Abstract
Although the alternative oxidase (AOX) has been proposed to play a role in fruit development, the function of AOX in fruit ripening is unclear. To gain further insight into the role of AOX in tomato fruit ripening, transgenic tomato plants 35S-AOX1a and 35S-AOX-RNAi were generated. Tomato plants with reduced LeAOX levels exhibited retarded ripening; reduced carotenoids, respiration, and ethylene production; and the down-regulation of ripening-associated genes. Moreover, no apparent respiratory climacteric occurred in the AOX-reduced tomato fruit, indicating that AOX might play an important role in climacteric respiration. In contrast, the fruit that overexpressed LeAOX1a accumulated more lycopene, though they displayed a similar pattern of ripening to wild-type fruit. Ethylene application promoted fruit ripening and anticipated ethylene production and respiration, including the alternative pathway respiration. Interestingly, the transgenic plants with reduced LeAOX levels failed to ripen after 1-methylcyclopropene (1-MCP) treatment, while such inhibition was notably less effective in 35S-AOX1a fruit. These findings indicate that AOX is involved in respiratory climacteric and ethylene-mediated fruit ripening of tomato.
Key words: Alternative oxidase, climacteric, ethylene, fruit ripening, tomato
Introduction
Fruit ripening is a complex, genetically programmed process that culminates in dramatic changes in colour, texture, flavour, and aroma (Alexander and Grierson, 2002; Klee, 2010). Fruit with different ripening mechanisms can be divided into two groups: climacteric fruit, in which ripening is accompanied by a peak in respiration and a concomitant burst of ethylene, and non-climacteric fruit, in which respiration shows no dramatic change and ethylene production remains at a very low level (White, 2002).
Ethylene has been identified as the major hormone that initiates and controls ripening in climacteric fruit, and its biosynthesis in plant tissues has been studied extensively (Srivastava and Handa, 2005; Argueso et al., 2007). Two systems of ethylene production have been defined in plants. System-1 represents basal ethylene in unripe fruit and vegetative tissues and is regulated in an autoinhibitory manner, whereas system-2 operates during the ripening of climacteric fruit and flower senescence and is autocatalytic (Barry and Giovannoni, 2007; Yokotani et al., 2009). Studies have shown that the suppression of ethylene production by knocking down the expression of 1-aminocyclopropane-1-carboxylate (ACC) oxidase (ACO) and ACC synthase (ACS) resulted in a strong ripening inhibition (Hamilton et al., 1990; Oeller et al., 1991). Conversely, application of ethylene to climacteric fruit at the mature stage stimulates system-2 ethylene biosynthesis, accelerating ripening (Nakatsuka et al., 1998).
It has been proposed that the alternative oxidase (AOX) pathway plays a role in fruit development (Kumar et al., 1990; Considine et al., 2001) but knowledge of its function is still lacking. The AOX branches from the cytochrome c oxidase (COX) pathway at the level of ubiquinone (UQ) and couples the oxidation of ubiquinol to the four-electron reduction of oxygen to water in a manner insensitive to cyanide (CN; an inhibitor of COX) (Vanlerberghe and McIntosh, 1997; Affourtit et al., 2001). AOX is encoded by a small nuclear gene family. In tomato, four isoforms of two types of AOX have shown different expression patterns. LeAOX1a and LeAOX1b transcripts were found in most tomato tissues, including leaves, root, flowers, and fruit. The LeAOX2 transcript was detected in carpels and roots, and the LeAOX1c transcript was expressed preferentially in roots but not in fruit (Holtzapffel et al., 2003; Fung et al., 2006).
In general, AOX is thought to play roles in heat production of thermogenic floral organs and cell adaptation under environmental stresses, such as inhibition of reactive oxidase species (ROS) formation and optimization of photosynthesis (Yoshida et al., 2008; Vanlerberghe et al., 2009; Zhang et al., 2010). To date, limited information exists on the contribution of the AOX pathway to fruit ripening. Some reports suggested that the expression of AOX and the CN-insensitive respiration of isolated mitochondria decreased during post-harvest ripening of tomato (Almeida et al., 1999; Costa et al., 1999; Sluse and Jarmuszkiewicz, 2000). Nevertheless, Holtzapffel et al. (2002) showed that AOX protein levels increased dramatically in tomato fruit ripened on the vine. Similar increases have been reported in mango and apple fruit in which climacteric bursts were associated with an enhanced CN-insensitive respiration (Duque and Arrabaca, 1999; Considine et al., 2001). In this regard, the role of AOX in climacteric fruit ripening requires further investigation.
In this study, a combination of chemical and transgenic approaches were undertaken to explore the role of AOX during tomato ripening. Here, evidence is provided that AOX plays an important role in the respiratory climacteric and may affect system-2 ethylene synthesis during tomato ripening. The relationships and possible mechanisms of these processes are discussed.
Materials and methods
Plant materials and growth conditions
Tomato (Solanum lycopersicum L. cv Hongyan) was used as the wild-type (WT) plant. Tomato seeds were surface-sterilized for 5min in 70% (v/v) ethanol, then for 8min in 10% (w/v) NaClO, followed by several washes in sterile distilled water. Tomato seeds were germinated and grown in artificial climate incubators (25 ºC day, 18 ºC night; 16h light, 8h dark) and transplanted into a greenhouse. The homozygous progeny of transgenic plants were selected and used for subsequent experiments.
Plasmid construction and tomato transformation
To generate the tomato overexpression LeAOX1a vector, the complete open reading frame (ORF) of AOX1a (accession no. AY034148) was amplified by reverse transcription-PCR (RT-PCR) using primers AOX1a-F (5’-TATTTGCCTTCTTCCTCAAGTTTC-3’) and AOX1a-R (5’-AAAAAGGAACAAAATAGTGACGGAC-3’), incorporating restri ction sites for BamHI and EcoRI at the product ends. The amplified 1231bp product was cloned into the pBI121 vector (Clontech, Palo Alto, CA, USA). The AOX1a insert was excised by BamHI and EcoRI, and cloned in the sense orientation driven by the Cauliflower mosaic virus (CaMV) 35S promoter (Fang et al., 1989), and was designated as 35S-AOX1a.
To construct AOX-RNAi vectors, the homologous sequence of the LeAOX1a (accession no. AY034148), LeAOX1b (accession no. AY034149), and LeAOX2 (accession no. AY324396) cDNA fragment was used for a double-stranded RNA interference (RNAi) trial. Sequences from AOX1a cDNA were isolated by RT-PCR using primers AOX1a-UF (5’-TGTATTTTTTCAGAGGAGATATGGT-3’), AOX1a-UR (5’-ATT- ATTAGTCGCTTAGTGATACCCA-3’), AOX1a-DF (5’-AGAGCAA- TGATGTTAGAGACAGTGG-3’), and AOX1a-DR (5’-GCTTAGT- GATACCCAAGTGGTGCTG-3’). An inverted repeat fragment of AOX1a was inserted downstream of the CaMV 35S promoter at the BamHI and EcoRI restriction sites of the modified PBI121. The construct AOX-RNAi was thus generated.
Transgenic plants were generated by Agrobacterium tumefaciens (strain EHA105)-mediated transformation according to the method described previously (Wang et al., 2009), and transformed lines were first selected for kanamycin (70mg l–1) resistance and then analysed by PCR to determine the presence of T-DNA. The primers designed to the NPTII (Kan resistance) marker of PBI121 for confirmation of integration were 5’-GAGAGGCTATTCGGCTATG-3’ and 5’-CTCAGAAGAACTCGTCAAGA-3’.
