‘Zhongshuang 9’ relative to control treatment (100%). Results are shown as means ±SE (n=3) and the asterisk indicates a statistically significant difference(*P <0.05, Student’s t-test). One of three independentexperiments with similar results is shown.
Fig. 6.
Infection of B. napus ‘Falcon’ and ‘Zhongshuang 9’ with S. sclerotiorum mycelium plugs at sites infiltrated with water or 0.5mM H2O2. Lesion sizes were measured at 31h post-infection. Results are shown as means ±SE (n=8) and asterisks indicate statistically significant differences compared with Falcon water treatment (***P <0.01, Students t-test). One of three independent experiments with similar results is shown.
Transgenic expression of BnGLP12:HA in A. thaliana increases tolerance to S. sclerotiorum
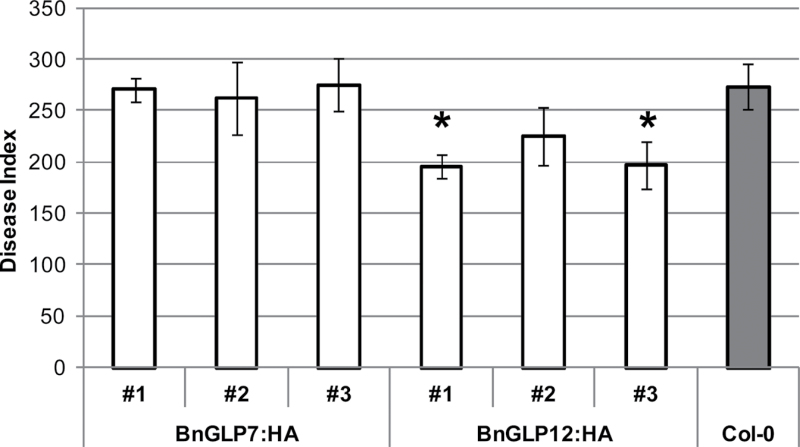
The experiments performed in this study so far strongly suggested that members of the germin-like family in B. napus with SOD activity contribute to reduce the spread of S. sclerotiorum. To confirm a direct link between the upregulation of GLPs and resistance to S. sclerotiorum, we transformed A. thaliana with BnGLP7:HA and BnGLP12:HA representing GLPs without and with SOD activity, respectively. A. thaliana is a host for S. sclerotiorum and is closely related to B. napus. Following infection with S. sclerotiorum, transgenic Arabidopsis expressing BnGLP7:HA did not reveal any difference in susceptibility compared with wild-type Col-0, while two of three plants expressing BnGLP12:HA were significantly (P <0.05) more resistant (Fig. 7).
Fig. 7.
Infection of A. thaliana with S. sclerotiorum. Transgenic plants expressing BnGLP7:HA or BnGLP12:HA under control of a 35S promoter compared with Col-0 Arabidopsis wild-type. Leaves were infected with drops of mycelia solution and evaluated after 3 d, as described in Materials and methods. Numbers denote independent transgenic lines. Results are shown as means ±SE of three pots with four plants each and asterisks indicate a significant difference at P <0.05 compared with Col-0 (Student’s t-test). Two independent experiments were carried out and showed the same trend.
Discussion
GLP family in B. napus
Germin-like proteins are present in many if not all plants and are encoded by gene families in the respective genomes. Here, we have presented for the first time the GLP family in rape(B. napus), which is represented by 14 BnGLPs (Fig. 1A, 1B). As complete sequence information of the B. napus genome is currently not available, our classification of GLPs was based on genomic sequences and ESTs that translate into proteins with a maximum of 65% amino acid identity. In this way, we excluded cultivar-specific variants of one gene, and probably also gene copies present in the B. napus genome due to genome duplication during evolution (Parkin et al., 2003) and the amphidiploid nature of the B. napus genome (Nagaharu, 1935). The BnGLPs possessed all three conserved germin sequence boxes defined by Bernier and Berna (2001) including the PxHxHxxxxE motive (Fig. 1A) essential for OXO activity, but exhibited only 30–48% amino acid sequence identity to the original HvOXO2 germin from wheat and thus are termed ‘germin-like’. Accordingly, all germins with proven OXO activity separated in a phylogenetic tree from the BnGLPs (Fig. 1B), implying that they also exhibit functional divergence. In the same analysis, the 14 BnGLPs clustered into four groups, but it was not clear whether proteins of one group shared common features.
