Abstract
Plasmodium ookinetes traverse midgut epithelial cells before they encounter the complement system in the mosquito hemolymph. We identified a heme peroxidase (HPX2) and NADPH oxidase 5 (NOX5) as critical mediators of midgut epithelial nitration and antiplasmodial immunity that enhance nitric oxide toxicity in Anopheles gambiae. We show that the two immune mechanisms that target ookinetes—epithelial nitration and thioester-containing protein 1 (TEP1)-mediated lysis—work sequentially and propose that epithelial nitration works as an opsonization-like system that promotes activation of the mosquito complement cascade.
Keywords: mosquito, nitration, NADPH oxidase, NOX5, peroxidase, nitric oxide, epithelial immunity, complement, TEP1, malaria
Mosquitoes become infected with Plasmodium when they ingest blood that contains gametocytes. Zygotes form within the lumen of the mosquito midgut and mature into motile ookinetes. To complete their development in the mosquito, ookinetes must traverse the midgut epithelium and avoid being detected and lysed by the mosquito complement system. Thioester-containing protein 1 (TEP1) is a member of the macroglobulin family in Anopheles gambiae with a structure similar to complement C3 protein in vertebrates (1). In the susceptible A. gambiae G3 strain, TEP1 lyses about 80% of Plasmodium berghei ookinetes that complete invasion as they encounter the mosquito hemolymph (2). TEP1 protein circulates as a stable complex in association with two proteins of the leucine-rich repeat family, LRIM1 and APL1C (3–5). The mechanism of TEP1 activation is unknown, and it is not clear what determines whether TEP1 will bind to the surface of a given parasite and lyse it.
Ookinete invasion of midgut epithelial cells causes irreversible damage that leads to apoptosis (6). Invaded cells mount defense responses such as expression of high levels of nitric oxide synthase (NOS). We previously proposed that the rate of cellular responses to invasion may be critical to Plasmodium survival (6). Biochemical studies in A. gambiae revealed extensive nitration in Plasmodium-invaded cells that appears to be a two-step process in which induction of NOS expression is followed by increased peroxidase activity (7). The peroxidase activity induced in Plasmodium-infected midguts is very stable (remaining active after glutaraldehyde fixation) and can catalyze protein nitration in vitro (7) in a reaction similar to that described for myeloperoxidase-mediated nitration in vertebrates (8, 9). In this study, we identify heme peroxidase (HPX2) and NADPH oxidase 5 (NOX5) as key enzymes induced in ookinete- invaded midgut cells of A. gambiae (G3) that—together with NOS—mediate protein nitration. The HPX2/NOX5 system potentiates nitric oxide (NO) toxicity and is critical for mosquitoes to mount an effective antiplasmodial response. We provide direct experimental evidence that epithelial nitration and TEP1-mediated lysis, which are often thought to be mutually exclusive (10), work sequentially and propose that epithelial nitration by HPX2/NOX5 modifies ookinetes and makes them “visible” to the mosquito complement system.
Bioinformatic analysis of the A. gambiae genome identified 18 genes containing heme-peroxidase (HPX) domains (11). Transcription of four of these putative heme peroxidases—HPX2, HPX7, HPX8, and double peroxidase (DBLOX)—and of the dual oxidase (Duox) gene is induced in the midgut in response to Plasmodium infection (7). We explored the participation of the four inducible peroxidases in antiplasmodial immunity. The role of Duox has been previously reported (12).
