Abstract
Universal stress protein (USP) appears to play an active role in the abiotic stress response, but their functions remain largely unknown in plants. A USP gene (SpUSP) was cloned from wild tomato (Solanum pennellii) and functionally characterized in cultivated tomato in the present study. The SpUSP transcript is abundantly accumulated in leaf stomata and its expression varied with the circadian rhythm. SpUSP was remarkably induced by dehydration, salt stress, oxidative stress, and the phytohormone abscisic acid (ABA) etc. This protein was predominantly localized in the nucleus and cell membrane. Overexpressing SpUSP increased drought tolerance of tomato in the seedling and adult stages. Under drought stress, the ABA content significantly increased in the SpUSP-overexpressing plants, which induced stomatal closure and reduced water loss, leading to the enhancement of drought tolerance. Based on the microarray data, a large number of chlorophyll a/b-binding proteins and photosystem-related genes were up-regulated in the SpUSP-overexpressing plants under drought conditions, which possibly enhanced the stomatal sensivitity to ABA and maintained the photosynthetic function. SpUSP overexpression also alleviated the oxidative damage accompanied by oxidative stress-responsive gene activation and osmolyte accumulation. Annexin (SGN-U314161) was found to interacte with SpUSP in the yeast two-hybrid method. This interaction was further confirmed by the bimolecular fluorescence complementation assay. The present study demonstrated that the annexin-interacting SpUSP plays important roles in the drought tolerance of tomato by influencing ABA-induced stomatal movement, increasing photosynthesis, and alleviating oxidative stress.
Key words: ABA, abiotic stress, annexin, Solanum pennellii, SpUSP, tomato
Introduction
Sessile plants have to cope with various environmental stresses during their life cycle (Takashi and Kazuo, 2010). Among the different abiotic stresses, drought is a major agronomic threat to crop growth and yield, especially in arid and semi-arid areas (Adams et al., 2009). In drought conditions, 80–90% of water loss occurs via stomata in the leaf epidermis. Although various stimuli affect stomatal closure, the phytohormone abscisic acid (ABA)-induced stomatal closure is considered as a crucial mechanism for preventing water loss from plants (Lyudmila et al., 2011). ABA is a key regulator involved in diverse developmental processes and responses to abiotic stress (Cutler et al., 2010). Drought and salt stress conditions dramatically increase the ABA level which, in turn, induce the expression of many stress-related genes and activate signal transduction pathways that lead to stomatal movement (Tuteja, 2007; Zou et al., 2010).
Stomatal movement affects CO2 assimilation and further affects photosynthesis. The light-harvesting chlorophyll a/b-binding proteins (LHCBs) fulfil a constitutive light-harvesting function for photosystem II (PSII) during photosynthesis (Roberto et al., 1997). Previous reports have shown that the members of the LHCB family play an important role in plant photosynthesis and adaptation to environmental stresses (Ganeteg et al., 2004; Kovacs et al., 2006), as well as in guard cell signalling in response to ABA (Xu et al., 2012). The absence of the Lhcb1 and Lhcb2 proteins affects photosynthesis and results in the decrease of light absorption by the leaf (Andersson et al., 2003). The down-regulation of LHCB members also reduces plant tolerance to environmental stresses and affects seed production (Ganeteg et al., 2004; Kovacs et al., 2006).
Drought and salinity can lead to oxidative stress, thus, plants accumulate different kinds of osmoprotective solutes to reduce oxidative damage (Mahajan and Tuteja, 2005). These adaptive changes are achieved through a series of stress-dependent signal transduction pathways involving different types of genes (Chinnusamy et al., 2004). Some genes involved in osmoregulation have been cloned, such as pyrroline-5-carboxylate synthetase (P5CS) (Choudhary et al., 2005), betaine aldehyde dehydrogenase (Jia et al., 2002), and trehalose-6-phosphate synthase (Li et al., 2011). Transgenic plants overexpressing P5CS have significantly increased proline levels and tolerance to drought and salt stress (Kishor et al., 1995; Vendruscolo et al., 2007). The osmotic-stress-related genes are largely regulated by specific transcription factors, such as the members of the APETELA2, bZIP, and MYB families (Jung et al., 2010; Yang et al., 2009; Shin et al., 2011).
A type of protein called universal stress protein (USP) appears to play a positive role in the abiotic stress response. This protein features with the conserved USP domain in plants together with other functional domains (Kvint et al., 2003). USP was first discovered in bacteria whose expression is enhanced when the cell is exposed to stress agents (Nystrom and Neidhardt, 1992). The six USP genes of Escherichia coli have different functions linked to motility, adhesion, and oxidative stress resistance. Among these genes, UspA and UspD are required in the defence against superoxide-generating agents (Nachin et al., 2005).
USP homologues are ubiquitous in plants and encoded by gene families, while the functions of USPs remain largely unknown. The 44 putative USP genes in Arabidopsis are divided into two groups: ATP binding and non-ATP binding (Kerk et al., 2003). Two Arabidopsis USP genes, At3g62550 and At3g53990, that encode an ATP-binding motif have recently been up-regulated in a drought microarray dataset. (Isokpehi et al., 2011). GUSP1 and GUSP2 have been detected in water-stressed leaves of Gossypium arboretum (Maqbool et al., 2009). The present study is the first to report the cloning and functional characterizations of a SpUSP gene in tomato. The results suggest that SpUSP plays an important role in abiotic stress tolerance together with annexin via an ABA-dependent way.
Materials and methods
Isolation of SpUSP cDNA and sequence analysis
Total RNA was extracted from the leaves of the wild tomato species Solanum pennellii LA716 using Trizol reagent (Invitrogen, USA). Reverse transcription PCR (RT-PCR) was performed using a reverse transcription kit (Toyobo, Japan). The cDNA of SpUSP was amplified via a PTC-100 programmable thermal cycler (MJ Research, USA) using the primers USP1-F and USP1-R (see Supplementary Table S1 at JXB online). The amplified PCR fragment was cloned into pMD18-T (TaKaRa, Japan), transformed into DH5α E. coli cells, and sequenced. Sequence and phylogenetic analysis were conducted as previously described by Yang et al. (2011). The cis-acting regulatory elements in the promoter region were analysed using the PlantCARE (Lescot et al., 2002) and PLACE databases (Higo et al., 1999).
Plant growth and stress treatments
For gene expression profiling analysis, 2-month-old uniformly developed tomato plants (LA716, S. pennellii) were grown in a greenhouse under a 14/10h light/dark regime at 25 °C and subjected to various stresses or hormone treatments. Salt stress was induced by watering the plants with 200mM NaCl solution. Drought stress was simulated by placing detached leaves on a filter paper in 70% relative humidity at 25 °C. Cold or hot stress conditions were imposed by transferring the plants to a growth chamber and holding the plants at 4 °C or 40 °C, respectively. Wounding was performed by pinching the leaves with forceps. For hormone treatments and oxidative stress, 100 μM ABA, 100 μM ethylene (ETH), 100 μM gibberellic acid (GA3), and 100 μM paraquat were directly sprayed onto tomato plants. After each treatment, leaves from different plants (three biological replicates) were collected and immediately frozen in liquid nitrogen and stored at –80 °C until RNA isolation.
Quantitative RT-PCR (qRT-PCR) analysis
Quantitative RT-PCR was performed on a LightCycler 480 system (Roche, Switzerland). The reaction mixture contained 5 μl of 2× SYBR Premix Ex Taq mix (TaKaRa, Japan), 0.5 μM each of forward and reverse primers, and 1 μl of 10-fold diluted first-strand cDNA. The PCR cycling regime comprised an initial denaturation step at 95 °C for 10min, followed by 40 cycles at 95 °C for 10 s, 60 °C for 20 s, and 72 °C for 20 s. The primers used were QUSP1-F and QUSP1-R (see Supplementary Table S1 at JXB online). The tomato β-actin gene (GenBank accession no. BT013524) was used as an internal control. The threshold cycle value was given by the program automatically. The gene expression data were analysed using the ΔΔCt method (Schmittgen and Livak, 2008).
Plasmid construction and production of SpUSP-overexpressing plants
SpUSP promoter::GUS was generated by fusing the promoter fragment of SpUSP (1.981kb) in front of the GUS coding sequence in the PV3P vector. The promoter fragment of SpUSP was amplified using the primer pairs of SpUSP Pro F/R with an attB site (see Supplementary Table S1 at JXB online). The fragment was then integrated to the PV3P vector by BP and LR reactions (Invitrogen, USA), and then transformed into the cultivated tomato. The tissues of positive transgenic plants were treated with pre-chilled 90% acetone for 20min, rinsed with chilled water, and incubated with chilled GUS stain at 37 °C overnight in the dark. An ethanol series was used to destain the tissues, and the expression patterns were analysed under a microscope.