The SODs BnGLP3 and BnGLP12 are induced in response to S. sclerotiorum infection
Gene expression and protein function analyses reported in the literature indicate a bias of germins and GLPs to participate in plant defence responses against pathogens, including the necrotrophic fungus S. sclerotiorum (reviewed by Lane, 2002; Dunwell et al., 2008). B. napus is also a host for S. sclerotiorum, and most commercially available rape varieties are highly susceptible to S. sclerotiorum, such as B. napus ‘Falcon’. In contrast, B. napus ‘Zhongshuang 9’ shows increased resistance against the biotrophic fungus V. longisporum (Eynck et al., 2009) and higher tolerance to S. sclerotiorum infection compared with B. napus ‘Falcon’, as shown in Fig. 2A. To investigate a possible involvement of the BnGLP family in response to S. sclerotiorum infection, we examined the regulation of the corresponding genes. Quantitative measurements of the 14 BnGLP genes in non-treated leaves revealed no differences a priori in transcript abundance between both B. napus varieties (Fig. 2B), indicating conserved gene regulation under normal growth conditions. We extended the analysis to 6h after S. sclerotiorum infection, assuming that early regulated genes would be directly connected to S. sclerotiorum invasion and probably essential for the success of plant defence. In both B. napus varieties, BnGLP3 was upregulated at 6h post-infection, while BnGLP12 transcripts increased in B. napus ‘Zhongshuang 9’ only (Fig. 2C). Similarly, Zhao et al. (2007) investigated gene expression changes in rape with a microarray from A. thaliana and found a germin-like gene to be upregulated 4–4.8-fold in a susceptible and a semi-tolerant variety at the earliest time point of 24h after S. sclerotiorum infection. The corresponding orthologue in B. napus (BnGLP7) was not upregulated under our experimental conditions; this may be due to the different time point chosen or non-specific hybridization of the microarray to other BnGLP members. In another experiment employing a B. napus-specific oligonucleotide chip, BnGLP3 was upregulated 11-fold at 78h after S. sclerotiorum infection in stem tissue of the susceptible B. napus ‘Westar’ but not in the partially resistant ‘Zhongyou 821’ cultivar (Zhao et al., 2009). It should be noted that, in B. napus, BnGLP3 is regulated following S. sclerotiorum infection in both susceptible and tolerant B. napus varieties, supporting a potential role in plant basal resistance, as suggested by Zimmermann et al. (2006) for barley GLPs in the compatible interaction with the powdery mildew fungus Blumeria gramins f. sp. hordei. The fact that BnGLP12 was only upregulated in the resistant ‘Zhongshuang 9’ following S. sclerotiorum infection strongly suggests that it has a role in plant resistance to S. sclerotiorum. Furthermore, we demonstrated that heterologous expression of BnGLP12:HA, but not of the SOD-inactive germin-like protein BnGLP7:HA, in A. thaliana increased tolerance to S. sclerotiorum (Fig. 7). This corroborates our interpretation of increased resistance against S. sclerotiorum through the upregulation of BnGLP12.
Germin proteins from wheat and barley form homohexameric complexes and degrade oxalic acid to H2O2 and CO2. Of the five BnGLPs tested here, both homologues of BnGLP3:HA and BnGLP12:HA as well as BnGLP8:HA migrated as higher-molecular-weight complexes of~170kDa in semi-native SDS-PAGE (Fig. 3A). This is equivalent to six times the monomer mass of ~28kDa, revealing the same complex stoichiometry and SDS stability as observed for germin proteins (Lane et al., 1992; Woo et al., 1998) and GLPs from P. patens (Nakata et al., 2004), barley (Christensen et al., 2004; Zimmermann et al., 2006), A. thaliana (Membré et al., 2000), and pea (Gucciardo et al., 2007). The absence of higher-molecular-weight complexes observed here for BnGLP8:HA and BnGLP10:HA was also shown for AtGER1 (Membré et al., 2000), revealing distinct biochemical properties with respect to either complex formation or complex stability.