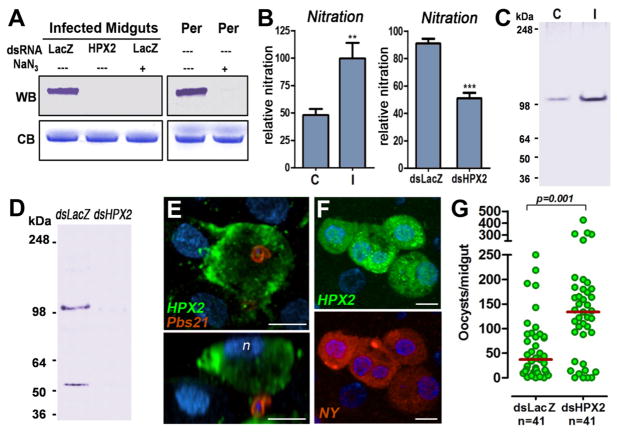
Transcriptional activation of the four HPX genes by Plasmodium berghei ookinete invasion 24 h post feeding was confirmed (13) (Fig. 1A), and their expression was efficiently silenced (70–90%) by systemic injection of dsRNA (Fig. 1B). The effect of silencing each of these enzymes on the peroxidase activity of glutaraldehyde-fixed midguts from mosquitoes fed on a healthy or a Plasmodium-infected mouse was measured to determine Plasmodium-induced enzymatic activity. HPX2 silencing reduced activity induced by infection by 90%, while silencing of HPX7, HPX8, and DBLOX had no effect (Fig. 1C). Midgut homogenates from mosquitoes injected with dsLacZ and infected with Plasmodium (Fig. 2A, left panels) and a commercial peroxidase (Fig. 2A, positive control, right panel) can catalyze bovine serum albumin nitration in vitro; however, nitration was no longer observed when the enzymatic activity was inhibited by addition of sodium azide or when HPX2 expression was silenced (Fig. 2A), indicating that HPX2 is the enzyme induced by Plasmodium infection that catalyzes protein nitration in vitro. An enzyme-linked immunosorbent assay (ELISA)-type assay test was developed to measure in vivo protein nitration using an anti-nitrotyrosine monoclonal antibody. As expected, midgut nitration increased significantly in response to Plasmodium infection (Fig. 2B). Furthermore, silencing HPX2 significantly reduced midgut nitration in Plasmodium- infected midguts (Fig. 2B).
Fig. 1.
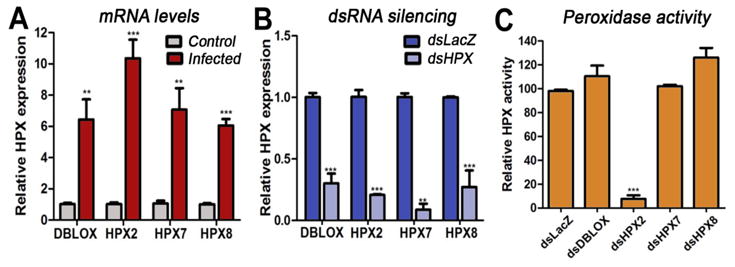
Expression and activity of midgut heme peroxidases (HPXs). (A) mRNA expression of double peroxidase (DBLOX), HPX2, HPX7, and HPX8 in the midguts of mosquitoes fed on a healthy (C, control) or a Plasmodium-infected (I, infected) mouse 24 h post-feeding (hpf); and (B) in the midguts of infected mosquitoes injected with dsLacZ or with dsRNA from each of the four HPXs 24 hpf. (C) Effect of silencing each HPX on Plasmodium-induced midgut peroxidase activity (mean ± SEM).
Fig 2.
Localization of HPX2 and effect of HPX2 silencing on nitration and Plasmodium infection. (A) In vitro bovine serum albumin (BSA) nitration by a commercial peroxidase (Per) or by peroxidase from Plasmodium-infected midguts. Inhibition of peroxidase activity by addition of sodium azide (NaN3) or silencing HPX2 prevent in vitro nitration. Western blot (WB) with an anti-nitrotyrosine antibody and Coomassie blue (CB) staining of BSA. (B) Effect of Plasmodium infection (left panel) and of silencing HPX2 (right panel) in Plasmodium-infected mosquitoes on in vivo midgut nitration (mean ± SEM). Western blot detection of HPX2 protein in midgut homogenates from (C) mosquitoes fed on a healthy (C, control) or a Plasmodium-infected (I, infected) mouse 24 h post feeding (hpf) and (D) Plasmodium-infected mosquitoes injected with dsLacZ control or dsHPX2 collected 24 hpf. (E, F) Immunofluorescent staining of Plasmodium-infected midguts 24 hpf. (E) Ookinetes stained with anti-Pbs21 antibodies (red) and HPX2 (green). Lower panel shows side view; n, nuclei of apoptotic cell (blue). (F) HPX2 (upper panel) and nitrotyrosine (lower panel) expression in Plasmodium-infected midguts m (bar = 10 μm). (G) Effect of HPX2 silencing on Plasmodium infection 7 days post feeding. Each circle represents the number of parasites in individual midguts (median = red line).
