Abstract
Gamete formation is an important step in the life cycle of sexually reproducing organisms. In flowering plants, haploid spores are formed after the meiotic division of spore mother cells. These spores develop into male and female gametophytes containing gametes after undergoing mitotic divisions. In the female, the megaspore mother cell undergoes meiosis forming four megaspores, of which one is functional and three degenerate. The megaspore then undergoes three mitotic cycles thus generating an embryo sac with eight nuclei. The embryo sac undergoes cellularization to form the mature seven-celled female gametophyte. Entry into and progression through meiosis is essential for megasporogenesis and subsequent megagametogenesis, but control of this process is not well understood. FOUR LIPS (FLP) and its paralogue MYB88, encoding R2R3 MYB transcription factors, have been extensively studied for their role in limiting the terminal division in stomatal development by direct regulation of the expression of cell cycle genes. Here it is demonstrated that FLP and MYB88 also regulate female reproduction. Both FLP and MYB88 are expressed during ovule development and their loss significantly increases the number of ovules produced by the placenta. Despite the presence of excess ovules, single and double mutants exhibit reduced seed set due to reduced female fertility. The sterility results at least in part from defective meiotic entry and progression. Therefore, FLP and MYB88 are important regulators of entry into megasporogenesis, and probably act via the regulation of cell cycle genes.
Key Words: Arabidopsis thaliana, female sterility, FLP, gametogenesis, MYB88, plant reproduction, transcription
Introduction
Plants are characterized by the alternation of haploid (gametophyte) and diploid (sporophyte) generations. In angiosperms, such as Arabidopsis thaliana, the gametophyte is short lived and develops within the sporophytic tissue of the flower. The female gametophyte, or embryo sac, develops within ovules contained in the ovary of the pistil. Ovule primordia develop from placental tissue in the ovary. Along the proximal–distal axis an ovule primordium consists of three distinct regions: the funiculus, the chalaza, and the nucellus. Within the nucellus the megaspore mother cell (MMC) is formed, which divides first meiotically and then mitotically to form the embryo sac (reviewed in Berger and Twell, 2011). The MMC divides meiotically to make four haploid megaspores, of which three degenerate and one, the proximal chalazal megaspore, continues to develop. This functional megaspore increases in size and undergoes a series of nuclear divisions to form an eight-nuclei embryo sac. Then cellularization takes place, dividing the embryo sac into seven cells with four cell types: three antipodal cells at the chalazal end, a diploid central cell, and two synergids and the egg cell at the micropylar end. Antipodal cells, which have no currently known function, degenerate before fertilization in Arabidopsis. The central cell, the egg cell, and the synergid cells form the female germ unit.
Proper specification of the MMC, entry into and completion of meiosis, control of mitotic divisions, and cell fate determination and differentiation are essential for development of the embryo sac. In Arabidopsis, a number of genes are known to regulate these processes. For example, mutations in a gene encoding a type I MADS-box transcription factor, AGL23, block the first nuclear division of the functional megaspore (Colombo et al., 2008). Mutations in the PROLIFERA (PRL) gene, encoding the DNA replication licensing factor subunit MCM7, arrest the embryo sac with one nucleus (Springer et al., 2000). Temporal and spatial regulation of cellularization of the syncytial embryo sac is critical for gametophytic development and cell fate. In the female sterile mutant hadad (hdd), the gametophyte becomes prematurely cellularized after the first or second gametophytic division (Moore et al., 1997). Simultaneously with cellularization, cell fate within the embryo sac is acquired and differentiation begins. MYB98 encodes an R2R3-MYB transcription factor specifically expressed in the synergid cells. myb98 mutants fail to form the filiform apparatus and are unable to guide the pollen tube to the micropyle (Punwani et al., 2007). However, although many genes are known that are involved in meiotic recombination and other aspects of the meiotic cycle (reviewed in Ma, 2006), how MMC entry into meiosis is controlled is still not known.
The atypical R2R3-MYB transcription factor FOUR LIPS (FLP) and its paralogue MYB88 have been well characterized for their partially redundant roles in stomatal patterning. FLP was first identified during a mutant screen (Yang and Sack, 1995), while MYB88 was identified by sequence similarity with FLP (Lai et al., 2005). These genes have overlapping functions in restricting the last precursor cell in the stomatal lineage, the guard mother cell (GMC), to a single symmetric division and also promoting guard cell differentiation. In loss-of-function flp-1 and flp-7 alleles, the GMC undergoes extra divisions resulting in clusters of four or more guard cells in contact with one another. flp-1; myb88 double mutants exhibit more severe stomatal phenotypes than the single flp-1 mutation alone, while single myb88 mutants show no discernible defects in stomata. In addition, the cells in stomatal clusters do not properly differentiate into guard cells. Thus, FLP/MYB88 are required both for limiting the GMC to a single symmetric division and for the subsequent differentiation of guard cells (Lai et al., 2005).
As DNA-binding transcription factors, FLP and MYB88 directly regulate the expression of a number of genes, including cell cycle genes (Xie et al., 2010a; Vanneste et al., 2011). FLP has been shown to bind to the promoter of CDKB1;1, a mitosis-inducing factor, and to reduce its expression. CDKB1;1 along with the closely related gene CDKB1;2 is required both for the last division in the stomatal pathway (Boudolf et al., 2004) and for the overproliferation of guard cells in flp mutants (Xie et al., 2010a). It has been proposed that FLP and MYB88 together regulate stomatal patterning by controlling cell cycle progression and terminal differentiation through multiple cell cycle targets (Xie et al., 2010a). In addition to CDKB1;1, FLP/MYB88 are also necessary to repress expression in a timely manner of the plant A2-type cyclin-encoding gene CYCA2;3 in newly formed guard cells to promote exit from the cell cycle (Vanneste et al., 2011).
Although their function in epidermal patterning is best described, FLP and MYB88 are also required for abiotic stress tolerance since flp-1; myb88 double mutants have reduced expression of stress-induced genes and are more susceptible to both drought and salt stress (Xie et al., 2010b). FLP/MYB88 positively and directly regulate the expression of at least some stress-responsive genes, suggesting that changes in the stress transcriptome in flp-1; myb88 mutants are not just an indirect effect of abnormal stomatal complexes. For example, FLP/MYB88 positively regulate the NAC019 gene, involved in response to dehydration, abscisic acid (ABA), and salt (Tran et al., 2004), by binding directly to its promoter (Xie et al., 2010b). FLP and MYB88 are likely to have other developmental functions as well since they are expressed in a wide variety of tissues. FLP is expressed in developing stomata, specifically in GMCs just before their symmetric division. Expression is also seen in organs without stomata, including the root (Lai et al., 2005). However, whether FLP and MYB88 have any developmental roles other than stomatal development has not been investigated.
Here, genetic and cell-biological evidence is provided that FLP and MYB88 function during female reproductive development in Arabidopsis. In loss-of-function flp single and flp; myb88 double mutants, the placenta produces extra ovule primordia, suggesting these genes normally restrict the proliferation of this tissue. However, seed set is reduced. The reduction is the result of defects in female megasporogenesis. The expressivity of the defect is influenced by genetic background. In a subset of mutant ovules the MMC either fails to divide meiotically or divides abnormally, leading to a lack of female gametophyte development. In contrast to the embryo sac defects, mutant pollen developed and functioned normally. Therefore, FLP and MYB88 function as positive transcriptional regulators of entry into megasporogenesis.
Materials and Methods
Plant Material and Growth Conditions
The following alleles of FLP were used in this study: flp-1, flp-7, flp-8, and SALK_033970. In addition, two double mutants were also analysed: flp-1; myb88 and flp-7; myb88. flp-7 and flp-8 are in the Landsberg erecta (L. er) background whereas flp-1, flp-1; myb88, SALK_033970, and flp-7; myb88 are all in the Columbia-0 (Col-0) background as previously reported (Lai et al., 2005). Genotyping was performed as described, using primer combinations listed in Supplementary Table S1 available at JXB online (primer sequences are given in Supplementary Table S2). Various female gametophyte-specific marker lines (Supplementary Table S3) were introgressed into the flp-7 background by crossing homozygous mutant plants to the marker lines and allowing the F1 to self-fertilize. PCR genotyping of the segregating F2 population was performed and the presence of the marker gene was identified by selecting seedlings on Murashige and Skoog (MS) plates containing kanamycin at 50 µg ml–1.
Arabidopsis seeds were cold treated for 3 d at 4 °C and germinated and grown on Fafard 2 mix soil (Fafard) under long-day (16h, 80 µmol m–2 s–1) irradiance either in controlled growth chambers (Enconair Ecological Chambers Inc., Manitoba, Canada) or in growth rooms with subirrigation at 22 °C with 60% relative humidity. Seeds grown on plates were sterilized with 70% ethanol followed by 10% (v/v) hypochlorite and 0.1% SDS, and placed on Petri dishes containing MS media (RP1 Corp.) with 1% plant agar with or without antibiotic. The plates were incubated in the dark at 4 °C for 5 d to achieve uniform germination and then moved to a CU-36L growth chamber (Percival Scientific Inc., Perry, IA, USA) and grown under long-day conditions (22 °C; 16h photoperiod) unless noted. Seedlings were transplanted to soil at ~2 weeks old.
