Abstract
The present study was carried out to investigate the role of reactive oxygen species (ROS) metabolism in symptom development and pathogenesis in Nicotiana benthamiana plants upon infection with two strains of Pepper mild mottle virus, the Italian (PMMoV-I) and the Spanish (PMMoV-S) strains. In this host, it has been shown that PMMoV-I is less virulent and plants show the capability to recover 21 d after inoculation. Analyses of oxidative stress biomarkers, ROS-scavenging enzyme activities, and antioxidant compounds were conducted in plants at different post-infection times. Only PMMoV-I stimulated a defence response through: (i) up-regulation of different superoxide dismutase isozymes; (ii) maintenance of adequate levels of three peroxiredoxins (2-Cys Prx, Prx IIC, and Prx IIF); and (iii) adjustments in the glutathione pool to maintain the total glutathione content. Moreover, there was an increase in the level of oxidized glutathione and ascorbate in the recovery phase of PMMoV-I-infected plants. The antioxidant response and the extent of oxidative stress in N. benthamiana plants correlates to: (i) the severity of the symptoms elicited by either strain of PMMoV; and (ii) the high capacity of PMMoV-I-infected plants for symptom recovery and delayed senescence, compared with PMMoV-S-infected plants.
Key words: Antioxidant defence, biotic stress, Pepper mild mottle tobamovirus, ROS, tobamovirus
Introduction
In some compatible virus–host plant interactions, oxidative stress is manifested as an increase in specific physiological parameters such as lipid peroxidation, protein oxidation, and electrolyte leakage, accumulation of hydrogen peroxide (H2O2), and an imbalance in the antioxidative systems at the subcellular level (Clarke et al., 2002; Hernández et al., 2004, 2006; Díaz-Vivancos et al., 2006). Furthermore, some authors have proposed that disturbances in the photosynthetic electron transport chain during viral pathogenesis enhance the generation of reactive oxygen species (ROS) in the chloroplast (Torres et al., 2006; Díaz-Vivancos et al., 2008).
The production of ROS is a common feature of incompatible and compatible plant–pathogen interactions (Bolwell et al., 1998, 2002; Torres et al., 2006). However, little attention has been devoted to resolving the actual role of ROS in symptom development and pathogenesis, in particular in compatible plant–virus interactions (Riedle-Bauer, 2000; Stone et al., 2000; Venisse et al., 2001). Contradictory results were reported in compatible plant–virus interactions regarding the levels of antioxidant enzymes (Riedle-Bauer, 2000; Hernández et al., 2001, 2004; Clarke et al., 2002). Clarke et al. (2002) proposed that a decline in antioxidant enzymes with a consequent increase in ROS may be necessary for the establishment of infection, replication, and spread of the virus. After a detailed study of resistant and susceptible cultivars of Prunus armeniaca inoculated with Plum pox virus (PPV), Hernández et al. (2006) suggested that ROS could activate defence genes and regulation of antioxidant enzymes could be of importance in determining susceptibility or resistance to the plant viruses. Riedle-Bauer (2000) studied oxidative stress in Cucumber mosaic virus-infected Cucumis sativus and Zucchini yellow mosaic virus-infected Cucurbita pepo plants, and concluded that virus-enhanced peroxidation via formation of ROS is involved in the development of both mosaic and yellowing symptoms in virus-infected tissues. In a review, De Gara et al. (2003) concluded that alterations in the activities of ROS-scavenging enzymes could be a key step in the activation of the phytopathogenic response.
In previous studies, it was shown that the oxygen-evolving complex (OEC) of photosystem II (PSII) was the target of the Pepper mild mottle tobamovirus (PMMoV) in the chloroplast. Nicotiana benthamiana plants infected with two strains of PMMoV—Italian (PMMoV-I) and Spanish (PMMoV-S) strains—exhibited an inhibition of PSII from 7 to 21 days post-inoculation (dpi) (Rahoutei et al., 2000). The accumulation levels of several chloroplastidic proteins involved in both the photosynthetic electron transport chain and the Benson–Calvin cycle decreased in N. benthamiana plants upon infection with PMMoV-S (Pérez-Bueno et al., 2004; Pineda et al., 2010).
The aim of this work is to gain further knowledge on the role of ROS metabolism in viral pathogenesis. Nicotiana benthamiana plants were infected with PMMoV-S and PMMoV-I. PMMoV-S is the most virulent strain, inducing more dramatic symptoms; in contrast, plants infected with PMMoV-I are able to recover from their symptoms. Analysis of oxidative stress biomarkers and quantification of ROS-scavenging capacity by different enzymes and antioxidant compounds were carried out in plants at different times post-infection. The impact of the oxidative stress in the chloroplast during the infection was also investigated by analysis of PSII protein composition. The results suggest that the antioxidant response, and the extent of oxidative stress, correlate with the differences in virulence between the two PMMoV strains analysed. Moreover, the antioxidant response elicited by PMMoV-I could be associated with the capacity for symptom recovery.
Materials and methods
Plants and treatments
Nicotiana benthamiana Gray plants were cultivated in a growth chamber at 100 μmol m–2 s–1 PAR (photosynthetically active radiation), generated by cool white fluorescent lamps, with a 16/8h light/dark photoperiod, a temperature of 23 ºC, and a relative humidity of 60–70%. Plants with 6–7 fully expanded leaves were inoculated in the three lower leaves (inoculated leaves), using 25 μl of inoculum per leaf (50 μg ml–1 PMMoV protein in 20mM sodium phosphate/biphosphate buffer, pH 7.0). Mock-inoculated plants treated with virus-free buffer were used as controls. The Italian and Spanish strains of PMMoV were isolated in Sicily (Italy) and Almería (Spain), respectively (Wetter et al., 1984; Alonso et al., 1989). Leaves were harvested at 7, 14, 21, and 28 dpi.
Visual symptoms are clearly established at 7 dpi with PMMoV-S or PMMoV-I. New leaves which developed after inoculation (S leaves) showed severe wrinkling and curling. No symptoms were observed in those leaves already developed at the time of the inoculation (AS leaves). Inoculated and AS leaves were not analysed in this study. At 14 dpi, stunting of the plants was evident, and symptoms were more severe in PMMoV-S-infected plants. Moreover, PMMoV-I-infected plants 21 dpi developed new non-curly leaves similar to corresponding leaves in control plants, and grew in height, during the so-called recovery phase of the infection (Pineda et al., 2008).
