Abstract
Cytokinins (CKs) are thought to play important roles in fruit development, especially cell division. However, the mechanisms and regulation of CK activity have not been well investigated. This study analysed CK concentrations and expression of genes involved in CK metabolism in developing tomato (Solanum lycopersicum) ovaries. The concentrations of CK ribosides and isopentenyladenine and the transcript levels of the CK biosynthetic genes SlIPT3, SlIPT4, SlLOG6, and SlLOG8 were high at anthesis and decreased immediately afterward. In contrast, trans-zeatin concentration and the transcript levels of the CK biosynthetic genes SlIPT1, SlIPT2, SlCYP735A1, SlCYP735A2, and SlLOG2 increased after anthesis. The expression of type-A response regulator genes was high in tomato ovaries from pre-anthesis to early post-anthesis stages. These results suggest that the CK signal transduction pathway is active in the cell division phase of fruit development. This study also investigated the effect of CK application on fruit set and development. Application of a synthetic CK, N-(2-chloro-pyridin-4-yl)-N’-phenylurea (CPPU), to unpollinated tomato ovaries induced parthenocarpic fruit development. The CPPU-induced parthenocarpic fruits were smaller than pollinated fruits, because of reduction of pericarp cell size rather than reduced cell number. Thus, CPPU-induced parthenocarpy was attributable to the promotion of cell division, not cell expansion. Overall, the results provide evidence that CKs are involved in cell division during development of tomato fruit.
Key words: CPPU, cytokinin, fruit development, Micro-Tom, parthenocarpy, tomato
Introduction
Because of its agronomic importance, fruit development has been the subject of extensive research. In the case of tomato (Solanum lycopersicum), early fruit development can be separated into three phases (Gillaspy et al., 1993). Phase I involves development of the ovary, pollination, fertilization, and fruit set. Phase II involves cell division that lasts for 7–14 days after pollination. Phase III involves cell expansion, which depends on genotype and is responsible for determining final fruit size.
Plant hormones play important roles in the development of tomato fruit (Gillaspy et al., 1993; Srivastava and Handa, 2005). Application of gibberellins (GAs) to unpollinated ovaries can induce fruit set in tomato (Fos et al., 2000; Serrani et al., 2007a), as can application of auxins (Abad and Monteiro, 1989; Ramin, 2003; Serrani et al., 2007a) and overexpression of indole-3-acetic acid (IAA) biosynthesis genes (Ficcadenti et al., 1999). Some of the genes involved in the biosynthesis and signal transduction pathways of these hormones during fruit development have recently been identified. For example, expression of the GA 20-oxidase gene was induced in tomato ovaries by pollination (Olimpieri et al., 2007; Serrani et al., 2007b) and parthenocarpic fruit development has been induced by manipulating the genes of the auxin or GA signal transduction pathway (Wang et al., 2005; Goetz et al., 2007; Martí et al., 2007; de Jong et al., 2009). These results indicate that GAs and auxins are the main compounds involved in tomato fruit set and development. Other plant hormones and effectors, such as abscisic acid and polyamines, are also involved in regulation of fruit development, but their roles remain unclear (Gillaspy et al., 1993; Alabadí et al., 1996; Nitsch et al., 2009).
Cytokinins (CKs) are plant hormones known to be key regulators of various aspects of plant growth and development, including cell division, leaf senescence, apical dominance, lateral root formation, stress tolerance, and nutritional signalling (Werner et al., 2003; Sakakibara, 2006; Argueso et al., 2009). Exogenous application of synthetic CKs, such as 6-benzylaminopurine (BA) and N-(2-chloro-pyridin-4-yl)-N’-phenylurea (CPPU), can induce fruit set and development in fruit crops such as grape, kiwifruit, melon, watermelon, apple, and pear (Hayata et al., 1995, 2000; Flaishman et al., 2001; Stern et al., 2003; Kim et al., 2006; Zabadal and Bukovac, 2006). Furthermore, endogenous levels of CKs have been linked with fruit growth (Gillaspy et al., 1993; Srivastava and Handa, 2005). Therefore, CKs may play important roles in fruit development, but the mechanisms and regulation of their activity have not been well investigated.
In plants, endogenous CK content is known to be spatially and temporally regulated by a fine balance between synthesis and catabolism (Hirose et al., 2008; Supplementary Fig. S1, available at JXB online). In many plant species, the initial step of CK biosynthesis is catalysed by adenosine phosphate-isopentenyltransferase (IPT), producing isopentenyladenine (iP) nucleotides as CK precursors (Kakimoto, 2001; Takei et al., 2001; Sakamoto et al., 2006). In Arabidopsis (Arabidopsis thaliana), the iP-nucleotides are converted into trans-zeatin (tZ) nucleotides by the cytochrome P450 monooxygenases, CYP735A1 and CYP735A2 (Takei et al., 2004). To become biologically active, CK nucleotides produced by IPTs and CYP735As must be converted to the free-base form. A CK-activating enzyme (LOG), which directly converts CK nucleotides to the active nucleobases, was recently identified in rice and Arabidopsis (Kurakawa et al., 2007; Kuroha et al., 2009). Inactivation of CKs occurs by degradation or conjugation, and cytokinin oxidase/dehydrogenase (CKX) catalyses the irreversible degradation of CKs in many plant species. CKX is a flavin adenine dinucleotide-containing oxidoreductase that selectively cleaves unsaturated N 6 side chains from tZ, iP, and their corresponding ribosides and it is primarily responsible for metabolic CK inactivation (Jones and Schreiber, 1997; Mok and Mok, 2001; Werner et al., 2006).
The present study investigated the roles and regulation of CKs in tomato fruit development, using Micro-Tom. Regardless of the presence of mutations that cause the dwarf size of this cultivar, it has been proven to be suitable as a standard genotype in tomato research, including the study of fruit development (Serrani et al., 2007a; Wang et al., 2009; Carvalho et al., 2011). First, endogenous CK contents of tomato fruits were quantified during various developmental stages, then the genes involved in CK biosynthesis and inactivation were isolated and their transcript levels during fruit development quantified. Finally, the effects of CK application on development of tomato fruit were investigated.
Materials and methods
Plant materials and growth conditions
Tomato plants (S. lycopersicum) cv. Micro-Tom and cv. Ailsa Craig were used in the experiments. Plants were grown, one per pot, with fertilized granulated soil (Kureha Corporation, Japan), in a phytotron (Koito Electric Industries, Japan) under a 14/10 light/dark cycle at 160 µmol m–2 s–1 and at 25 °C (light) and 20 °C (dark). For gene expression analysis, flowers were emasculated 2 d before anthesis to prevent self-pollination, then pollinated manually at anthesis.