Ethylene and 1-MCP treatments
Mature green (MG) fruit of uniform sizes were collected, washed with water, and air-dried. For ethylene treatment, fruit were incubated in 500 µl l–1 ethephon (ET) solution in a closed container at room temperature for 12h, and then air-dried and placed at 25±1 ºC. For 1-MCP treatment, fruit were placed in 20 litre containers and exposed to 0.5 µl l–1 1-MCP gas (SmartFresh™, 0.14% a.i., Rohm and Haas, Philadelphia, PA, USA) for 12h at room temperature. Immediately following 1-MCP treatment, fruit were removed from the chambers and stored at 25±1 ºC. Control fruit were treated with deionized water instead of ET or 1-MCP. Three replicates each of 20 fruit were used for each treatment.
Real-time quantitative RT-PCR
Total RNA extraction and qRT-PCR were performed as previously described (Xu et al., 2012). Three replicates were performed for each experiment. ACTIN1 and histone H4 genes were used as internal controls (German et al., 2002; Galpaz et al., 2008). The qRT-PCR primers are listed in Supplementary Table S1 available at JXB online. All the mRNA data were expressed as a percentage of the corresponding ACTIN1 transcript levels.
Respiration measurements
Oxygen consumption was measured using Clark-type electrodes (Hansatech, King’s Lynn, UK) based on the methods of Møller et al. (1988) with some modifications. Fruit pulp (0.05g; adjacent to the peel) was weighed and cut into small pieces, then pre-treated with 5ml of deionized water for 15min in order to minimize the effect of wound-induced respiration. Measurements were performed at 25 ºC in a final volume of 2ml of 2mM phosphate buffer (pH 6.8), and the cuvette was tightly closed to prevent diffusion of oxygen from the air. KCN at a final concentration of 1mM was used to inhibit the COX pathway, and 100 µM n-propyl gallate (nPG) was used to inhibit the AOX pathway. Total respiration (Vt) is defined as the O2 uptake rate without any inhibitor. AOX pathway respiration (Valt) was defined as the O2 uptake rate in the presence of KCN that was sensitive to nPG. Residual respiration (Vres) is defined as the O2 uptake in the presence of both 1mM KCN and 100 µM nPG. COX pathway respiration (Vcyt) was calculated by the formula: Vcyt=Vt–Valt–Vres.
Ethylene production
A whole tomato fruit was placed in a 10cm × 10cm closed container at 25±1 ºC, 85% relative humidity for 2h. Then, a 1ml gas sample from the headspace of each container was injected into a flame ionization detection gas chromatograph (Agilent 6890 Series GC system, UK) equipped with an activated alumina stainless steel column. The carrier gas (helium) flow rate was 0.5ml s−1. The detector and injector were operated at 100 ºC and the oven temperature was 50 ºC.
ATP and HCN measurements
ATP was extracted from tomato tissues and quantified as previously described (Zheng et al., 2009). A 1g aliquot of tomato tissue was finely sliced and put into 5ml of acetone, and then kept in a boiling water bath for 5min. A 3ml aliquot of 20mM TRIS-HCl buffer at pH 7.6 was added and samples were heated in a boiled water bath for 10min, and then immediately cooled in an ice bath. The extract was centrifuged at 3000 g for 10min, and the supernatant was collected. Bioluminescence was measured with the ATP Bioluminescent Assay Kit (Sigma, St Louis, MO, USA) using an SHG-D Bioluminescence Meter (Analytical and Testing Center, Chengdu, China).
Hydrogen cyanide (HCN) was measured by the method of Dicenta et al. (2002). A 1g aliquot of pulp was processed with 0.2g of β-glucosidase (Sigma) in 4ml of acetate buffer (pH 5.5) in a cylindrical glass vessel for 2h at 35 ºC. The HCN released was collected by microdiffusion in 1ml of 0.2M NaOH, located in a very small glass collector in the interior of the vessel. HCN content was estimated at 580nm using a spectrophotometer (TU1800 spectrophotometer, P-general Limited Comp., Beijing, China).
Statistical analysis
Data were analysed by one-way analysis of variance (ANOVA) and means were compared by a Dunnett’s test at P < 0.05.
Results
LeAOX gene expression and CN-insensitive respiration during fruit ripening
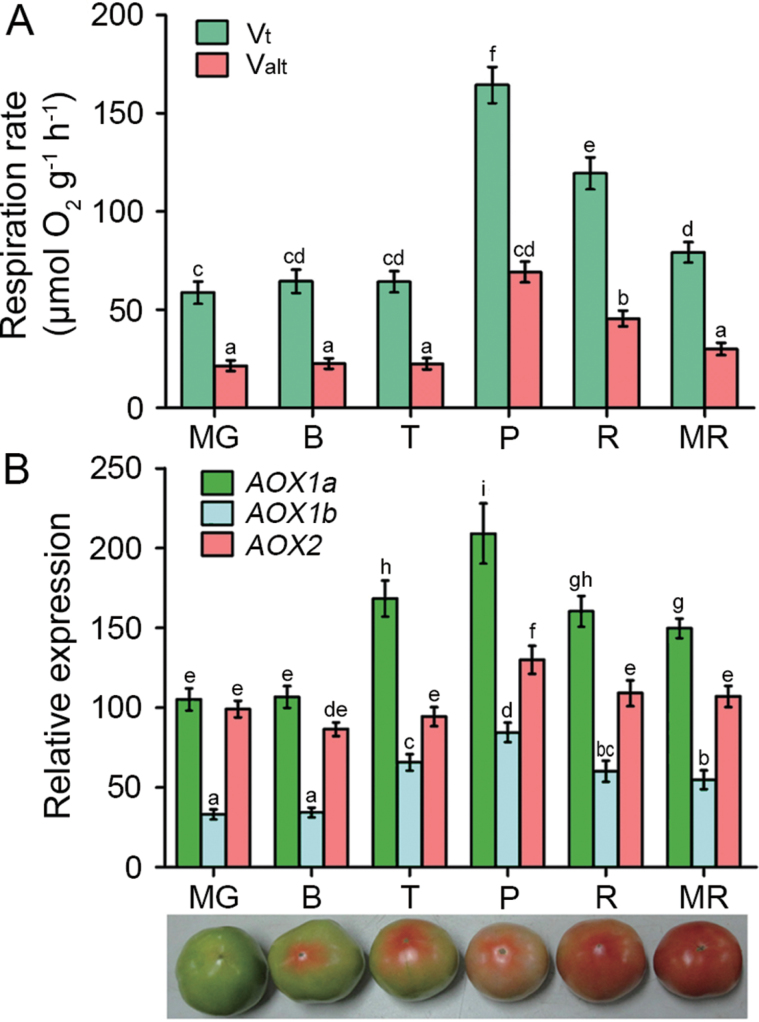
As shown in Fig. 1A, the total respiration (Vt) changed little from the mature green (MG) to the turning (T) stage. It peaked at the pink (P) stage and subsequently decreased. CN-insensitive respiration (Valt) followed a pattern similar to that of Vt. Valt increased 3-fold from the MG to the P stage when it accounted for 40% of the Vt at the P stage (Fig. 1A). The results suggest that the AOX pathway respiration may contribute to the respiration climacteric during fruit ripening.
Fig. 1.