PCR-based Genotyping
Genomic DNA was extracted as previously described (Teotia and Lamb, 2009). Primer combinations and primer sequences are shown in Supplementary Tables S1 and S2 at JXB online, respectively. PCR was done using Biolase Red DNA Polymerase (Bioline) on a conventional PCR machine (Bio-Rad-iCycler Thermal Cycler). flp-1, flp-7, and er alleles were identified by derived cleaved amplified polymorphic sequences (dCAPS; see Supplementary Table S1) (Neff et al., 1998, 2002). The flp-1 allele was genotyped as described (Xie et al., 2010a).
Seed Set and Fertility Analysis
In order to analyse the seed set of the mutants and wild type, plants were grown under long-day conditions. Seeds of various genotypes were sown in 4 inch round pots. Four plants per genotype were used for comparing the percentage seed set. The total number of ovules and the number of seeds present in the first 15 siliques on the primary inflorescence only were counted, as previously described (Alvarez and Smyth, 1999). The percentage seed set was calculated by taking into account the total number of ovules and the number of seeds present in a silique. Statistical significance of the values was calculated using a Student’s t-test.
Reciprocal crosses were done to test male and female fertility by emasculating and hand pollinating 15 flowers of each genotype, as previously described (Unte et al., 2003). The number of seeds made in each silique was counted after the siliques were fully matured. Statistical significance of the values was calculated as above.
Aniline Blue Staining of Pollen Tubes and Mucilage Staining of Seeds
Aniline blue staining of pistils was done according to Jiang et al. (2005). Flowers were emasculated just prior to pollination (late stage 12) and were grown for another 18–24h to allow transmitting tract and ovule development to finish. Pistils were hand pollinated and grown for a further 24h to allow pollen tube growth. Pollinated pistils were fixed in a solution of ethanol:acetic acid (3:1) for 2h at room temperature, washed three times with ddH2O, softened in 8M NaOH overnight, and washed in ddH2O several times before staining. Pistils were stained in aniline blue solution (0.1% aniline blue in 0.1M K2HPO4–KOH buffer, pH 11) for 3h in the dark. The stained pistils were observed and photographed with a Nikon Eclipse 80i fluorescence microscope.
Mucilage production by flp-7 and flp-1; myb88 seeds was analysed according to Debeaujon et al. (2000). In brief, seeds were soaked in 0.03% ruthenium red (w/v) for 15min and washed in water before being mounted on a slide and observed using a Nikon Digital Sight DS-5M camera on a Nikon SMZ800 dissecting microscope.
Ovule Clearing and Differential Interference Contrast (DIC) Optics
Definitions for floral, ovule, and gametophyte development stages were as described in Smyth et al. (1990), Christensen et al. (1997), and Schneitz et al. (1995). Embryo sacs were collected from the entirety of the primary inflorescence and also secondary inflorescences. To examine mature ovules, flowers were emasculated just prior to pollination (late stage 12) and were grown for another 24h. Ovule clearing and microscopy was done according to Rodrigo-Peiris et al. (2011), with some modifications. Pistils were opened along the carpel margins and fixed in a solution of 9:1 absolute ethanol:glacial acetic acid overnight at 4 °C followed by washing in 90% ethanol for 1h, and stored in 70% ethanol until examination. Ovules were cleared in chloral hydrate (8g of chloral hydrate and 2ml of ddH2O to each ml of glycerol) on microscope slides for 2h before microscopic analysis with DIC optics on a Nikon Eclipse 90i Microscope. Pictures were taken using the attached Nikon camera and analysed with NIS elements Advanced Research software version 3.0.
β-Glucuronidase (GUS) Staining
Histochemical staining for GUS activity was performed as described (Jefferson et al., 1987). In brief, ovules were collected from pistils at different stages of flower development. The ovules were incubated in GUS staining buffer (50mM sodium phosphate buffer pH 7.0, 10mM EDTA, 2mM potassium ferricyanide, 2mM potassium ferrocyanide, 0.1% Triton X-100, and 2mM 5-bromo-4-chloro-3-indolyl-β-d-glucuronic acid) at 37 °C for 72h. Ovules were then washed in 90% ethanol for 1h and stored in 70% ethanol until examination. Photographs were taken using a Nikon Digital Sight DS-5M camera attached to a Nikon Eclipse 80i compound microscope.
Confocal Microscopy of Ovules
Ovules were collected from pistils at different stages of flower development and were incubated in 0.5% propidium iodide in 50mM phosphate buffer at room temperature or in 10 µg ml–1 of propidium iodide. Ovules were observed using either a Nikon D-Eclipse C1si or Nikon Eclipse 80i confocal microscope at excitation wavelengths of 488nm and 543nm. Emission was collected at 620–720nm and 488–562nm to visualize propidium iodide and green fluorescence protein (GFP) fluorescence, respectively.
Results
FLP and MYB88 are Expressed in the Flower
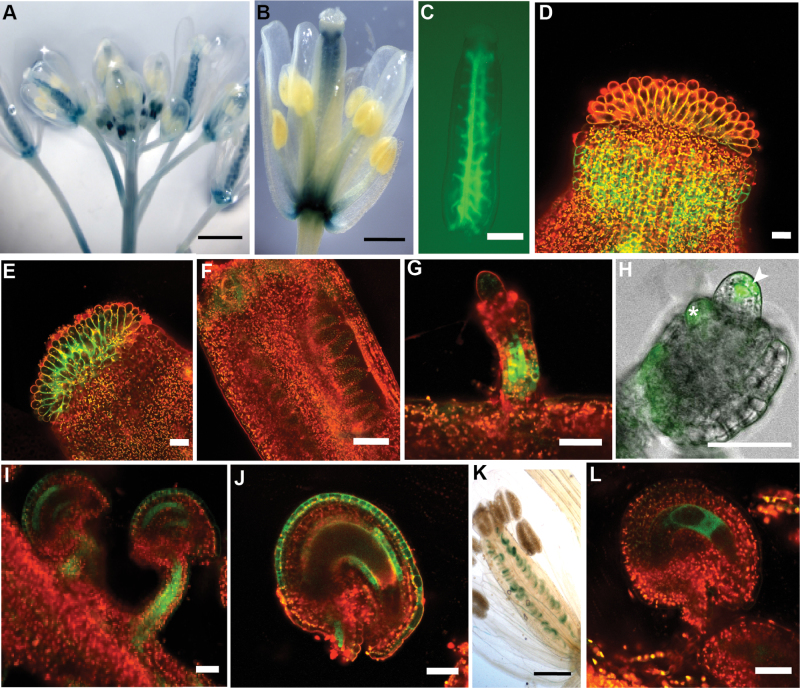
To characterize FLP and MYB88 expression during reproductive development, transgenic plants containing either a pFLP::GUS-GFP construct (Lai et al., 2005) or a pMYB88::GUS-GFP construct (Vanneste et al., 2011; a kind gift of Steffen Vanneste) were used. FLP expression was detected in unopened flower buds, at the bases of sepals, petals, and stamens and in the receptacle of carpels (Fig. 1A, 1B, and data not shown). pFLP::GUS-GFP is strongly expressed in the placenta within the ovary (Fig. 1C). Additionally, expression of FLP can be seen in both the style (Fig. 1D) and stigma (Fig. 1E) of the pistil. During ovule development, FLP has a dynamic expression pattern. During early stages of ovule development (before integuments are morphologically distinct), FLP expression was barely detectable (Fig. 1F). However, once integument outgrowth has begun, strong FLP expression is seen in the funiculus (Fig. 1G), which persists into later stages (Fig. 1H, 1I). Notably, the FLP promoter drives expression in the nucellus in younger ovules, where it is specifically expressed in the MMC and in epidermal cells (Fig. 1H). FLP is also expressed in the integuments, starting at a low level when they initiate (Fig. 1H). Later in ovule development it is expressed in the endothelial layer (the adaxial layer of the inner integument) and the outer layer of the outer integument, which will form the mucilage-containing seed coat cells (Fig. 1I, 1J). In contrast, little to no expression was seen in older anthers (Fig. 1B), consistent with Genevestigator data (Zimmermann et al., 2004, 2005). MYB88 is expressed at a much lower level than FLP (Lai et al., 2005). However, MYB88 expression is detected in ovules (Fig. 1K) and in the embryo sac of stage 13 flowers (Fig. 1L).
Fig. 1.
FLP and MYB88 are expressed in reproductive organs. (A–J) Micrographs of pFLP::GUS-GFP transgenic plants. (A and B) GUS staining. (C–J) GFP fluorescence visualized using confocal microscopy. (A) FLP expression in whole inflorescence. (B) FLP is expressed in the carpel. (C) Expression of FLP can be seen in the placenta. (D) FLP is expressed in the style. (E) FLP is expressed in the stigmatic tissue. (F) During early ovule development, before integument initiation, little FLP expression is detectable within the ovule. (G) Expression of FLP can be seen in the funiculus as the integuments are initiating. (H) FLP is expressed in the nucellus, including epidermal cells (arrowhead) and the MMC. FLP is also expressed in initiating integuments (asterisk) and the funiculus. (I) FLP expression persists in the funiculus and is seen in the integuments of older ovules. (J) In stage 13 ovules, FLP is expressed in both the endothelial layer and the outer layer of the outer integument. (K and L) Micrographs of pMYB88::GUS-GFP transgenic plants. (K) GUS staining showing MYB88 expression in ovules. (L) In stage 13 ovules, MYB88 is expressed in the embryo sac.