Quantification of CKs
CKs were extracted and purified according to the method of Dobrev and Kamínek (2002) with some modifications. About 1g fruit material was homogenized in liquid nitrogen and placed in 10ml cold (–20 °C) methanol/water/formic acid (15:4:1, v/v/v). Deuterium-labelled CKs (Olchemim) were added to the extract to serve as internal standards. After overnight extraction at –20 °C, solids were separated by centrifugation and re-extracted for 30min in 10ml of the same extraction solution. To remove interfering compounds, the extract was first passed through an Oasis HLB column (200mg, Waters), equilibrated with 1M formic acid. The column was further washed with 5ml extraction solvent. The combined eluate was evaporated and then reconstituted with 5ml of 1M formic acid. The hormone-containing fraction was passed through an Oasis MCX column (150mg, Waters) equilibrated with 1M formic acid. To separate CKs from IAA and abscisic acid, the column was washed and eluted stepwise with the solutions indicated in Dobrev and Kamínek (2002). Solvents were evaporated at 40 °C under vacuum. Samples were then dissolved in water/methanol/acetic acid (80:19.95:0.05, v/v/v) and analysed by HPLC coupled with a tandem quadrupole mass spectrometer (MS/MS). The HPLC/MS/MS system consisted of a Prominence 20A Series HPLC (Shimadzu), equipped with a 3200 QTrap LC/MS/MS System (AB Sciex), using an electrospray interface.
The purified samples were injected onto a Shim-pack XR-ODS column (2.2 µm, 75×2.0mm; Shimadzu) at 45 °C and eluted at a flow rate of 0.2ml min−1. For chromatographic separation, the mobile phase A consisted of water/methanol/acetic acid (80:19.95:0.05, v/v/v) and the mobile phase B was methanol. The initial conditions were 100% A, changing linearly to 80% A and 20% B in 10min, changing to 50% A and 50% B in 5min, changing to 100% B in 5min, and finally maintained at 100% B for 5min. The column was equilibrated with the starting composition of the mobile phase for 10min before each analytical run. Quantification was obtained by multiple reaction monitoring of the protonated intact precursor ion [M+H]+ and a specific product ion, using the following mass transitions: [2H5]tZ, 225.2 > 137.0; tZ, 220.2 >136.1; [2H5]trans-zeatin riboside (tZR), 357.2 > 225.1; tZR, 352.2 > 220.1; [2H6]iP, 210.2 > 137.1; iP, 204.2 > 136.1; [2H6]isopentenyladenosine (iPR), 342.2 > 210.1; iPR, 336.2 > 204.1; [2H3]dihydrozeatin (DZ), 225.2 > 136.1; DZ, 222.2 >136.1;[2H3]dihydrozeatin riboside (DZR), 357.2 > 225.1; and DZR, 354.2 >222.1. Data were analysed using Analyst version 1.4.2 (AB Sciex). Concentrations were calculated on the basis of the peak areas for the endogenous compounds, relative to areas for the internal standards. The recovery values were 74% for tZ, 92% for tZR, 85% for iP, 64% for iPR, 78% for DZ, and 96% for DZR. Three biological replicates were analysed for all samples.
Isolation of CK metabolic genes from tomato
The IPT, CYP735A, LOG, and CKX sequences of tomato were identified by searching the databases at NCBI (http://www.ncbi.nlm.nih.gov), SOL Genomics Network (SGN; http://solgenomics.net/tools/blast/index.pl), Tomato Gene Index (http://compbio.dfci.harvard.edu/tgi), MiBASE (http://www.kazusa.or.jp/jsol/microtom/indexj.html), and Tomato SBM (http://www.kazusa.or.jp/tomato/), using predicted amino acid sequences from Arabidopsis and rice genes as query sequences. RACE (Rapid Amplification of cDNA Ends) was performed to identify the sequences of 5’ and 3’ regions of the genes using a Marathon cDNA amplification kit and an Advantage 2 PCR kit (Clontech). The sequence information was used to design primers for the coding regions of each gene family (Supplementary Table S1). cDNA clones were amplified by reverse-transcription PCR, using total RNA from tomato samples. Amplified products were cloned into the pCR-Blunt II-TOPO vector (Invitrogen) and sequenced.
Phylogenetic analysis
The Deduced amino acid sequences of CK metabolic genes were aligned with the homologous proteins in Arabidopsis and rice, using clustal w version 2.0.12 (http://clustalw.ddbj.nig.ac.jp/top-j.html) in the default setting. The alignment results were edited and marked using boxshade version 3.21 (http://www.ch.embnet.org/software/BOX_form.html). The phylogenetic trees were constructed using the neighbour-joining method in clustal w version 2.0.12 with bootstrap analysis based on 1000 replicates to evaluate the reliability of different phylogenetic groups. Tree files were viewed and edited using NJplot software (http://pbil.univ-lyon1.fr/software/njplot.html).
Quantitative real-time PCR analysis
Total RNA was isolated from tomato samples, using an RNeasy Plant Mini Kit (Qiagen). Genomic DNA was eliminated, using an RNase Free DNase I kit (Qiagen), according to the manufacturer’s instructions. cDNA was synthesized from total RNA, using a Transcriptor First Strand cDNA Synthesis Kit (Roche Applied Science), according to the manufacturer’s instructions. Quantitative real-time PCR of SlIPT, SlCYP735A, SlLOG, and SlCKX was carried out using the Universal ProbeLibrary system (Roche Applied Science), according to the manufacturer’s instructions. Primers and probes for each gene assay were designed using Universal ProbeLibrary ProbeFinder software (https://www.roche-applied-science.com/sis/rtpcr/upl/index.jsp?id=uplct_030000; Roche Applied Science). The SAND family protein gene (SAND, SGN-U573169) was used as an internal control for normalization of gene expression (Expósito-Rodríguez et al., 2008). Quantitative real-time PCR of SAND and tomato type-A response regulator genes (TRR) was carried out using the LightCycler 480 SYBR Green I Master system (Roche Applied Science), according to the manufacturer’s instructions. The sequences of the primer pairs for each gene are shown in Supplementary Table S2. DNA from plasmids containing cDNA clones was used to generate standard curves by serial dilution. For Universal ProbeLibrary assays, reactions were carried out under the following conditions: 95 °C for 5min and 45 cycles at 95 °C for 10 s, 60 °C for 30 s, and 72 °C for 1 s. For SYBR Green assays, reactions were carried out under the following conditions: 95 °C for 5min and 45 cycles at 95 °C for 10 s, 60 °C for 10 s, and 72 °C for 10 s. Melting curves from 65 to 98 °C were used to confirm the presence of single products. Three biological replicates were analysed in each case.
CK application
CPPU (Tokyo Chemical Industry), tZ (Wako Pure Chemical Industries), kinetin (Sigma-Aldrich), and BA (Wako Pure Chemical Industries) were applied to unpollinated ovaries in 20 µl of 10% ethanol and 0.1% Tween 20. Flower emasculation was carried out 2 d before anthesis to prevent self-pollination. Equal volumes of solvent solution were applied to control ovaries. Fruits were weighed 20 d after treatment.
Light microscopy
Ovaries and fruit tissue sections were fixed in formalin/acetic acid/alcohol, dehydrated using a tertiary butyl alcohol series, and embedded in Paraplast Plus (McCormick Scientific). The sections were sliced into 8 µm thicknesses, stained with 0.1% toluidine blue-O, and observed by microscopy (DM2000, Leica). All microscopic measurements were performed on nine independent sections (three per fruit), using imaging software (Leica Application Suite; Leica). The number of cell layers was estimated by counting the cells along a line across the pericarp, perpendicular to the epidermis and endocarp.