Changes in the respiration and expression of LeAOX genes during tomato ripening. (A) Total respiration (Vt) and CN-insensitive respiration (Valt) were determined at different ripening stages. (B) Relative expression profiles of LeAOX1a, LeAOX1b, and LeAOX2 during fruit ripening obtained by quantitative RT-PCR. MG, mature green; B, breaker; T, turning; P, pink; R, red; and MR, mature red. Data are the means ±SD of three independent experiments. Significant differences (P < 0.05) are denoted by different letters.
To identify the possible involvement of the tomato AOX genes, their expression at different ripening stages was measured. LeAOX1a expression increased significantly at the T stage and peaked at the P stage, whereas LeAOX1b and LeAOX2 were expressed at a relatively low level during fruit ripening, although their expression pattern was similar to that of LeAOX1a (Fig. 1B). These data suggest that LeAOX1a might play a dominant role in the AOX pathway respiration.
LeAOX1a expression is stimulated by ethylene and inhibited by 1-MCP treatment
To investigate whether AOX expression is ethylene dependent, MG fruit were treated with ET or 1-MCP. As shown in Supplementary Fig. S1A at JXB online, the respiratory climacteric of the ET-treated fruit occurred 2 d earlier than in the control fruit. Also, CN-insensitive respiration was up-regulated and peaked at the same time as the respiratory climacteric following ET treatment (Supplementary Fig. S1B). 1-MCP treatment had the opposite effect on fruit respiration, in that both Vt and Valt were markedly suppressed and their peaks occurred later (Supplementary Fig. S1B).
Notably, LeAOX1a was significantly anticipated when ET was applied to MG fruit compared with the control fruit (Supplementary Fig. S1C). In contrast, 1-MCP treatment reduced the transcript levels of LeAOX1a, suggesting that its expression is ethylene regulated. No alterations in LeAOX1b or LeAOX2 transcript levels were observed upon the exposure to ET, although they were repressed by the 1-MCP treatment (Supplementary Fig. S1D, E at JXB online).
Effect of AOX overexpression and suppression on fruit ripening
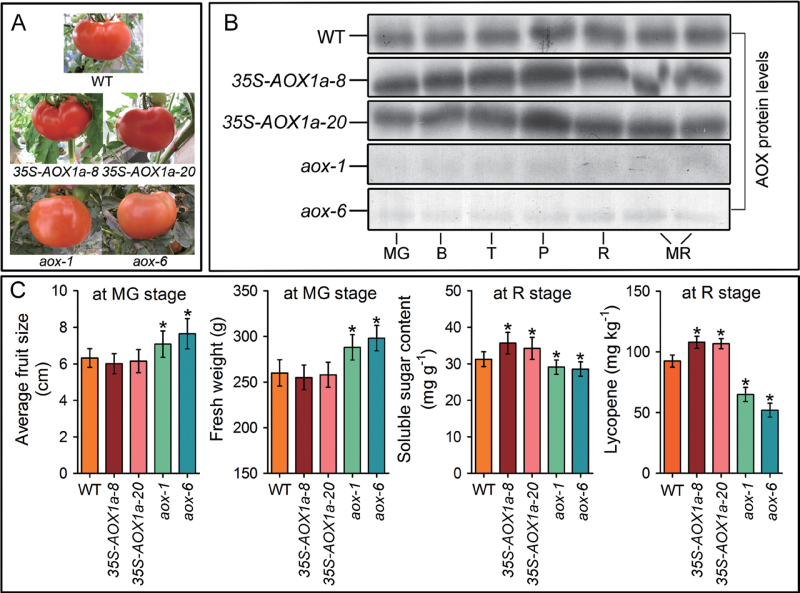
To gain further insight into LeAOX function, transgenic tomato plants overexpressing LeAOX1a (35S-AOX1a) or suppressing LeAOX (AOX-RNAi) were generated. As shown in Supplementary Fig. S2A at JXB online, all eight 35S-AOX1a transgenic lines that were tested showed increased levels of LeAOX1a, and the 35S-AOX1a-8 and 35S-AOX1a-20 lines showed the greatest expression levels. In contrast, there were no significant differences in the expression of LeAOX1b and LeAOX2 between the transgenic and WT plants. Among the six AOX-RNAi transgenic lines, lines 1 and 6 exhibited the most severe LeAOX reduction (90% for the LeAOX1a transcript and ~50% for the LeAOX1b and LeAOX2 transcripts) (Supplementary Fig. S2B). Therefore, the 35S-AOX1a-8, 35S-AOX1a-20, aox-1, and aox-6 transgenic lines were selected for further experiments.
AOX overexpression or down-regulation did not result in noticeable changes in flowering time but affected ripening (Supplementary Fig. S3 at JXB online). The AOX-RNAi fruit had a longer ripening time [from the MG to the red (R) stage], and, on average, the fruit was larger than control and AOX-overexpressing tomatoes (Fig. 2). Reduction of AOX increased fresh weight and reduced soluble solids and lycopene upon ripening (Fig. 2C). AOX silencing also led to prolonged post-harvest ripening (Fig. 3A). These fruit had reduced peel colour (hue angle), loss of firmness, and water loss in comparison with WT and 35S-AOX1a tomatoes (Supplementary Fig. S4). In contrast, the 35S-AOX1a fruit reached maturity first during on-vine or off-vine ripening and accumulated more lycopene content at the R stage when compared with WT fruit (Fig. 2).
Fig. 2.
Physiological, morphological, and metabolic characterization of the wild-type (WT) and transgenic fruit. (A) Representative red fruit of WT and transgenic plants in the T2 generation. (B) Changes in the AOX protein (~33kDa) levels during fruit ripening in WT and transgenic fruit. (C) Average fruit size and fresh weight of the WT and transgenic fruit at the MG (mature green) stage were measured, respectively. Soluble sugar and lycopene content of the WT and transgenic fruit at the R (red) stage were measured, respectively. Data are the means ±SD of three independent experiments. The asterisks indicate statistically significant differences between the WT and transgenic fruit (P < 0.05).
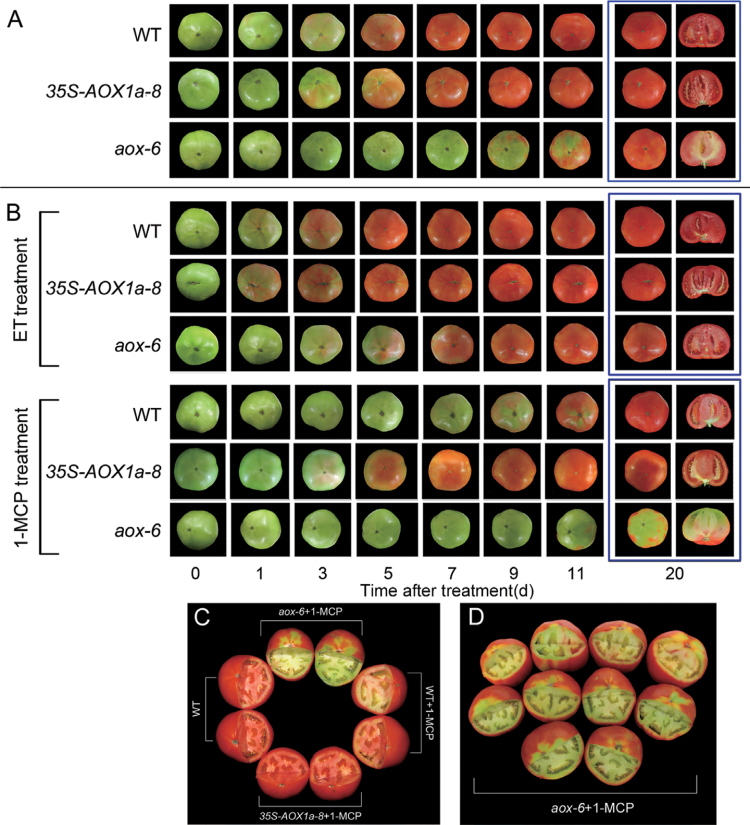
Fig. 3.