The GLPs examined to date do not possess an OXO activity, but some members have been shown to produce H2O2 through a SOD activity. The BnGLP proteins investigated here did not show any OXO activity, but higher-molecular-weight complexes of BnGLP3:HA and BnGLP12:HA, including their homologues, were able to dismutate superoxide to H2O2 through SOD activity (Fig. 3B, 3C). Despite the complex formation of BnGLP8:HA, no SOD activity could be detected as for monomeric BnGLP7:HA and BnGLP10:HA. Thus, BnGLP7, BnGLP8 and BnGLP10 probably fulfil different functions in the plant compared with BnGLP3 and BnGLP12. We also expressed BnGLP10 as native protein to exclude a negative effect of the HA tag, but were again not able to detect SOD activity (data not shown). Remarkably, of the five BnGLPs tested, those that showed SOD activity were also transcriptionally induced in response to S. sclerotiorum infection. Despite equivalent complex formation and SOD activity of BnGLP3:HA and BnGLP12:HA, BnGLP12:HA was soluble in Tris buffer (pH 7.5) during protein extraction from plant tissue, while BnGLP3 required the addition of a reducing agent and a strong detergent to become fully soluble (Fig. 4). This indicated that BnGLP3 is associated with cellular structures via reducing/oxidizing and hydrophobic interactions and is not redundant with BnGLP12 in the plant. Thus, infection with S. sclerotiorum results in quantitative and qualitative differences in SOD activation between susceptible B. napus ‘Falcon’ and the tolerant B. napus ‘Zhongshuang 9’.
Early formation of H2O2 restricts S. sclerotiorum pathogenesis
In line with the induction of SOD-active BnGLPs, we also measured an increase in H2O2 in rape leaves at 6h after S. sclerotiorum infection (Fig. 5). Both varieties responded with an increase in H2O2, but B. napus ‘Zhongshuang 9’ significantly exceeded the H2O2 amounts of the susceptible ‘Falcon’ cultivar, correlating with the additional induction of BnGLP12 in ‘Zhongshuang 9’. This oxidative burst at the early state of S. sclerotiorum-infected rape leaves confirmed results Xu et al. (2009) who also found higher H2O2 levels in transgenic rape expressing a glucose oxidase and this plant also had restrained S. sclerotiorum lesion formation compared with a susceptible rape variety. Production of plant-derived H2O2 has been reported for compatible and incompatible plant pathogen interactions acting as a direct antimicrobial compound, to trigger signal transduction pathways that occasionally lead to a hypersensitive response or to foster cell-wall fortification (reviewed in Shetty et al., 2008). This functional diversity probably relates to the site of H2O2 generation, the timing and the amount of H2O2 produced. The BnGLP proteins are predicted to posses a secretion signal (Petersen et al., 2011), and this is in agreement with experimental data that localized GLPs to the cell wall (Irshad et al., 2008; Banerjee et al., 2010; Komatsu et al., 2010). Thus, BnGLP3 and BnGLP12 are likely to participate in the S. sclerotiorum-induced apoplastic formation of H2O2 and may act in concert with NADPH oxidases and peroxidases, which are known to execute the apoplastic oxidative burst in response to pathogen stress in different species (Torres, 2010). The target of BnGLP-derived H2O2 is unclear, but the work of Banerjee et al. (2010) suggests a role in cell-wall reinforcement. The authors expressed rice germin-like protein1 in transgenic tobacco and correlated its SOD activity with hyper-accumulation of H2O2 and enhanced cross-linkage of cell-wall components after infection with Fusarium solani, which led to higher tolerance against this fungal pathogen. Similarly, we observed a positive effect of H2O2 on the resistance of B. napus ‘Falcon’ to S. sclerotiorum by the infiltration of 0.5mM H2O2 prior to infection (Fig. 6). B. napus ‘Zhongshuang 9’ did not respond with increased S. sclerotiorum resistance, indicating that the naturally stronger induction of H2O2 production in B. napus ‘Zhongshuang 9’ in response to S. sclerotiorum infection (Fig. 4) is sufficient for H2O2-triggered defences at the applied concentration and may explain the increased resistance to S. sclerotiorum compared with B. napus ‘Falcon’. In A. thaliana, L’Haridon et al. (2011) induced H2O2 formation by wounding leaves or exogenously applied H2O2 and by this increased the resistance against the necrotrophic pathogen Botrytis cinerea. Moreover, the authors showed that the wound-induced reactive oxygen species formation and resistance against B. cinerea were independent of the NADPH oxidases AtRBOHD and AtRBOHF, substantiating our interpretation of BnGLP proteins as part of an oxidative burst and subsequent increase in rape resistance against necrotrophic S. sclerotiorum.