Accession numbers
Sequence data can be found in the GenBank/EMBL or SGN databases under the following accession numbers: tomato sequences: SlIPT1 (AB690812), SlIPT2 (AB690813), SlIPT3 (AK329766), SlIPT4 (AB690814), SlIPT5 (AB690815), SlIPT6 (AK324787), SlCYP735A1 (AB690816), SlCYP735A2 (AB690817), SlLOG1 (AK319846), SlLOG2 (AK320492), SlLOG3 (AK322121), SlLOG4 (AK322980), SlLOG5 (AK323270), SlLOG6 (AB690818), SlLOG7 (AB690819), SlLOG8 (AB690820), SlCKX1 (AB690821), SlCKX2 (AB690822), SlCKX3 (AK323363), SlCKX4 (AB690823), SlCKX5 (AB690824), SlCKX6 (AB690825), SlCKX7 (AB690826), SlCKX8 (AB690827), TRR3/4 (SGN-U577676), TRR8/9a (SGN-U572841), TRR8/9b (SGN-U572839), TRR16/17 (SGN-U601012), TRR7/15 (AK329138), and SAND (SGN-U316474).
Results
Changes in CK levels during tomato fruit development
To investigate the relationship between endogenous levels of CKs and tomato fruit development, the concentrations of CK bases and their corresponding ribosides were measured in developing Micro-Tom ovaries (Fig. 1). tZ concentration increased after anthesis, reaching its highest level 5 days after anthesis (DAA), and then slowly decreased. In contrast, iP concentration was relatively high before and at anthesis (–2 and 0 DAA) and decreased after anthesis. DZ concentration was undetectable before anthesis, increased from 0 to 5 DAA, and then decreased. The amounts of CK ribosides (tZR, iPR, and DZR) before anthesis were very high compared to CK bases, reached their highest levels at 0 DAA, and then decreased drastically after anthesis.
Fig. 1.
Endogenous levels of cytokinins in tomato ovaries before, at, and after anthesis. DAA = days after anthesis; tZ = trans-zeatin; tZR = trans-zeatin riboside; iP = isopentenyladenine; iPR = isopentenyladenosine; DZ = dihydrozeatin; DZR = dihydrozeatin riboside. Values are mean ± SE (n = 3).
CK metabolic genes isolated from tomato
To obtain homologous sequences of CK biosynthesis and inactivation pathways in tomato, the tomato DNA databases were screened using amino acid sequences predicted from Arabidopsis and rice genes as probes. To isolate the cDNA sequences of tomato CK metabolic genes, total RNA was prepared from various organs and reverse-transcription-PCR was performed with specific primers for each predicted gene. Deduced amino acid sequence alignments were determined for all tomato CK metabolic genes (Supplementary Figs. S2–S5), and sequences were compared with orthologues from Arabidopsis and rice (Supplementary Figs. S6–S9).
Six IPT-like cDNA sequences were isolated and designated SlIPT1–6 (Supplementary Fig. S2). The deduced amino acid sequence lengths were 323–449 residues, with the exception of SlIPT5, which contained a predicted premature stop codon. The similarities of the sequences within the gene family ranged from 17.23 to 51.38%. SlIPT6 showed high similarity to AtIPT9 (60.80%) and OsIPT10 (54.20%), suggesting that it might play a role in tRNA isoprenylation and the production of cis-zeatin-type CKs (Supplementary Fig. S6; Miyawaki et al., 2006).
Two CYP735A-like cDNA sequences were isolated and designated SlCYP735A1 and SlCYP735A2 (Supplementary Fig. S3). Both had deduced amino acid sequence lengths of 516 residues, and the two sequences had 95.16% similarity (Supplementary Fig. S7).
Eight LOG-like cDNA sequences were isolated and designated SlLOG1–8 (Supplementary Fig. S4). The deduced amino acid sequence lengths were 191–234 residues, and the similarities of the sequences within the gene family ranged from 59.80 to 92.66%. Comparison with other LOG-like proteins in Arabidopsis (AtLOG1–9) and rice (OsLOG, OsLOGL1–10) showed that SlLOG1–3 and SlLOG6–8 belong to clade I and SlLOG4 and SlLOG5 belong to clade II (Supplementary Fig. S8).
Eight CKX-like cDNA sequences were isolated and designated SlCKX1–8 (Supplementary Fig. S5). Several splice variants were amplified for SlCKX6 and SlCKX8, and all sequences contained predicted premature stop codons. Therefore, SlCKX6 and SlCKX8 were excluded from further studies. The deduced amino acid sequence lengths of the SlCKX genes were 519–543 residues, and the similarities of sequences within the gene family ranged from 30.48 to 83.81% (Supplementary Fig. S9).
Expression patterns of CK metabolic genes in tomato
The expression patterns of CK metabolic genes varied among tomato organs (Supplementary Fig. S10). SlIPT1 and SlIPT2 were mainly expressed in flowers, flower buds, and young fruits. SlIPT3 and SlIPT4 were expressed in all tested organs, but expression was low in young fruits. SlIPT6 (putative tRNA-IPT) was constitutively expressed in all tested organs.
SlCYP735A1 was mainly expressed in roots, flowers, and young fruits. SlCYP735A2 was expressed in leaves, roots, and young fruits.
SlLOG1 was mainly expressed in leaves. SlLOG2 was mainly expressed in flowers and flower buds. SlLOG4 was highly expressed in roots. SlLOG8 was mainly expressed in flowers. SlLOG3, SlLOG5, SlLOG6, and SlLOG7 were expressed in all tested organs, but the expression was low in young fruits.
SlCKX1 was expressed in all tested organs, but expression was low in young fruits. SlCKX2 was mainly expressed in flowers. SlCKX3 was highly expressed in roots. SlCKX4 was mainly expressed in leaves, flowers, flower buds, and young fruits. SlCKX5 was expressed in all tested organs. SlCKX7 was mainly expressed in young fruits.
Expression of CK metabolic and response genes during tomato fruit development
To investigate the relationship between CK metabolism and tomato fruit development, the expression profiles of CK metabolic genes in ovaries of developing fruits were examined (Fig. 2). Expression of SlIPT1 was low before anthesis (–2 DAA), gradually increased after pollination, peaked at 5 DAA, decreased at 10 DAA, and then increased again at 20 DAA. Unpollinated ovaries showed consistently low expression of SlIPT1. Expression of SlIPT2 was relatively high before anthesis, gradually increased after pollination, peaked at 5 to 10 DAA, and then decreased. Expression of SlIPT3 and SlIPT4 was high from –2 to 3 DAA, and then sharply decreased. Expression of SlIPT6 (putative tRNA-IPT) was relatively high and did not change during early fruit development (data not shown).
Fig. 2.
Expression of CK metabolic and response genes during tomato fruit development. Real-time PCR was performed with cDNA prepared from pollinated ovaries (Poll.) at 1, 3, 5, 10, 15, and 20 days after anthesis (black bars), and unpollinated ovaries (Unpoll.) at –2, 0, 1, and 3 days after anthesis (grey bars). Expression levels are normalized to SAND expression levels. Values are mean ± SE (n = 3).
Expression of SlCYP735A1 was low before anthesis, increased sharply to 1 DAA, and then slowly decreased. Expression of SlCYP735A2 was low before and at anthesis, gradually increased after pollination, peaked at 5 DAA, decreased until 15 DAA, and increased again at 20 DAA. Unpollinated ovaries showed consistently low expression of SlCYP735A1 and SlCYP735A2.
Expression of SlLOG2 was low before anthesis, gradually increased after pollination, peaked at 3 DAA, and then decreased. Unpollinated ovaries showed consistently low expression of SlLOG2. Expression of SlLOG6 was high from –2 to 3 DAA, then sharply decreased. Expression of SlLOG8 was low before anthesis, increased sharply at anthesis, and then decreased. Expression of other LOG genes did not change during early fruit development (data not shown).