Post-harvest ripening of wild-type (WT) and transgenic fruit with or without 500 µl l–1 ET or 0.5 µl l–1 1-MCP treatment. (A) Representative ripening of WT, 35S-AOX1a, and AOX-RNAi tomatoes. (B) Representative ripening of WT, 35S-AOX1a, and AOX-RNAi tomatoes in response to ET or 1-MCP treatment. (C and D) Representative phenotypes of the 1-MCP-treated WT, 35S-AOX1a, and AOX-RNAi tomatoes after 30 d of storage.
Changing AOX levels affects ethylene production and respiration
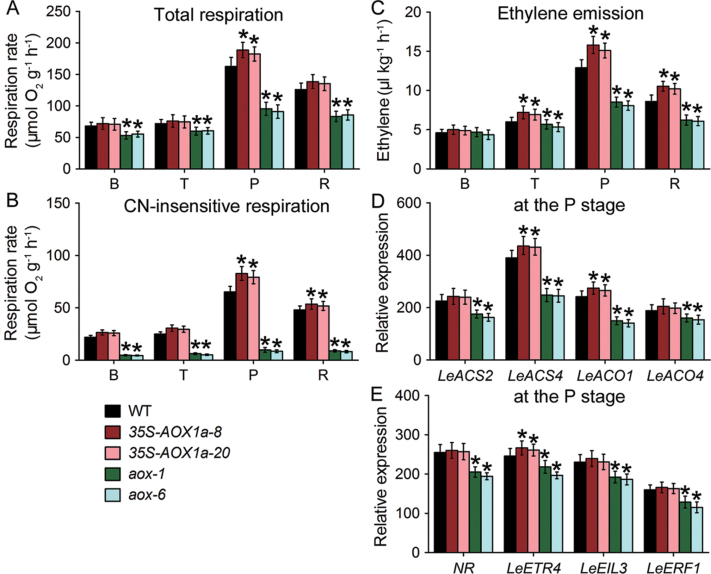
No respiratory climacteric was found during the ripening of AOX-RNAi fruit (Fig. 4). The respiration pattern of 35S-AOX1a fruit was similar to that of WT fruit, although the climacteric peak of 35S-AOX1a fruit was higher than that of WT fruit (Fig. 4). Consistent with the changes in CN-insensitive respiration during ripening, the AOX protein levels were altered in the transgenic fruit. The expression of AOX in WT and 35S-AOX1a fruit peaked at the P stage, and then decreased slightly, whereas it was barely detectable in AOX-RNAi fruit throughout ripening (Fig. 2B). Interestingly, ethylene production was higher in 35S-AOX1a fruit than in WT fruit, whereas it was suppressed in AOX-RNAi fruit (Fig. 4), especially at the P stage, suggesting that the down-regulation of AOX influences ethylene synthesis.
Fig. 4.
Changes in the respiration and ethylene emission during ripening in wild-type (WT) and transgenic fruit. Total respiration (A), CN-insensitive respiration (B), and ethylene production (C) were measured at the B (breaker), T (turning), P (pink), and R (red) stage in WT and transgenic fruit. (D and E) Quantitative RT-PCR analyses of the transcript levels of ethylene synthesis- (LeACS2, LeACS4, LeACO1, and LeACO4) and ethylene signal transduction- (NR, LeETR4, LeEIL3 and LeERF1) related genes at the P stage. Data are the means ±SD of three independent experiments. The asterisks indicate statistically significant differences between the WT and transgenic fruit (P < 0.05).
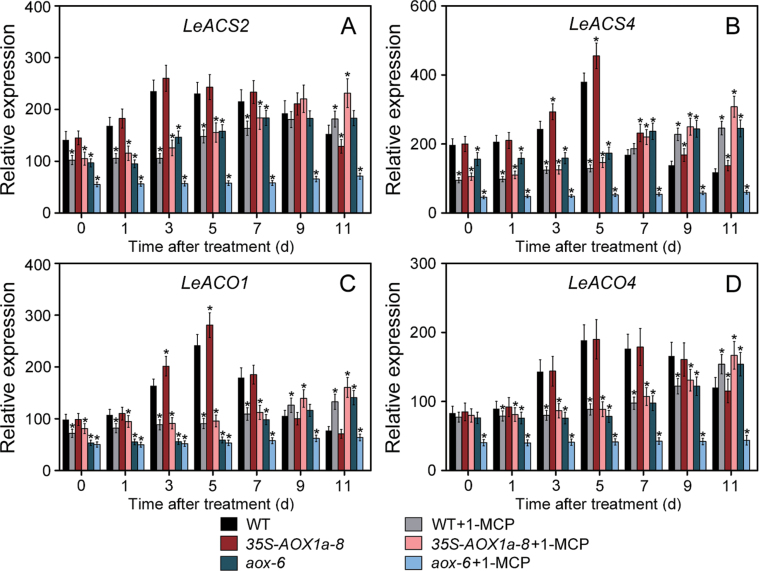
The characterization of the expression of ethylene biosynthesis genes indicated that the ACC synthase4 (LeACS4) mRNA in AOX-RNAi fruit was substantially repressed at climacteric (P stage), as compared with WT fruit, and the LeACS2 transcript was repressed by 20% (Fig. 4D). Moreover, AOX-repressed fruit showed dramatic reductions in the transcript level of ACC oxidase1 (LeACO1), which was repressed by >40%, whereas LeACO4 expression was repressed to an extent similar to that of the expression of LeACS2 (Fig. 4D). In contrast, the mRNA levels of these genes were slightly higher in AOX1a-overexpressing fruit than in WT fruit at the P stage (Fig. 4D). Therefore, the ethylene reduction in AOX-RNAi fruit may be attributed to the down-regulation of the key genes in ethylene biosynthesis, especially via the down-regulation of LeACS4 and LeACO1 expression. In addition, there was a reduced accumulation in the transcript levels of a number of ethylene-regulated genes, including polygalacturonase (LePG) and the carotenoid synthesis enzyme, phytoene synthase1 (LePSY1); these results are consistent with the reduced ethylene levels in AOX-silenced fruit (Supplementary Fig. S5 at JXB online).
The results also showed that several genes that are involved in ethylene signal transduction were altered in the transgenic fruit. Transcripts of NR (LeETR3), LeETR4, LeEIL3, and LeERF1 were slightly up-regulated in the 35S-AOX1a fruit compared with WT fruit, but were suppressed in AOX-RNAi fruit (Fig. 4E), suggesting that AOX might affect the flux through the ethylene signalling pathway during ripening.
AOX-silenced fruit fail to ripen after 1-MCP treatment
The interactions between ethylene and AOX were further studied following the application of ET or 1-MCP. ET application at the MG stage promoted fruit ripening, as WT and 35S-AOX1a fruit reached ripening at 5 d after the treatment (Fig. 3B). This treatment also promoted ripening in AOX-RNAi fruit, which were fully ripe 5–7 d earlier than the water-treated control fruit. It is important to note that ET treatment recovered the impaired ripening phenotype but did not recover the ripening rate of AOX-silenced fruit to an extent similar to that of the ET-treated WT fruit. In contrast, fruit ripening was significantly delayed by the 1-MCP treatment. However, ripening of 35S-AOX1a was less affected by the 1-MCP treatment compared with WT fruit, which were fully ripe after 11 d of storage (Fig. 3B). When AOX-RNAi fruit was treated with 1-MCP, ripening was nearly blocked (Fig. 3C, 3D).