Loss of FLP and MYB88 Reduces Female Fertility
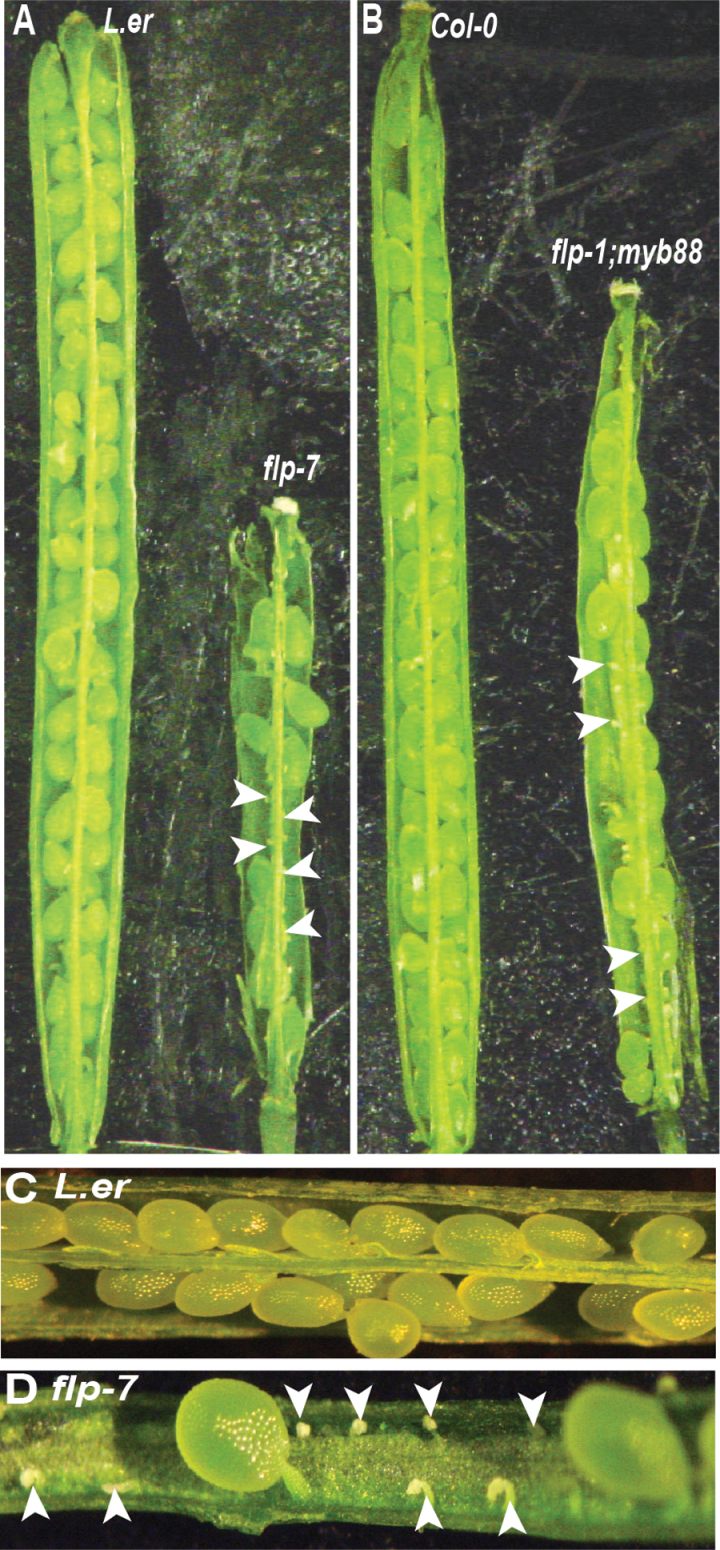
To investigate the roles of FLP and MYB88 in reproductive development, fruit size and seed set were examined in different flp alleles and double mutants with myb88. The siliques of both flp-7 and flp-1; myb88 appeared shorter than their respective wild types (Fig. 2A, 2B). Mutant siliques contain small, white ovules that appear to be either aborted or not fertilized (Fig. 2A, 2B, 2D; Table 1), consistent with the presence of shorter siliques. The seed set of flp and myb88 mutants was then compared with that of their respective wild type (L. er or Col-0). All examined flp and flp; myb88 plants except flp-1 have significantly reduced seed set (Table 1). Interestingly, flp-7, flp-8, SALK_033970 (which is a loss-of-function allele in the FLP locus), and flp-7; myb88 have significantly more ovules than the wild type, but still have reduced seed set (Table 1). This suggests that the ovary and placenta are larger in pistils of flp mutants, but that the reduced fertility leads to smaller fruits. These results show that FLP and MYB88 regulate reproductive development. None of the available flp alleles is RNA null, although the myb88 allele is a knockdown (Lai et al., 2005). This makes it difficult to assess the extent to which FLP function is lost in these backgrounds. For example, both flp-1 and flp-7 mutations are in the 3’ splice site AG of introns (3 and 4, respectively) and both have been shown to cause splicing errors (Lai et al., 2005). These errors are predicted to result in premature translational stops within the MYB domain (in the third R2 helix in flp-1 and after the first R3 helix in flp-7), thus producing truncated proteins likely to have disrupted activity. Despite the fact that these alleles are predicted to produce similar truncation products, the stomatal defects seen in flp-7 are more severe than those of flp-1 (Lai et al., 2005). As flp-7 (L. er) and flp-1; myb88 (Col-0) display significantly reduced seed set and are in two different genetic backgrounds, they were selected for further studies.
Fig. 2.
Fertility is lowered in flp-7 and flp-1; myb-88 plants. Dissecting microscope images of siliques. (A) flp-7 fruit are shorter than those of the wild type and contain aborted ovules (arrowheads). (B) flp-1; myb88 fruit are shorter than those of the wild type and contain aborted ovules. (C) L. er silique containing many seeds. (D) flp-7 silique containing a few seeds and several aborted ovules (arrowheads).
Table 1.
flp and flp; myb88 mutants have reduced fertility that is influenced by genetic background
| Genotype | Ovules per siliquea | Seeds per siliquea | Aborted ovules per siliquea,b | Seed set |
|---|---|---|---|---|
| L. er | 45±0.6 | 42±1.0 | 3±0.6 | 94% |
| L. er/pER::ER | 58±0.8 | 53±0.7 | 5±0.3 | 92% |
| flp-7 | 61±0.9** | 4±0.7** | 57±1.3** | 6% |
| flp-7/pER::ER | 54±1.4*** | 17±0.5**** | 40±1.3**** | 31% |
| flp-8 | 66±0.8** | 46±2.1 | 20±0.6** | 70% |
| Col-0 | 48±0.5 | 39±1.5 | 9±1.5 | 80% |
| flp-1 | 47±0.7 | 34±1.7 | 13±1.6 | 72% |
| SALK_033970/flp | 54±0.8** | 37±3.4 | 17±2.7* | 63% |
| flp-1; myb88 | 49±0.7 | 14±2.4** | 35±2.1** | 27% |
| flp-7; myb88 | 68±0.7** | 47±3.1* | 21±3.2** | 69% |
a Values are means ±SE (n = 60) or means ±SE of n = 60 for three independent transgenic lines (180 siliques in total) for L. er/pER::ER and flp-7/pER::ER.
b Defined as small white ovules with no evidence of embryo or seed development.
*Values that are significantly different from the wild type at P < 0.05. **Values that are significantly different from the wild type at P < 0.01. ***Values that are significantly different from L. er/pER::ER at P < 0.05. ****Values that are significantly different from L. er/pER::ER at P < 0.01.
The strong expression of FLP in integuments (Fig. 1I, 1J) suggests that this gene may be involved in development of the seed coat. Mature seeds of flp-7 and flp-1; myb88 were compared with their respective wild types. Although some minor variability in seed shape was observed in the mutant seeds (Supplementary Fig. S1 at JXB online), particularly flp-7, no noticeable germination defects were observed (data not shown). In addition, mucilage was produced by both flp-7 and flp-1; myb88 seeds (Supplementary Fig. S1), suggesting that differentiation of the seed coat is not significantly altered.
To determine whether the reduced seed set is due to male and/or female infertility, reciprocal crosses were made using flp-7 or flp-1; myb88 homozygous plants and the wild type. Both flp-7 and flp-1; myb88 siliques harboured fewer seeds than their respective wild types when pollinated with either wild-type or mutant pollen (Table 2), indicating that female fertility is compromised. flp-7 pollen, when used to fertilize wild-type pistils, resulted in slightly reduced seed set (Table 2). However, the fertility of flp-1; myb88 pollen was comparable with that of the wild type. Taken together, these results suggest that FLP and MYB88 function in female reproduction.
Table 2.