Expression of SlCKX1 was high before anthesis, then gradually decreased. Expression of SlCKX3 was low before anthesis, gradually increased after pollination, peaked at 10 DAA, then decreased. Unpollinated ovaries showed consistently low expression of SlCKX3. Expression of SlCKX4 was low before anthesis, gradually increased until 1 DAA, decreased at 5 DAA, then increased at 20 DAA. Expression of SlCKX7 was low before anthesis, increased sharply at 0 DAA, decreased at 10 DAA, then increased at 15 DAA. Expression of other CKX genes did not change during early fruit development (data not shown).
This study also examined the expression of TRR genes, which have been used as markers of CK signalling (Shani et al., 2010). Expression levels of five genes [TRR3/4, TRR8/9a, TRR8/9b, TRR16/17, and TRR7/15 (AK329138)] were high in ovaries from –2 to 5 DAA, and then slowly decreased (Fig. 2).
Effect of CK application on tomato fruit development
To analyse the role of CKs in tomato fruit development, the effects of four different types of CKs (tZ, BA, kinetin, and CPPU) on fruit set and growth were examined in unpollinated ovaries of Micro-Tom. As previously reported (Serrani et al., 2007a), untreated Micro-Tom ovaries neither abscised nor grew. Applications of tZ, BA, and kinetin did not have any visible effect on fruit growth; however, application of CPPU induced growth (Fig. 3A). Parthenocarpic fruit growth was observed in all ovaries tested at 1000 and 10,000ng CPPU ovary–1, with maximum response at 10,000ng ovary–1. The weight of fruits treated with 10,000ng CPPU ovary–1 was about half that of pollinated fruits (Fig. 3B).
Fig. 3.
Response of unpollinated tomato ovaries to N-(2-chloro-pyridin-4-yl)-N’-phenylurea (CPPU) treatment. (A) Cross-sections of pollinated fruits and fruits treated with different amounts of CPPU. (B) Dose response of unpollinated tomato ovaries to CPPU treatment (mean ± SE, n = 8). The figure in parentheses indicates that only three fruit developed. (C) Seed of pollinated fruit. (D) Degenerated ovule (arrow) of CPPU-treated fruit. (E) Whole pollinated (Poll.) and CPPU-treated (10,000ng ovary–1) fruits, collected 20 d after anthesis or treatment. Arrow indicates enlarged pedicel and calyx. Bars, 1cm (A, B, E) and 1mm (C, D) (this figure is available in colour at JXB online).
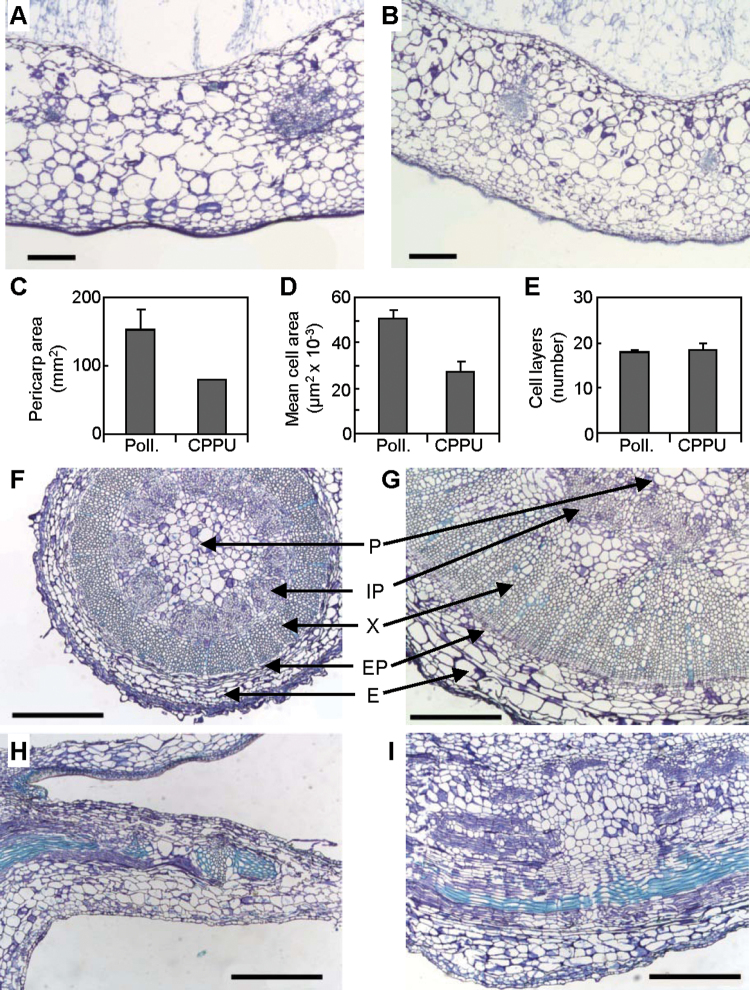
Ovaries of CPPU-treated fruits exhibited morphology similar to that of pollinated fruits, with the exception of size reduction and aborted seed development (Fig. 3A). In CPPU-treated ovaries, the locules were filled with jelly-like tissue; some ovules were observed, but their growth was extremely limited (Fig. 3C, 3D). The small size of cross-sectional areas of CPPU-treated fruits was the result of smaller pericarp areas (Fig. 4C; Supplementary Fig. S11A, B). Cells of CPPU-treated pericarps were about half the size of cells of pollinated fruits (Fig. 4A, 4B, 4D), while the number of cell layers was almost the same (Fig. 4E). Therefore, the size reduction of CPPU-treated ovaries appeared to be due to smaller cell size.
Fig. 4.
Effects of N-(2-chloro-pyridin-4-yl)-N’-phenylurea (CPPU) on tomato fruit histology. (A, B) Transverse sections of ovaries of pollinated (A) and CPPU-treated (B) fruits. (C–E) Pericarp surface area (C), cross-sectional area of pericarp cells (D), and number of cell layers (E) in pericarps of pollinated (Poll.) and CPPU-treated fruits (mean ± SD, n = 9). (F, G) Transverse sections of pedicels of pollinated (F) and CPPU-treated (G) fruits. (H, I) Longitudinal sections of calyxes of pollinated (H) and CPPU-treated (I) fruits. E, epidermis and primary cortex; EP, external phloem; IP, internal phloem; P, pith; X, xylem. Bars, 500 µm (this figure is available in colour at JXB online).
The most remarkable change observed in the external morphology of CPPU-treated fruits was enlargement of pedicels and calyxes (Fig. 3E). These organs were more than twice as wide in CPPU-treated fruits than in pollinated fruits. The cross-sectional areas of external phloem, xylem, and internal phloem tissues were greatly increased in the pedicel (Fig. 4F, 4G; Supplementary Fig. S11C, D, Supplementary Table S3), and the tissues surrounded by xylem were enlarged in the calyx (Fig. 4H, 4I; Supplementary Fig. S11E, F). The size of cells in these tissues was greater in CPPU-treated fruits than in pollinated fruits (Fig. 4F–I). Thus, the effect of CPPU on cell growth varied among organs.