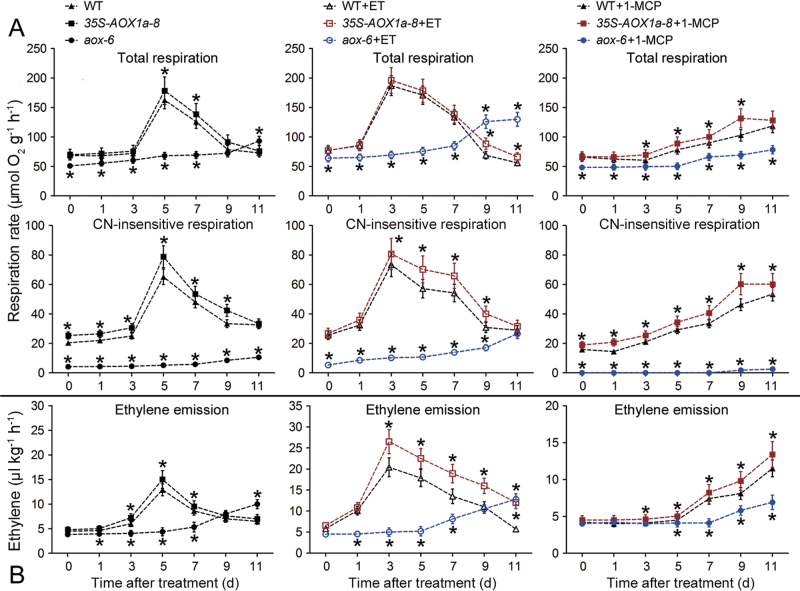
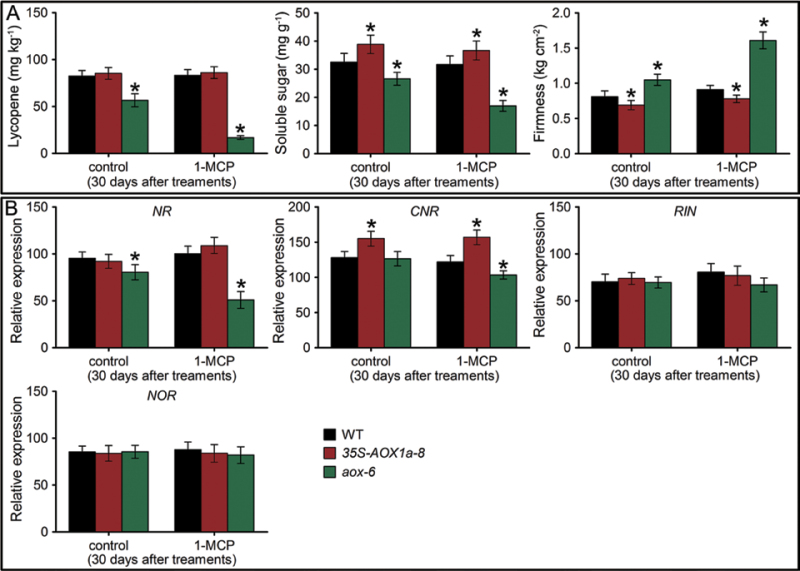
Consistent with the observed fruit ripening, the ET treatment significantly promoted total respiration, CN-insensitive respiration, and ethylene emission in WT and 35S-AOX1a fruit, while only a slight increase occurred in AOX-RNAi fruit (Fig. 5), supporting the idea that AOX plays an important role in ethylene autocatalysis. 1-MCP treatment delayed respiration and ethylene peaks in WT and AOX1a-overexpressing fruit (Fig. 5). Indeed, ethylene production was further suppressed in 1-MCP-treated AOX-RNAi fruit. In agreement with the observed lower ethylene production, several key genes involved in ethylene biosynthesis in AOX-RNAi fruit were significantly down-regulated after the 1-MCP treatment (Fig. 6). In addition, characterization of the ripening metabolism indicated that the lycopene accumulation and the soluble sugar content were substantially repressed in 1-MCP-treated AOX-RNAi fruit, which still maintained higher fruit firmness after 30 d of storage than the 1-MCP-treated WT and AOX1a-overexpressing fruit (Fig. 7A).
Fig. 5.
Dynamic changes in the respiration (A) and ethylene production (B) during post-harvest ripening in wild-type (WT) and transgenic fruit with or without 500 µl l–1 ET or 0.5 µl l–1 1-MCP treatment. Data are the means ±SD of three independent experiments. The asterisks indicate statistically significant differences between the WT and transgenic fruit (P < 0.05).
Fig. 6.
Changes in the transcript levels of ethylene synthesis-related genes in wild-type (WT) and transgenic fruit during post-harvest ripening in response to 0.5 µl l–1 1-MCP treatment. Relative expression profiles of LeACS2 (A), LeACS4 (B), LeACO1 (C), and LeACO4 (D) in 1-MCP-treated WT and transgenic fruit obtained by quantitative RT-PCR. Data are the means ±SD of three independent experiments. The asterisks indicate statistically significant differences from the water-treated WT fruit (P < 0.05).
Fig. 7.
Fruit quality and ripening-related gene comparison of the 1-MCP-treated wild-type (WT) and transgenic fruit. (A) Comparison of the lycopene content, soluble sugar content, and fruit firmness in WT and transgenic fruit 30 d after 1-MCP treatment. (B) Comparison of the transcript levels of NR, CNR, RIN, and NOR in WT and transgenic fruit 30 d after 1-MCP treatment. Data are the means ±SD of three independent experiments. The asterisks indicate statistically significant differences between the WT and transgenic fruit (P < 0.05).
To confirm these results further, the expression profiles of regulatory genes previously described as involved in the ripening of tomato fruit, such as RIPENING INHIBITOR (RIN), COLORLESS NON RIPENING (CNR), NON-RIPENING (NOR), and NEVER RIPE (NR) were analysed (Tigchelaar et al., 1978; Wilkinson et al., 1995; Vrebalov et al., 2002; Manning et al., 2006). NR and CNR were down-regulated in 1-MCP-treated AOX-RNAi fruit, and their transcript levels were lower in AOX-RNAi fruit than in WT and 35S-AOX1a fruit, especially for the expression of NR (Fig. 7B). These results are consistent with the notion that NR expression in tomato fruit is positively regulated by ethylene (Wilkinson et al., 1995; Nakatsuka et al., 1998). No difference in the expression of RIN and NOR was observed between WT and transgenic fruit in response to 1-MCP (Fig. 7B).