To overexpress SpUSP in tomato, the 35S promoter was employed to drive the target gene. A 550bp BamHI–XhoI fragment containing the SpUSP cDNA was cloned into the plant binary vector pMV, replacing the GUS reporter gene. The construct was introduced into the Agrobacterium tumefaciens strain LBA4404. Genetic transformation was performed on a drought-sensitive tomato cultivar ZS6 (S. lycopersicum) as previously described by Ouyang et al. (2005). Kanamycin-resistant plants were further confirmed through PCR using 35S-F (a forward primer in the 35S promoter) and USP1S-R (a reverse primer specific for SpUSP) (see Supplementary Table S1 at JXB online). The kanamyicn spraying test was used in the genetic segregation analysis (Weide et al., 1989), and three single-copy homozygous T3 lines (OE44, OE45, and OE69) were used for further study.
Stress tolerance assays in the transgenic tomato plants
To investigate the functions of SpUSP in stress tolerance, stress assays were conducted at the germination and adult-plant stages including three transgenic lines (OE44, OE54, and OE69) and one Wt line. Approximately 30 seedlings at uniform developmental stages for each line were transferred to half-strength Murashige and Skoog (MS) solid medium supplemented with either 200mM mannitol, 100mM NaCl, or 3 μM ABA. After growing for 10 d, the length and weight of the seedlings were measured. In addition, adult OE and Wt plants were drought stressed in the identical 2.0 l preweighed pots. Water was withheld until the plants began to wilt, then the plants were reirrigated, and their ability to recover was investigated. All measurements were conducted with six replicates.
Meanwhile, some stress-related biochemical markers were examined under drought stress at 25% and well-watered conditions at 100% field capacity (FC). The drought-stress treatment was initiated at the four-leaf stage. After 9 d of treatment, the chlorophyll contents were determined at 6-d intervals according to Wellburn (1994). Two months later, the dry and fresh weights, soluble sugars, proline, and malondialdehyde (MDA) were determined using previously described methods (Dubois et al., 1956; Bates et al., 1973). All measurements were conducted with three replicates.
To investigate the oxidative tolerance of SpUSP, excised 5h leaves from well-watered transgenic and Wt lines were stained using the diaminobenzidine (DAB) method according to Orozco-Cardenas and Ryan (1999). The oxidative tolerance of SpUSP was further studied by spraying 100 μM of paraquat (inducer of reactive oxygen species, ROS) onto well-watered plants for 24h. The oxidative damage of the leaves was then determined using the above-mentioned DAB staining method.
Subcellular localization of SpUSP
To detect the subcellular localisation of SpUSP, a green fluorescent protein (GFP) reporter system was constructed. The GFP gene and full-length cDNA of SpUSP without stop codon were amplified using the high-fidelity Taq polymerase (KOD plus, Toyobo) with primers GFPsub-F/GFPsub-R (with KpnI) and USPsub-F (with XbaI)/USPsub-R, respectively (see Supplementary Table S1 at JXB online). Overlapping PCR was conducted to assemble the resulting PCR products, and the chimeric fragment (SpUSP::GFP) yield was cloned into pMV through the restriction sites XbaI and KpnI to replace the GUS reporter gene. The resulting GFP reporter vector was designated as 35S::SpUSP::GFP. A GFP control vector (35S::GFP) was also constructed, and then transformed into the tobacco protoplast using a Biolistic PDS-1000/He Particle Delivery System (Bio-Rad, USA). After incubation for 12–18h at 25±2 °C, the GFP signals were detected under a Zeiss LSM510 confocal laser scanning microscope (Carl Zeiss, Germany).
Yeast two-hybrid (Y2H) screening and bimolecular fluorescence complementation (BiFC)
Y2H screens were performed using BD Matchmaker Library Construction and Screening kits (Clontech, USA) according to the manufacturer’s instructions. SpUSP was used as bait to screen the interacting proteins with the yeast library of tomato Ailsa Craig, and 40 randomly selected positive clones were sequenced and analysed. The interactions were further confirmed in vivo using the BiFC method as previously described by Walter et al. (2004). The cDNAs of SpUSP and Annexin (Unigene U314161), named AnnSp2 based on the report of Lu et al. (2012), were amplified with the primers listed in Supplementary Table S1 at JXB online and cloned into the vectors pUC-SPYCE and pUC-SPYNE, respectively. Then, the BiFC constructs were bombarded into tobacco cells using a gene gun. Photographs were taken 12–18h after transformation using a LSM510 confocal microscope.
ABA content and water loss assays
The ABA content was determined according to the method described by Seiler et al. (2011) with some modifications. After 7 d of exposure to drought and well-watered conditions, about 1g of leaf sample was ground and extracted several times in 10ml of extraction buffer (90% methanol+5% acetic acid+5% water), then the supernatant was collected by centrigugation and evaporated to dryness. The dried samples were redissolved in pure methanol and filtered. The filtrate was used for subsequent quantification through the chromatography using a Shimadzu UFLC syetem. Each sample analysis was repeated three times.
A water-loss assay was conducted as follows. Young fully expanded leaves of the transgenic and Wt lines were detached and placed on a filter paper under white florescent light and weighed periodically every 1h for 5h. The percentage of decreases in the fresh weight was expressed as the percentage water loss, and the stomatal conductance and transpiration rate were detected using the PP Systems-CIRAS-2 Portable Photosynthesis System according to the manufacturer’s protocol. The stomatal aperture was measured as previously described by Zou et al. (2010).
Microarray hybridization analysis
When the OE SpUSP and Wt lines reached the six-leaf stage, drought stress was imposed by withholding water but the control plants were watered as usual. The total RNA extracted from the third leaf was sent for microarray hybridization using the tomato TOM2 oligo microarray, including 12 000 elements (CapitalBio Corp., China). The unigenes with changes higher or lower than 2-fold were considered as differentially expressed genes. These unigenes were converted to their corresponding probe ID and annotated using the online software Plant MetGenMAP (http://bioinfo.bti.cornell.edu/cgi-bin/MetGenMAP/home.cgi) (Joung et al., 2009). Some unannotated unigenes were further analysed using the National Center for Biotechnology Information database (http://www.ncbi.nlm.nih.gov).
Results
Structure of the SpUSP gene
Based on previous microarray results (Gong et al., 2010), a differential expression drought-responsive USP gene (SGN-U214690, http://solgenomics.net) was identified. The unigene information and full-length cDNA from KafTom (http://www.pgb.kazusa.or.jp/kaftom/) were successfully employed to design the gene amplification primer USP1-F/R (see Supplementary Table S1 at JXB online) and obtain the full-length cDNA of SpUSP. This cDNA was 572bp in length and included a 438bp open reading frame, a 91bp 5’-untranslated region, and a 43bp 3’-untranslated region. A BLAST homology search of the tomato genome database (http://solgenomics.net/) revealed that the SpUSP gene was located in chromosome 1. The genomic DNA of SpUSP was cloned using the same primers amplified for cDNA to investigate the gene structure. The sequence alignments between the genomic DNA and cDNA showed that the gene contained three exons separated by two introns (see Supplementary Fig. S1 at JXB online). SpUSP was predicted to encode a protein of 145 amino acids, with a molecular weight of 16.2kDa and an isoelectric point of 5.9. A protein BLAST search of GenBank using SpUSP as a probe revealed the best homologue from grape (GenBank accession no. XP_002266746) with 48% amino acids identities, and 44% identities from Arabidopsis (GenBank accession no. NP_566108). The alignment of the 13 best hits (one from each species) showed that a conserved USP domain was present in all sequences (see Supplementary Fig. S2 at JXB online). The predicted secondary structure of SpUSP had conserved ATP-binding regions and other binding sites, and showed very similar distributions of α-helices and β-strands to that described for the crystal structure of MJ0577.