Polyclonal antibodies to recombinant HPX2 detect a major protein band (100 kDa under native conditions) that is present at higher levels in Plasmodium-infected midguts (Fig. 2C), and a second 55-kDa band is occasionally observed (Fig. 2D). The signal from both bands (100 and 55 kDa) is greatly reduced when HPX2 is silenced (Fig. 2D). HPX2 is highly expressed in ookinete-invaded cells undergoing apoptosis (Fig. 2E), and nitrotyrosine signal is detected in damaged cells protruding into Plasmodium-infected midguts that express high levels of HPX2 (Fig. 2F and Fig. S1); however, the level of HPX2 and nitrotyrosine signal is very weak in uninvaded cells (Fig. 2F and Fig. S1) and in the midguts of mosquitoes fed on a healthy mouse (Fig. S1). HPX2 silencing increased the intensity of Plasmodium infection by 3.6 fold (P = 0.001) (Fig. 2G), while HPX8 and DBLOX silencing did not affect intensity of infection, and HPX7 silencing reduced infection to a moderate degree (P = 0.054) (Fig. S2). Together, these findings indicate that HPX2-mediated nitration in invaded midgut cells is a major determinant of Plasmodium survival.
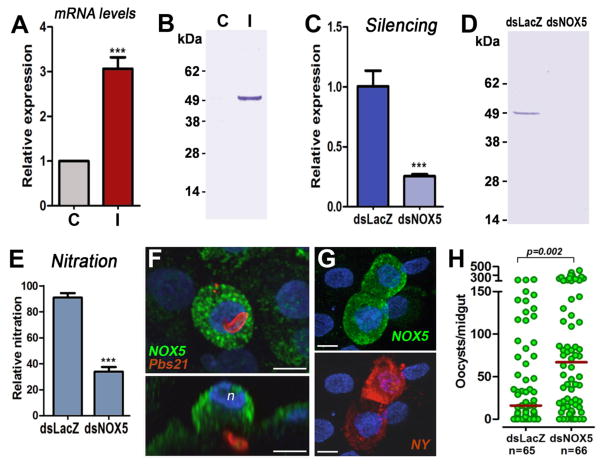
Heme peroxidases require a source of hydrogen peroxide to be enzymatically active. A single member of the NADPH oxidase (NOX) family, a putative ortholog of the vertebrate NOX5, was identified in A. gambiae by BLAST analysis (AGAP008072) (14). NOX5 mRNA (Fig. 3A) and protein (Fig. 3B) expression was highly induced in the midgut in response to Plasmodium infection, and expression was efficiently silenced at the mRNA (Fig. 3C) and protein (Fig. 3D) levels by dsNOX5 injection. NOX5 silencing significantly reduced nitration in Plasmodium-infected midguts (Fig. 3E). NOX5 protein is highly expressed in cells damaged by ookinete invasion (Fig. 3F), and nitrotyrosine staining is detected in damaged cells that express high levels of NOX5 (Fig. 3G and Fig. S3). In contrast, the staining is very weak in uninvaded cells (Fig. 3G and Fig. S3) or in the midguts of mosquitoes fed on a healthy mouse (Fig. S3). Furthermore, NOX5 silencing significantly increased the intensity of Plasmodium infection (P = 0.002) (Fig. 3H). Double silencing of HPX2 and NOX5 had the same effect as silencing either HPX2 or NOX5 alone (Fig. S4), indicating that they mediate the same antiplasmodial response. We also explored the possibility of a functional link between HPX2 and TEP1. Co-silencing HPX2 and TEP1 had the same effect on Plasmodium infection as silencing TEP1 alone, suggesting that the antiplasmodial effect of HPX2 is mediated by TEP1 (Fig. 4A).