The effects of CPPU (10,000ng ovary–1) was also examined using unpollinated ovaries of Ailsa Craig (AC, a non-dwarf tomato cultivar). Unlike Micro-Tom ovaries, all untreated AC ovaries abscised during 5–10 DAA. CPPU had similar, but weaker, effects on AC ovaries, compared to its effects on Micro-Tom (Supplementary Fig. S12). No CPPU-treated AC ovaries abscised, but some did not grow. The weight of CPPU-treated fruits was about one-fifth that of pollinated fruits (Supplementary Fig. S12B). Similar to Micro-Tom, CPPU-treated AC ovaries showed enlargement of pedicels and calyxes (Supplementary Fig. S12C), but jelly-like tissue was almost absent, probably because of reduction of the locular area (Supplementary Fig. S12A).
Discussion
Regulation of CK metabolism
CKs are thought to play important roles in fruit development. However, the molecular mechanisms of the regulation of CKs have not been well investigated in fruit crops. The present study, as far as is known for the first time, describes a complete set of CK metabolic genes in tomato and regulation of their expression in early fruit development.
Concentrations of different CK forms showed different temporal patterns during early tomato fruit development (Fig. 1). The levels of tZR, iPR, DZR, and iP were high in ovaries at anthesis. Transcript levels of the CK biosynthetic genes SlIPT3, SlIPT4, SlLOG6, and SlLOG8 were also high at anthesis, then decreased sharply after anthesis (Fig. 2). The transcript level of SlCYP735A1 showed a slight increase at anthesis (Fig. 2). These results indicate that unpollinated ovaries synthesize CK from adenosine phosphates to CK nucleobases. After anthesis, concentrations of iP and CK ribosides immediately decreased, probably because of degradation or conversion to other CK forms. Elevated expression of the CKX genes SlCKX1 and SlCKX7 in early developing ovaries (Fig. 2) might be involved in the degradation of these CKs.
In contrast to iP and CK ribosides, the levels of tZ and DZ in tomato ovaries continued to increase after anthesis, reached maxima at 5 DAA, then slowly decreased (Fig. 1). Similarly, transcript levels of SlIPT1, SlIPT2, SlCYP735A1, SlCYP735A2, and SlLOG2 increased after pollination, and moderate to high expression of these genes was detected at 1–5 DAA (Fig. 2). Surprisingly, the transcript level of SlCYP735A1 at 1 DAA was about 10-times higher in ovaries than in roots (Fig. 2, Supplementary Fig. S10), suggesting that CK trans-hydroxylase activity was strongly induced, and that tZ-type CK was specifically synthesized in pollinated ovaries. Roots are generally thought to be major sites of CK biosynthesis, and root-synthesized CKs are thought to act as long-distance signals (Hirose et al., 2008). The importance of root-synthesized CKs in tomato was reported by Ghanem et al. (2011), who showed that the root-localized induction of CK biosynthesis improved shoot growth and fruit yield in salt-stressed tomato. On the other hand, locally synthesized CKs also play important roles in plant development, such as promoting axillary bud outgrowth (Tanaka et al., 2006). The spatial expression patterns of CK metabolic genes in ovaries indicated that locally synthesized CKs are important in tomato fruit development, as well as root-synthesized CKs.
The expression patterns of tomato TRR genes in developing fruits showed that the expression of five TRR genes was high in ovaries from –2 to 5 DAA and then slowly decreased (Fig. 2). Together with temporal changes in CK contents, these results suggest that the CK signal transduction pathway is active during pre-anthesis and early post-anthesis stages.
Fluctuations in CK concentrations during fruit development were previously observed in wild-type tomato (Solanum pimpinellifolium Mill.) by Bohner and Bangerth (1988), using radioimmunoassays. They reported that tZ-type CK levels in ovaries increased 4 d after pollination and that iP-type CK levels were high at anthesis. The results of that study and the present study indicate two peaks of CK accumulation during early fruit development. The first peak, at anthesis, is not linked to pollination, because the transcripts of several CK biosynthetic genes were upregulated without pollination (Fig. 2). High CK concentrations were also observed at anthesis in kiwifruit ovaries, with a decrease immediately after anthesis (Lewis et al., 1996). It was suggested that, prior to fertilization, factors produced by the sporophytic tissue surrounding the developing ovary are required to trigger and maintain cell division in the fruit primordia, until the ovary reaches mature size (Gillaspy et al., 1993). Furthermore, Bohner and Bangerth (1988) reported that cell division prior to anthesis is critical in determining fruit size in wild-type tomato. Therefore, high CK levels might be necessary for the growth and/or maintenance of unpollinated ovaries until successful pollination, after which the CKs might be inactivated.
The concentrations of CK ribosides (tZR, iPR) in unpollinated ovaries were about 10-times those of the corresponding CK bases (Fig. 1). Although the binding activity of CK ribosides is lower than that of CK bases, CK ribosides have relatively high binding activity to some CK receptors, and they are considered to have a genuine biological activity (Spíchal et al., 2004). There are three CK receptors in Arabidopsis (AHK2, AHK3, and CRE1/AHK4); each receptor has specific roles in CK-regulated processes, which depend on its ligand preferences and gene expression pattern (Stolz et al., 2011). Therefore, it is possible that the high concentrations of CK ribosides and their interactions with specific receptors have important roles in the growth of unpollinated ovaries.
The second peak in CK concentrations in developing tomato fruit occurred 5 d after anthesis. The peak in tZ content corresponded with phase II cell division, suggesting that tZ is involved in cell division in ovaries after pollination. An increase in CK content after anthesis has also been observed in other plant species, including maize, kiwifruit, white lupine, and Helleborus niger (Lewis et al., 1996; Emery et al., 2000; Tarkowski et al., 2006; Brugière et al., 2008; Rijavec et al., 2011). Interestingly, iP content in ovaries decreased after anthesis, whereas tZ content increased (Fig. 1). SlCYP735As might be key enzymes regulating these CKs. These results indicate that tZ, but not iP, plays an important role in early tomato fruit development after anthesis. tZ and iP are known to be the most common active CKs in plants, but the physiological role of different side-chain structures remains unclear. Further analysis of SlCYP735As will elucidate the role of different types of CKs in tomato fruit development.
Relatively high levels of tZR and DZR in tomato ovaries (Fig. 1), and upregulation of several CK metabolic genes (Fig. 2), indicated a third peak in CK concentrations at 20 DAA or later. Similar peaks were observed in wild-type tomato and kiwifruit (Bohner and Bangerth, 1988; Lewis et al., 1996), but are not likely to influence cell division.
Effects of CK application
CPPU application to unpollinated Micro-Tom ovaries induced parthenocarpic fruit development (Fig. 3A). However, CPPU-treated fruits were consistently smaller than pollinated fruits (Fig. 3B), and pericarp cells were also smaller in CPPU-treated fruits, although the number of cell layers was similar (Fig. 4D, 4E). Thus, CPPU-induced parthenocarpic fruit development appeared to be due to the promotion of cell division, rather than cell expansion. These findings strongly suggest that CKs are involved in cell division during tomato fruit development. Serrani et al. (2007a) reported that auxin- or GA-induced parthenocarpic tomato fruits had cell sizes equal to or larger than those of pollinated fruits. They suggested that auxin was primarily involved in cell division and GA in cell expansion. The small size of CPPU-treated fruits, especially in the case of AC fruits, might be due to the importance of cell expansion in determining final fruit size. Other plant hormones, such as auxins and GAs, might be necessary for further fruit development.