Expression pattern of SpUSP in tomato
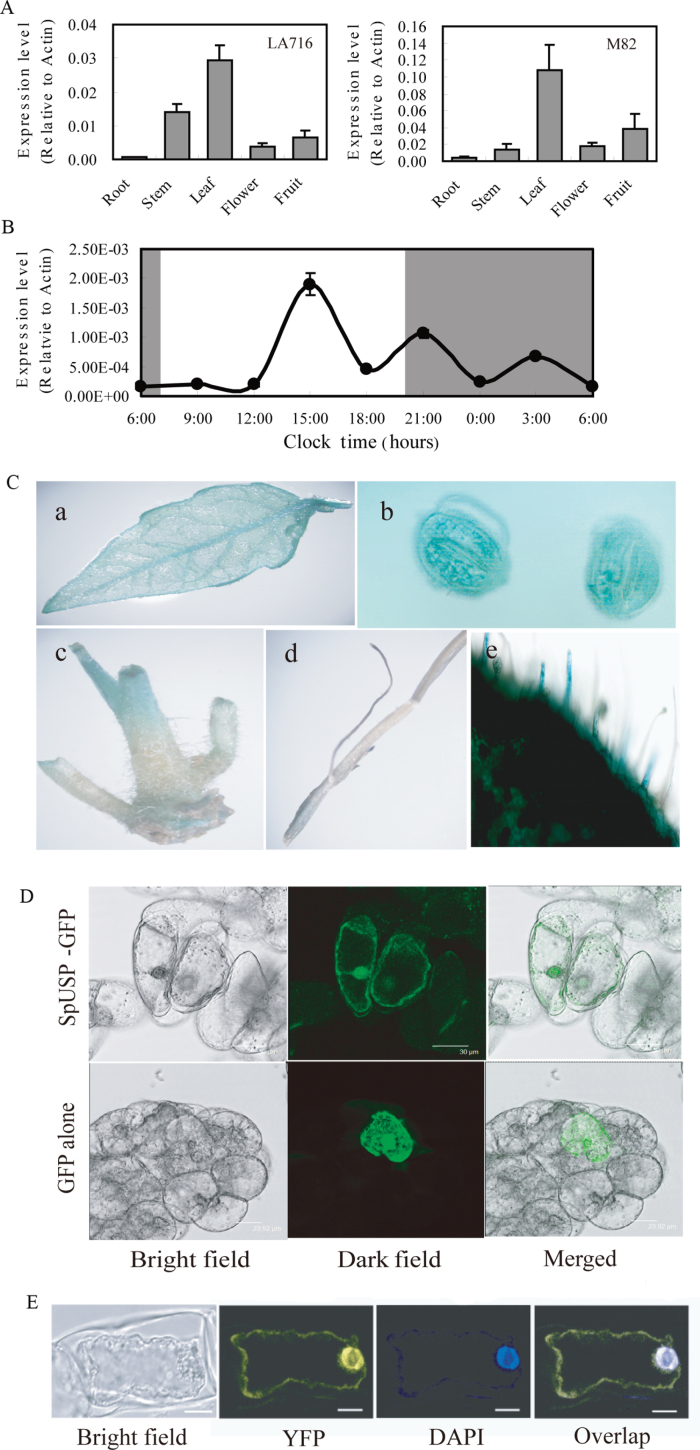
Quantitative RT-PCR was employed to investigate the expression profile of SpUSP in tomato tissues. SpUSP was highly expressed in the leaf but barely in the root, although SpUSP expression was detected in all organs tested (Fig. 1A). Similar expression patterns were found in the wild relative S. pennellii LA716 and in cultivated tomato M82. A relatively higher expression level was detected in the stem of LA716 than M82 compared with other tissues. Noticeably, during the day/night cycles, the SpUSP transcripts exhibited maximum expression in the afternoon, some fluctuations at night, and minimumal expression in the morning (Fig. 1B).
Fig. 1.
Expression patterns of SpUSP. (A) Tissue profiling analysis of SpUSP in different organs of wild tomato LA716 (Solanum pennellii) and cultivated tomato M82 (S. lycopersicum) using qRT-PCR. (B) Expression pattern of SpUSP during a 24h period. Leaf samples were collected every 3h for 24h starting from 06.00h. (C) Expression patterns of SpUSP via GUS staining: (a) leaf, (b) stoma, (c) stem, (d) root, and (e) trichome. (D) Subcellular localization of SpUSP. The photographs were taken under bright light, in the dark field for the GFP-derived green fluorescence and merged respectively. (E) Interaction of SpUSP with annexin via BiFC. The photographs were taken under bright light, in the dark field for YFP-derived green fluorescence, staining with DAPI and overlap, respectively. Scale bars=10 μm.
Further investigation using the SpUSP promoter::GUS system showed that GUS fluorescence was mainly detected in leaves with very low expression in roots. These results agree with the expression pattern of SpUSP examined using qRT-PCR. High GUS fluorescence was also detected in the stomata and trichomes of the leaf epidermis (Fig. 1C). In addition, the subcellular localization of SpUSP protein was determined. The green fluorescence of 35S::SpUSP::GFP was exclusively detected in the nucleus and cell membrane, whereas the cells transformed with the vector containing GFP alone displayed fluorescence throughout the cells (Fig. 1D). The promoter of SpUSP was cloned and analysed. Some cis-acting regulatory elements involved in the light response, circadian control, and the phytohormone response, such as ABA, MeJA, GA3, and abiotic stress (e.g. MYB binding site and heat stress response), were found in the SpUSP promoter region (see Supplementary Fig. S3 at JXB online).
SpUSP expression was induced by various stress conditions and hormones
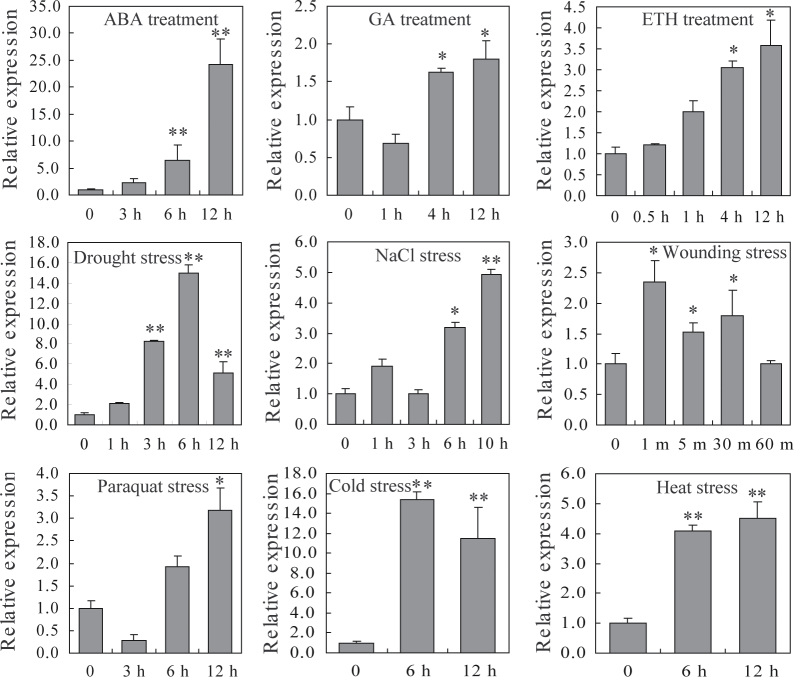
The expression patterns of SpUSP were investigated under different stress and hormone treatments using qRT-PCR assays. The SpUSP gene was significantly induced by some stress conditions (Fig. 2). Under drought stress, the SpUSP transcripts initially accumulated to 15-fold after 6h and then decreased after 12h. Under high salt stress (200mM NaCl), SpUSP expression increased after 1, 6, and 10h, but was unchanged after 3h. Wounding resulted in a quick increase and then a decrease in the expression level of SpUSP. Interestingly, after 100 μM paraquat (oxidation-inducing agent) treatment, the SpUSP transcripts declined within 3h and then increased after 12h. Subjecting the tomato plants to cold stress (4 °C) resulted in the accumulation of SpUSP transcripts by 15-fold after 6h. On the other hand, heat stress (40 °C) up-regulated the expression by approximately 4-fold after 6h and stabilized it after 12h. Regarding phytohormone treatments, the SpUSP transcripts rapidly increased up to 24-fold within 12h with ABA (100 μM), gradually increased with ETH (100 μM), and had a limited effect with GA3 (100 μM). Overall, the ABA and drought stresses exerted the strongest effects on all hormones or stressors.
Fig. 2.
Expression levels of SpUSP in tomato leaves under phytohormones and different stress conditions. The leaves of 2-month-old plants were used for RNA extraction in LA716 (Solanum pennellii) after treatment with 100 μM ABA, 100 μM GA, and 100 μM ETH, drought, 200mM NaCl, wounding, 100 μM paraquat, 4 °C cold, 40 °C heat, respectively. All samples were collected at the indicated time points (‘h’ and ‘m’ refer to hours and minutes after treatment, respectively) from three biological replicates. Single (*P <0.05) and double (**P <0.01) asterisks denote statistically significant differences between the stress treatment and the 0h control. The β-actin gene (BT013524) was used as an internal control in the qRT-PCR.