Enzymatic assays
All enzyme extractions were performed at 4 °C. Leaves (1g fresh weight) were homogenized in 2ml of ice-cold medium containing 50mM potassium phosphate/biphosphate pH 7.8, 0.1mM EDTA, 5mM cysteine, 1% (w/v) polyvinylpolypyrrolidone (PVPP), 0.1mM phenylmethylsulphonyl fluoride (PMSF), and 0.2% (v/v) Triton X-100. For the ascorbate peroxidase (APX) activity assay, 20mM sodium ascorbate was added to the extraction buffer. The extracts were filtered through two layers of nylon cloth and centrifuged at 8000 g for 20min at 4 °C. The supernatant was then filtered through Sephadex G-50M PD10 columns (Amersham Pharmacia Biotech., Wien, Austria) equilibrated with the extraction buffer.
Catalase (CAT; EC 1.11.1.6) activity was determined spectrometrically by following the dismutation of H2O2 at 240nm for 1min, as described by Aebi (1984). Activities of enzymes in the ascorbate–glutathione (ASC–GSH) cycle were determined as described by Jiménez et al. (1998).
Isoenzymes of superoxide dismutase (SOD; EC 1.15.1.1; Cu/Zn-SOD, Mn-SOD, and Fe-SOD) were separated by PAGE in 10% (w/v) polyacrylamide gels. SOD activity bands were detected in gels by photochemical nitroblue tetrazolium (NBT) staining according to Beauchamp and Fridovich (1971), loading 100 μg of protein in each well. To identify Cu/Zn-SOD, Mn-SOD, and Fe-SOD isoenzymes, their activities were specifically inhibited by using 2mM KCN and 5mM H2O2 prior to staining of gels. KCN inhibits Cu/Zn-SODs, while H2O2 inactivates both Cu/Zn-SODs and Fe-SODs. The isozyme activities were quantified on an image analyser (GeneTools; Syngene, Cambridge, UK).
Determination of H2O2 and oxidative stress parameters
The H2O2 concentration in the leaf was determined immediately after isolation using 4-aminoantipyrine and phenol as donor substrates (Frew et al., 1983).
The extent of lipid peroxidation in leaves was estimated by quantification of thiobarbituric acid-reactive substances (TBARS) according to Cakmak and Horst (1991). Protein oxidation, quantified as carbonylated protein (CO-protein) content, was measured by reaction with 2,4-dinitrophenylhydrazine, as described by Levine et al. (1994) and modified by Prasad (1996).
Lipoxygenase (LOX; 1.13.11.12) activity was assayed by linoleic acid reaction, following the formation of a diene by LOX-catalysed hydroxyperoxidation according to Suurmejier et al. (1998).
Ascorbate and glutathione determination
For the extraction of total ascorbate and total glutathione, leaves were homogenized in 5% (w/v) metaphosphoric acid and 10% (w/v) perchloric acid in a 1mM β-phenanthroline disulphonic acid solution. The resulting acid extract was incubated for 30min at 4 °C in the dark and centrifuged at 12 000 g for 10min (Jiménez et al., 1997). ASC and DHA (dehydroascorbate) levels in the supernatant were determined by high-performance liquid chromatography (HPLC), as described by Castillo and Greppin (1988). GSH and GSSG (reduced and oxidized glutathione, respectively) derivatization of the supernatant was carried out as previously described (Fariss and Reed, 1987), and HPLC analysis was conducted as described in Asensi et al. (1994).
Protein extraction, SDS–PAGE, and western blotting
Frozen leaves from different treatments were ground in liquid nitrogen. Proteins were extracted from 50mg of leaf tissue ground in liquid N2 by adding 200 μl of extraction buffer [100mM Tris–HCl pH 6.8, 5mM PMSF, 4% SDS, 30% glycerol, 200mM dithiothreitol (DTT)].
Gel electrophoresis was performed on a 15% (w/v) polyacrylamide gel with a 6% (w/v) stacking gel, loading 30 μg of protein in each well. Western blotting was carried out according to Berzal-Herranz et al. (1995). For protein immunodetection, rabbit antisera against the OEC proteins, light-harvesting complex of PSII (LHCII; a kind gift from Dr P. Böger. University of Konstanz, Germany), D1 (Agrisera, Sweden), and peroxiredoxins (Prxs) (anti 2-Cys Prx, Prx IIC, and Prx IIF were gifts from Dr Lázaro Paniagua) were used. The antigen–antibody complex was detected with goat anti-rabbit alkaline phosphatase–IgG (Sigma Aldrich) using NBT/5-bromo-4-chloro-3-indolyl phosphate (NBT/BCIP, Roche) as substrate. Protein was determined with Bio-Rad Protein Assay (Bio-Rad, USA). The western blot filters were photographed in a Chemi Doc XRS (Bio-Rad, USA), and quantification of the bands was carried out by Photoshop CS5 (Adobe).
Statistical analysis
Values presented are means ±SD of at least three replicates. When analysis of variance (ANOVA) showed significant treatment effects, Duncan’s multiple range test was applied to compare the means at P < 0.05 using SPSS software.
Results
Changes in PSII protein pattern due to viral infection
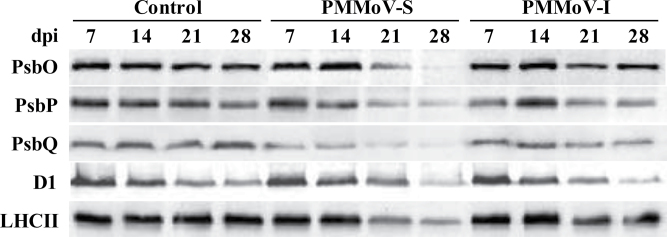
Figure 1 shows representative results of the immunodetection of the most significant PSII subunits during pathogenesis. Levels of the three OEC proteins (PsbO, PsbP, and PsbQ) were reduced in PMMoV-S-infected plants to different extents relative to the control. At 21 dpi the levels of PsbO and PsbP were reduced to 40% of control values and those of PsbQ to 20%. The accumulation of these three proteins decreased dramatically at 28 dpi. In PMMoV-I-infected plants, however, the OEC protein levels showed a smaller decrease at 21 dpi, down to 70–60% of the control values for PsbO and PsbP, and to 80% in the case of PsbQ. Interestingly, in the recovery phase, the levels of PsbO and PsbP were comparable with those in control plants.
Fig. 1.
Western blots of PsbO, PsbP, PsbQ, D1, and LHCII proteins in extracts from control and symptomatic leaves from PMMoV-infected N. benthamiana plants carried out at different dpi.
The accumulation levels of the D1 protein decreased to 50% of control levels in plants infected by both viral strains at the end of the infection period. This was accompanied by diminished levels of LHCII, to 25% and 75% of the controls in PMMoV-S- and PMMoV-I-infected plants, respectively. In the recovery phase (28 dpi) of PMMoV-I-infected plants, the content on most of the PSII subunits analysed was similar or even higher than that found at 21 dpi. This contrasts with the further decrease found at 28 dpi in PMMoV-S-infected plants.