ATP and HCN contents correlate with ethylene production and AOX levels
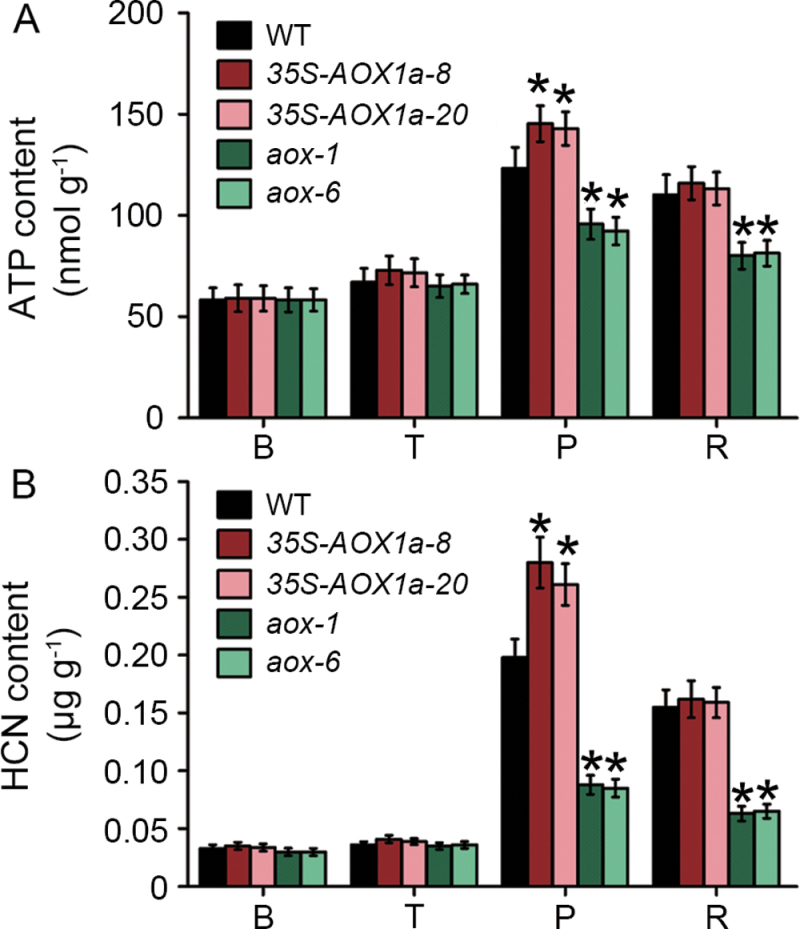
As shown in Fig. 8A, the ATP content increased at the T stage and peaked at the P stage during the ripening of WT and 35S-AOX1a fruit; this result was consistent with the changes in total respiration (Fig. 4A). AOX-RNAi fruit had a normal basal level of ATP, which however did not show a similar changing pattern to WT and 35S-AOX1a fruit. In contrast, at the P stage, the ATP levels in AOX-RNAi fruit were significantly lower than in WT and 35S-AOX1a fruit (Fig. 8A). Notably, the HCN content was very low before the climacteric, abundant at the climacteric, and then rapidly diminished (Fig. 8B). Compared with WT fruit, the peak HCN content was higher in 35S-AOX1a fruit but lower in AOX-RNAi fruit.
Fig. 8.
Changes in the ATP (A) and HCN (B) levels during ripening in wild-type (WT) and transgenic tomatoes. Data are the means ±SD of three independent experiments. The asterisks indicate statistically significant differences between the WT and transgenic fruit (P < 0.05). B, breaker stage; T, turning stage; P, pink stage; R, red stage.
Discussion
The data presented here demonstrate that AOX plays an important role in controlling tomato ripening. The overexpression of LeAOX1a did not change the pattern of fruit ripening, but it contributed to offsetting the inhibitory effect of the ethylene perception inhibitor, 1-MCP. In contrast, the reduction of AOX expression affected ethylene action and delayed ripening. Thus, AOX might be a newly identified component of the currently known regulatory network that controls fleshy fruit ripening.
Although several studies have revealed that AOX may play a role in the respiration climacteric (Duque and Arrabaca, 1999) or post-climacteric senescent processes during fruit ripening (Considine et al., 2001), no detailed study on tomato fruit has used transgenic plants or mutants; therefore, the precise role of AOX in respiratory climacteric is still to be determined. It was shown that in mango the activity of AOX peaks after climacteric respiration, which contributed to fruit senescence, rather than the respiratory climacteric (Considine et al., 2001). However, studies in apple fruit demonstrated that climacteric increases in respiration during fruit ripening were linked to increased AOX capacity and that AOX was induced at the climacteric during post-harvest storage (Duque and Arrabaca, 1999). The present data are consistent with the latter observation. In WT fruit, the respiratory climacteric occurred at the P stage (or after 5 d post-tharvest storage), concomitant with a burst of CN-insensitive respiration (Fig. 1; Supplementary Fig. S1 at JXB online). Furthermore, ET treatment anticipated total respiration and the capacity of the AOX pathway; while in the AOX-silenced fruit, no apparent respiratory climacteric occurred during on-vine or post-harvest ripening (Figs 4, 45). Even after the ET treatment, the respiratory pattern of the AOX-silenced fruit was not restored to that of the WT fruit (Fig. 5). These results indicate that the AOX pathway is an important component in achieving the respiration peak and that the role of AOX in the tomato respiratory climacteric cannot be substituted by ET treatment. Indeed, previous studies demonstrated that AOX activity in vivo may be regulated in a feed-forward fashion by upstream respiratory carbon metabolism; for example, intramitochondrial pyruvate, a potent activator of the AOX, was proved to stimulate the AOX capacity (Millar et al., 1993; Day and Wiskich, 1995; Pastore et al., 2001). Moreover, it was found that glycolysis is stimulated at the climacteric peak: rapid flux through the glycolytic pathway will lead to increased pyruvate production and accumulation of intramitochondrial reducing power, and these metabolic conditions are likely to result in an enhanced activity of the AOX (Duque et al., 1999). These findings reveal the importance of AOX in climacteric burst and fruit ripening.
Despite no direct connection between AOX and ethylene being found in previous studies, this work suggests that AOX plays an important role in climacteric ripening, probably through interactions with the ethylene pathway. The present data showed that AOX-RNAi fruit had a long shelf life during post-harvest storage and delayed on-vine fruit ripening (Fig. 3; Supplementary Fig. S3 at JXB online). Furthermore, no apparent ethylene climacteric was observed during the AOX-RNAi fruit ripening, and ET treatment did not fully restore the ripening rate of the AOX-silenced fruit (Fig. 3B). These observations suggest that AOX plays a partial role in ethylene signal transduction and might be necessary for ethylene autocatalysis. In fact, the overexpression of LeAOX1a led to slightly higher ethylene production during ripening, especially at the peak time (Fig. 4).
It should be noted that AOX-silenced fruit do not show ethylene or respiration bursts but can reach the R stage, indicating that the climacteric is dispensable for ripening to occur in tomato. More interestingly, the 1-MCP-treated AOX-RNAi fruit failed to ripen. It thus seems likely that both AOX and ethylene contribute to fruit ripening and that the inhibition of both AOX and ethylene is required to halt tomato ripening completely. Expression analyses of ripening genes such as NR, CNR, RIN, and NOR revealed that NR and CNR mRNA accumulation was reduced in the 1-MCP-treated AOX-RNAi fruit (Fig. 7). Considering that the NR gene acts downstream of the ethylene pathway but CNR acts upstream (Adams-Phillips et al., 2004; Barry and Giovannoni, 2007), the reduction of these transcripts implicates that AOX has some unexpected roles in fruit ripening. Further work will be necessary to understand the cross-regulation between the AOX pathway and ripening-associated transcription factors, since both ethylene-dependent and ethylene-independent regulatory pathways co-exist to coordinate the ripening process in climacteric fruit (Alba et al., 2000; Pech et al., 2008).