OE of SpUSP increased abiotic stress tolerance in tomato
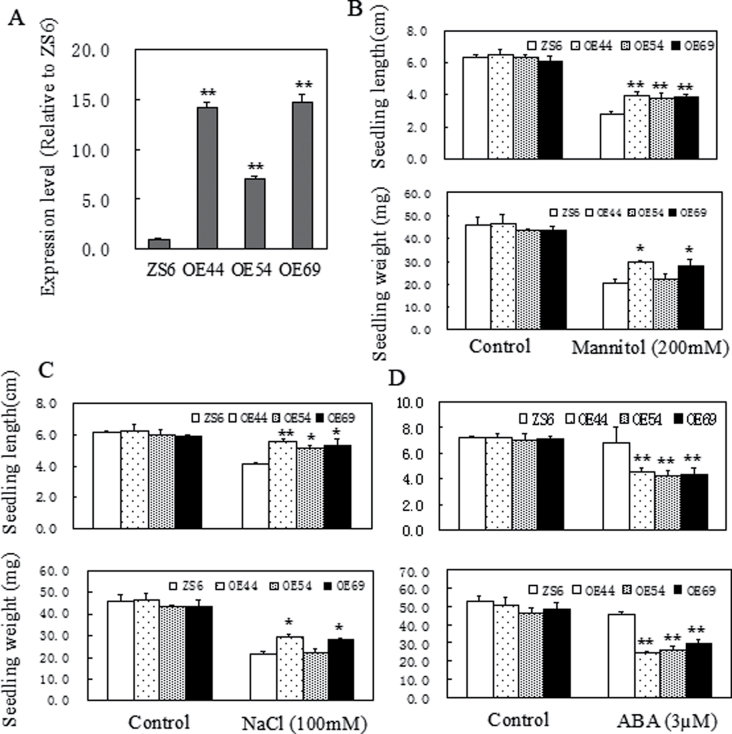
To evaluate the abiotic tolerance of SpUSP, three homozygous OE lines of SpUSP (OE44, OE54, and OE69) with a high transcript level were selected (Fig. 3A). Abiotic stress tolerance tests were performed with mannitol (200mM) or NaCl (100mM) in MS medium at the seedling stages. ZS6 was used as the control. Under mannitol stress, the seedling length decreased by 52% for Wt and only by 28–34% for the three SpUSP OE lines. The seedling weight decreased by 56% for Wt and only by 34–47% for the OE lines (Fig. 3B; see Supplementary Fig. S4 at JXB online). Under NaCl stress, the seedling length decreased by 32% for Wt and 15–20% for the OE lines, whereas seedling weight decreased by 53% for Wt and 30–41% for the OE lines (Figs 3C). Under ABA (3 μM) treatment, the seedling length and weight of the OE lines decreased by 30%, whereas those of the Wt line decreased by approximately 10% (Fig. 3D; see Supplementary Fig. S5 at JXB online). After the different treatments, significant differences were found in the seedling length and weight between the OE and Wt lines, except for a few cases.
Fig. 3.
Growth performances of OE SpUSP and wild-type seedlings treated with mannitol, salt, and ABA stresses. (A) Analysis of SpUSP transcriptional expression via qRT-PCR in overexpressing (OE44, OE54, and OE69) and wild-type (ZS6) lines. Seedling lengths and weights of transgenic and wild-type lines after treatment with 200mM mannitol (B), 100mM NaCl (C), and 3 μM ABA (D), and without stress as a control. The seedlings were grown in half-strength MS medium. The data shown are the mean ±SE (n= 6). Single (*P <0.05) and double (**P <0.01) asterisks denote statistically significant differences between transgenic and wild-type lines.
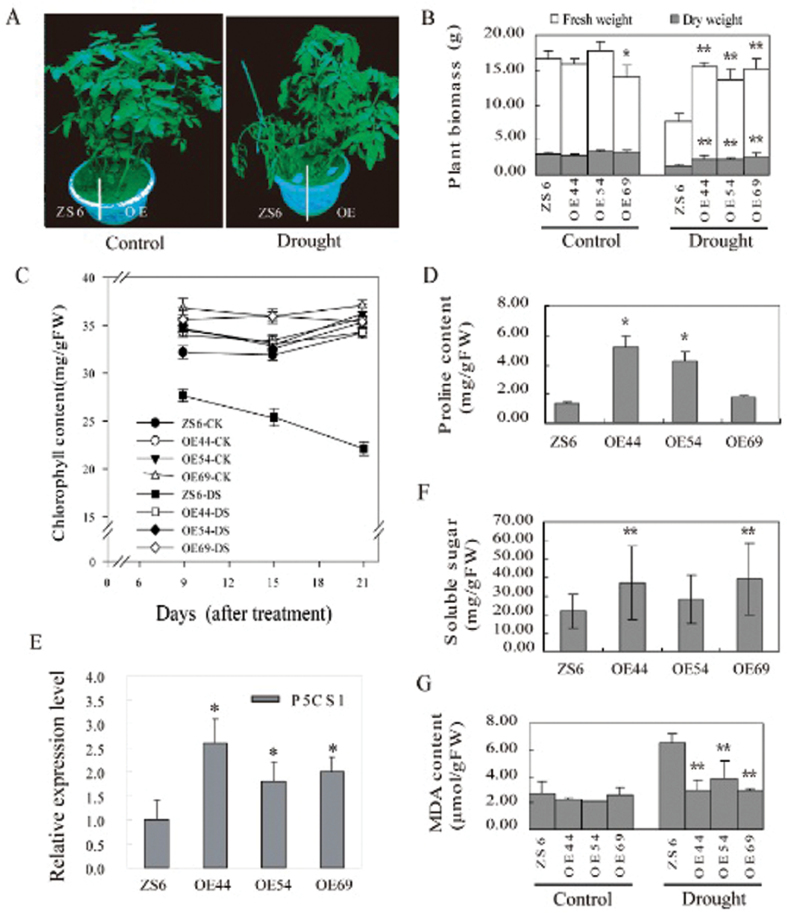
The SpUSP OE and Wt lines were also challenged with drought stress by withholding watering in adult plants. After 3 weeks without watering, the three SpUSP OE lines (OE44, OE54, and OE69) showed only mild wilting, whereas severe wilting occurred in the Wt plants (Fig. 4A). After undergoing three cycles of drought and recovering, approximately 80% of the SpUSP-overexpressing plants survived, whereas all Wt plants died.
Fig. 4.
SpUSP overexpression enhances drought tolerance in tomato. (A) Drought tolerance tests for SpSUP-overexpressing (OE44, OE54, and OE69) and wild-type ZS6 plants grown in the same pot. The phenotypes under well-watered (‘Control’) and drought-stress conditions (‘Drought’) are shown.. (B) Effects of drought on the fresh and dry weights of the OE and wild-type lines. (C) Chlorophyll contents in plant leaves under drought stress (‘DS’) or normal condition (‘CK’). (D) Proline accumulation in plant leaves under drought stress. (E) Relative expression levels of P5CS1 in the OE and wild-type lines under drought stress via qRT-PCR. (F) Soluble sugar content in plant leaves under drought stress. (G) MDA content in plant leaves under stress (‘Drought’) or normal condition (‘Control’). The data shown are the mean ±SE (n=3). Single (*P <0.05) and double (**P <0.01) asterisks denote statistically significant differences between the transgenic and wild-type lines under drought stress.
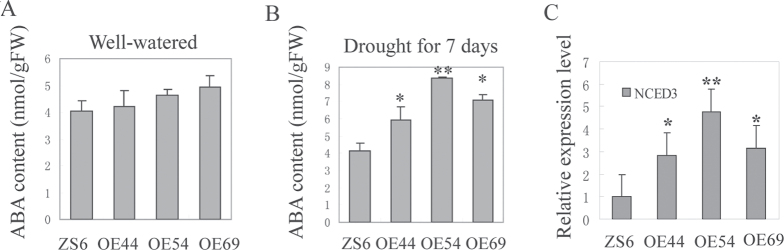
Elevated ABA contents increased stomatal closure in drought condition
The endogenous ABA content of 2-month-old SpUSP-overexpressing and Wt plants in well-watered or without watering conditions for 7 d were measured. No significant difference was found between the transgenic and Wt plants under the well-watered conditions. Under drought stress, the ABA content in the transgenic lines increased from 53% to 126%, whereas that in the Wt plants increased by only 20% (Fig. 5A, 5B). The expression of NCED3, a key gene in ABA biosynthesis, was also assessed under drought stress. Its expression was significantly higher in the transgenic lines than in the Wt plants (Fig. 5C).
Fig. 5.
Endogenous ABA content in SpUSP-overexpressing and wild-type 2 month-old plants measured by HLPC. (A) ABA content in well-watered condition. (B) ABA content after withholding water for 7 d. (C) Relative expression level of NCED3 in OE and wild-type (ZS6) lines under drought stress via qRT-PCR. Variance analysis was performed to determine significant differences (*P <0.05 and **P <0.01) between the ZS6 and OE lines.