Oxidative stress biomarkers
The levels of lipid peroxidation, protein oxidation, and ROS accumulation are oxidative stress parameters commonly used as biomarkers to assess the extent of cell damage produced under different stress conditions.
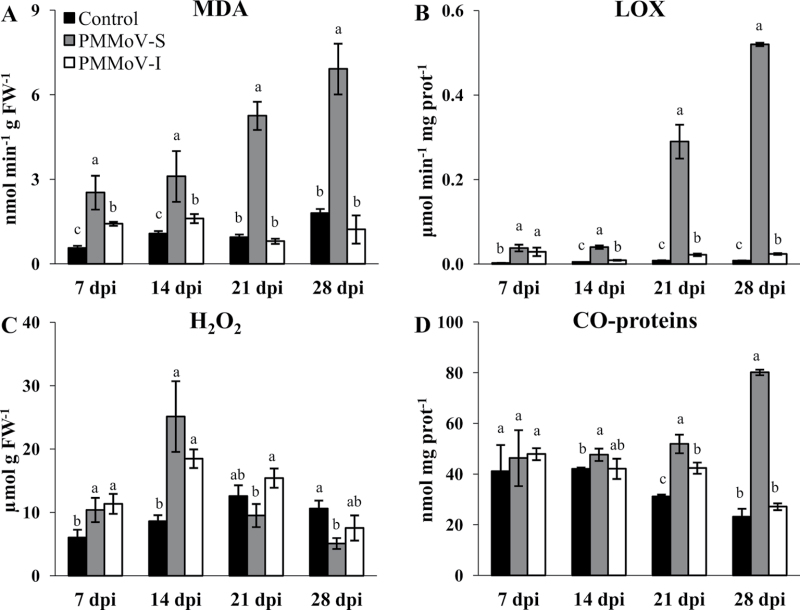
Malondialdehyde (MDA) is a breakdown product of peroxidation of membrane lipids. Already at 7 dpi the level of MDA increased 3-fold in PMMoV-S-infected plants compared with control values and continued increasing throughout the infection. In plants infected with PMMoV-I, the least virulent strain, the increase in the level of MDA accumulation was lower, ~1.5-fold of the control, and was apparent only at 7 and 14 dpi (Fig. 2A); between 21 and 28 dpi, no significant differences were found with respect to the control.
Fig. 2.
Changes in MDA content (A), lipoxygenase activity (B), H2O2 (C), and carbonylated proteins (D) in control and symptomatic leaves from PMMoV-infected plants as a function of dpi. Different letters in the same block indicate significant differences between means (P < 0.05) according to Duncan’s test.
The LOX activity increased greatly during the infection with PMMoV-S, especially towards the end of the period analysed, reaching a value ~30 times larger than that of the control. In contrast, this activity only showed a small increase (2- to 3-fold) in PMMoV-I-infected plants at 21 and 28 dpi (Fig. 2B).
A remarkably high content of H2O2 was observed at 7 and 14 dpi in leaves of PMMoV-S- and PMMoV-I-infected plants relative to the control levels (Fig. 2C). During the recovery phase of plants inoculated with the PMMoV-I strain, the values were comparable with those of the control, whereas leaves of PMMoV-S-inoculated plants showed lower levels at the same number of dpi. These results correlated with the data obtained by 3,3’-diaminobenzidine (DAB) staining of leaf tissue (data not shown).
ROS may cause irreversible oxidation of proteins by carbonylation, resulting in structural and/or functional alterations. In PMMoV-S-infected plants, protein oxidation increases by 60% at 21 dpi, compared with the control, whereas in PMMoV-I-infected plants the increase is only 40%. At 28 dpi, the accumulation of CO-proteins was very high (3- to 4-fold) in plants infected with the PMMoV-S strain; however, in PMMoV-I-infected plants protein oxidation decreased to control levels during the recovery phase (Fig. 2D).
ROS-scavenging capacity
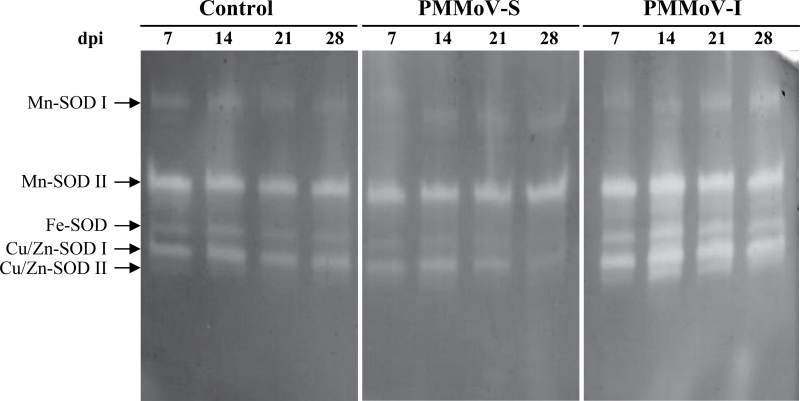
To investigate the role of ROS-scavenging metabolism during pathogenesis in N. benthamiana infected with PMMoV, the activities of several antioxidant enzymes were determined. SOD activities were analysed on non-denaturing electrophoresis gels (Fig. 3). Five isozymes were detected: two Mn-SODs (named Mn-SOD I and Mn-SOD II, in order of increasing mobility), one Fe-SOD, and two Cu/Zn-SODs (Cu/Zn-SOD I and II, in order of increasing mobility). The most remarkable change in Mn-SODs was found in PMMoV-I-infected plants, with the enhancement of isoform II activity throughout the infection, reaching 2-fold control values at 28 dpi; the activity of isoform I increased by 1.2-fold only during the last infection steps (21–28 dpi). Fe-SOD and Cu/Zn-SOD I activities decreased throughout the PMMoV-S infection, reaching around 70% and 50%, respectively, of control levels in the last infection steps. In contrast, PMMoV-I induced an increase in Fe-SOD and Cu/Zn-SOD I activities by 2- and 1.5-fold, respectively. Cu/Zn-SOD II activity increased drastically (4- to 6-fold) throughout the infection with PMMoV-I.
Fig. 3.
Separation of SOD isoforms by non-denaturating gel electrophoresis in samples from control and symptomatic leaves from PMMoV-infected N. benthamiana plants at different dpi.