Taken together, we have established here the family of GLPs in B. napus represented by 14 BnGLP members. Gene expression profiling of this family and biochemical characterization of selected members suggested that the SODs BnGLP3 and BnGLP12 are involved in early rape defence against S. sclerotiorum by the initiation of an oxidative burst. We also showed that H2O2, either produced in vivo or applied exogenously, correlated with increased resistance against S. sclerotiorum, providing a link to the functions of BnGLP3 and BnGLP12 in rape defence. Beyond the B. napus/S. sclerotiorum system investigated here, GLPs in different species relate to increased resistance including insects (Lou and Baldwin, 2006; Collins et al., 2010), nematodes (Knecht et al., 2010), microbes (Wei et al., 1998; Schweizer et al., 1999; Hückelhoven et al., 2001; Ficke et al., 2004; Zimmermann et al., 2006; Godfrey et al., 2007; Manosalva et al., 2009; Shetty et al., 2009; Banerjee and Maiti, 2010) and tobacco mosaic virus (Park et al., 2004), indicating basal functions of GLPs in plant resistance. Whether GLPs are also involved in R-protein-mediated or non-host resistance and what the immediate consequences of GLP derived reactive oxygen species formation are remain to be investigated.
Supplementary Material
Supplementary data
Supplementary data can be found at JXB online.
Fig. S1. Differential transcription of the β-tubulin reference gene between mock- and S. sclerotiorum-treated leaf samples ofB. napus ‘Falcon’ and ‘Zhongshuang 9’.
Table S1. Primer combinations for qPCR analysis of the BnGLP gene family and β-tubulin as a reference gene.
Table S2. Primer combinations for full-length gene cloning into the pDONR201 Gateway vector.
Acknowledgements
This work was funded by the Federal Ministry of Education and Research, Germany (BMBF, grant no. 0315637-B). The authors thank the Robert Bosch Stiftung for travel grants.
Glossary
Abbreviations:
- EST
expressed sequence tag
- GLP
germin-like protein
- OXO
oxalate oxidase
- PDA
potato dextrose agar
- qPCR
quantitative real-time PCR
- SE
standard error
- SOD
superoxide dismutase.
References
- Banerjee J, Das N, Dey P, Maiti MK. 2010. Transgenically expressed rice germin-like protein1 in tobacco causes hyper-accumulation of H2O2 and reinforcement of the cell wall components Biochemical and Biophysical Research Communications 402 637–643 [DOI] [PubMed] [Google Scholar]
- Banerjee J, Maiti MK. 2010. Functional role of rice germin-like protein1 in regulation of plant height and disease resistance Biochemical and Biophysical Research Communications 394 178–183 [DOI] [PubMed] [Google Scholar]
- Barceló A. 1998. Hydrogen peroxide production is a general property of the lignifying xylem from vascular plants Annals of Botany 82 97–103 [Google Scholar]
- Beauchamp C, Fridovich I. 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels Analytical Biochemistry 44 276–287 [DOI] [PubMed] [Google Scholar]
- Berna A, Bernier F. 1999. Regulation by biotic and abiotic stress of a wheat germin gene encoding oxalate oxidase, a H2O2-producing enzyme Plant Molecular Biology 39 539–549 [DOI] [PubMed] [Google Scholar]
- Bernier F, Berna A. 2001. Germins and germin-like proteins: plant do-all proteins. But what do they do exactly? Plant Physiology and Biochemistry 39 545–554 [Google Scholar]
- Bolton MD, Thomma BPHJ, Nelson BD. 2006. Sclerotinia sclerotiorum (Lib.) de Bary: biology and molecular traits of a cosmopolitan pathogen Molecular Plant Pathology 7 1–16 [DOI] [PubMed] [Google Scholar]
- Breen J, Bellgard M. 2010. Germin-like proteins (GLPs) in cereal genomes: gene clustering and dynamic roles in plant defence Functional and Integrative Genomics 10 463–476 [DOI] [PubMed] [Google Scholar]
- Caliskan M. 2009. Salt stress causes a shift in the localization pattern of germin gene expression Genetics and Molecular Research 8 1250–1256 [DOI] [PubMed] [Google Scholar]