Relationships between AOX and ethylene probably exist. ATP, which is generated during respiration and is required for ethylene biosynthesis (Yang and Hoffman, 1984; Genard and Gouble, 2005) and fruit metabolism (Barry and Giovannoni, 2007), is a possible candidate, although AOX respiration is uncoupled from ATP generation. It is known that AOX can allow carbon flow through glycolysis and the citric acid cycle by removing excess carbohydrates and avoiding the over-reduction of the electron transport chain as well (Borecky and Vercesi, 2005). Therefore, it seems that the AOX pathway respiration increases rapidly and is accompanied by the respiratory climacteric to enable high turnover rates of carbon, thus allowing the large amount of ATP that is needed for system-2 ethylene synthesis and a series of ethylene-regulated ripening processes to be produced. Increased ethylene, in turn, further induces the CN-insensitive respiration by itself or by its co-product CN (Yip and Yang, 1988). CN has been reported to activate the AOX genes transcriptionally in tobacco and maize (Ederli et al., 2006). Moreover, CN treatment causes a rise in respiration and the ripening response in many fruit very similar to that evoked by ethylene (Solomos and Laties, 1974, 1976; Tucker and Laties, 1984). Therefore, it is possible that increased ethylene biosynthesis during fruit ripening results in increased HCN levels, which in turn induces AOX expression and triggers CN-insensitive respiration. Here, an interesting observation was that the HCN content accumulated extensively at the climacteric during wild-type fruit ripening (Fig. 8), which was consistent with the burst of ethylene production (Fig. 4). In the AOX-silenced fruit, the detectable HCN content was lower than that in the wild-type fruit. It should be pointed out that, although β-cyanoalanine synthase (β-CAS), which detoxifies HCN, is localized mainly in the mitochondria (Millenaar and Lambers, 2003; Ebbs et al., 2010), the HCN content that is produced during ethylene synthesis might be high enough to induce AOX expression and promote CN-insensitive respiration at the climacteric. In fact, whether CN acts as a signal molecule, or only as a toxic byproduct of ethylene metabolism, needs to be determined in further studies.
Supplementary data
Supplementary data are available at JXB online.
Figure S1. Response of respiration and LeAOX genes to ET or 1-MCP treatment during post-harvest ripening in tomato.
Figure S2. Transcript levels of LeAOX in the transgenic plants.
Figure S3. Representative phenotypes of wild-type and transgenic plants in the T1 generation.
Figure S4. Changes in the hue angle, fruit firmness, and water loss during ripening in wild-type and transgenic tomatoes.
Figure S5. Changes in the expression of LePG and LePSY1 during ripening in wild-type and transgenic fruit.
Supplementary Material
Acknowledgements
This work was supported by the National Nature Science Foundation of China (91017004, 31070210, 30970214, and J1103518), the National Key Basic Research ‘973’ Program of China (2009CB118500), the Sichuan and Chengdu Nature Science Foundation (2010JQ0080 and 11DXYB097JH-027), and the PhD Programs Foundation of Ministry of Education of China (20110181110059).
References
- Adams-Phillips L, Barry C, Giovannoni J. 2004. Signal transduction systems regulating fruit ripening Trends in Plant Science 9 331–338 [DOI] [PubMed] [Google Scholar]
- Affourtit C, Krab K, Moore AL. 2001. Control of plant mitochondrial respiration Biochimica et Biophysica Acta 1504 58–69 [DOI] [PubMed] [Google Scholar]
- Alba R, Cordonnier-Pratt MM, Pratt LH. 2000. Fruit-localized phytochromes regulate lycopene accumulation independently of ethylene production in tomato Plant Physiology 123 363–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander L, Grierson D. 2002. Ethylene biosynthesis and action in tomato: a model for climacteric fruit ripening Journal of Experimental Botany 53 2039–2055 [DOI] [PubMed] [Google Scholar]
- Almeida AM, Jarmuszkiewicz W, Khomsi H, Arruda P, Vercesi AE, Sluse FE. 1999. Cyanide-resistant, ATP-synthesis-sustained, and uncoupling-protein-sustained respiration during postharvest ripening of tomato fruit Plant Physiology 119 1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argueso CT, Hansen M, Kieber JJ. 2007. Regulation of ethylene biosynthesis Journal of Plant Growth Regulation 26 92–105 [Google Scholar]
- Barry CS, Giovannoni JJ. 2007. Ethylene and fruit ripening Journal of Plant Growth Regulation 26 143–159 [Google Scholar]
- Borecky J, Vercesi AE. 2005. Plant uncoupling mitochondrial protein and alternative oxidase: energy metabolism and stress Bioscience Reports 25 271–286 [DOI] [PubMed] [Google Scholar]
- Considine MJ, Daley DO, Whelan J. 2001. The expression of alternative oxidase and uncoupling protein during fruit ripening in mango Plant Physiology 126 1619–1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa ADT, Nantes IL, Jezek P, Leite A, Arruda P, Vercesi AE. 1999. Plant uncoupling mitochondrial protein activity in mitochondria isolated from tomatoes at different stages of ripening Journal of Bioenergetics and Biomembranes 31 527–533 [DOI] [PubMed] [Google Scholar]
- Day DA, Wiskich JT. 1995. Regulation of alternative oxidase activity in higher plants Journal of Bioenergetics and Biomembranes 27 379–385 [DOI] [PubMed] [Google Scholar]
- Dicenta F, Martínez-Gómez P, Grane N, Martin M, León A, Cánovas J, Berenguer V. 2002. Relationship between cyanogenic compounds in kernels, leaves, and roots of sweet and bitter kernelled almonds Journal of Agricultural and Food Chemistry 50 2149–2152 [DOI] [PubMed] [Google Scholar]
- Duque P, Arrabaca JD. 1999. Respiratory metabolism during cold storage of apple fruit. II. Alternative oxidase is induced at the climacteric Physiologia Plantarum 107 24–31 [Google Scholar]
- Duque P, Barreiro MG, Arrabaca JD. 1999. Respiratory metabolism during cold storage of apple fruit. I. Sucrose metabolism and glycolysis Physiologia Plantarum 107 14–23 [Google Scholar]
- Ebbs SD, Kosma DK, Nielson EH, Machingura M, Baker AJ, Woodrow IE. 2010. Nitrogen supply and cyanide concentration influence the enrichment of nitrogen from cyanide in wheat (Triticum aestivum L.) and sorghum (Sorghum bicolor L.) Plant, Cell and Environment 33 1152–1160 [DOI] [PubMed] [Google Scholar]
- Ederli L, Morettini R, Borgogni A, Wasternack C, Miersch O, Reale L, Ferranti F, Tosti N, Pasqualini S. 2006. Interaction between nitric oxide and ethylene in the induction of alternative oxidase in ozone-treated tobacco plants Plant Physiology 142 595–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang RX, Nagy F, Sivasubramaniam S, Chua NH. 1989. Multiple cis regulatory elements for maximal expression of the cauliflower mosaic virus 35S promoter in transgenic plants The Plant Cell 1 141–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fung RWM, Wang CY, Smith DL, Gross KC, Tao Y, Tian M. 2006. Characterization of alternative oxidase (AOX) gene expression in response to methyl salicylate and methyl jasmonate pre-treatment and low temperature in tomatoes Journal of Plant Physiology 163 1049–1060 [DOI] [PubMed] [Google Scholar]
- Galpaz N, Wang Q, Menda N, Zamir D, Hirschberg J. 2008. Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content The Plant Journal 53 717–730 [DOI] [PubMed] [Google Scholar]