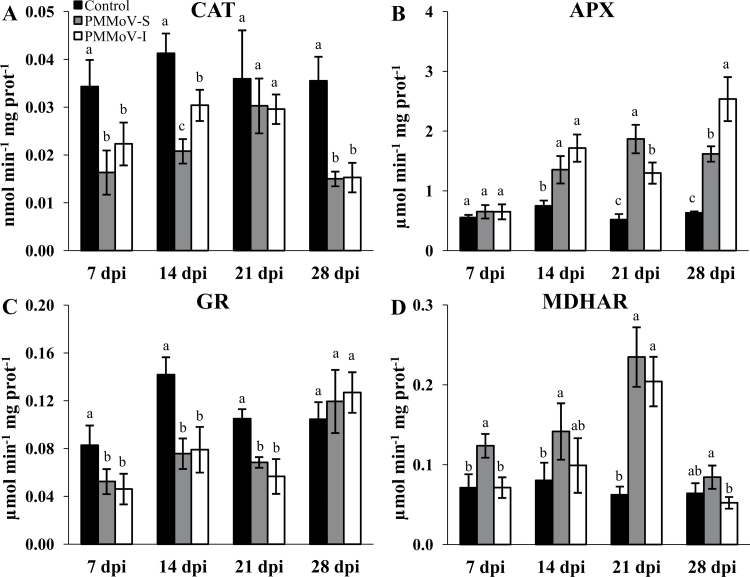
The activity of CAT was found to be lower in the infected plants compared with controls (Fig. 4A). In contrast, the APX activities increased from 14 dpi onwards in plants inoculated with both virus strains (Fig. 4B), the increase being highest (~4-fold) in the case of PMMoV-I-infected plants at 28 dpi. Glutathione reductase (GR) activities were lower in plants infected with both virus strains (Fig. 4C) but increased back to control levels at 28 dpi. Monodehydroascorbate reductase (MDHAR) activity (Fig. 4D) showed an increase in PMMoV-S-infected plants from 7 dpi until 21 dpi, and then decreased to reach values similar to control plants at 28 dpi. In PMMoV-I-infected plants, a significant increase in this enzyme activity was only observed at 21 dpi.
Fig. 4.
Changes in enzyme activities of CAT (A), APX (B), GR (C), and MDHAR (D) in control and symptomatic leaves from PMMoV-infected N. benthamiana plants as a function of dpi. Different letters in the same block indicate significant differences between means (P < 0.05) according to Duncan’s test.
Prxs, the most recently identified group of H2O2-decomposing antioxidant enzymes, were also investigated during pathogenesis. Representative data obtained by immunodetection of Prxs (2-Cys Prx, Prx IIC, and Prx IIF) are shown in Fig. 5. The infected plants contained less plastid 2-Cys Prx towards the last stages of the infection: 70% of control values at 21 dpi with both viral strains, and 60% and 40% of control values with strains PMMoV-S and PMMoV-I, respectively, at 28 dpi (Fig. 5). The levels of the cytosolic Prx IIC were negligible in the last infection steps with PMMoV-S. In PMMoV-I-infected plants, however, Prx IIC decreased until 21 dpi but increased up to 1.5-fold of the control values in the recovery phase (28 dpi). The accumulation levels of the mitochondrial Prx IIF decreased to 75% of the control at 21 dpi and 40% at 28 dpi during the infection with the PMMoV-S strain, whereas it increased by 1.5-fold at 28 dpi with the PMMoV-I strain.
Fig. 5.
Western blots of Prx proteins in extracts from control and symptomatic leaves from PMMoV-infected N. benthamiana plants carried out at different dpi.
Ascorbate and glutathione content
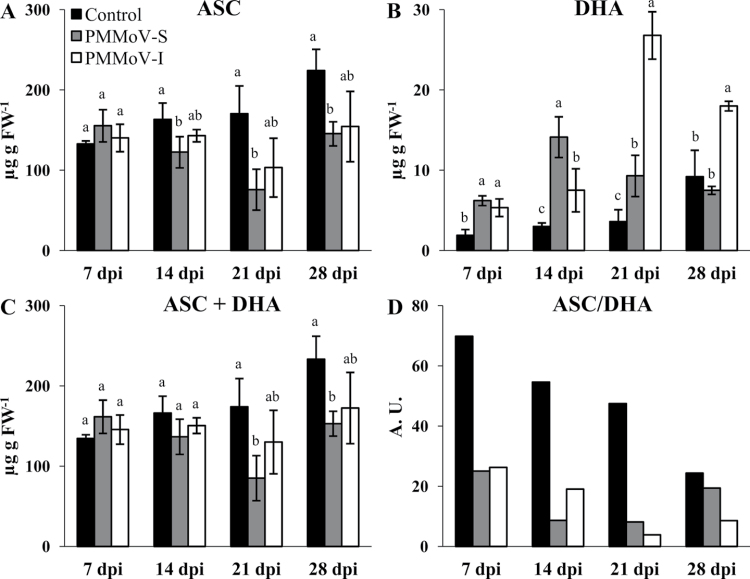
A decrease in ASC content was observed only in plants infected with PMMoV-S from 14 dpi (Fig. 6A). However, DHA increased from 7 dpi onwards (Fig. 6B): PMMoV-S-infected plants showed a maximum increase in DHA content, ~4-fold of the control values, at 14 dpi. In PMMoV-I-infected plants, the maximum DHA accumulation, by 7-fold of control values, was reached at 21 dpi. At 28 dpi it remained high in PMMoV-I-infected plants, whereas PMMoV-S-infected plants showed similar values to those of control plants. In consequence, the size of the ascorbate pool (ASC+DHA) was only decreased in PMMoV-S-inoculated plants towards the end of the infection (21 and 28 dpi) and no significant effect was found at any time point in those plants inoculated with the PMMoV-I strain (Fig. 6C). Moreover, the ascorbate pool was more oxidized in plants infected by both viral strains although at 28 dpi PMMoV-S inoculated plants showed values similar to those in the control.
Fig. 6.
Quantification of reduced (ASC) and oxidized (DHA) ascorbate (A, B) in control and symptomatic leaves from PMMoV-infected N. benthamiana plants at different dpi. (C, D) Ascorbate pool size and redox state. Different letters in the same block indicate significant differences between means (P < 0.05) according to Duncan’s test.