- Carter C, Graham RA, Thornburg RW. 1998. Arabidopsis thaliana contains a large family of germin-like proteins: characterization of cDNA and genomic sequences encoding 12 unique family members Plant Molecular Biology 38 929–943 [DOI] [PubMed] [Google Scholar]
- Cheeseman JM. 2006. Hydrogen peroxide concentrations in leaves under natural conditions Journal of Experimental Botany 57 2435–2444 [DOI] [PubMed] [Google Scholar]
- Chen X, Wang ML, Holbrook C, Culbreath A, Liang X, Brenneman T, Guo B. 2011. Identification and characterization of a multigene family encoding germin-like proteins in cultivated peanut (Arachis hypogaea L.) Plant Molecular Biology Reporter 29 389–403 [Google Scholar]
- Christensen AB, Thordal-Christensen H, Zimmermann G, Gjetting T, Lyngkjaer MF, Dudler R, Schweizer P. 2004. The germinlike protein GLP4 exhibits superoxide dismutase activity and is an important component of quantitative resistance in wheat and barley Molecular Plant–Microbe Interactions 17 109–117 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. 1998. Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana The Plant Journal 16 735–743 [DOI] [PubMed] [Google Scholar]
- Collins RM, Afzal M, Ward DA, Prescott MC, Sait SM, Rees HH, Tomsett AB. Differential proteomic analysis of Arabidopsis thaliana genotypes exhibiting resistance or susceptibility to the insect herbivore, Plutella xylostella . PLoS ONE. 2010;5:e10103. doi: 10.1371/journal.pone.0010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong X, Ji R, Guo X, Chen H, Dong C, Liu Y, Hu Q, Liu S. 2008. Expressing a gene encoding wheat oxalate oxidase enhances resistance to Sclerotinia sclerotiorum in oilseed rape (Brassica napus) Planta 228 331–340 [DOI] [PubMed] [Google Scholar]
- Dumas B, Sailland A, Cheviet JP, Freyssinet G, Pallett K. 1993. Identification of barley oxalate oxidase as a germin-like protein Comptes Rendus de l’Académie des Sciences Série III 316 793–798 [PubMed] [Google Scholar]
- Dunwell JM, Gibbings JG, Mahmood T, Saqlan Naqvi SM. 2008. Germin and germin-like proteins: evolution, structure, and function Critical Reviews in Plant Sciences 27 342–375 [Google Scholar]
- Effendi Y, Rietz S, Fischer U, Scherer GFE. 2011. The heterozygous abp1/ABP1 insertional mutant has defects in functions requiring polar auxin transport and in regulation of early auxin-regulated genes The Plant Journal 65 282–294 [DOI] [PubMed] [Google Scholar]
- Eynck C, Koopmann B, Karlovsky P, von Tiedemann A. 2009. Internal resistance in winter oilseed rape inhibits systemic spread of the vascular pathogen Verticillium longisporum Phytopathology 99 802–811 [DOI] [PubMed] [Google Scholar]
- Fan Z, Gu H, Chen X, Song H, Wang Q, Liu M, Qu LJ, Chen Z. 2005. Cloning and expression analysis of Zmglp1, a new germin-like protein gene in maize Biochemical and Biophysical Research Communications 331 1257–1263 [DOI] [PubMed] [Google Scholar]
- Felsenstein J. 1989. Phylogeny inference package (version 3.2) Cladistics 5 164–166 [Google Scholar]
- Fernández P, Paniego N, Lew S, Hopp HE, Heinz RA. Differential representation of sunflower ESTs in enriched organ-specific cDNA libraries in a small scale sequencing project. BMC Genomics. 2003;4:40. doi: 10.1186/1471-2164-4-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficke A, Gadoury DM, Seem RC, Godfrey D, Dry IB. 2004. Host barriers and responses to Uncinula necator in developing grape berries Phytopathology 94 438–445 [DOI] [PubMed] [Google Scholar]
- Godfrey D, Able AJ, Dry IB. 2007. Induction of a grapevine germin-like protein (VvGLP3) gene is closely linked to the site of Erysiphe necator infection: a possible role in defense? Molecular Plant–Microbe Interactions 20 1112–1125 [DOI] [PubMed] [Google Scholar]
- Gucciardo S, Wisniewski J, Brewin NJ, Bornemann S. 2007. A germin-like protein with superoxide dismutase activity in pea nodules with high protein sequence identity to a putative rhicadhesin receptor Journal of Experimental Botany 58 1161–1171 [DOI] [PubMed] [Google Scholar]