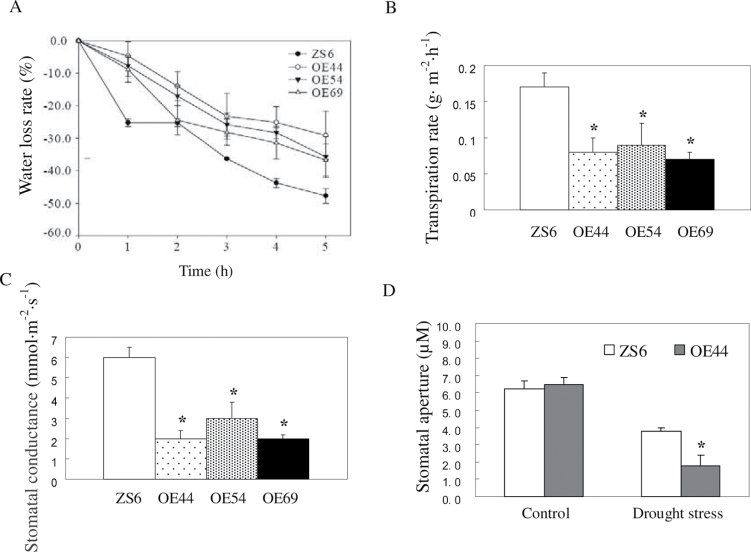
In addition, a drought test was performed in vitro to investigate whether the elevated ABA content affected stomatal closure. The third leaf from the bottom was detached and stored for over 5h at room temperature. The water loss rate was assayed in the Wt and three transgenic lines in vitro every hour for 5h. The water loss rate of the transgenic plant leaves was slower than that of the Wt plant leaves (Fig. 6A). The transpiration rate and stomatal conductance of the transgenic plants were significantly reduced compared with those of the Wt (Fig. 6B, 6C). Furthermore, the stomatal apertures of OE44 and Wt were investigated. Under drought stress, the stomatal aperture of the OE44 lines was significantly smaller than that of the Wt line (Fig. 6D). These results further confirm the notion that SpUSP functions in drought stress by regulating stomatal movements to reduce water loss.
Fig. 6.
Water loss test for the detached leaves excised from the wild type and transgenic plants. Variance analysis was performed to determine significant differences (*P <0.05) between the detached leaves of the ZS6 and OE lines under drought stress. (A) Water loss test of the detached leaves for 5h. The detached leaves were collected from mature plants from at the same position and placed on a Wattman paper at room temperature. The mean weight was measured every hour. (B) Transpiration rate and (C) stomatal conductance of the detached leaves. The two parameters were detected after the detached leaves were placed on a filter paper and exposed under white florescent light for 5h. (D) Stomatal aperture of transgenic and wild-type plants under drought stress. Leaves were detached from 2-month-old transgenic (OE44) and wild-type (ZS6) plants and subjected to drought stress for 5h in vitro. The lower surfaces of the leaves with or without stress were examined under a microscope.
SpUSP improves photosynthesis and alleviates oxidative stress during drought stress
The chlorophyll content and biomass of the SpUSP OE lines grown under optimal (at 100% FC) and reduced watering regimes (at 25% FC) were measured. Under the optimal watering regime, the biomass and chlorophyll contents did not differ between the transgenic and Wt plants. However, reduced watering for one month severely affected the biomass and chlorophyll contents of the Wt plants. The biomass of Wt plants was decreased by approximately 60%, whereas that of the transgenic plants was decreased by only about 25% (Fig. 4B). The decrease in biomass was significantly different between the OE lines and ZS6. Under drought stress, the chlorophyll content of the Wt plants significantly decreased with prolonged drought duration, whereas that of the transgenic plants remained at a high level (Fig. 4C).
Small biomolecules, including proline, soluble sugars, and MDA, are important indicators of plant oxidative stresses. Under drought stress, the proline concentrations in the transgenic lines OE44 and OE54 were 3–4-fold higher than those in the control, whereas no significant difference was observed under well-watered conditions (Fig. 4D). The expression level of P5CS, a key gene in proline biosynthesis, was significantly higher in the SpUSP-OE plants than the Wt (Fig. 4E). Similarly, the concentration of soluble sugars increased by up to several fold in the transgenic plants, especially for the OE44 and OE69 lines (Fig. 4F). By contrast, MDA increased significantly in the drought-stressed Wt compared with the SpUSP-OE plants (Fig. 4G).
Under oxidative stress, ROS can cause cell damage. To determine whether the ectopically expressed SpUSP affected ROS production, leaves of the transgenic and Wt lines were detached. After 5h of drought in vitro, the Wt leaves appeared to wilt more than the transgenic ones. DAB staining showed the Wt plants accumulated more hydrogen peroxide than the transgenic ones under drought stress (see Supplementary Fig. S6A at JXB online). When paraquat was spread directly onto the leaves to induce ROS production, although the leaves showed deeper brown spots than those under drought stress in both transgenic and Wt plants, the leaves of the transgenic plants accumulated much less hydrogen peroxide compared with the Wt plants (see Supplementary Fig. S6B at JXB online). Hence, SpUSP can alleviate excess ROS-induced oxidative damage and thus improve oxidative stress tolerance.
SpUSP interacted with annexin in vivo
The SpUSP full-length cDNA was used as the bait in the Y2H to explore its interacting proteins. After screening, 20 sequences were obtained from 40 positive clones encoded with 11 distinct entities. The database search revealed that seven of them were related to abiotic stress (see Supplementary Table S2 at JXB online). Based on the role of Arabidopsis AnnAt1 in drought tolerance (Konopka-Postupolska et al., 2009), the annexin gene AnnSp2 was selected as the top candidate for interaction with SpUSP. AnnSp2 has more than 98% similarity with the annexin p35 gene (Lim et al., 1998) in tomato, and has the highest similarity of 71% with AnnAt2 in Arabidopsis. To confirm the SpUSP–AnnSp2 interaction, BiFC was performed using tobacco cells. The cells co-transfected with SpUSP–YFPC and AnnSp2–YFPN displayed strong yellow fluorescence that was mainly accumulated in the nucleus and plasmalemma (Fig. 1E). Hence, the interaction between SpUSP and AnnSp2 occurred in both the nucleus and cell membrane, in agreement with the result of SpUSP subcellular localization.
Overall gene expression changes in SpUSP-overexpressing plants
The gene expression profile of the SpUSP-overexpressing lines was compared with that of the Wt under both normal and drought-stress conditions using the tomato TOM2 microarray (12 000 probes). Under normal conditions, 259 genes were detected to have more than a 2-fold change in the SpUSP-overexpressing line OE44 (123 genes were up-regulated and 136 were down-regulated; see Supplementary Table S3 at JXB online) compared with the Wt. However, under the drought stress condition, 856 genes were detected to have more than a 2-fold change (479 genes were up-regulated and 377 were down-regulated; see Supplementary Table S4 at JXB online).
Many of the differentially expressed genes were functionally unknown. However, some photosystem-related genes were up-regulated (see Supplementary Table S5 at JXB online), involved in the entire photosystem process, including the main photosynthetic apparatus components (27 chlorophyll a/b-binding proteins and 11 photosystems I and II reaction centre subunits or proteins). The expression levels of seven ATP synthases and a cytochrome P450 gene were also changed. Few changes were observed in the microarray data of the well-watered tomato.
Several abiotic stress-responsive genes were also changed based on the microarray results, such as L-ascorbate oxidase (SGN-U219080), osmotin-like protein (SGN-U213934 and SGN- U212927), two NADP-malic enzymes (SGN-U232389 and SGN- U213228), calcium-dependent protein kinase 4 (SGN-U224039), aquaporin (SGN-U221263), MYB (SGN-U226315), ERD1-like (SGN-U216637), HSP (SGN-U220044), serine/threonine protein kinase (SGN-U225125), and bZIP transcription factor (SGN-U213708).
Discussion
USP, first identified in E. coli (Nystrom and Neidhardt, 1992), reportedly plays an important role in stress adaptation (Nachin et al., 2005). The present study explored the function of the SpUSP gene in the abiotic stress of tomato. SpUSP has low sequence similarity with the USPs of other species, the highest being only 48% with a USP of grape (GenBank accession no. XP_002266746), thus implying it is a novel USP gene. In plants, only a few homologues of the USP family have been isolated (Chou et al., 2007; Maqbool et al., 2009), and their functions remain unclear. The present study demonstrates that SpUSP plays a critical role in abiotic stress, particularly in drought tolerance in tomato, as supported by data from overexpressing lines.
SpUSP expression is regulated by various stress and photoperiodic conditions
SpUSP expression is regulated in response to abiotic stress conditions and several hormones, specifically for ABA. ABA mediates the core signalling network in the plant abiotic-stress response (Cutler et al., 2010). The ABA-responsive element (ABRE) widely exists in the promoters of ABA-induced genes. These promoters function in ABA-dependent gene expression (Yamaguchi-Shinozaki and Shinozaki, 2005). An ABRE element was also found in the SpUSP promoter region, and ABA treatment in vitro induced high expression levels of SpUSP. This result indicates that SpUSP is probably ABA dependent. Other abiotic stress-response elements such as the heat shock element and recognition sites for MYB were also found in the SpUSP promoter region. These cis-elements are the core elements for stress response (Urao et al., 1993). Surprisingly, SpUSP expression changed with the circadian rhythm and coincided with stomatal movement in a 1 d cycle. A cis-acting regulatory element involved in the circadian control (circadian CAACAGCATC) was found in its promoter region. Several other genes involved in stomatal movement have been affected by photoperiodic rhythms such as AnnAt1 and AnnAt4 (Huh et al., 2010), PHOT1 and PHOT2 (Kinoshita et al., 2001), and AtMYB60 (Cominelli et al., 2005).