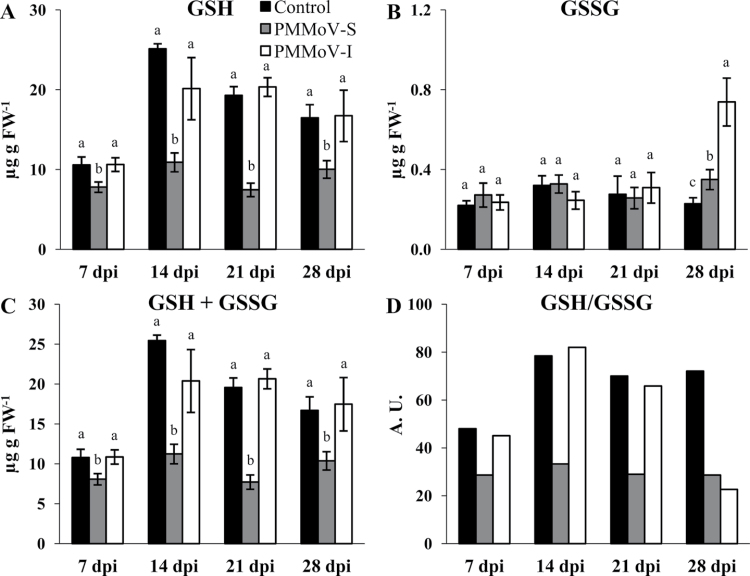
Significant changes in GSH content were observed only in PMMoV-S-infected plants (Fig. 7A). On the other hand, the accumulation level of GSSG was only affected at 28 dpi, increasing slightly in the case of PMMoV-S and drastically in the case of PMMoV-I (Fig. 7B). However, the glutathione pool size was only decreased in plants inoculated with the PMMoV-S strain throughout the infection, and no significant effect was observed in the case of the PMMoV-I strain (Fig. 7C). Interestingly, in PMMoV-S-inoculated plants the glutathione pool is more oxidized at any analysed time point relative to the control. In contrast, the redox state of the glutathione pool was unaffected in plants inoculated with PMMoV-I until 21 dpi and was more oxidized in the recovery phase (Fig. 7D) due to an increase in the levels of GSSG (Fig. 7B).
Fig. 7.
Quantification of reduced (GSH) and oxidized (GSSG) glutathione (A, B) in control and symptomatic leaves from PMMoV-infected N. benthamiana plants at different dpi. (C, D) Glutathione pool size and redox state. Different letters in the same block indicate significant differences between means (P < 0.05) according to Duncan’s test.
Discussion
The decrease in PSII electron transport efficiency during PMMoV infection reported by Rahoutei et al. (2000) could enhance the production of ROS, such as singlet oxygen (1O2; Foyer and Harbison, 1994). ROS can oxidize chloroplastidic proteins, especially PSII subunits such as D1 (particularly sensitive to oxidants), and inhibit their de novo synthesis, thus inhibiting partial reactions of photosynthesis and eventually leading to further photoinhibition (Takahashi and Murata, 2008). A decrease in the contents of PSII proteins was observed in infected plants (Fig. 1). The extent of the decrease in D1 and OEC proteins during the infection with PMMoV-I, relative to the effects of PMMoV-S, was consistent with the lower virulence of the PMMoV-I strain.
In N. benthamiana plants inoculated with PMMoV-S and PMMoV-I, there is an increase in the levels of H2O2 (Fig. 2C). Similar effects were reported during infection of Pisum sativum with Plum pox virus (Díaz-Vivancos et al., 2008). Stomatal closure and limitation of CO2 uptake during PMMoV pathogenesis (Chaerle et al., 2006) might drive a photorespiratory burst of O2 – and H2O2 that could be considered as the first line of defence against the pathogen, and could also be involved in oxidative modifications (Malolepsza and Rózalska 2005; Torres et al., 2006; Shetty et al., 2007). Histochemical H2O2 staining (data not shown) confirmed that the accumulation of H2O2 is an early event during pathogenesis, being evident even before the symptoms appeared (7 dpi).
Membrane degradation related to lipid peroxidation and protein oxidation is a marker of senescence (Elstner, 1990; Jiménez et al., 1998; Vanacker et al., 2006). The accumulation levels of LOX mRNA as well as an increase in LOX activity have been reported for several plant–pathogen systems (Koch et al., 1992; Melan et al., 1993; Gullner et al., 2010). In the present work, the dramatic increase in LOX activity during the last stages of PMMoV-S infection may account for the enhanced lipid oxidation. The remarkable rise in oxidation of lipids and proteins in PMMoV-S-infected plants and the substantial increase in LOX activity, especially at the last time points analysed (Fig. 2), together with previous results on lipid peroxidation (Rahoutei et al., 1999; Sajnani et al., 2007), inhibition of PSII (Rahoutei et al., 2000), and the ultrastructure of chloroplasts from PMMoV-infected leaves (Pérez-Bueno et al., 2006), are consistent with an accelerated senescence in PMMoV-S-infected plants. In contrast, PMMoV-I-infected plants exhibited slight changes in LOX activity as well as in lipid (7 and 14 dpi) and protein (21 dpi) oxidation. During the recovery phase in PMMoV-I-inoculated plants, the biomarkers of oxidative stress analysed showed values comparable with those of the control (Fig. 2).
Although the primary oxidative burst following pathogen recognition occurs in the apoplast (De Gara et al., 2003; Díaz-Vivancos et al., 2006; Torres et al., 2006), ROS produced in other cell compartments may also play a role in defence. Uncoupling or inhibition of the photosynthetic machinery in the chloroplasts and photorespiration associated with chloroplasts, peroxisomes, and mitochondria can lead to high ROS levels (Torres et al., 2006). Plants infected with PMMoV-I showed a higher capacity to eliminate O2 – than those infected with PMMoV-S, due to higher activities of all SOD isoenzymes, particularly Cu/Zn-SOD II; in contrast, in PMMoV-S-infected plants, all isoenzymes (except Cu/Zn-SOD II) show decreased activities between 7 and 21 dpi. The higher MDA and CO-protein levels measured in PMMoV-S-infected plants could be due to a higher accumulation of O2 – associated with the decreased SOD activities in different cell compartments. These data are consistent with previous reports suggesting that SODs played an important role in regulating ROS levels in different cell compartments during senescence and viral pathogenesis (del Río et al., 1998; Riedle-Bauer, 2000; Hernández et al., 2001).
The APX activity is thought to play the most essential role in ROS scavenging. At 14 dpi, the H2O2-scavenging capacities of APX and CAT seemed to be overwhelmed, with a consequent increase in foliar H2O2 (Fig. 2C). At 21 and 28 dpi, infected plants showed a decrease in H2O2 content, with respect to the values at 14 dpi, that could be related to an increase in APX activity (Fig. 4B). The total APX activity analysed here was the sum of contributions by APX isozymes located in the chloroplast (stroma and bound to thylakoid membranes), mitochondrion, microbodies, and cytosol, whereas CAT is found in the mitochondrion, peroxisomes, and cytosol. The expression of APXs increases under different abiotic stress conditions and is related to tolerance (reviewed by Gill and Tutej, 2010). Moreover, the decrease in CAT activity reported here was also found in other host–pathogen systems (Clarke et al., 2002; Yi et al., 2003; Hernández et al., 2006).
The combined rate of ASC production by reduction of DHA plus de novo synthesis could not match the rate of ASC oxidation in PMMoV-infected leaves; this resulted in a significant rise in DHA content and a decrease in the ASC/DHA ratio during the infection with both viral strains. The increase in MDHAR activity was described in other host–virus compatible interactions (Hernández et al., 2006). ASC has been linked to leaf senescence (Jiménez et al., 1998; Foyer, 2004; Vanacker et al., 2006).