- Hu X. 2003. Overexpression of a gene encoding hydrogen peroxide-generating oxalate oxidase evokes defense responses in sunflower Plant Physiology 133 170–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hückelhoven R, Dechert C, Trujillo M, Kogel KH. 2001. Differential expression of putative cell death regulator genes in near-isogenic, resistant and susceptible barley lines during interaction with the powdery mildew fungus Plant Molecular Biology 47 739–748 [DOI] [PubMed] [Google Scholar]
- Hurkman WJ, Tao HP, Tanaka CK. 1991. Germin-like polypeptides increase in barley roots during salt stress Plant Physiology 97 366–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inohara N, Shimomura S, Fukui T, Futai M. 1989. Auxin-binding protein located in the endoplasmic reticulum of maize shoots: molecular cloning and complete primary structure Proceedings of the National Academy of Sciences, USA 86 3564–3568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irshad M, Canut H, Borderies G, Pont-Lezica R, Jamet E. A new picture of cell wall protein dynamics in elongating cells of Arabidopsis thaliana: confirmed actors and newcomers. BMC Plant Biology. 2008;8:94. doi: 10.1186/1471-2229-8-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Y, Han G, He H, Li J. 2009. Differential regulation of proteins and phosphoproteins in rice under drought stress Biochemical and Biophysical Research Communications 379 133–138 [DOI] [PubMed] [Google Scholar]
- Knecht K, Seyffarth M, Desel C, Thurau T, Sherameti I, Lou B, Oelmüller R, Cai D. 2010. Expression of BvGLP-1 encoding a germin-like protein from sugar beet in Arabidopsis thaliana leads to resistance against phytopathogenic fungi Molecular Plant–Microbe Interactions 23 446–457 [DOI] [PubMed] [Google Scholar]
- Komatsu S, Kobayashi Y, Nishizawa K, Nanjo Y, Furukawa K. 2010. Comparative proteomics analysis of differentially expressed proteins in soybean cell wall during flooding stress Amino Acids 39 1435–1449 [DOI] [PubMed] [Google Scholar]
- Lane BG, Cuming AC, Frégeau J, Carpita NC, Hurkman WJ, Bernier F, Dratewka-Kos E, Kennedy TD. 1992. Germin isoforms are discrete temporal markers of wheat development. Pseudogermin is a uniquely thermostable water-soluble oligomeric protein in ungerminated embryos and like germin in germinated embryos, it is incorporated into cell walls European Journal of Biochemistry 209 961–969 [DOI] [PubMed] [Google Scholar]
- Lane BG, Dunwell JM, Ray JA, Schmitt MR, Cuming AC. 1993. Germin, a protein marker of early plant development, is an oxalate oxidase Journal of Biological Chemistry 268 12239–12242 [PubMed] [Google Scholar]
- Lane BG. 2002. Oxalate, germins, and higher-plant pathogens IUBMB Life 53 67–75 [DOI] [PubMed] [Google Scholar]
- L’Haridon F, Besson-Bard A, Binda M, et al. A permeable cuticle is associated with the release of reactive oxygen species and induction of innate immunity. PLoS Pathogens. 2011;7:e1002148. doi: 10.1371/journal.ppat.1002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li CX, Liu SY, Sivasithamparam K, Barbetti MJ. New sources of resistance to Sclerotinia stem rot caused by Sclerotinia sclerotiorum in Chinese and Australian Brassica napus and B. juncea germplasm screened under Western Australian conditions. Australasian Plant Pathology. 2009;38:149. [Google Scholar]
- Liang H, Maynard CA, Allen RD, Powell WA. 2001. Increased Septoria musiva resistance in transgenic hybrid poplar leaves expressing a wheat oxalate oxidase gene Plant Molecular Biology 45 619–629 [DOI] [PubMed] [Google Scholar]
- Liu S, Wang H, Zhang J, Fitt BD, Xu Z, Evans N, Liu Y, Yang W, Guo X. 2005. In vitro mutation and selection of doubled-haploid Brassica napus lines with improved resistance to Sclerotinia sclerotiorum Plant Cell Reports 24 133–144 [DOI] [PubMed] [Google Scholar]