Enlargement of calyxes and pedicels was observed in CPPU-treated fruits (Fig. 3E) and was due to the promotion of both cell division and cell expansion (Fig. 4F–I). Similar morphological changes were reported by Kataoka et al. (1994), who observed that a mixture of synthetic auxin and CPPU reduced auxin-induced puffiness in tomato fruits and enlarged the size of calyxes and pedicels. He and Saedler (2007) found that application of BA and GA3 induced enlargement of the calyx (inflated calyx syndrome) in Physalis floridana (Solanaceae) and suggested that CK was involved in cell division while GA was involved in cell expansion. Therefore, the effect of CPPU on cell expansion in tomato calyxes and pedicels might be indirect and other plant hormones, such as GA, might be involved.
Significant enlargement of pedicels, which involved abscission zones, indicated that this area is highly sensitive to CKs. Several CK biosynthetic and response genes were specifically expressed in fruit abscission zones in Arabidopsis (Miyawaki et al., 2004; Hirose et al., 2008; Kuroha et al., 2009). Thus, CKs might play roles in growth and/or maintenance of pedicels. Auxin accumulates in pedicels during early tomato fruit development and is thought to prevent premature abscission of ovaries (Nishio et al., 2010). CK application to developing Arabidopsis tissues leads to upregulation of auxin biosynthesis (Jones et al., 2010). CKs can also affect auxin distribution via regulation of PIN auxin efflux transporters in various plant tissues (Pernisová et al., 2009; Růžička et al., 2009). Thus, one possible role of CKs in fruit development is to modulate auxin biosynthesis and/or polar auxin transport to prevent abscission of ovaries.
Microscopic analysis revealed enlargement of external and internal phloem and xylem of pedicels (Fig. 4F, 4G). The growth of tomato fruit depends on transport of water, nutrients, and assimilates from other parts of the plant via the xylem and phloem of the pedicel (Ho et al., 1987; Van Ieperen et al., 2003). Furthermore, CKs are central regulators of cambial activity, which produces xylem and phloem through cell division (Matsumoto-Kitano et al., 2008; Nieminen et al., 2008). Thus, CKs might play roles in regulation of cambial development of pedicel, which influences the transport of water, nutrients, and assimilates to fruit.
The application of CKs other than CPPU (tZ, BA, and kinetin) to unpollinated tomato ovaries did not induce parthenocarpic fruit development under the present experimental conditions. Similar results were observed in muskmelon, watermelon, and kiwifruit (Hayata et al., 1995, 2000; Ohara et al., 1997). Phenylurea derivatives, such as CPPU and thidiazuron, are known to exhibit higher activities than purine CKs (Shudo 1994). Therefore, compared to other CKs, CPPU seems to have much more consistent and stronger effect on fruit set and growth in several fruit crops.
In summary, the results of the present study demonstrated that CKs are involved in early tomato fruit development (Fig. 5). High levels of CKs (CK ribosides and iP) are accumulated in ovaries prior to pollination and might be involved in the growth of unpollinated ovaries. After pollination, concentrations of these CKs decrease, while tZ is increased by the upregulation of CK biosynthetic genes (SlIPT1, SlIPT2, SlCYP735A1, SlCYP735A2, and SlLOG2). tZ might be involved in early fruit development through promotion of cell division and, based on effects of CPPU application, might function as a signal affecting fruit set and development. Future studies, such as modification of CK contents in ovaries by genetic manipulation and analysis of crosstalk with other plant hormones, will be needed to better understand the mechanism involving CKs in the regulation of fruit development.
Fig. 5.
Proposed model for the roles and regulation of cytokinins (CKs) in tomato fruit development. Before pollination, high levels of CKs are accumulated in ovaries by the expression of CK biosynthetic genes (SlIPT3, SlIPT4, SlLOG6, and SlLOG8). These CKs are involved in the growth of unpollinated ovaries, and their concentrations are decreased after pollination by the expression of SlCKX genes (SlCKX1 and SlCKX7). Concentration of trans-zeatin (tZ) is increased after pollination through the upregulation of other CK biosynthetic genes (SlIPT1, SlIPT2, SlCYP735A1, SlCYP735A2, and SlLOG2). tZ promotes cell division during early fruit development. Concentrations of auxin and gibberellin (GA) also increase after pollination. These hormones are involved in the cell division and expansion, which determine final fruit size. CKs also promote the growth of pedicel and influence the transport of water, nutrients, and assimilates to fruit. The question mark and dotted arrow indicate that CKs might also modulate auxin biosynthesis and/or polar auxin transport, which prevent abscission of ovaries.
Supplementary material
Supplementary material are available at JXB online.
Supplementary Table Primer sequences used for amplification
Supplementary Table Primer sequences used for quantitative real-time PCR analyses
Supplementary Table Areas of various pedicel tissue layers, measured on cross-sections of pollinated and CPPU-treated fruits
Supplementary Fig. S1. Scheme of CK metabolic pathway
Supplementary Fig. S2. Multiple alignment of deduced amino acid sequences of SlIPT genes.
Supplementary Fig. S3. Multiple alignment of deduced amino acid sequences of SlCYP735A genes
Supplementary Fig. S4. Multiple alignment of deduced amino acid sequences of SlLOG genes.
Supplementary Fig. S5. Multiple alignment of deduced amino acid sequences of SlCKX genes.
Supplementary Fig. S6. Phylogenetic tree of IPT proteins
Supplementary Fig. S7. Phylogenetic tree of CYP735A proteins
Supplementary Fig. S8. Phylogenetic tree of LOG proteins
Supplementary Fig. S9. Phylogenetic tree of CKX proteins
Supplementary Fig. S10. Expression of CK metabolic genes in various organs of tomato
Supplementary Fig. S11. Effects of CPPU on tomato fruit histology
Supplementary Fig. S12. Response of unpollinated Ailsa Craig ovaries to CPPU application
Supplementary Material
Acknowledgements
The authors thank Ayako Jike and Setsuko Sanagawa for technical assistance. This work was supported by the grant ‘Research Project for Molecular Evaluation of Parthenocarpy on Horticultural Crops’ funded by the National Agriculture and Food Research Organization (NARO), Japan.