Differences between the infections with the two viral strains were most striking in the glutathione pool and the GSH/GSSG ratio. In contrast to PMMoV-S-infected plants, PMMoV-I-infected plants maintained their total glutathione content throughout the infection and the GSH/GSSG ratio at values similar to the controls until 21 dpi; at 28 dpi, GSSG increased dramatically. Modulation of cellular glutathione content has been described to transmit information through diverse signalling mechanisms (Gómez et al., 2004); glutathione has been implicated in a mechanism to protect proteins from oxidative damage and regulation of defence-related genes (Mou et al., 2003; Pavet et al., 2005). Artificial elevation of cellular GSH and activation of GSH-related enzymes can markedly suppress necrotic disease symptoms and in some cases also virus multiplication (Gullner et al., 1999).
In addition to CAT and APX, Prxs can also act as H2O2-decomposing enzymes in different cell organelles. Prxs reduce alkyl hydroperoxides and peroxynitrite and work as redox sensor proteins (Barranco-Medina et al., 2008). The 2-Cys Prx is thought to play a key role in the antioxidant defence of the chloroplast (Dietz et al., 2006; Kim et al., 2009). Thus, the lower levels of the chloroplastidic 2-Cys Prx during the infection with PMMoV-S could lead to subsequent photoinhibition in the chloroplast. On the other hand, the decrease in the mitochondrial Prx IIF content, found only after 21 and 28 dpi of inoculation with PMMoV-S, may influence the levels of H2O2 in this organelle.
In contrast to PMMoV-S, the stable levels of Prx IIF during the infection with PMMoV-I and the observed increase in the cytosolic Prx IIC during the recovery phase could contribute to the delay in the senescence process in the PMMoV-I-infected plants. An up-regulation of Prx IIC protein has been reported under abiotic stress such as high salinity (Horling et al., 2003), whereas Prx IIF expression could be rather stable upon abiotic stress conditions (Gama et al., 2007). Three Prx proteins in poplar (Prx IIC, Prx IIF, and Prx Q) were affected during compatible and incompatible interactions with two different races of the fungus Melampsora laricii populina (Rouhier et al., 2004; Gama et al., 2007): in the compatible interaction, the content of Prx IIF increased and the content of Prx IIC and Prx Q decreased, in the first hours of infection. The opposite effect was found in the incompatible interaction. Moreover, the expression of 2-Cys Prx did not change upon infection in these experimental systems. The physiological meaning of these variations is still uncertain, but it appears clear that Prxs are important players in plant stress defence and plant–pathogen interactions.
Conclusions
The extent of the oxidative stress in N. benthamiana caused by infection with PMMoV correlates to the virulence of the strain. Furthermore, the capacity of PMMoV-I-infected plants to recover from their symptoms contrasts with the early senescence of PMMoV-S-infected plants. Plants inoculated with PMMoV-I, but not with PMMoV-S, exhibited: up-regulation of all SOD isozymes; maintenance of adequate levels of chloroplastidic 2-Cys Prx, cytosolic Prx IIC, and mitochondrial Prx IIF; and adjustments in the glutathione pool to maintain the levels of total glutathione content throughout the infection. A remarkable increase in the GSSG and DHA content was noteworthy in the recovery phase. It is suggested that these combined defence responses allow PMMoV-I-infected plants to recover from the symptoms and delay senescence compared with PMMoV-S-infected plants.
Acknowledgements
This research was supported by grants from the Spanish Government (AGL2008-00214 to MB and BFU2008-00745 and SENECA 04553/GERM/06 to FS) and FEDER Funds. AH was recipient of an AECID fellowship. The authors are very grateful to Drs Isabel García-Luque and Maite Serra (Centro Investigaciones Biológicas, CSIC, Madrid) for providing the PMMoV strains and antibodies against the viral coat protein, and to Drs Peter Böger (University Kontanz, Germany) and Juan J. Lázaro (EEZ, CSIC, Granada) for the gift of antisera against LHCII and Prxs, respectively. Thanks are also due to Dr Espen Granum (EEZ, CSIC, Granada) for helpful comments and proofreading of this manuscript.
Glossary
Abbreviations:
- ASC
ascorbate
- ASC–GSH cycle
ascorbate–glutathione cycle
- APX
ascorbate peroxidase
- CAT
catalase
- CP
viral coat protein
- CO-protein
carbonylated protein
- 2-Cys Prx
2-cysteine plastid peroxiredoxin
- DAB
3,3’-diaminobenzidine
- D1
PsbA protein PSII reaction centre
- DHA
dehydroascorbate
- DHAR
dehydroascorbate reductase
- dpi
days post-inoculation
- GR
glutathione reductase
- GSH and GSSG
reduced and oxidized glutathione
- H2O2
hydrogen peroxide
- LHCII
major light-harvesting complex of PSII
- LOX
lipoxygenase
- MDA
malondialdehyde
- MDHAR
monodehydroascorbate reductase
- O2–
superoxide radical
- OEC
oxygen-evolving complex
- PAR
photosynthetically active radiation
- PMMoV-S and PMMoV-I
Spanish and Italian strains of the Pepper mild mottle virus
- Prx
peroxiredoxin
- PSII
photosystem II
- PsbO, PsbP, and PsbQ
33, 24, and 16kDa extrinsic proteins of the photosystem II oxygen-evolving complex
- ROS
reactive oxygen species
- SOD
superoxide dismutase
References
- Aebi M. 1984. Catalase in vitro Methods in Enzymology 105 121–126 [DOI] [PubMed] [Google Scholar]
- Alonso E, García-Luque I, Ávila-Rincón MJ, Wicke B, Serra MT, Díaz-Ruíz JR. 1989. A Tobamovirus causing heavy losses in protected pepper crops in Spain Journal of Phytopathology 125 67–76 [Google Scholar]
- Asensi M, Sastre J, Pallardó FV, García de la Asunción J, Estrela JM, Viña J. 1994. A high-performance liquid chromatography method for measurement of oxidized glutathione in biological samples Analytical Biochemistry 217 323–328 [DOI] [PubMed] [Google Scholar]
- Barranco-Medina S, Krell T, Bernier-Villamor L, Sevilla F, Lázaro JJ, Dietz KJ. 2008. Hexameric oligomerization of mitochondrial peroxiredoxin PrxIIF and formation of an ultrahigh affinity complex with its electron donor thioredoxin Trx-o Journal of Experimental Botany 59 3259–3269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C, Fridovich I. 1971. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels Analytical Biochemistry 44 276–287 [DOI] [PubMed] [Google Scholar]