- Livingstone DM. 2005. Enhancing resistance to Sclerotinia minor in peanut by expressing a barley oxalate oxidase gene Plant Physiology 137 1354–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Y, Baldwin IT. 2006. Silencing of a germin-like gene in Nicotiana attenuata improves performance of native herbivores Plant Physiology 140 1126–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manosalva PM, Davidson RM, Liu B, Zhu X, Hulbert SH, Leung H, Leach JE. 2009. A germin-like protein gene family functions as a complex quantitative trait locus conferring broad-spectrum disease resistance in rice Plant Physiology 149 286–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei J, Qian L, Disi JO, Yang X, Li Q, Li J, Frauen M, Cai D, Qian W. 2011. Identification of resistant sources against Sclerotinia sclerotiorum in Brassica species with emphasis on B. oleracea Euphytica 177 393–399 [Google Scholar]
- Membré N, Bernier F, Staiger D, Berna A. 2000. Arabidopsis thaliana germin-like proteins: common and specific features point to a variety of functions Planta 211 345–354 [DOI] [PubMed] [Google Scholar]
- Minic Z, Jamet E, San-Clemente H, Pelletier S, Renou JP, Rihouey C, Okinyo DP, Proux C, Lerouge P, Jouanin L. Transcriptomic analysis of Arabidopsis developing stems: a close-up on cell wall genes. BMC Plant Biology. 2009;9:6. doi: 10.1186/1471-2229-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaharu U. 1935. Genome analysis in Brassica with special reference to the experimental formation of B. napus and peculiar node of fertilization Japanese Journal of Botany 389–452 [Google Scholar]
- Nakagawa T, Kurose T, Hino T, Tanaka K, Kawamukai M, Niwa Y, Toyooka K, Matsuoka K, Jinbo T, Kimura T. 2007. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation Journal of Bioscience and Bioengineering 104 34–41 [DOI] [PubMed] [Google Scholar]
- Nakata M, Shiono T, Watanabe Y, Satoh T. 2002. Salt stress-induced dissociation from cells of a germin-like protein with Mn-SOD activity and an increase in its mRNA in a moss, Barbula unguiculata Plant and Cell Physiology 43 1568–1574 [DOI] [PubMed] [Google Scholar]
- Nakata M, Watanabe Y, Sakurai Y, Hashimoto Y, Matsuzaki M, Takahashi Y, Satoh T. 2004. Germin-like protein gene family of a moss, Physcomitrella patens, phylogenetically falls into two characteristic new clades Plant Molecular Biology 56 381–395 [DOI] [PubMed] [Google Scholar]
- Park C, An J, Shin Y, Kim K, Lee B, Paek K. Molecular characterization of pepper germin-like protein as the novel PR-16 family of pathogenesis-related proteins isolated during the resistance response to viral and bacterial infection. Planta. 2004;219 doi: 10.1007/s00425-004-1290-x. [DOI] [PubMed] [Google Scholar]
- Parkin IAP, Sharpe AG, Lydiate DJ. 2003. Patterns of genome duplication within the Brassica napus genome Genome 46 291–303 [DOI] [PubMed] [Google Scholar]
- Petersen TN, Brunak S, von Heijne G, Nielsen H. 2011. SignalP 4.0: discriminating signal peptides from transmembrane regions Nature Methods 8 785–786 [DOI] [PubMed] [Google Scholar]
- Robert S, Kleine-Vehn J, Barbez E, et al. 2010. ABP1 mediates auxin inhibition of clathrin-dependent endocytosis in Arabidopsis Cell 143 111–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd S. (2003). Expressed sequence tags: alternative or complement to whole genome sequences? Trends in Plant Science 8 321–329 [DOI] [PubMed] [Google Scholar]
- Schopfer P. 1996. Hydrogen peroxide-mediated cell-wall stiffening in vitro in maize coleoptiles Planta 1996 43–49 [Google Scholar]
- Schweizer P, Christoffel A, Dudler R. 1999. Transient expression of members of the germin-like gene family in epidermal cells of wheat confers disease resistance The Plant Journal 20 541–552 [DOI] [PubMed] [Google Scholar]
- Shetty NP, Jensen JD, Knudsen A, Finnie C, Geshi N, Blennow A, Collinge DB., Jørgensen HJL. 2009. Effects of β-1,3-glucan from Septoria tritici on structural defence responses in wheat Journal of Experimental Botany 60 4287–4300 [DOI] [PubMed] [Google Scholar]
- Shetty NP, Jørgensen HJL, Jensen JD, Collinge DB, Shetty HS. 2008. Roles of reactive oxygen species in interactions between plants and pathogens European Journal of Plant Pathology 121 267–280 [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. 2011. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods Molecular Biology and Evolution 28 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice Nucleic Acids Research 22 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres MA. 2010. ROS in biotic interactions Physiologia PLANTARUM 138 414–429 [DOI] [PubMed] [Google Scholar]