Loss of FLP and MYB88 compromises female fertility
| Female parent | Male parent | Seeds per crossed siliquea |
|---|---|---|
| L. er | L. er | 43.7±6.1** |
| flp-7 | 37.8±25.0* | |
| flp-7 | L. er | 14.6±6.2** |
| flp-7 | 14.9±7.5** | |
| Col-0 | Col-0 | 40.3±4.7 |
| flp-1; myb88 | 42.6±6.3 | |
| flp-1; myb88 | Col-0 | 7.3±9.4** |
| flp-1; myb88 | 2.1±4.7** |
a Values are the mean ±SE (n = 15).
*Values that are significantly different from the wild type at P < 0.05. **Values that are significantly different from the wild type at P < 0.01.
Embryo Sac Development is Altered by Loss of FLP and MYB88
The aborted ovules seen in siliques of flp mutants could be caused by defects in ovule and/or embryo sac development or by lack of fertilization. Stigmatic and transmitting tract tissues in the carpel are required for proper pollen tube growth and fertilization. Mutants with reduced growth of these tissues have reduced fertility due to poor pollen tube growth (Heisler et al., 2001; Gremski et al., 2007). FLP is strongly expressed in the stigma and style of the pistil (Fig. 1D, 1E), suggesting that it could function in these tissues. In order to examine whether pollen tube growth is affected in flp mutants, carpels were stained with aniline blue 24h after pollination. In both L. er and flp-7 pistils, pollen tubes grew throughout the transmitting tract and into ovules (Supplementary Fig. S2 at JXB online), indicating that the reduced seed set in flp alleles is not due to defects in either the stigma or transmitting tract.
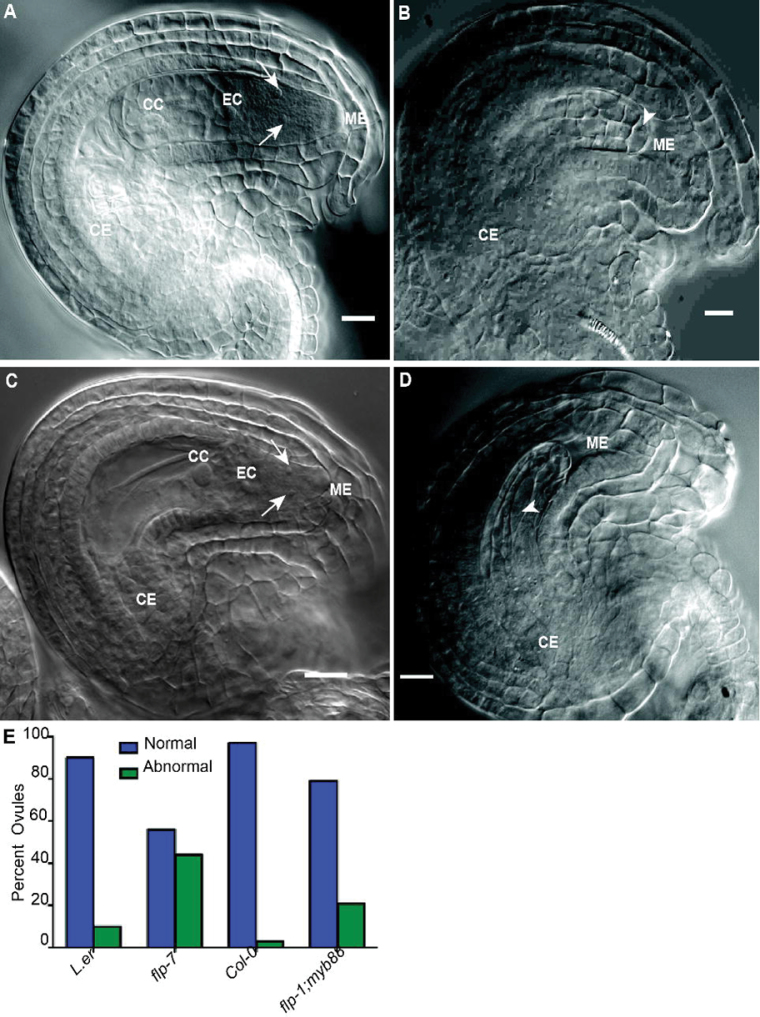
Since pollen tubes were able to travel to the ovules, the reduced fertility seen in flp-7 and flp-1; myb88 plants is probably due to ovule and/or female gametophyte defects. Therefore, ovules were examined 2 d after emasculating flowers at stage 13 (according to Smyth et al., 1990) to determine whether ovule development proceeded normally. The morphology of mutant ovules at female gametophyte developmental stage 7 (FG7; Christensen et al., 1997) appeared normal, with fully developed outer and inner integuments and well-differentiated proximal–distal polarity (Fig. 3B, 3D), suggesting that ovule development is intact in these mutants. The FG7 embryo sacs of flp-7 and flp-1; myb88 were then compared with their respective wild types, L. er and Col-0. Ninety percent of L. er (n=281) and 97% of Col-0 (n=607) ovules display four visible nuclei, corresponding to the egg cell, central cell, and two synergids (Fig. 3E). Although Polygonum-type embryo sacs, such as those found in Arabidopsis, contain seven cells of four types (Yadegari and Drews, 2004), in Arabidopsis the three antipodal cells have degenerated by FG7 (Christensen et al., 1997). In contrast to the wild type, only 56% of flp-7 (n=337) and 78% of flp-1; myb88 (n=589) embryo sacs had four discernible cells. Strikingly, 44% and 22% of the mutant ovules appeared to contain cellular structures harbouring one or more prominent large cells and did not resemble mature female gametophytes (Fig. 3B, 3D). Another difference between the abnormal flp-7 and flp-1; my88 ovules and wild-type ovules was also observed. In the wild type, the growth and expansion of the embryo sac is accompanied by the degeneration of cells in the proximal nucellar region. However, in the abnormal flp ovules, these proximal cells persist (Fig. 3B, 3D).
Fig. 3.
Loss of FLP and/or MYB88 leads to abnormal nucellar structures. (A–D) DIC micrographs of FG7 ovules containing mature female gametophytes. (A) Col-0. (B) flp-1; myb88. (C) L. er. (D) flp-7. (E) Quantification of embryo sac defects. CC, central cell; CE, chalazal end of the embryo sac; EC, egg cell; ME, micropylar end of the embryo sac. Arrows indicate synergid cells, and arrowheads indicate large cells found in abnormal flp ovules in the region where an embryo sac would normally form.
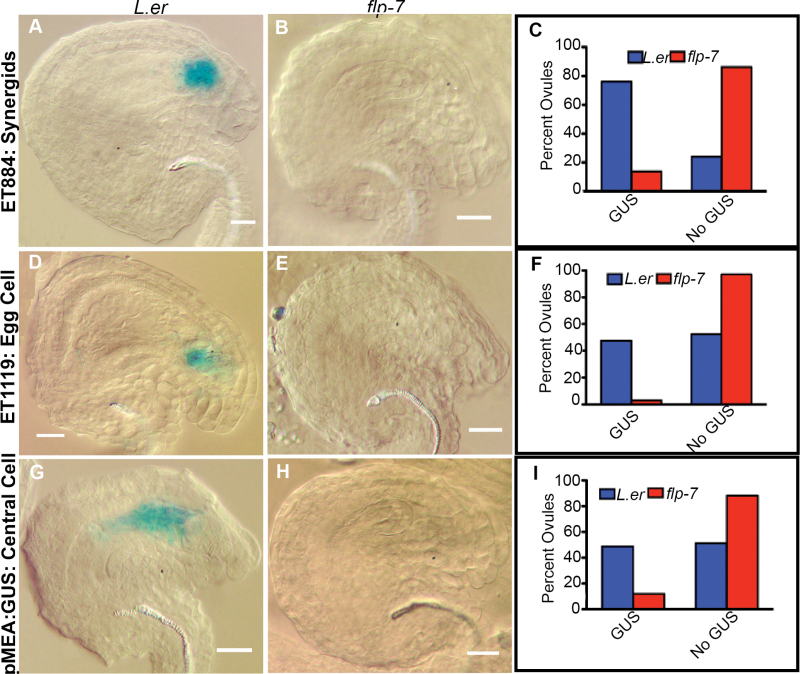
In order to identify and compare the cell types present in embryo sacs at FG7 in wild-type and mutant ovules, specific markers were crossed into the flp-7 mutant background. ET884 has been shown to drive expression in both synergids at the micropylar end of the wild-type embryo sac (Gross-Hardt et al., 2007). However, the expression of this marker was observed in fewer flp-7 ovules compared with the wild type (Fig. 4C). In the wild type, 76% of the observed ovules (n=361) had ET884 expression, while in flp-7 only 14% of the observed ovules (n=356) expressed the synergid maker. No expression of ET884 was observed in those flp-7 ovules in which no embryo sac could be distinguished (Fig. 4B). The egg cell-specific marker ET1119 (Gross-Hardt et al., 2007) was also examined. Relatively few flp-7 ovules sampled expressed this marker (3%, n=430) (Fig. 4F). In contrast, 48% of wild-type ovules sampled (n=250) displayed ET1119 expression while the remainder did not. ET1119 expression was never seen in ovules with abnormal embryo sac development (Fig. 4E). Finally, expression of two markers of central cells, pMEA::GUS (Gross-Hardt et al., 2007) and AGL61::GFP (Steffen et al., 2008), was analysed in wild-type and flp-7 ovules. Consistent with the above results, the expression of the central cell markers was less frequent in flp-7 than in wild-type ovules (Fig. 4I; Supplementary Fig. S3 at JXB online). pMEA::GUS expression in the wild type was found in 49% of ovules (n=279) but only in 12% (n=243) of flp-7 ovules. No pMEA::GUS expression was seen in ovules containing abnormal embryo sacs (Fig. 4H). Results with AGL61::GFP were similar (Supplementary Fig. S3). Taken together, these data suggest that the aborted flp-7 ovules lack differentiated female gametophytes and thus are incapable of being fertilized.