SpUSP-mediated ABA signals enhance drought resistance by reducing the stomatal opening
Plants can reduce water loss through stomatal closure and transpiration inhibition. Stomatal movement is generally regulated via the ABA signalling pathway (Jung et al., 2008; Zou et al., 2010). ABA treatment stimulates stomatal closure in leaves and enhances resistance to drought in myb96-overexpressing Arabidopsis (Seo et al., 2009). A positive correlation is found between the ABA level and enhanced abiotic stress tolerance (Lee et al., 2006). In the present study, the increased ABA content and transcripts of SpUSP in the stomata lead to less stomatal aperture, suggesting that SpUSP has a function in regulating stomatal opening and thus improves drought resistance by ABA. The transcript accumulations of some genes in guard cells also mediate abiotic stress responses by ABA (Zhang et al., 2007; Jung et al., 2008; Seo et al., 2009). Recently LHCB members have been identified as new players in ABA signalling in stomatal movement in Arabidopsis. The down-regulation of any member of the LHCB family reduces the responsiveness of stomatal movement to ABA thereby resulting in decrease in drought tolerance in Arabidopsis thaliana (Xu et al., 2012). The up-regulation of many LHCB members in SpUSP-OE lines (see Supplementary Table S5 at JXB online) probably contributes to enhanced stomatal sensitivity to ABA.
Interestingly, exogenous ABA application reduced the seedling size of the SpUSP overexpressing lines compared with the Wt, indicating that SpUSP may be related to ABA signalling in seedling growth regulation. The seed germination of the SpUSP overexpression lines was also significantly inhibited in ABA-containing media. The sensitivity of seed germination and growth to ABA has also been reported in Arabidopsis showing enhanced drought tolerance due to the OE of MYB15 and MYB96 (Ding et al., 2009; Seo et al., 2009).
Abiotic stress tolerance of SpUSP is involved in the improvement of photosynthesis
Under drought stress, stomatal closure reduces CO2 availability, energy balance has been recognized as a key component of cell function under a limited supply of CO2 (Lawlor and Tezara, 2009; Pfannschmidt et al., 2009). LHCB is the main photosynthetic component that can mediate the distribution of excitation energy between photosystems I and II to balance photosynthesis (Asada, 2006). In the present microarray data, many chlorophyll a/b-binding proteins were up-regulated under drought stress in the SpUSP-OE lines. LHCB regulation is considered to be one of the most important mechanisms of plants in modulating chloroplast functions (Pruneda-Paz and Kay, 2010; Thines and Harmon, 2010). These up-regulated LHCBs possibly keep the PSII antenna complex intact and ensure its functional involvement in photosynthesis.
Several ATP synthase subunits that can enhance ATP production and provide chemical energy for plant growth were up-regulated. The importance of ATP synthase in photosynthetic regulation is well recognized (Wu et al., 2007). SpUSP is predicted to contain ATP binding residues similar to the bacterial MJ0577-type proteins (Zarembinski et al., 1998), suggesting SpUSP is an ATP-mediated molecular switch. UspA protein is described as an autophosphorylating serine and threonine phosphoprotein that uses either GTP or ATP as phosphate donors in E. coli (Freestone et al., 1997). Phopshorylation may be related to the function of the STK-N domain predicted in SpUSP. Thus, the regulation of LHCB and ATP may be involved in the improvement of photosynthesis in the SpUSP OE lines.
Overexpressing SpUSP alleviates oxidative stress in tomato
Drought is a kind of oxidative stress in plants. Some osmoprotectants including proline, soluble sugars, and oxidative-stress biohazards can be used as biochemical markers to indicate the oxidative condition. Proline accumulates in plants as an osmoprotectant under a wide range of biotic and abiotic stresses (Ashraf and Harris, 2004; Verbruggen and Hermans, 2008). In the present study, the amount of free proline in transgenic lines was higher than that in non-transgenic plants under drought stress (Fig. 4D). The high proline content may be involved in the enhanced expression of the P5CS1 gene. In drought stress, the elevated expression of P5CS1 improves drought tolerance in tobacco and Arabidopsis (Huh et al., 2010; Ziaf et al., 2011). In addition, SpUSP was also significantly induced by paraquat, an inducer of ROS. DAB staining results under drought and paraquat stress conditions suggest that SpUSP plays an important role in alleviating oxidative stress.
Oxidation-related genes have been extensively studied under drought stress at the molecular level (Allen and Tresini, 2000). In the present microarray results, some genes involved in alleviating oxidative stress were changed, such as osmotin-like proteins (SGN-U213934 and SGN-U212927) that reportedly participate in defence response in potato (Castillo et al., 2005). Two NADP-malic enzymes (SGN-U232389 and SGN-U213228) whose homologues from rice confer salt tolerance in transgenic Arabidopsis (Cheng and Long, 2007) were changed. Some other changed genes such as ERD1-like (SGN-U216637), HSP (SGN-U220044), serine/threonine protein kinase (SGN-U225125), and bZIP transcription factor (SGN-U213708) are all involved in the alleviation of oxidative stress (Ziaf et al., 2011; Jiang et al., 2009; Mao et al., 2010; Orellana et al., 2010).
SpUSP functions together with annexin in drought-stress signalling
Annexins have been considered as targets of Ca2+ signals in eukaryotic cells and deemed to play an important role in plant stress responses (Mortimer et al., 2008; Laohavisit et al., 2009). Increased calcium influx and cytoplasmic Ca2+ are important in guard cell ABA signal transduction (Mcainsh et al., 1990; Roelfsema and Hedrich, 2010). The overexpression of annexin (AnnAt1) helps eliminate ROS and improve drought tolerance in Arabidopsis (Konopka-Postupolska et al., 2009). In the present study, SpUSP was also induced by ABA and paraquat (inducer of ROS), and the OE of SpUSP showed stronger antioxidative ability and drought tolerance. These functions of SpUSP agree well with those attributed to annexin. Thus, the interaction between the two proteins is suggested to be indeed dependable, and the stomatal closure induced by SpUSP probably involves Ca2+ signalling, although further confirmation is needed.
How does SpUSP work in drought-tolerance improvement?
The mechanism of the SpUSP:AnnSp2 complex in alleviating drought stress may be highly complex. When the tomato plants were exposed to the abiotic stresses of drought and salt, the ABA content significantly increased in SpUSP OE plants. Drought tolerance mediated by elevated ABA may be attributed to the following aspects. First, OE SpUSP increases the expression of LHCB under drought stress, which probably enhances stomatal sensitivity to elevated ABA. The stomatal aperture is then reduced, which is likely to be the major factors contributing to the drought tolerance of SpUSP. Second, under less stomatal aperture condition, the higher expression of LHCBs and photosystem-related genes keeps the PSII antenna complex intact and maintains normal photosynthesis in SpUSP OE plants. Finally, SpUSP complex proteins activate some stress-responsive genes, which lead to the accumulation of some osmoprotective solutes to alleviate oxidative stress by eliminating ROS production. Altogether, these various mechanisms result in enhanced drought tolerance in tomato SpUSP OE lines.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Table S1 List of primers used in this study.
Supplementary Table S2 SpUSP-interacting proteins identified by yeast two-hybrid screening.
Supplementary Table S3. A complete list of genes with expression levels that changed more than 2-fold under a well-watered condition in transgenic plants overexpressing SpUSP, as compared with the non-transformed wild-type control.
Supplementary Table S4. A complete list of genes with expression levels that changed more than 2-fold under drought stress in transgenic plants overexpressing SpUSP, as compared with the non-transformed wild-type control.
Supplementary Table S5. A list of genes that underwent changes in association with photosynthesis under drought stress in SpUSP-overexpressing plants compared with the non-transformed wild-type control.
Supplementary Fig. S1. Structure of the SpUSP gene.
Supplementary Fig. S2. Multiple sequence alignment and phylogenetic tree analysis of USPs and USP-like proteins from different species.
Supplementary Fig. S3. Cis-acting element analysis of SpUSP promoter.
Supplementary Fig. S4. SpUSP overexpression improves the growth performance of seedlings under osmotic stress.
Supplementary Fig. S5. SpUSP overexpression affects the growth performance of seedlings under ABA treatment.
Supplementary Fig. S6. Oxidative stress assay on the leaves of transgenic and wild-type plants
Supplementary Material
Acknowledgements
This work was supported by the National Program on the Development of Basic Research in China (grant no. 2011CB100600) and the National Natural Science Foundation of China (grant nos. 31000912 and 30871712).