- Walz A, Zingen-Sell I, Loeffler M, Sauer M. 2008. Expression of an oxalate oxidase gene in tomato and severity of disease caused by Botrytis cinerea and Sclerotinia sclerotiorum Plant Pathology 57 453–458 [Google Scholar]
- Wang H, Liu G, Zheng Y, Wang X, Yang Q. 2004. Breeding of the Brassica napus cultivar Zhongshuang 9 with high-resistance to Sclerotinia sclerotiorum and dynamics of its important defense enzyme activity Scientia Agricultura Sinica 2004 23–28 [Google Scholar]
- Wei Y, Zhang Z, Andersen CH, Schmelzer E, Gregersen PL, Collinge DB, Smedegaard-Petersen V, Thordal-Christensen H. 1998. An epidermis/papilla-specific oxalate oxidase-like protein in the defence response of barley attacked by the powdery mildew fungus Plant Molecular Biology 36 101–112 [DOI] [PubMed] [Google Scholar]
- Whittaker MM, Whittaker JW. 2002. Characterization of recombinant barley oxalate oxidase expressed by Journal of Biological Inorganic Chemistry 7 136–145 [DOI] [PubMed] [Google Scholar]
- Witte C, Noël LD, Gielbert J, Parker JE, Romeis T. 2004. Rapid one-step protein purification from plant material using the eight-amino acid StrepII epitope Plant Molecular Biology 55 135–147 [DOI] [PubMed] [Google Scholar]
- Woo E, Marshall J, Bauly J, Chen JG, Venis M, Napie RM, Pickersgill RW. 2002. Crystal structure of auxin-binding protein 1 in complex with auxin EMBO Journal 21 2877–2885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo EJ, Dunwell JM, Goodenough PW, Marvier AC, Pickersgill RW. 2000. Germin is a manganese containing homohexamer with oxalate oxidase and superoxide dismutase activities Nature Structural Biology 7 1036–1040 [DOI] [PubMed] [Google Scholar]
- Woo EJ, Dunwell JM, Goodenough PW, Pickersgill RW. 1998. Barley oxalate oxidase is a hexameric protein related to seed storage proteins: evidence from X-ray crystallography FEBS Letters 437 87–90 [DOI] [PubMed] [Google Scholar]
- Xu Q, Liu S, Zou Q, Guo XL, Dong XY, Li PW, Song DY, Chen H, Zhao YD. 2009. Microsensor in vivo monitoring of oxidative burst in oilseed rape (Brassica napus L.) leaves infected by Sclerotinia sclerotiorum Analytica Chimica Acta 632 21–25 [DOI] [PubMed] [Google Scholar]
- Yamahara T, Shiono T, Suzuki T, Tanaka K, Takio S, Sato K, Yamazaki S, Satoh T. 1999. Isolation of a germin-like protein with manganese superoxide dismutase activity from cells of a moss, Barbula unguiculata Journal of Biological Chemistry 274 33274–33278 [DOI] [PubMed] [Google Scholar]
- Yang H, Kaur N, Kiriakopolos S, McCormick S. 2006. EST generation and analyses towards identifying female gametophyte-specific genes in Zea mays L Planta 224 1004–1014 [DOI] [PubMed] [Google Scholar]
- Yin K, Han X, Xu Z, Xue H. 2009. Arabidopsis GLP4 is localized to the Golgi and binds auxin in vitro Acta Biochimica et Biophysica Sinica 41 478–487 [DOI] [PubMed] [Google Scholar]
- Zhao J, Buchwaldt L, Rimmer SR, Sharpe A, McGregor L, Bekkaoui D, Hegedus D. 2009. Patterns of differential gene expression in Brassica napus cultivars infected with Sclerotinia sclerotiorum Molecular Plant Pathology 10 635–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Meng J. 2003. Genetic analysis of loci associated with partial resistance to Sclerotinia sclerotiorum in rapeseed (Brassica napus L.) Theoretical and Applied Genetics 106 759–764 [DOI] [PubMed] [Google Scholar]
- Zhao J, Wang J, An L, Doerge RW, Chen ZJ, Grau CR, Meng J, Osborn TC. 2007. Analysis of gene expression profiles in response to Sclerotinia sclerotiorum in Brassica napus Planta 227 13–24 [DOI] [PubMed] [Google Scholar]
- Zimmermann G, Baumlein H., Mock H-P, Himmelbach A., Schweizer P. 2006. The multigene family encoding germin-like proteins of barley. regulation and function in basal host resistance Plant Physiology 142 181–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.