Fig. 4.
flp-7 morphologically abnormal ovules do not contain differentiated female gametophytes. (A, B, D, E, G, H) Micrographs of GUS-stained ovules. (A) In L. er embryo sacs, ET884 is expressed in the synergids. (B) Abnormal flp-7 ovules do not express this marker. (C) Quantification of expression. (D) In L. er embryo sacs, ET1119 expression is found in the egg cell. (E) Abnormal flp-7 ovules do not express this marker. (F) Quantification of expression. (G) In L. er embryo sacs, pMEA:GUS is expressed in the central cell. (H) Abnormal flp-7 ovules do not express this marker. (I) Quantification of expression.
Comparison of Structural Development of the Female Gametophyte in the flp-7 Mutant and Wild Type
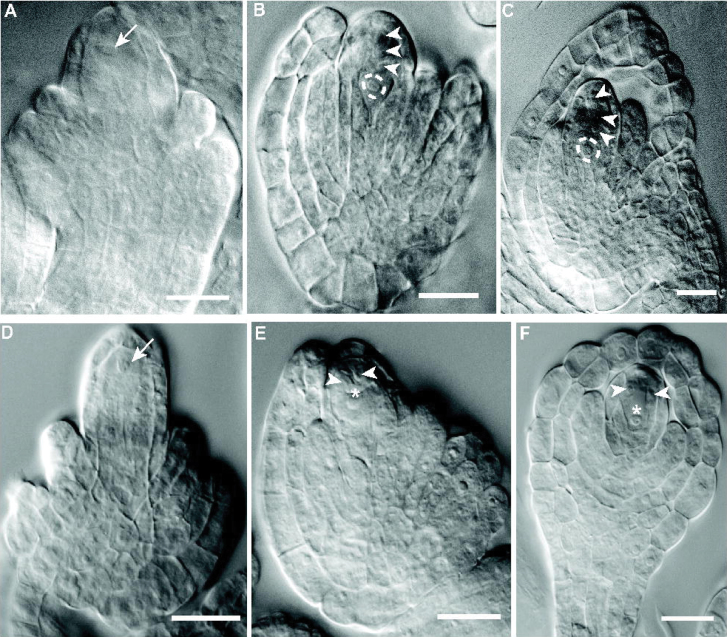
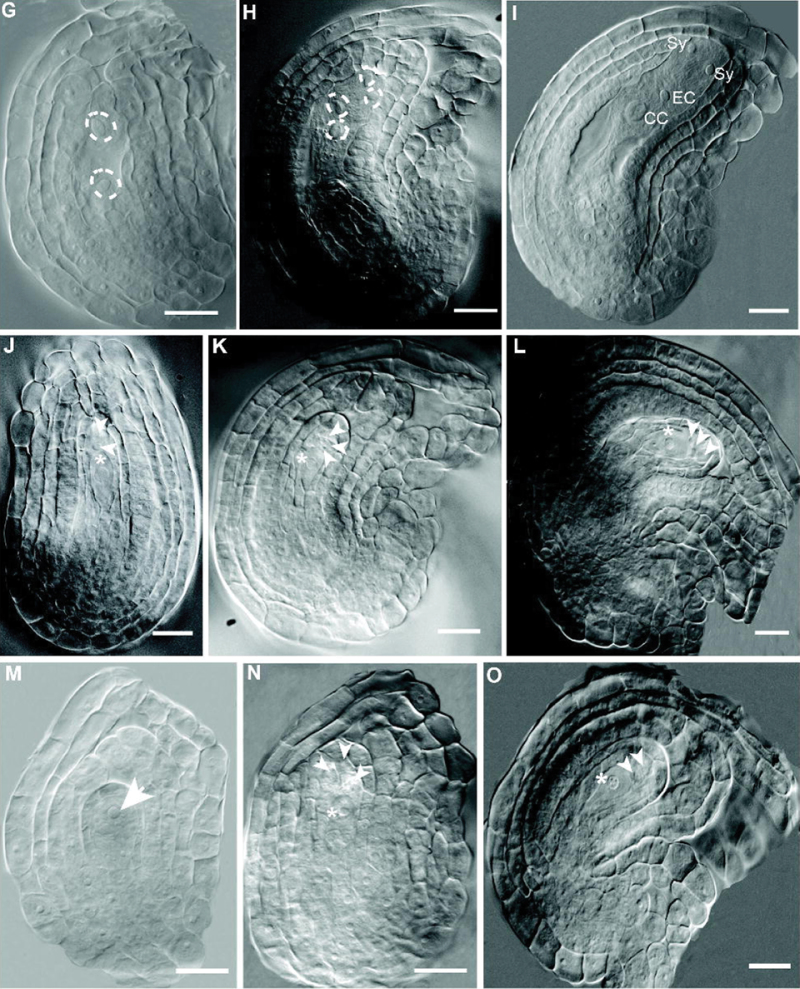
To investigate the basis of the flp-7 phenotype and the origin of the large cells seen in FG7 ovules of the mutant, a stage wise comparison of development was carried out by examining cleared ovules using DIC microscopy. Stages of ovule and female gametophyte development are summarized in Supplementary Table S4 at JXB online, based on Smyth et al. (1990) and Schneitz et al. (1995). Early stages were normal in flp-7, such as the appropriate initiation of ovule primordia (data not shown). In addition, both wild-type and flp-7 pre-meiotic ovules (stage 2-III of ovule development; see Supplementary Table S4) displayed clearly differentiated MMCs (Fig. 5A, 5D). In L. er wild type stage 2-V, after meiosis, a clear tetrad of megaspores can be seen (Fig. 5B, Table 3). However, many of the flp-7 ovules at comparable stages contain an abnormal number of cells in the region where the megaspores should be, including one large cell (Fig. 5E, Table 3). Normally during stage 3-I, the three non-functional megaspores begin to degenerate, leaving a mononuclear embryo sac (Fig. 5C). However, in similarly staged flp-7 ovules, no cellular degeneration was found (Fig. 5F, Table 3). At stage 3-II, the embryo sac normally undergoes its first mitotic division producing a cell with two nuclei (Fig. 5G). Abnormal flp-7 ovules display no such nuclear division and contain a single larger MMC- or megaspore-like cell as well as no (Fig. 5M), two (Fig. 5J), or three (Fig. 5N) other cells nearby. By stage 3-IV, the wild-type female gametophyte contains four nuclei (Fig. 5H), but these were not present in flp-7 ovules (Fig. 5K). At stage 3-VI the embryo sac begins differentiation and cellularization in L. er (Fig. 5I). However, in abnormal flp-7 ovules, instead of an embryo sac forming, a single large cell can be seen and is associated with a variable number of other cells (Fig. 5L, 5O). These results suggest that the MMC meiosis either did not occur or was abnormal.
Fig. 5.
Stages of female gametophyte development in the wild type and flp-7 mutant. Ovule stages were determined from the development of the sporophyte using the nomenclature of Schneitz et al. (1995); see Supplementary Table S4 at JXB online. (A–O) DIC micrographs of ovules at different stages. (A, B, C, G, H, I) L. er. (D, E, F, J, K, L, M, N, O) flp-7. (A, D) Stage 2-III (pre-meiotic) with large MMCs near the tip of the nucellus (arrows). (B) Stage 2-V with tetrad of four megaspores (primary megaspore indicated by a dashed circle) in L. er. (C) At stage 3-I, the three non-functional megaspores begin to degenerate in L. er. (E) Varying numbers of cells are seen in flp-7 ovules. Here, three cells can be seen (two toward the apex, arrowheads; and a larger cell more basally, asterisk). (F) The abnormal cells formed in flp-7 ovules do not appear to be degenerating. (G) At stage 3-II, the first mitotic division of the megaspore to give rise to a two-nuclei (circled by dashed lines) female gametophyte in L. er. (J, M, N) No corresponding nuclear division can be seen in abnormal stage 3-II flp-7ovules; instead, a large cell remains with either zero (M), two (J), or three (N) other cells attached. (H) At stage 3-IV, a female gametophyte with four nuclei (dashed circles) is seen in L. er. In addition, the other cells of the nucellus have begun degenerating. (K) No such structures were seen in flp-7 ovules; instead the abnormal cells remain. (I) By stage 3-VI, the embryo sac has begun differentiating and cellularizing in L. er. (L, O) In flp-7 a single large cell is associated with a variable number of other cells (three in L, two in O). Asterisks indicate MMC-like/functional megaspore-like cells seen in flp-7 ovules. Arrowheads indicate megaspores in L. er and cells associated with the MMC-like/functional megaspore-like cell in flp-7
Table 3.