- Genard M, Gouble B. 2005. ETHY. A theory of fruit climacteric ethylene emission Plant Physiology 139 531–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- German MA, Dai N, Chmelnitsky I, Sobolev I, Salts Y, Barg R, Schaffer AA, Granot D. 2002. LeFRK4, a novel tomato (Lycopersicon esculentum Mill.) fructokinase specifically expressed in stamens Plant Science 163 607–613 [Google Scholar]
- Hamilton AJ, Lycett GW, Grierson D. 1990. Antisense gene that inhibits synthesis of the hormone ethylene in transgenic plants Nature 346 284–287 [Google Scholar]
- Holtzapffel RC, Castelli J, Finnegan PM, Millar AH, Whelan J, Day DA. 2003. A tomato alternative oxidase protein with altered regulatory properties Biochimica et Biophysica Acta 1606 153–162 [DOI] [PubMed] [Google Scholar]
- Holtzapffel RC, Finnegan PM, Millar AH, Badger MR, Day DA. 2002. Mitochondrial protein expression in tomato fruit during on-vine ripening and cold storage Functional Plant Biology 29 827–834 [DOI] [PubMed] [Google Scholar]
- Klee HJ. 2010. Improving the flavor of fresh fruits: genomics, biochemistry, and biotechnology New Phytologist 187 44–56 [DOI] [PubMed] [Google Scholar]
- Kumar S, Patil BC, Sinha SK. 1990. Cyanide resistant respiration is involved in temperature rise in ripening mangoes Biochemical and Biophysical Research Communications 168 818–822 [DOI] [PubMed] [Google Scholar]
- Møller IM, Bérczi A, Plas LHW, Lambers H. 1988. Measurement of the activity and capacity of the alternative pathway in intact plant tissues: identification of problems and possible solutions Physiologia Plantarum 72 642–649 [Google Scholar]
- Manning K, Tör M, Poole M, Hong Y, Thompson AJ, King GJ, Giovannoni JJ, Seymour GB. 2006. A naturally occurring epigenetic mutation in a gene encoding an SBP-box transcription factor inhibits tomato fruit ripening Nature Genetics 38 948–952 [DOI] [PubMed] [Google Scholar]
- Millar AH, Wiskich JT, Whelan J, Day DA. 1993. Organic acid activation of the alternatlve oxidase of plant mitochondria FEBS Letters 329 259–262 [DOI] [PubMed] [Google Scholar]
- Millenaar F, Lambers H. 2003. The alternative oxidase: in vivo regulation and function Plant Biology 5 2–15 [Google Scholar]
- Nakatsuka A, Murachi S, Okunishi H, Shiomi S, Nakano R, Kubo Y, Inaba A. 1998. Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening Plant Physiology 118 1295–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeller PW, Lu MW, Taylor LP, Pike DA, Theologis A. 1991. Reversible inhibition of tomato fruit senescence by antisense RNA Science 254 437–439 [DOI] [PubMed] [Google Scholar]
- Pastore D, Trono D, Laus MN, Di Fonzo N, Passarella S. 2001. Alternative oxidase in durum wheat mitochondria. Activation by pyruvate, hydroxypyruvate and glyoxylate and physiological role Plant and Cell Physiology 42 1373–1382 [DOI] [PubMed] [Google Scholar]
- Pech JC, Bouzayen M, Latche A. 2008. Climacteric fruit ripening: ethylene-dependent and independent regulation of ripening pathways in melon fruit Plant Science 175 114–120 [Google Scholar]
- Sluse FE, Jarmuszkiewicz W. 2000. Activity and functional interaction of alternative oxidase and uncoupling protein in mitochondria from tomato fruit Brazilian Journal of Medical and Biological Research 33 259–268 [DOI] [PubMed] [Google Scholar]
- Solomos T, Laties GG. 1974. Similarities between the actions of ethylene and cyanide in initiating the climacteric and ripening of avocados Plant Physiology 54 506–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomos T, Laties GG. 1976. Effects of cyanide and ethylene on the respiration of cyanide-sensitive and cyanide-resistant plant tissues Plant Physiology 58 47–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A, Handa AK. 2005. Hormonal regulation of tomato fruit development: a molecular perspective Journal of Plant Growth Regulation 24 67–82 [Google Scholar]
- Tigchelaar E, McGlasson W, Buescher R. 1978. Genetic regulation of tomato fruit ripening HortScience 13 508–513 [Google Scholar]
- Tucker ML, Laties GG. 1984. Interrelationship of gene expression, polysome prevalence, and respiration during ripening of ethylene and/or cyanide-treated avocado fruit Plant Physiology 74 307–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanlerberghe GC, Cvetkovska M, Wang J. 2009. Is the maintenance of homeostatic mitochondrial signaling during stress a physiological role for alternative oxidase? Physiologia Plantarum 137 392–406 [DOI] [PubMed] [Google Scholar]
- Vanlerberghe GC, McIntosh L. 1997. ALTERNATIVE OXIDASE: from gene to function Annual Review of Plant Physiology and Plant Molecular Biology 48 703–734 [DOI] [PubMed] [Google Scholar]
- Vrebalov J, Ruezinsky D, Padmanabhan V, White R, Medrano D, Drake R, Schuch W, Giovannoni J. A MADS-box gene necessary for fruit ripening at the tomato ripening-inhibitor (rin) locus. Science. 2002;296:343. doi: 10.1126/science.1068181. [DOI] [PubMed] [Google Scholar]
- Wang A, Yamakake J, Kudo H, Wakasa Y, Hatsuyama Y, Igarashi M, Kasai A, Li T, Harada T. 2009. Null mutation of the MdACS3 gene, coding for a ripening-specific 1-aminocyclopropane-1-carboxylate synthase, leads to long shelf life in apple fruit Plant Physiology 151 391–399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White PJ. 2002. Recent advances in fruit development and ripening: an overview Journal of Experimental Botany 53 1995–2000 [DOI] [PubMed] [Google Scholar]
- Wilkinson JQ, Lanahan MB, Yen HC, Giovannoni JJ, Klee HJ. 1995. An ethylene-inducible component of signal transduction encoded by Never-ripe Science 270 1807–1809 [DOI] [PubMed] [Google Scholar]
- Xu F, Zhang DW, Zhu F, Tang H, Lv X, Cheng J, Xie HF, Lin HH. 2012. A novel role for cyanide in the control of cucumber (Cucumis sativus L.) seedlings response to environmental stress Plant, Cell and Environment(in press) [DOI] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE. 1984. Ethylene biosynthesis and its regulation in higher plants Annual Review of Plant Physiology 35 155–189 [Google Scholar]
- Yip WK, Yang SF. 1988. Cyanide metabolism in relation to ethylene production in plant tissues Plant Physiology 88 473–476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokotani N, Nakano R, Imanishi S, Nagata M, Inaba A, Kubo Y. 2009. Ripening-associated ethylene biosynthesis in tomato fruit is autocatalytically and developmentally regulated Journal of Experimental Botany 60 3433–3442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida K, Watanabe C, Kato Y, Sakamoto W, Noguchi K. 2008. Influence of chloroplastic photo-oxidative stress on mitochondrial alternative oxidase capacity and respiratory properties: a case study with Arabidopsis yellow variegated 2 Plant and Cell Physiology 49 592–603 [DOI] [PubMed] [Google Scholar]
- Zhang DW, Xu F, Zhang ZW, Chen YE, Du JB, Jia SD, Yuan S, Lin HH. 2010. Effects of light on cyanide-resistant respiration and alternative oxidase function in Arabidopsis seedlings Plant, Cell and Environment 33 2121–2131 [DOI] [PubMed] [Google Scholar]
- Zheng CF, Jiang D, Liu FL, Dai TB, Liu WC, Jing Q, Cao WX. 2009. Exogenous nitric oxide improves seed germination in wheat against mitochondrial oxidative damage induced by high salinity Environmental and Experimental Botany 67 222–227 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.