- Berzal-Herranz A, De La Cruz A, Tenllado F, Díaz-Ruíz JR, López L, Sanz AI, Vaquero C, Serra MT, García-Luque I. 1995. The Capsicum L3 gene-mediated resistance against the tobamoviruses is elicited by the coat protein Virology 209 498–505 [DOI] [PubMed] [Google Scholar]
- Bolwell GP, Bindschedler LV, Blee KA, Butt VS, Davies DR, Gardner SL, Gerrish C, Minibayeva F. 2002. The apoplastic oxidative burst in response to biotic stress in plants: a three component system Journal of Experimental Botany 53 1367–1376 [PubMed] [Google Scholar]
- Bolwell GP, Davies DR, Gerrish C, Auh C-K, Murphy TM. 1998. Comparative biochemistry of the oxidative burst produced by rose and French bean cells reveals two distinct mechanisms Plant Physiology 116 1379–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak I, Horst WJ. 1991. Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase and peroxidase activities in root tip of soybean (Glycine max) Physiologia Plantarum 83 463–468 [Google Scholar]
- Castillo FJ, Greppin H. 1988. Extracellular ascorbic acid and enzyme activities related to ascorbic acid metabolism in Sedum album L. leaves after ozone exposure Environmental and Experimental Botany 28 231–238 [Google Scholar]
- Chaerle L, Pineda M, Romero-Aranda R, Van Der Straeten D, Barón M. 2006. Robotized thermal and chlorophyll fluorescence imaging of Pepper mild mottle virus infection in Nicotiana benthamiana Plant and Cell Physiology 47 1323–1336 [DOI] [PubMed] [Google Scholar]
- Clarke SF, Guy PL, Burritt DJ, Jameson PE. 2002. Changes in the activities of antioxidant enzymes in response to virus infection and hormone treatment Physiologia Plantarum 114 157–164 [DOI] [PubMed] [Google Scholar]
- De Gara L, De Pinto MC, Tommasi F. 2003. The antioxidant systems vis-à-vis reactive oxygen species during plant–pathogen interaction Plant Physiology and Biochemistry 41 863–870 [Google Scholar]
- del Río LA, Pastori GM, Palma JM, Sandalio LM, Sevilla F, Corpas FJ, Jiménez A, López-Huertas E, Hernández JA. 1998. The activated oxygen role of peroxysomes in senescence Plant Physiology 116 1195–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Vivancos P, Clemente-Moreno MJ, Rubio M, Olmos E, García JA, Martínez-Gómez P, Hernández JA. 2008. Alteration in the chloroplast metabolism leads to ROS accumulation in pea plants in response to plum pox virus Journal of Experimental. Botany 59 2147–2160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Vivancos P, Rubio M, Mesonero V, Periago PM, Barceló A, Martínez-Gómez P, Hernández JA. 2006. The apoplastic antioxidant system in Prunus: response to plum pox virus Journal of Experimental Botany 57 3813–3824 [DOI] [PubMed] [Google Scholar]
- Dietz KJ, Jacob S, Oelze ML, Laxa M, Tognetti V, De Miranda SMN, Baier M, Finkemeier I. 2006. The function of peroxiredoxins in plant organelle redox metabolism Journal of Experimental Botany 57 1697–1709 [DOI] [PubMed] [Google Scholar]
- Elstner EF 1990. Der Sauerstoff Mannheim:Bl-Wissenschafts-Verlag; 418–422 [Google Scholar]
- Fariss MW, Reed DJ. 1987. High-performance liquid chromatography of thiols and disulfides: dinitrophenol derivatives Methods in Enzymology 143 101–109 [DOI] [PubMed] [Google Scholar]
- Foyer CH. 2004. The role of ascorbic acid in defence networks and signalling in plants. In: Asard H, May JM, Smirnoff N, eds. Vitamin C: its functions and biochemistry in animals and plants Oxford: BIOS Scientific Publishers; 65–82 [Google Scholar]
- Foyer CH, Harbison J. 1994. Oxygen metabolism and the regulation of photosynthetic electron transport. In: Foyer CH, Mullineaux P, eds. Causes of photooxidative stresses and amelioration of defense systems in plants Boca Raton, FL: CRC Press; 1–42 [Google Scholar]
- Frew J, Jones P, Scholes G. 1983. Spectrophotometric determination of hydrogen peroxide and organic hydroperoxides at low concentrations in aqueous solution Analytica Chimica Acta 155 130–150 [Google Scholar]
- Gama F, Keech O, Eymery F, Finkemeier I, Gelhaye E, Gardestrom P, Dietz KJ, Rey P, Jacquot JP, Rouhier N. 2007. The mitochondrial type II peroxiredoxin from poplar Physiologia Plantarum 129 196–206 [Google Scholar]
- Gill SS, Tuteja N. 2010. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants Plant Physiology and Biochemistry 48 909–930 [DOI] [PubMed] [Google Scholar]
- Gómez LD, Noctor G, Knight MR, Foyer CH. 2004. Regulation of calcium signalling and gene expression by glutathione Journal of Experimental Botany 55 1851–1859 [DOI] [PubMed] [Google Scholar]
- Gullner G, Tobia I, Fodor J, Komives T. 1999. Elevation of glutathione level and activation of glutathione-related enzymes affect virus infection in tobacco Free Radical Research 31 155–161 [DOI] [PubMed] [Google Scholar]
- Gullner G, Künstler A, Király L, Pogány M, Tobia I. 2010. Up-regulated expression of lipoxygenase and divinyl ether synthase genes in pepper leaves inoculated with Tobamoviruses Physiological and Molecular Plant Pathology 74 387–393 [Google Scholar]
- Hernández JA, Diaz-Vivancos P, Rubio M, Olmos E, Ros-Barceló A, Martínez-Gómez P. 2006. Long-term PPV infection produces an oxidative stress in a susceptible apricot cultivar but not in a resistant cultivar Physiologia Plantarum 126 140–152 [Google Scholar]
- Hernández JA, Rubio M, Olmos E, Ros-Barceló A, Martínez-Gómez P. 2004. Oxidative stress induced by long-term Plum Pox virus infection in peach (Prunus persica L. cv. GF305) Physiologia Plantarum 122 486–495 [Google Scholar]
- Hernández JA, Talavera JM, Martínez-Gómez P, Dicenta F, Sevilla F. 2001. Response of antioxidative enzymes to plum pox virus in two apricot cultivars Physiologia Plantarum 111 313–321 [DOI] [PubMed] [Google Scholar]