Phenotypic analysis of MMC division products during ovule development in the wild type and mutants
| Ovule stagea | Genotype | One cellb | Two cells | Four cells | >4 cells | Total ovules observed |
|---|---|---|---|---|---|---|
| 3-I | L. er | 57% (99) | 31% (54) | 10% (17) | 2% (3) | 173 |
| flp-7 | 68% (186) | 15% (40) | 17% (47) | NOc | 273 | |
| 3-II | L. er | 3% (5) | 49% (77) | 40% (63) | 8% (13) | 158 |
| flp-7 | 39% (18) | 41% (19) | 13% (6) | 7% (3) | 46 | |
| 3-IV | L. er | 6% (3) | NO | 55% (27) | 39% (19) | 49 |
| flp-7 | 54% (41) | NO | 21% (16) | 25% (19) | 76 | |
| 3-VI | L. er | 6% (12) | 1% (2) | 67% (137) | 26% (54) | 205 |
| flp-7 | 58% (82) | NO | 28% (40) | 13% (19) | 141 |
a Stages according to Schneitz et al. (1995).
b The number in parentheses indicates the number of ovules observed.
c NO, none observed.
The lowered expressivity of embryo sac defects in flp-1; myb88 mutants made a detailed developmental study of embryo sac defects difficult. However, examination of mature embryo sacs of this genotype reveals that many (22%) contained abnormal structures that do resemble embryo sacs but rather contain a large cell resembling the MMC in position and size (Fig. 3D). This suggests that, similar to flp-7, flp-1; myb88 MMCs undergo abnormal meiosis.
The Expressivity of Female Gametophytic Defects Caused by Loss of FLP and/or MYB88 is Influenced by Genetic Background
Although a number of flp alleles and allelic combinations with myb88 show significant reductions in fertility from their wild types, there is variability in the expressivity of the phenotype, with seed set varying from 6% to 72% (Table 1), although the penetrance is complete (data not shown). Examination of the pattern of severity reveals that flp alleles in the L. er ecotype background exhibit more phenotypic severity than those in Col-0 (Table 1). In addition, crossing flp-7 into the Col-0 background reduces the severity of the fertility defect (Table 1), supporting the hypothesis that either the L. er background harbours enhancers of the flp phenotype or Col-0 has suppressors of the flp phenotype, or both.
Many genetic differences exist between L. er and Col-0, including both single nucleotide polymorphisms and larger scale indels (Schmid et al., 2003; Ziolkowski et al., 2009). A prominent difference is the presence of a mutated ERECTA (ER) locus in L. er. ER and its family members, ERECTA LIKE1 (ERL1) and ERECTA LIKE2 (ERL2), encode leucine-rich repeat (LRR) domain-containing receptor-like kinases. They function in the stomatal patterning along with the TOO MANY MOUTHS (TMM) LRR receptor-like protein (Nadeau and Sack, 2002; Shpak et al., 2005). ER and its family members act upstream of FLP and MYB88. ER is the most important gene in its family and masks the functions of its paralogues. The ER family members also function in female reproductive development (Pillitteri et al., 2007). In er-105; erl1-2; erl2-1/+ ovules, cell proliferation in integuments is reduced, gametophytes abort, and cyclin A-encoding genes are misregulated. It was hypothesized that the mutant er locus in L. er influences the expressivity of flp alleles and may sensitize the ovule to loss of FLP function. To test these hypotheses, the er allele was complemented in the flp-7 mutant background by transforming L. er and flp-7 plants with an ER genomic rescue construct (a kind gift of Dr Keiko Torii). Complementing the defect in er in the flp-7 background increased the seed set from 6% to 31% (Table 1), suggesting that this mutation accounts for some of the increased expressivity of female gametophyte defects in flp-7 compared with flp-1; myb88. Restoration of wild-type ER activity also reduced the number of ovules per silique (Table 1) and reduced the severity of the stomatal defect (data not shown). However, ER complementation did not restore fertility to wild-type levels, suggesting that other genetic differences account for the remaining difference in expressivity between L. er and Col-0.
Discussion
In all sexually reproducing organisms, haploid gametes are formed after meiosis, either directly as in animals or indirectly after mitotic divisions as in plants. Therefore, meiosis is the key step leading to gametogenesis (van Werven and Amon, 2011). In angiosperms, although there is sexual dimorphism between the MMC and the male microspore mother cell, most mutations affecting meiosis affect both sexes. However, not all genes implicated in meiosis affect both male and female reproductive development (Ma, 2006). Broadly speaking, genes that have roles in megasporogenesis and megagametogenesis can be divided into sporophytic-acting and gametophytic-acting groups. Sporophytically acting genes with specific roles in regulating gametogenesis exhibit defects in spore and/or gamete formation but not in sporophytic parts of the ovule (Schneitz et al., 1997).
In this work, it was shown that a variety of loss-of-function, although not RNA null, flp alleles alone and in combination with myb88 exhibit reduced seed set due to reduced female fertility (Fig. 2, Tables 1, 2). A defect in female but not male fertility is consistent with the low expression of FLP and MYB88 in stamens and pollen (Zimmermann et al., 2004, 2005; Lai et al., 2005; this study). The female infertility of flp and flp; myb88 mutants is not due to major defects in overall ovule morphology (Fig. 3B, 3D). In addition, mucilage is produced normally by mutant seed coats (Supplementary Fig. S1 at JXB online), suggesting that seed coat differentiation is relatively normal. However, examination of mature ovules revealed that abnormal flp-7 and flp-1; myb88 ovules did not contain embryo sacs; rather, they contained cellular structures with a larger single cell located close to the micropylar end of the ovule (Fig. 3B, 3D). These structures do not contain differentiated synergids, egg cells, or central cells (Fig. 4; Supplementary Fig. S3). Examination of earlier stages in ovule development revealed that these abnormal ovules arise due to a partially expressed defect in entry into meiosis by the MMC and/or abnormal meiosis and lack of megaspore differentiation (Fig. 5). Several lines of evidence support this interpretation. First, mature mutant ovules harboured a single large cell that resembles an MMC or a megaspore in position and size and which was accompanied by variable numbers of cells that appeared to arise from the same division (Figs 3, 5). Secondly, the retention of proximal nucellar cells in abnormal flp-7 and flp-1; myb88 ovules suggests that embryo sacs are absent, consistent with phenotypes seen before in other embryo sac absent mutants, including those with defects in meiosis of the MMC (Siddiqi et al., 2000). Thirdly, the smaller of the presumed division products of the MMC seen in flp mutants are positioned nearer the antipodal end of the ovule, where the non-functional megaspores are positioned in the wild type, while the large cell is nearer to the chalazal end, where the functional megaspore operates (Figs 3, 5). However, these smaller cells do not degenerate as normally the smaller megaspores would. Similar observations have been reported in Arabidopsis mutants where meiosis is incomplete or otherwise abnormal (Chen et al., 2011). Taken together, these results suggest that the paralogous FLP and MYB88 transcription factors function in the ovule to control entry into and progression through megasporogenesis, although they do not appear to function in male reproductive development, consistent with expression analysis.
FLP and MYB88 are required to limit cell divisions in the stomatal lineage; flp and flp; myb88 double mutant GMCs undergo extra divisions, resulting in stomatal clusters (Yang and Sack, 1995; Lai et al., 2005; Xie et al., 2010a). They perform this function by regulating expression of cell cycle genes (Xie et al., 2010a; Vanneste et al., 2011). FLP directly represses expression of the CDKB1;1 gene (encoding a cyclin-dependent kinase that promotes entry into mitosis) after the symmetric division of the GMC, thereby preventing further division and forming a functional two-celled stoma (Xie et al., 2010a). The A2-type cyclin CYCA2;3 can form a functional complex with CDKB1;1 (Boudolf et al., 2009) and its expression is also directly and coordinately repressed by FLP/MYB88 in young guard cells (Vanneste et al., 2011). It has been demonstrated here that a larger number of ovules are formed in flp and flp; myb88 mutants (Table 1). The molecular events that regulate development of the placenta, from which ovules are formed, is not well understood; however, an increase in the number of ovules suggests either increased or extended proliferation to create a larger placenta or more entry into organogenesis by cells of this tissue. It is possible that the placental phenotype seen in flp; myb88 mutants might also be due to lack of repression of similar cell cycle genes they repress during stomatal development. Alternatively, it has been shown that down-regulation of cytokinin signalling and metabolism takes place within the stomatal lineage and that this might be important for the transition from proliferation to differentiation (Pillitteri et al., 2011). Mutations in the CKX3 and CKX5 genes, which encode cytokinin oxidase/dehydrogenase enzymes functioning in degradation of this hormone, lead to increased proliferation in the placenta, supernumerary ovules, and increased seed set (Bartrina et al., 2011), suggesting that levels of cytokinin are important to regulate the size of this tissue. Therefore, FLP and MYB88 might also influence ovule number by regulating cytokinin signalling or homeostasis. Orthologues of FLP and MYB88 in seed crop plants could be involved in control of ovule number (and therefore yield) and would be interesting targets for modification in such crops.