References
- Adams HD, Guardiola-Claramonte M, Barron-Gafford GA, Villegas JC, Breshears DD, Zou CB, Troch PA, Huxman TE. 2009. Temperature sensitivity of drought-induced tree mortality portends increased regional die-off under global-change-type drought Proceedings of the National Academy of Sciences, USA 106 7063–7066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RG, Tresini M. 2000. Oxidative stress and gene regulation Free Radical Biology and Medicine 28 463–499 [DOI] [PubMed] [Google Scholar]
- Andersson J, Wentworth M, Walters RG, Howard CA, Ruban AV, Horton P, Jansson S. 2003. Absence of the Lhcb1 and Lhcb2 proteins of the light-harvesting complex of the photosystem II: effects on photosynthesis, grana stacking and fitness The Plant Journal 35 350–361 [DOI] [PubMed] [Google Scholar]
- Asada K. 2006. Production and scavenging of reactive oxygen species in chloroplasts and their functions Plant Physiology 141 391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M, Harris PJC. 2004. Potential biochemical indicators of salinity tolerance in plants Plant Science 166 3–16 [Google Scholar]
- Bates LS, Waldren RP, Teare ID. 1973. Rapid determination of free proline for water studies Plant and Soil 39 205–207 [Google Scholar]
- Castillo Ruiz RA, Herrera C, Ghislain M, Gebhardt C. 2005. Organization of phenylalanine ammonia lyase (PAL), acidic PR-5 and osmotin-like (OSM) defence-response gene families in the potato genome Molecular Genetics and Genomics 274 168–179 [DOI] [PubMed] [Google Scholar]
- Cheng Y, Long M. 2007. A cytosolic NADP-malic enzyme gene from rice (Oryza sativa L.) confers salt tolerance in transgenic Arabidopsis Biotechnology Letters 29 1129–1134 [DOI] [PubMed] [Google Scholar]
- Chinnusamy V, Schumaker K, Zhu JK. 2004. Molecular genetic perspectives on cross-talk and specificity in abiotic stress signalling in plants Journal of Experimental Botany 55 225–236 [DOI] [PubMed] [Google Scholar]
- Chou MX, Wei XY, Chen DS, Zhou JC. 2007. A novel nodule-enhanced gene encoding a putative universal stress protein from Astragalus sinicus Journal of Plant Physiology 164 764–772 [DOI] [PubMed] [Google Scholar]
- Choudhary NL, Sairam R, Tyagi A. 2005. Expression of Δ1-pyrroline-5-carboxylate synthetase gene during drought in rice (Oryza sativa L.) Indian Journal of Biochemistry and Biophysics 42 366–370 [PubMed] [Google Scholar]
- Cominelli E, Galbiati M, Vavasseur A, Conti L, Sala T, Vuylsteke M, Leonhardt N, Dellaporta SL, Tonelli C. 2005. A guard-cell-specific MYB transcription factor regulates stomatal movements and plant drought tolerance Current Biology 15 1196–1200 [DOI] [PubMed] [Google Scholar]
- Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. 2010. Abscisic acid: emergence of a core signaling network Annual Review of Plant Biology 61 651–679 [DOI] [PubMed] [Google Scholar]
- Ding ZH, Li SM, An XL, Liu X, Qin HJ, Wang DW. 2009. Transgenic expression of MYB15 confers enhanced sensitivity to abscisic acid and improved drought tolerance in Arabidopsis thaliana Journal of Genetics and Genomics 36 17–29 [DOI] [PubMed] [Google Scholar]
- Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F. 1956. Colorimetric method for determination of sugars and related substances Analytical Chemistry 38 350–356 [Google Scholar]
- Freestone P, Nyström T, Trinei M, Norris V. 1997. The universal stress protein, UspA, of Escherichia coli is phosphorylated in response to stasis Journal of Molecular Biology 274 318–324 [DOI] [PubMed] [Google Scholar]
- Ganeteg U, Kulheim C, Andersson J, Jansson S. 2004. Is each light-harvesting complex protein important for plant fitness? Plant Physiology 134 502–509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong P, Zhang J, Li H, et al. 2010. Transcriptional profiles of drought-responsive genes in modulating transcription signal transduction, and biochemical pathways in tomato Journal of Experimental Botany 61 3563–3575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higo K, Ugawa Y, Iwamoto M, Korenaga T. 1999. Plant cis-acting regulatory DNA elements (PLACE) database: 1999 Nucleic Acids Research 27 297–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh SM, Noh EK, Kim HG, Jeon BW, Bae K, Hu HC, Kwak JM, Park OK. 2010. Arabidopsis annexins annAt1 and annAt4 interact with each other and regulate drought and salt stress responses Plant and Cell Physiology 51 1499–1514 [DOI] [PubMed] [Google Scholar]
- Isokpehi RD, Simmons SS, Cohly HH, Ekunwe SI, Begonia GB, Ayensu WK. 2011. Identification of drought-responsive universal stress proteins in viridiplantae Bioinformatics and Biology Insights 5 41–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia GX, Zhu ZQ, Chang FQ, Li YX. 2002. Transformation of tomato with the BADH gene from Atriplex improves salt tolerance Plant Cell Reports 21 141–146 [Google Scholar]
- Jiang C, Xu J, Zhang H, Zhang X, Shi J, Li M, Ming F. 2009. A cytosolic class I small heat shock protein, RcHSP17.8, of Rosa chinensis confers resistance to a variety of stresses to Escherichia coli, yeast and Arabidopsis thaliana Plant, Cell and Environment 32 1046–1059 [DOI] [PubMed] [Google Scholar]
- Joung JG, Corbett AM, Fellman SM, Tieman DM, Klee HJ, Giovannoni JJ, Fei ZJ. 2009. Plant MetGenMAP: an integrative analysis system for plant systems biology Plant Physiology 151 1758–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung C, Seo JS, Han SW, Koo YJ, Kim CH, Song SI, Nahm BH, Choi YD, Cheong JJ. 2008. Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis Plant Physiology 146 623–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KH, Seo YS, Walia H, Cao P, Fukao T, Canlas PE, Amonpant F, Bailey-Serres J, Ronald PC. 2010. The submergence tolerance regulator Sub1A mediates stress-responsive expression of AP2/ERF transcription factors Plant Physiology 152 1674–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerk D, Bulgrien J, Smith DW, Gribskov M. 2003. Arabidopsis proteins containing similarity to the universal stress protein domain of bacteria Plant Physiology 131 1209–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K. 2001. phot1 and phot2 mediate blue light regulation of stomatal opening Nature 414 656–660 [DOI] [PubMed] [Google Scholar]
- Kishor P, Hong Z, Miao GH, Hu C, Verma D. 1995. Overexpression of [delta]-pyrroline-5-synthetase increases proline production and confers osmotolerance in transgenic plants Plant Physiology 108 1387–1394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konopka-Postupolska D, Clark G, Goch G, Debski J, Floras K, Cantero A, Fijolek B, Roux S, Hennig J. 2009. The role of annexin 1 in drought stress in Arabidopsis Plant Physiology 150 1394–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs L, Damkjær J, Kereiche S, Ilioaia C, Ruban AV, Boekema EJ, Jansson S, Horton P. 2006. Lack of the light-harvesting complex CP24 affects the structure and function of the grana membranes of higher plant chloroplasts The Plant Cell 18 3106–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvint K, Nachin L, Diez A, Nystrom T. 2003. The bacterial universal stress protein: function and regulation Current Opinion Microbiology 6 140–145 [DOI] [PubMed] [Google Scholar]
- Laohavisit A, Mortimer JC, Demidchik V, et al. 2009. Zea mays annexins modulate cytosolic free Ca2+ and generate a Ca2+-permeable conductance The Plant Cell 21 479–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DW, Tezara W. 2009. Causes of decreased photosynthetic rate and metabolic capacity in water-deficient leaf cells: a critical evaluation of mechanisms and integration of processes Annals of Botany 103 561–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KH, Piao HL, Kim HY, Choi SM, Jiang F, Hartung W, Hwang I, Kwak JM, Lee IJ. 2006. Activation of glucosidase via stress-induced polymerization rapidly increases active pools of abscisic acid Cell 126 1109–1120 [DOI] [PubMed] [Google Scholar]
- Lescot M, Dehais P, Thijs G, Marchal K, Moreau Y, Van de Peer Y, Rouze P, Rombauts S. 2002. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences Nucleic Acids Research 30 325–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li HW, Zang BS, Deng XW, Wang XP. 2011. Overexpression of the trehalose-6-phosphate synthase gene OsTPS1 enhances abiotic stress tolerance in rice Planta 234 1007–1018 [DOI] [PubMed] [Google Scholar]
- Lim EK, Roberts MR, Bowles DJ. 1998. Biochemical characterization of tomato annexin p35 The Journal of Biological Chemistry 273 34920–34925 [DOI] [PubMed] [Google Scholar]