References
- Abad M, Monteiro AA. 1989. The use of auxins for the production of greenhouse tomatoes in mild-winter conditions – a review Scientia Horticulturae 38 167–192 [Google Scholar]
- Alabadí D, Agüero MS, Pérez-Amador MA, Carbonell J. 1996. Arginase, arginine decarboxylase, ornithine decarboxylase and polyamines in tomato ovaries – changes in unpollinated ovaries and parthenocarpic fruits induced by auxin or gibberellin Plant Physiology 112 1237–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso CT, Ferreira FJ, Kieber JJ. 2009. Environmental perception avenues: the interaction of cytokinin and environmental response pathways Plant, Cell and Environment 32 1147–1160 [DOI] [PubMed] [Google Scholar]
- Bohner J, Bangerth F. 1988. Cell number, cell-size and hormone levels in semi-isogenic mutants of Lycopersicon pimpinellifolium differing in fruit size Physiologia Plantarum 72 316–320 [Google Scholar]
- Brugière N, Humbert S, Rizzo N, Bohn J, Habben JE. 2008. A member of the maize isopentenyl transferase gene family Zea mays isopentenyl transferase 2(ZmIPT2 )encodes a cytokinin biosynthetic enzyme expressed during kernel development Plant Molecular Biology 67 215–229 [Google Scholar]
- Carvalho RF, Campos ML, Pino LE, Crestana SL, Zsöegöen A, Lima JE, Benedito VA, Peres LEP. Convergence of developmental mutants into a single tomato model system: ‘Micro-Tom’ as an effective toolkit for plant development research. Plant Methods. 2011;7:18. doi: 10.1186/1746-4811-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong M, Wolters-Arts M, Feron R, Mariani C, Vriezen WH. 2009. The Solanum lycopersicum auxin response factor 7 (SlARF7) regulates auxin signaling during tomato fruit set and development The Plant Journal 57 160–170 [DOI] [PubMed] [Google Scholar]
- Dobrev PI, Kamínek M. 2002. Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction Journal of Chromatography A 950 21–29 [DOI] [PubMed] [Google Scholar]
- Emery RJN, Ma QF, Atkins CA. 2000. The forms and sources of cytokinins in developing white lupine seeds and fruits Plant Physiology 123 1593–1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expósito-Rodríguez M, Borges AA, Borges-Pérez A, Pérez JA. Selection of internal control genes for quantitative real-time RT-PCR studies during tomato development process. BMC Plant Biology. 2008;8:131. doi: 10.1186/1471-2229-8-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ficcadenti N, Sestili S, Pandolfini T, Cirillo C, Rotino GL, Spena A. 1999. Genetic engineering of parthenocarpic fruit development in tomato Molecular Breeding 5 463–470 [Google Scholar]
- Flaishman MA, Shargal A, Stern RA. 2001. The synthetic cytokinin CPPU increases fruit size and yield of ‘Spadona’ and ‘Costia’ pear (Pyrus communis L.). Journal of Horticultural Science and Biotechnology 76 145–149 [Google Scholar]
- Fos M, Nuez F, García-Martínez JL. 2000. The gene pat-2, which induces natural parthenocarpy, alters the gibberellin content in unpollinated tomato ovaries Plant Physiology 122 471–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanem ME, Albacete A, Smigocki AC, et al. 2011. Root-synthesized cytokinins improve shoot growth and fruit yield in salinized tomato (Solanum lycopersicum L.) plants Journal of Experimental Botany 62 125–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillaspy G, Ben-David H, Gruissem W. 1993. Fruits – a developmental perspective The Plant Cell 5 1439–1451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetz M, Hooper LC, Johnson SD, Rodrigues JCM, Vivian-Smith A, Koltunow AM. 2007. Expression of aberrant forms of AUXIN RESPONSE FACTOR8 stimulates parthenocarpy in Arabidopsis and tomato Plant Physiology 145 351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayata Y, Niimi Y, Inoue K, Kondo S. 2000. CPPU and BA, with and without pollination, affect set, growth, and quality of muskmelon fruit HortScience 35 868–870 [Google Scholar]
- Hayata Y, Niimi Y, Iwasaki N. 1995. Synthetic cytokinin-1-(2-chloro-4-pyridyl)-3-phenylurea (CPPU) promotes fruit-set and induces parthenocarpy in watermelon Journal of the American Society for Horticultural Science 120 997–1000 [Google Scholar]
- He C, Saedler H. 2007. Hormonal control of the inflated calyx syndrome, a morphological novelty, in Physalis The Plant Journal 49 935–946 [DOI] [PubMed] [Google Scholar]
- Hirose N, Takei K, Kuroha T, Kamada-Nobusada T, Hayashi H, Sakakibara H. 2008. Regulation of cytokinin biosynthesis, compartmentalization and translocation Journal of Experimental Botany 59 75–83 [DOI] [PubMed] [Google Scholar]
- Ho LC, Grange RI, Picken AJ. 1987. An analysis of the accumulation of water and dry-matter in tomato fruit Plant, Cell and Environment 10 157–162 [Google Scholar]
- Jones B, Gunnerås SA, Petersson SV, Tarkowski P, Graham N, May S, Dolezal K, Sandberg G, Ljung K. 2010. Cytokinin regulation of auxin synthesis in Arabidopsis involves a homeostatic feedback loop regulated via auxin and cytokinin signal transduction The Plant Cell 22 2956–2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones RJ, Schreiber BMN. 1997. Role and function of cytokinin oxidase in plants Plant Growth Regulation 23 123–134 [Google Scholar]
- Kakimoto T. 2001. Identification of plant cytokinin biosynthetic enzymes as dimethylallyl diphosphate: ATP/ADP isopentenyltransferases Plant and Cell Physiology 42 677–685 [DOI] [PubMed] [Google Scholar]
- Kataoka K, Date S, Goto T, Asahira T. 1994. Reducing of tomato puffiness in auxin-induced parthenocarpic fruits by forchlorfenuron (1-(2-chloro-4-pyridyl)-3-phenylurea) Journal of the Japanese Society for Horticultural Science 63 61–66 [Google Scholar]
- Kim JG, Takami Y, Mizugami T, Beppu K, Fukuda T, Kataoka I. 2006. CPPU application on size and quality of hardy kiwifruit Scientia Horticulturae 110 219–222 [Google Scholar]
- Kurakawa T, Ueda N, Maekawa M, Kobayashi K, Kojima M, Nagato Y, Sakakibara H, Kyozuka J. 2007. Direct control of shoot meristem activity by a cytokinin-activating enzyme Nature 445 652–655 [DOI] [PubMed] [Google Scholar]
- Kuroha T, Tokunaga H, Kojima M, Ueda N, Ishida T, Nagawa S, Fukuda H, Sugimoto K, Sakakibara H. 2009. Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis The Plant Cell 21 3152–3169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DH, Burge GK, Schmierer DM, Jameson PE. 1996. Cytokinins and fruit development in the kiwifruit (Actinidia deliciosa). I. Changes during fruit development Physiologia Plantarum 98 179–186 [Google Scholar]
- Martí C, Orzáez D, Ellul P, Moreno V, Carbonell J, Granell A. 2007. Silencing of DELLA induces facultative parthenocarpy in tomato fruits The Plant Journal 52 865–876 [DOI] [PubMed] [Google Scholar]
- Matsumoto-Kitano M, Kusumoto T, Tarkowski P, Kinoshita-Tsujimura K, Václavíková K, Miyawaki K, Kakimoto T. 2008. Cytokinins are central regulators of cambial activity Proceedings of the National Academy of Sciences, USA 105 20027–20031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki K, Matsumoto-Kitano M, Kakimoto T. 2004. Expression of cytokinin biosynthetic isopentenyltransferase genes in Arabidopsis: tissue specificity and regulation by auxin, cytokinin, and nitrate The Plant Journal 37 128–138 [DOI] [PubMed] [Google Scholar]