- Horling F, Lamkemeyer P, Konig J, Finkemeier I, Kandlbinder A, Baier M, Dietz KJ. 2003. Divergent light-, ascorbate-, and oxidative stress-dependent regulation of expression of the peroxiredoxin gene family in Arabidopsis Plant Physiology 131 317–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez A, Hernández JA, del Río LA, Sevilla F. 1997. Evidence for the presence of the ascorbate–glutathione cycle in mitochondria and peroxisomes of pea leaves Plant Physiology 114 275–284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez A, Hernández JA, Pastori GM, del Río LA, Sevilla F. 1998. Role of the ascorbate–glutathione cycle of mitochondria and peroxisomes in the senescence of pea leaves Plant Physiology 118 1327–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SU, Jang HH, Lee JR, et al. 2009. Oligomerization and chaperone activity of a plant 2-Cys peroxiredoxin in response to oxidative stress Plant Science 177 227–232 [Google Scholar]
- Koch E, Meier BM, Eiben HG, Slusarenko A. 1992. A lipoxigenase from leaves of tomato (Lycopersicon esculentumn Mill.) is induced in response to plant pathogenic Pseudomonas Plant Physiology 99 571–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RL, Williams JA, Stadtman ER, Shacter E. 1994. Carbonyl assays for determination of oxidatively modified proteins Methods in Enzymology 233 346–363 [DOI] [PubMed] [Google Scholar]
- Malolepsza U, Rózalaska S. 2005. Nitric oxide and hydrogen peroxide in tomato resistance. Nitric oxide modulates hydrogen peroxide level in o-hydroxyethylorutin-induced resistance to Botrytis cinerea in tomato Plant Physiology and Biochemistry 43 623–635 [DOI] [PubMed] [Google Scholar]
- Melan MA, Dong X, Endara ME, Davis KR, Ausubel FM, Peterman TK. 1993. An Arabidopsis thaliana lipoxygenase gene can be induced by pathogens, abscisic acid, and methyl jasmonate Plant Physiology 101 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mou Z, Fan W, Dong X. 2003. Inducers of plant systemic acquired resistance regulate NPR1 function through redox changes Cell 27 935–944 [DOI] [PubMed] [Google Scholar]
- Pavet V, Olmos E, Kiddle G, Mowla S, Kumar S, Antoniw J, Alvarez ME, Foyer CH. 2005. Ascorbic acid deficiency activates cell death and disease resistance responses in arabidopsis Plant Physiology 139 1291–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Bueno ML, Rahoutei J, Sajnani C, García-Luque I, Barón M. 2004. Proteomic analysis of the oxygen-evolving complex of photosystem II under biotec stress: studies on Nicotiana benthamiana infected with tobamoviruses Proteomics 4 418–425 [DOI] [PubMed] [Google Scholar]
- Pérez-Bueno ML, Ciscato M, vande Ven M, García-Luque I, Valcke R, Barón M. 2006. Imaging viral infection. Studies on Nicotiana benthamiana plants infected with the Pepper mild mottle tobamovirus Photosynthesis Research 90 11–123 [DOI] [PubMed] [Google Scholar]
- Pineda M, Sajnani C, Barón M. 2010. Changes induced by the pepper mild mottle tobamovirus on the chloroplast proteome of Nicotiana benthamiana Photosynthesis Research 103 31–45 [DOI] [PubMed] [Google Scholar]
- Pineda M, Soukupová J, Matouš K, Barón M, Nedbal L. 2008. Conventional and combinatorial chlorophyll fluorescence imaging of tobamovirus-infected plants Photosynthetica 46 441–451 [Google Scholar]
- Prasad TK. 1996. Mechanisms of chilling-induced oxidative stress injury and tolerance in developing maize seedlings: changes in antioxidant system, oxidation of proteins and lipids, and protease activities The Plant Journal 10 1017–1026 [Google Scholar]
- Rahoutei J, Barón M, García-Luque I, Droppa M, Neményi A, Horvath G. 1999. Effect of tobamovirus infection on the thermoluminiscence characteristics of chloroplast from infected plants Zeitschrift für Naturforschung 54 634–639 [Google Scholar]
- Rahoutei J, García-Luque I, Barón M. 2000. Inhibition of photosynthesis by viral infection: effect on PSII structure and function Physiologia Plantarum 110 286–292 [Google Scholar]
- Riedle-Bauer M. 2000. Role of reactive oxygen species and antioxidant enzymes in systemic virus infections of plants Journal of Phytopathology 148 297–302 [Google Scholar]
- Rouhier N, Gelhaye E, Gualberto JM, et al. 2004. Poplar peroxiredoxin Q. A thioredoxin-linked chloroplast antioxidant functional in pathogen defense Plant Physiology 134 1027–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajnani C, Zurita JZ, Roncel M, Ortega JM, Barón M, Ducruet JM. 2007. Changes in photosynthetic metabolism induced by tobamovirus infection in Nicotiana benthamiana studied in vivo by chlorophyll thermoluminescence New Phytologist 175 120–130 [DOI] [PubMed] [Google Scholar]
- Shetty NP, Mehrabi R, Lutken H, Haldrup A, Kema GH, Collenge DP, Jorgenson HJ. 2007. Role of hydrogen peroxide during the interaction between the hemibiotrophic fungal pathogen Septoria tritici and wheat New Phytologist 174 637–637 [DOI] [PubMed] [Google Scholar]
- Stone JM, Heard JE, Asai T, Ausubel FM. 2000. Simulation of fungal-mediated cell death by Fumonisin B1 and selection of Fumonisin B1-resistant (fbr) Arabidopsis mutants The Plant Cell 12 1811–1822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suurmejier CNSP, Pérez-Gilabert M, Van Der Hijden HTWM, Veldink GA, Viegenthart JFG. 1998. Purification, product characterization and kinetic properties of soluble tomato lipoxygenase Plant Physiology and Biochemistry 36 657–663 [Google Scholar]
- Takahashi S, Murata N. 2008. How do environmental stresses accelerate photoinhibition? Trends in Plant Science 13 178–182 [DOI] [PubMed] [Google Scholar]
- Torres MA, Jones JDG, Dangl JL. 2006. Reactive oxygen species signaling in response to pathogens Plant Physiology 141 373–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanacker H, Sandalio LM, Jiménez A, et al. 2006. Roles for redox regulation in leaf senescence of pea plants grown on different sources of nitrogen nutrition Journal of Experimental Botany 57 1735–1745 [DOI] [PubMed] [Google Scholar]
- Venisse JS, Gullner G, Brisset MN. 2001. Evidence for the involvement of an oxidative stress in the initiation of infection of pear by Erwinia amylovora Plant Physiology 125 2164–2172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter C, Conti M, Altschuh D, Tabillion R, van Regenmortel MHV. 1984. Pepper mild mottle virus, a tobamovirus infecting pepper cultivars in Sicily Phytopathology 74 405–410 [Google Scholar]
- Yi S, Yu S, Choi D. 2003. Involvement of hydrogen peroxide in repression of catalase in TMV-infected resistant tobacco Molecules and Cells 15 364−–369 [PubMed] [Google Scholar]