The role of cell cycle genes in controlling Arabidopsis meiosis is not well understood. In mammals, type A1 cyclins have been shown to function in the meiotic cell cycle (Wolgemuth, 2011). This appears be true for Arabidopsis. CYCA1;2/TARDY ASYNCHRONOUS MEIOSIS (TAM) is required for entry into both the first and second meiotic divisions (d’Erfurth et al., 2010). Such genes may be potential targets of FLP/MYB88. Suppression of mitotic genes such as CDKB1;1 and/or CYCA2;3 by FLP/MYB88 could also be necessary to allow entry into meiosis. This would be consistent with the necessity to suppress these genes for entry into the endocycle (Boudolf et al., 2009) and is also consistent with the finding that meiotic arrest can be caused by a failure to regulate CDK activity appropriately (Bulankova, 2010). It is reasonable to hypothesize that FLP and MYB88 function to regulate expression of cell cycle genes controlling meiotic entry and progression. During both stomatal development (Lai et al., 2005; Xie et al., 2010a) and placenta development (this study), FLP and MYB88 appear to inhibit expression of genes that positively regulate cell division. Based on the defects seen in meiosis in flp mutants (Fig. 5), they would appear to promote meiotic division, but might also do this by repression of mitotic promoting factors.
Loss of FLP or FLP/MYB88 function does not lead to complete loss of female fertility. This incomplete expressivity could be due to a number of factors. The FLP alleles available are not RNA nulls (Lai et al., 2005). Therefore, it is possible that none of them is functionally null and there is residual FLP function present that is able to support meiosis in many ovules. Alternatively, other as yet unidentified genes could be partially functionally redundant with FLP/MYB88, allowing some female fertility in the flp; myb88 mutants. However, it is clear that genetic background impacts the expressivity of the loss of FLP and/or MYB88 function. Although all the flp alleles examined have reduced seed set, the flp-7 allele in the L. er ecotype is the most severe, even more than the similar flp-1 allele in Col-0 or flp-7; myb88 in which flp-7 has been introgressed into the Col-0 background (Table 1). Interestingly, the flp-7 stomatal phenotype is also stronger than that of flp-1 (Lai et al., 2005). Differences between genetic backgrounds in mutant penetrance, expressivity, and/or phenotype have been documented in Arabidopsis previously (e.g. Sedbrook et al., 2004; Sugliani et al., 2009). Many differences are present between L. er and Col-0 (Schmid et al., 2003; Ziolkowski et al., 2009), including in the ER gene. ER and its family members ERL1 and ERL2 have diverse roles coordinating cell proliferation with differentiation (Torii et al., 1996; Shpak et al., 2003, 2004, 2005; Pillitteri et al., 2007; Hord et al., 2008). ER family members act in complexes with TMM in GMCs (Lee et al., 2012) and act synergistically in enforcing stomatal patterning by interacting with the secreted peptides EPIDERMAL PATTERNING FACTORS (EPFs) (Hara et al., 2007, 2009; Hunt and Gray, 2009; Lee et al., 2012). These peptides function upstream of FLP/MYB88 in the stomatal lineage (Shpak et al., 2005). ER family members also ensure the proper growth of integuments and the progression of the mitotic cell cycle in the female gametophyte in a dosage-dependent manner (Pillitteri et al., 2007). The mutant er allele found in the L. er background might increase sensitivity to loss of FLP function. Indeed, when this mutation was complemented in the flp-7 background, ovule number was reduced, seed set was improved (Table 1), and the stomatal defects were ameliorated (data not shown). The smaller number of ovules which form when ER function is restored is expected since ER also can control ovule number per fruit (Alonso-Blanco et al., 1999). The present results imply that ER also regulates entry of the MMC into meiosis. Complementation results support the hypothesis that loss of ER function provides a sensitized background for the loss of other genes, such as FLP, that function in common pathways. However, additional genetic changes must also contribute to the differential phenotypic expressivity, since ER complemented flp-7 plants still display more severe phenotypes than similar mutations in the Col-0 background.
Supplementary Data
Supplementary data are available at JXB online.
Supplementary Figure S1. Mucilage production is intact in flp-7 and flp-1; myb88 seeds.
Supplementary Figure S2. Pollen tube growth is not inhibited in flp-7 pistils.
Supplementary Figure S3. Abnormal flp-7 ovules do not express AGL61:GFP.
Supplementary Table S1. Primer combinations used for PCR genotyping.
Supplementary Table S2. Primers used in this study.
Supplementary Table S3. Female gametophyte markers used in this study.
Supplementary Table S4. Stages of floral, ovule, and gametophyte development.
Supplementary Material
Acknowledgements
The authors thank Dr Zidian Xie for advice and sharing of data before publication, Qin Lei for technical assistance, and two anonymous reviewers, members of the Lamb lab, and Dr Iris Meier (Ohio State University) for discussions. Dr Steffen Vanneste (Ghent University), Dr Ueli Grossniklaus (University of Zurich), and Dr Gary Drews (University of Utah) provided seeds, and Dr Keiko Torii provided the ERp::ER plasmid used for complementation experiments. This work was supported in part by a grant from the National Science Foundation to RSL and funds from The Ohio State University.
References
- Alonso-Blanco C, Blankestijn-de Vries H, Hanhart CJ, Koornneef M. 1999. Natural allelic variation at seed size loci in relation to other life history traits of Arabidopsis thaliana Proceedings of the National Academy of Sciences USA 96 4710–4717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez J, Smyth DR. 1999. CRABS CLAW and SPATULA, two Arabidopsis genes that control carpel development in parallel with AGAMOUS Development 126 2377–2386 [DOI] [PubMed] [Google Scholar]
- Bartrina I, Otto E, Strnad M, Werner T, Schmulling T. 2011. Cytokinin regulates the activity of reproductive meristems, flower organ size, ovule formation, and thus seed yield in Arabidopsis thaliana. The Plant Cell 23 69–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger F, Twell D. 2011. Germline specification and function in plants Annual Review of Plant Biology 62 461–484 [DOI] [PubMed] [Google Scholar]
- Boudolf V, , Barroco R, Engler Jde A, Verkest A, Beeckman T, Naudts M, Inze D, De Veylder L. 2004. B1-type cyclin-dependent kinases are essential for the formation of stomatal complexes in Arabidopsis thaliana The Plant Cell 16 945–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudolf V, Lammens T, Boruc J, et al. 2009. CDKB1;1 forms a functional complex with CYCA2;3 to suppress endocycle onset. Plant Physiology 150 1482–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulankova P R-KN, Nowack MK, Schnittger A, Riha K. 2010. Meiotic progression in Arabidopsis is governed by complex regulatory interactions between SMG7, TDM1, and the meiosis I-specific cyclin TAM. The Plant Cell 22 3791–3803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Higgens JD, Hui JTL, Li J, Franklin FCH, Berger F. 2011. Retinoblastoma protein is essential for early meiotic events in Arabidopsis. EMBO Journal 30 744–755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen CA, King EJ, Jordan JR, Drews GN. 1997. Megagametogenesis in Arabidopsis wild type and the Gf mutant Sexual Plant Reproduction 10 49–64 [Google Scholar]
- Colombo M, Masiero S, Vanzulli S, Lardelli P, Kater MM, Colombo L. 2008. AGL23, a type I MADS-box gene that controls female gametophyte and embryo development in Arabidopsis. The Plant Journal 54 1037–1048 [DOI] [PubMed] [Google Scholar]
- Debeaujon I, Leon-Kloosterziel KM, Koornneef M. 2000. Influence of the testa on seed dormancy, germination and longevity in Arabidopsis Plant Physiology 22 403–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- d’Erfurth I, Cromer L, Jolivet S, Girard C, Horlow C, Sun Y, To JP, Berchowitz LE, Copenhaver GP, Mercier R. 2010. The cyclin-A CYCA1;2/TAM is required for the meiosis I to meiosis II transition and cooperates with OSD1 for the prophase to first meiotic division transition PLoS Genetics 6, e1000989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gremski K, Ditta G, Yanofsky MF. 2007. The HECATE genes regulate female reproductive tract development in Arabidopsis thaliana. Development 134 3593–3601 [DOI] [PubMed] [Google Scholar]
- Gross-Hardt R, Kagi C, Baumann N, Moore JM, Baskar R, Gagliano WB, Jurgens G, Grossniklaus U. 2007. LACHESIS restricts gametic cell fate in the female gametophyte of Arabidopsis. PLoS Biology 5, e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Kajita R, Torii KU, Bergmann DC, Kakimoto T. 2007. The secretory peptide gene EPF1 enforces the stomatal one-cell-spacing rule. Genes and Development 21 1720–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara K, Yokoo T, Kajita R, Onishi T, Yahata S, Peterson KM, Torii KU, Kakimoto T. 2009. Epidermal cell density is autoregulated via a secretory peptide, EPIDERMAL PATTERNING FACTOR 2 in Arabidopsis leaves Plant and Cell Physiology 50 1019–1031 [DOI] [PubMed] [Google Scholar]