- Miyawaki K, Tarkowski P, Matsumoto-Kitano M, Kato T, Sato S, Tarkowska D, Tabata S, Sandberg G, Kakimoto T. 2006. Roles of Arabidopsis ATP/ADP isopentenyltransferases and tRNA isopentenyltransferases in cytokinin biosynthesis Proceedings of the National Academy of Sciences, USA 103 16598–16603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok DWS, Mok MC. 2001. Cytokinin metabolism and action Annual Review of Plant Physiology and Plant Molecular Biology 52 89–118 [DOI] [PubMed] [Google Scholar]
- Nieminen K, Immanen J, Laxell M, et al. 2008. Cytokinin signaling regulates cambial development in poplar Proceedings of the National Academy of Sciences, USA 105 20032–20037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio S, Moriguchi R, Ikeda H, Takahashi H, Fujii N, Guilfoyle TJ, Kanahama K, Kanayama Y. 2010. Expression analysis of the auxin efflux carrier family in tomato fruit development Planta 232 755–764 [DOI] [PubMed] [Google Scholar]
- Nitsch LMC, Oplaat C, Feron R, Ma Q, Wolters-Arts M, Hedden P, Mariani C, Vriezen WH. 2009. Abscisic acid levels in tomato ovaries are regulated by LeNCED1 and SlCYP707A1 Planta 229 1335–1346 [DOI] [PubMed] [Google Scholar]
- Ohara H, Izawa J, Kimura S, Hiroi N, Matsui H, Hirata N, Takanashi E. 1997. Induction of fruit set and growth of parthenocarpic ‘Hayward’ kiwifruit with plant growth regulators Journal of the Japanese Society for Horticultural Science 66 467–473 [Google Scholar]
- Olimpieri I, Siligato F, Caccia R, Mariotti L, Ceccarelli N, Soressi GP, Mazzucato A. 2007. Tomato fruit set driven by pollination or by the parthenocarpic fruit allele are mediated by transcriptionally regulated gibberellin biosynthesis Planta 226 877–888 [DOI] [PubMed] [Google Scholar]
- Pernisová M, Klíma P, Horák J, et al. 2009. Cytokinins modulate auxin-induced organogenesis in plants via regulation of the auxin efflux Proceedings of the National Academy of Sciences, USA 106 3609–3614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramin AA. 2003. Effects of auxin application on fruit formation in tomato growing under stress temperatures in the field Journal of Horticultural Science and Biotechnology 78 706–710 [Google Scholar]
- Rijavec T, Jain M, Dermastia M, Chourey PS. 2011. Spatial and temporal profiles of cytokinin biosynthesis and accumulation in developing caryopses of maize Annals of Botany 107 1235–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Růžička K, Šimášková M, Duclercq J, Petrášek J, Zažímalová E, Simon S, Friml J, Van Montagu MCE, Benková E. 2009. Cytokinin regulates root meristem activity via modulation of the polar auxin transport Proceedings of the National Academy of Sciences, USA 106 4284–4289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakibara H. 2006. Cytokinins: activity, biosynthesis, and translocation Annual Review of Plant Biology 57 431–449 [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Sakakibara H, Kojima M, Yamamoto Y, Nagasaki H, Inukai Y, Sato Y, Matsuoka M. 2006. Ectopic expression of KNOTTED1-like homeobox protein induces expression of cytokinin biosynthesis genes in rice Plant Physiology 142 54–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrani JC, Fos M, Atarés A, García-Martínez JL. 2007a. Effect of gibberellin and auxin on parthenocarpic fruit growth induction in the cv Micro-Tom of tomato Journal of Plant Growth Regulation 26 211–221 [Google Scholar]
- Serrani JC, Sanjuán R, Ruiz-Rivero O, Fos M, García-Martínez JL. 2007b. Gibberellin regulation of fruit set and growth in tomato Plant Physiology 145 246–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shani E, Ben-Gera H, Shleizer-Burko S, Burko Y, Weiss D, Ori N. 2010. Cytokinin regulates compound leaf development in tomato The Plant Cell 22 3206–3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shudo K. 1994. Chemistry of phenylurea cytokinins.In: Mok DWS, Mok MC, editors, Cytokinins: chemistry activity and function Boca Raton: CRC Press, pp 35–42
- Spíchal L, Rakova NY, Riefler M, Mizuno T, Romanov GA, Strnad M, Schmülling T. 2004. Two cytokinin receptors of Arabidopsis thaliana CRE1/AHK4 and AHK3, differ in their ligand specificity in a bacterial assay Plant and Cell Physiology 45 1299–1305 [DOI] [PubMed] [Google Scholar]
- Srivastava A, Handa AK. 2005. Hormonal regulation of tomato fruit development: a molecular perspective Journal of Plant Growth Regulation 24 67–82 [Google Scholar]
- Stern RA, Ben-Arie R, Neria O, Flaishman M. 2003. CPPU and BA increase fruit size of ‘Royal Gala’(Malus domestica) apple in a warm climate Journal of Horticultural Science and Biotechnology 78 297–302 [Google Scholar]
- Stolz A, Riefler M, Lomin SN, Achazi K, Romanov GA, Schmuelling T. 2011. The specificity of cytokinin signalling in Arabidopsis thaliana is mediated by differing ligand affinities and expression profiles of the receptors The Plant Journal 67 157–168 [DOI] [PubMed] [Google Scholar]
- Takei K, Sakakibara H, Sugiyama T. 2001. Identification of genes encoding adenylate isopentenyltransferase, a cytokinin biosynthesis enzyme, in Arabidopsis thaliana Journal of Biological Chemistry 276 26405–26410 [DOI] [PubMed] [Google Scholar]
- Takei K, Yamaya T, Sakakibara H. 2004. Arabidopsis CYP735A1 and CYP735A2 encode cytokinin hydroxylases that catalyze the biosynthesis of trans Zeatin Journal of Biological Chemistry 279 41866–41872 [DOI] [PubMed] [Google Scholar]
- Tanaka M, Takei K, Kojima M, Sakakibara H, Mori H. 2006. Auxin controls local cytokinin biosynthesis in the nodal stem in apical dominance The Plant Journal 45 1028–1036 [DOI] [PubMed] [Google Scholar]
- Tarkowski P, Tarkowská D, Novák O, Mihaljević S, Magnus V, Strnad M, Salopek-Sondi B. 2006. Cytokinins in the perianth, carpels, and developing fruit of Helleborus niger L Journal of Experimental Botany 57 2237–2247 [DOI] [PubMed] [Google Scholar]
- Van Ieperen W, Volkov VS, Van Meeteren U. 2003. Distribution of xylem hydraulic resistance in fruiting truss of tomato influenced by water stress Journal of Experimental Botany 54 317–324 [DOI] [PubMed] [Google Scholar]
- Wang H, Jones B, Li ZG, Frasse P, Delalande C, Regad F, Chaabouni S, Latché A, Pech JC, Bouzayen M. 2005. The tomato Aux/IAA transcription factor IAA9 is involved in fruit development and leaf morphogenesis The Plant Cell 17 2676–2692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Schauer N, Usadel B, Frasse P, Zouine M, Hernould M, Latché A, Pech JC, Fernie AR, Bouzayen M. 2009. Regulatory features underlying pollination-dependent and -independent tomato fruit set revealed by transcript and primary metabolite profiling The Plant Cell 21 1428–1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner T, Köllmer I, Bartrina I, Holst K, Schmülling T. 2006. New insights into the biology of cytokinin degradation Plant Biology 8 371–381 [DOI] [PubMed] [Google Scholar]
- Werner T, Motyka V, Laucou V, Smets R, Van Onckelen H, Schmülling T. 2003. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity The Plant Cell 15 2532–2550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabadal TJ, Bukovac MJ. 2006. Effect of CPPU on fruit development of selected seedless and seeded grape cultivars HortScience 41 154–157 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.