- Lu YE, Ouyang B, Zhang JH, Wang TT, Lu C, Han QQ, Zhao SN, Ye ZB, Li HX. 2012. Genomic organization, phylogenetic comparison and expression profiles of annexin gene family in tomato (Solanum lycopersicum) Gene 499 14–24 [DOI] [PubMed] [Google Scholar]
- Lyudmila VD, Yulia SB, Ekaterina VK, Dmitry LS, Martin RM, Alistair MH, Igor DV. 2011. cGMP-dependent ABA-induced stomatal closure in the ABA-insensitive Arabidopsis mutant abi1-1 New Phytologist 191 57–69 [DOI] [PubMed] [Google Scholar]
- Mahajan S, Tuteja N. 2005. Cold, salinity and drought stresses: an overview Archives of Biochemistry and Biophysics 444 139–158 [DOI] [PubMed] [Google Scholar]
- Mao X, Zhang H, Tian S, Chang X, Jing R. 2010. TaSnRK2.4, an SNF1-type serine/threonine protein kinase of wheat (Triticum aestivum L.), confers enhanced multistress tolerance in Arabidopsis Journal of Experimental Botany 61 683–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maqbool A, Zahur M, Husnain T, Riazuddin S. 2009. GUSP1 and GUSP2, two drought-responsive genes in Gossypium arboreum have homology to universal stress proteins Plant Molecular Biology Reporter 27 109–114 [Google Scholar]
- Mcainsh MR, Brownlee C, Hetherington AM. 1990. Abscisic acid-induced elevation of guard cellcytosolic Ca2+ precedes stomatal closure Nature 343 186–188 [Google Scholar]
- Mortimer JC, Laohavisit A, Macpherson N, Webb A, Brownlee C, Battey NH, Davies JM. 2008. Annexins: multifunctional components of growth and adaptation Journal of Experimental Botany 59 533–544 [DOI] [PubMed] [Google Scholar]
- Nachin L, Nannmark U, Nystrom T. 2005. Differential roles of the universal stress proteins of Escherichia coli in oxidative stress resistance, adhesion, and motility Journal of Bacteriology 187 6265–6272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nystrom T, Neidhardt FC. 1992. Cloning, mapping and nucleotide sequencing of a gene encoding a universal stress protein in Escherichia coli Molecular Microbiology 6 3187–3198 [DOI] [PubMed] [Google Scholar]
- Orellana S, Yanez M, Espinoza A, Verdugo I, Gonzalez E, Ruiz-Lara S, Casaretto JA. 2010. The transcription factor SlAREB1 confers drought, salt stress tolerance and regulates biotic and abiotic stress-related genes in tomato Plant, Cell and Environment 33 2191–2208 [DOI] [PubMed] [Google Scholar]
- Orozco-Cardenas M, Ryan CA. 1999. Hydrogen peroxide is generated systemically in plant leaves by wounding and systemin via the octadecanoid pathway Proceedings of the National Academy of Sciences, USA 96 6553–6557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang B, Chen YH, Li HX, Qian CJ, Huang SL, Ye ZB. 2005. Transformation of tomato with osmotin and chitinase genes and their resistance to Fusarium wilt Journal of Horticultural Science and Biotechnology 80 517–522 [Google Scholar]
- Pfannschmidt T, Bräutigam K, Wagner R, Dietzel L, Schröter Y, Steiner S, Nykytenko A. 2009. Potential regulation of gene expression in photosynthetic cells by redox and energy state: approaches towards better understanding Annals of Botany 103 599–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruneda-Paz JL, Kay SA. 2010. An expanding universe of circadian networks in high plants Trends in Plant Sciences 15 259–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberto B, Dorianna S, Roberta C. 1997. Novel aspects of chlorophyll a/b-binding proteins Physiologia Plantarum 100 769–779 [Google Scholar]
- Roelfsema MR, Hedrich R. 2010. Making sense out of Ca2+ signals: their role in regulating stomatal movements Plant, Cell and Environment 33 305–321 [DOI] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative C(T) method Nature Protocols 3 1101–1108 [DOI] [PubMed] [Google Scholar]
- Seiler C, Harshavardhan VT, Rajesh K, Reddy PS, Strickert M, Rolletschek H, Scholz U, Wobus U, Sreenivasulu N. 2011. ABA biosynthesis and degradation contributing to ABA homeostasis during barley seed development under control and terminal drought-stress conditions Journal of Experimental Botany 62 2615–2632 [DOI] [PubMed] [Google Scholar]
- Seo PJ, Xiang F, Qiao Mg, Park JY, Lee YN, Kim SG, Lee YH, Park WJ, Park CM. 2009. The MYB96 transcription factor mediates abscisic acid signaling during drought stress response in Arabidopsis Plant Physiology 151 275–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin D, Moon SJ, Han S, et al. 2011. Expression of StMYB1R-1, a novel potato single MYB-like domain transcription factor, increases drought tolerance Plant Physiology 155 421–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takashi H, Kazuo S. 2010. Research on plant abiotic stress responses in the post-genome era: past, present and future The Plant Journal 61 1041–1052 [DOI] [PubMed] [Google Scholar]
- Thines B, Harmon FG. 2010. Four easy pieces: mechanisms underlying circadian regulation of growth and development Current Opinion in Plant Biology 14 1–7 [DOI] [PubMed] [Google Scholar]
- Tuteja N. 2007. Mechanisms of high salinity tolerance in plants Methods in Enzymology 428 419–438 [DOI] [PubMed] [Google Scholar]
- Urao T, Yamaguchi-Shinozaki K, Urao S, Shinozaki K. 1993. An Arabidopsis myb homolog is induced by dehydration stress and its gene product binds to the conserved MYB recognition sequence The Plant Cell 5 1529–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendruscolo EC, Schuster I, Pileggi M, Scapim CA, Molinari HB, Marur CJ, Vieira LG. 2007. Stress-induced synthesis of proline confers tolerance to water deficit in transgenic wheat Journal of Plant Physiology 164 1367–1376 [DOI] [PubMed] [Google Scholar]
- Verbruggen N, Hermans C. 2008. Proline accumulation in plants: a review Amino Acids 35 753–759 [DOI] [PubMed] [Google Scholar]
- Walter M, Chaban C, Schutze K, et al. 2004. Visualization of protein interactions in living plant cells using bimolecular fluorescence complementation The Plant Journal 40 428–438 [DOI] [PubMed] [Google Scholar]
- Weide R, Koornneef M, Zabel P. 1989. A simple, non-destructive spraying assay for the detection of an active kanamycin resistance gene in transgenic tomato plants Theoretical and Applied Genetics 78 169–172 [DOI] [PubMed] [Google Scholar]
- Wellburn AR. 1994. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution Journal of Plant Physiology 144 307–313 [Google Scholar]
- Wu G, Ortiz-Flores G, Ortiz-Lopez A, Ort DR. 2007. A point mutation in the atpC1 raises the redox potential of the Arabidopsis chloroplast ATP synthase g-subunit regulatory disulphide above the range of thioredoxin modulation Journal of Biological Chemistry 282 36782–36789 [DOI] [PubMed] [Google Scholar]
- Xu YH, Liu R, Yan L, Liu ZQ, Jiang SC, Shen YY, Wang XF, Zhang DP. 2012. Light-harvesting chlorophyll a/b-binding proteins are required for stomatal response to abscisic acid in Arabidopsis Journal of Experimental Botany 63 1095–1106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. 2005. Organization of cis-acting regulatory elements in osmotic- and cold-stress-responsive promoters Trends in Plant Science 10 88–94 [DOI] [PubMed] [Google Scholar]
- Yang O, Popova OV, Süthoff U, Lüking I, Dietz KJ, Golldack D. 2009. The Arabidopsis basic leucine zipper transcription factor AtbZIP24 regulates complex transcriptional networks involved in abiotic stress resistance Gene 436 45–55 [DOI] [PubMed] [Google Scholar]
- Yang R, Deng C, Ouyang B, Ye Z. 2011. Molecular analysis of two salt-responsive NAC-family genes and their expression analysis in tomato Molecular Biology Reports 38 857–863 [DOI] [PubMed] [Google Scholar]
- Zarembinski TI, Hung LW, Mueller-Dieckmann HJ, Kim KK, Yokota H, Kim R, Kim SH. 1998. Structure-based assignment of the biochemical function of a hypothetical protein: a test case of structural genomics Proceedings of the National Academy of Sciences, USA 95 15189–15193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Yang C, Li Y, Zheng N, Chen H, Zhao Q, Gao T, Guo H, Xie Q. 2007. SDIR1 is a RING finger E3 ligase that positively regulates stress-responsive abscisic acid signaling in Arabidopsis The Plant Cell 19 1912–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziaf K, Loukehaich R, Gong P, Liu H, Han Q, Wang T, Li H, Ye Z. 2011. A multiple stress-responsive gene ERD15 from Solanum pennellii confers stress tolerance in tobacco Plant and Cell Physiology 52 1055–1067 [DOI] [PubMed] [Google Scholar]
- Zou JJ, Wei FJ, Wang C, Wu JJ, Ratnasekera D, Liu WX, Wu WH. 2010. Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca2+- mediated stomatal regulation in response to drought stress Plant Physiology 154 1232–1243 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.