- Heisler MG, Atkinson A, Bylstra YH, Walsh R, Smyth DR. 2001. SPATULA, a gene that controls development of carpel margin tissues in Arabidopsis, encodes a bHLH protein. Development 128 1089–1098 [DOI] [PubMed] [Google Scholar]
- Hord CL, Sun YJ, Pillitteri LJ, Torii KU, Wang H, Zhang S, Ma H. 2008. Regulation of Arabidopsis early anther development by the mitogen-activated protein kinases, MPK3 and MPK6, and the ERECTA and related receptor-like kinases Molecular Plant 1 645–658 [DOI] [PubMed] [Google Scholar]
- Hunt L, Gray JE. 2009. The signaling peptide EPF2 controls asymmetric cell divisions during stomatal development Current Biology 19 864–869 [DOI] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. 1987. GUS fusions: β-glucuronidase as a sensitive and versatile gene fusion marker. EMBO Journal 6 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Yang SL, Xie LF, Puah CS, Zhang XQ, Yang WC, Sundaresan V, Ye D. 2005. VANGUARD1 encodes a pectin methylesterase that enhances pollen tube growth in the Arabidopsis style and transmitting tract . The Plant Cell 17 584–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai LB, Nadeau JA, Lucas J, Lee EK, Nakagawa T, Zhao L, Geisler M, Sack FD. 2005. The Arabidopsis R2R3 MYB proteins FOUR LIPS and MYB88 restrict divisions late in the stomatal cell lineage. The Plant Cell 17 2754–2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Kuroha T, Hnilova M, Khatayevich D, Kanaoka MM, McAbee JM, Sarikaya M, Tamerler C, Torii KU. 2012. Direct interaction of ligand–receptor pairs specifying stomatal patterning. Genes and Development 26 126–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma H. 2006. A molecular portrait of Arabidopsis meiosis Arabidopsis Book 4, e0095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JM, Calzada JP, Gagliano W, Grossniklaus U. 1997. Genetic characterization of hadad, a mutant disrupting female gametogenesis in Arabidopsis thaliana Cold Spring Harbor Symposia on Quantitative Biology 62 35–47 [PubMed] [Google Scholar]
- Nadeau JA, Sack FD. 2002. Control of stomatal distribution on the Arabidopsis leaf surface Science 296 1697–1700 [DOI] [PubMed] [Google Scholar]
- Neff MM, Neff JD, Chory J, Pepper AE. 1998. dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: experimental applications in Arabidopsis thaliana genetics. The Plant Journal 14 387–392 [DOI] [PubMed] [Google Scholar]
- Neff MM, Turk E, Kalishman M. 2002. Web-based primer design for single nucleotide polymorphism analysis. Trends in Genetics 18 613–615 [DOI] [PubMed] [Google Scholar]
- Pillitteri LJ, Bemis SM, Shpak ED, Torii KU. 2007. Haploinsufficiency after successive loss of signaling reveals a role for ERECTA-family genes in Arabidopsis ovule development Development 134 3099–3109 [DOI] [PubMed] [Google Scholar]
- Pillitteri LJ, Peterson KM, Horst RJ, Torii KU. 2011. Molecular profiling of stomatal meristemoids reveals new component of asymmetric cell division and commonalities among stem cell populations in Arabidopsis. The Plant Cell 23 3260–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punwani JA, Rabiger DS, Drews GN. 2007. MYB98 positively regulates a battery of synergid-expressed genes encoding filiform apparatus localized proteins The Plant Cell 19 2557–2568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo-Peiris T, Xu XM, Zhao Q, Wang HJ, Meier I. 2011. RanGAP is required for post-meiotic mitosis in female gametophyte development in Arabidopsis thaliana Journal of Experimental Botany 62 2705–2714 [DOI] [PubMed] [Google Scholar]
- Schmid KJ, Sorensen TR, Stracke R, Torjek O, Altmann T, Mitchell-Olds T, Weisshaar B. 2003. Large-scale identification and analysis of genome-wide single-nucleotide polymorphisms for mapping in Arabidopsis thaliana Genome Research 13 1250–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneitz K, Hulskamp M, Kopczak SD, Pruitt RE. 1997. Dissection of sexual organ ontogenesis: a genetic analysis of ovule development in Arabidopsis thaliana. Development 124 1367–1376 [DOI] [PubMed] [Google Scholar]
- Schneitz K, Hulskamp M, Pruitt RE. 1995. Wild type ovule development in Arabidopsis thailana: a light microscope study of cleared whole mount tissue The Plant Journal 7 731–749 [Google Scholar]
- Sedbrook JC, Ehrhardt DW, Fisher SE, Scheible WR, Somerville CR. 2004. The Arabidopsis sku6/spiral1 gene encodes a plus end-localized microtubule-interacting protein involved in directional cell expansion. The Plant Cell 16 1506–1520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak ED, Berthiaume CT, Hill EJ, Torii KU. 2004. Synergistic interaction of three ERECTA-family receptor-like kinases controls Arabidopsis organ growth and flower development by promoting cell proliferation. Development 131 1491–1501 [DOI] [PubMed] [Google Scholar]
- Shpak ED, Lakeman MB, Torii KU. 2003. Dominant-negative receptor uncovers redundancy in the Arabidopsis ERECTA leucine-rich repeat receptor-like kinase signaling pathway that regulates organ shape. The Plant Cell 15 1095–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpak ED, McAbee JM, Pillitteri LJ, Torii KU. 2005. Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science 309 290–293 [DOI] [PubMed] [Google Scholar]
- Siddiqi I, Ganesh G, Grossniklaus U, Subbiah V. 2000. The dyad gene is required for progression through female meiosis in Arabidopsis. Development 127 197–207 [DOI] [PubMed] [Google Scholar]
- Smyth DR, Bowman JL, Meyerowitz EM. 1990. Early flower developmentin Arabidopsis. The Plant Cell 2 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer PS, Holding DR, Groover A, Yordan C, Martienssen RA. 2000. The essential Mcm7 protein PROLIFERA is localized to the nucleus of dividing cells during the G(1) phase and is required maternally for early Arabidopsis development Development 127 1815–1822 [DOI] [PubMed] [Google Scholar]
- Steffen JG, Kang IH, Portereiko MF, Lloyd A, Drews GN. 2008. AGL61 interacts with AGL80 and is required for central cell development in Arabidopsis Plant Physiology 148 259–268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugliani M, Rajjou L, Clerkx EJ, Koornneef M, Soppe WJ. 2009. Natural modifiers of seed longevity in the Arabidopsis mutants abscisic acid insensitive3-5 (abi3-5) and leafy cotyledon1-3 (lec1-3). New Phytologist 184 898–908 [DOI] [PubMed] [Google Scholar]
- Teotia S, Lamb RS. 2009. The paralogous genes RADICAL-INDUCED CELL DEATH1 and SIMILAR TO RCD ONE1 have partially redundant functions during Arabidopsis development. Plant Physiology 151 180–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii KU, Mitsukawa N, Oosumi T, Matsuura Y, Yokoyama R, Whittier RF, Komeda Y. 1996. The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extra cellular leucine-rich repeats. The Plant Cell 8 735–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran LS, Nakashima K, Sakuma Y, Simpson SD, Fujita Y, Maruyama K, Fujita M, Seki M, Shinozaki K, Yamaguchi-Shinozaki K. 2004. Isolation and functional analysis of Arabidopsis stress-inducible NAC transcription factors that bind to a drought-responsive cis-element in the early responsive to dehydration stress 1 promoter. The Plant Cell 16 2481–2498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unte US, Sorensen AM, Pesaresi P, Gandikota M, Leister D, Saedler H, Huijser P. 2003. SPL8, an SBP-box gene that affects pollen sac development in Arabidopsis The Plant Cell 15 1009–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanneste S, Coppens F, Lee E, et al. 2011. Developmental regulation of CYCA2s contributes to tissue-specific proliferation in Arabidopsis EMBO Journal 30 3430–3441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Werven FJ, Amon A. 2011. Regulation of entry into gametogenesis Philosophical Transactions of the Royal Society B: Biological Sciences 366 3521–3531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolgemuth DJ. 2011. Function of the A-type cyclins during gametogenesis and early embryogenesis. Results and Problems in Cell Differentiation 53 391–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Lee E, Lucas JR, Morohashi K, Li D, Murray JA, Sack FD, Grotewold E. 2010a. Regulation of cell proliferation in the stomatal lineage by the Arabidopsis MYB FOUR LIPS via direct targeting of core cell cycle genes. The Plant Cell 22 2306–2321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Li D, Wang L, Sack FD, Grotewold E. 2010b. Role of the stomatal development regulators FLP/MYB88 in abiotic stress responses. The Plant Journal 64 731–739 [DOI] [PubMed] [Google Scholar]
- Yadegari R, Drews GN. 2004. Female gametophyte development The Plant Cell 16Suppl, S133–S141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Sack FD. 1995. The too many mouths and four lips mutations affect stomatal production in Arabidopsis The Plant Cell 7 2227–2239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hennig L, Gruissem W. 2005. Gene-expression analysis and network discovery using Genevestigator. Trends in Plant Science 10 407–409 [DOI] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W. 2004. GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox Plant Physiology 136 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziolkowski PA, Koczyk G, Galganski L, Sadowski J. 2009. Genome sequence comparison of Col and Ler lines reveals the dynamic nature of Arabidopsis chromosomes Nucleic Acids Research 37 3189–3201 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.