Fig. 3.
Expression and localization of NOX5 and effect of NOX5 silencing on Plasmodium infection. (A) Midgut NOX5 mRNA and (B) protein expression 24 h post-feeding (hpf) in mosquitoes fed on a healthy (C, control) or a Plasmodium-infected (I, infected) mouse. (C) Midgut NOX5 mRNA and (D) protein expression 24 hpf on mosquitoes injected with dsLacZ or dsNOX5. (E) Effect of NOX5 silencing on in vivo midgut protein nitration (mean ± SEM). (F, G) Immunofluorescence staining of midguts 24 hpf on a Plasmodium-infected mouse. (F) Ookinetes stained with Pbs21 (red) and NOX5 (green). Lower panel, side view; n, nuclei of apoptotic cell (blue). (G) Nitrotyrosine (red) and NOX5 (green) expression in cells of Plasmodium-infected midguts undergoing apoptosis (bar = 10 μm). (H) Effect of NOX5 silencing on Plasmodium infection 7 dpf. Each circle represents the number of parasites in individual midguts (median = red line).
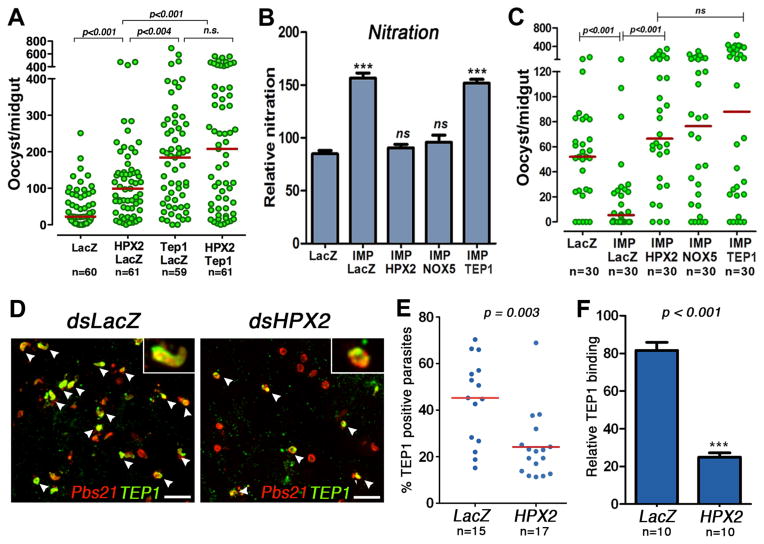
Fig. 4.
Midgut nitration and complement activation. (A) Effect of double silencing heme peroxidase 2 (HPX2) and thioester-containing protein 1 (TEP1) on Plasmodium infection 7 days post feeding (dpf). (B) Effect of silencing IMPer or co-silencing NADPH 5 (NOX5), HPX2, or TEP1 on in vivo protein nitration and (C) Plasmodium infection 7 dpf. Each circle represents the number of parasites in an individual midgut; the horizontal lines indicate medians. Effect of HPX2 silencing on (D) TEP1 staining (green) on ookinetes labeled with anti-Pbs21 antibody (red), (E) percentage of parasites on individual midguts that stain positive for TEP1, and (F) Plasmodium-induced TEP1 binding (difference in binding to infected and uninfected midguts) 28–30 hours post-feeding. Arrowheads indicate examples of TEP1-positive parasites.
We have previously shown that disruption of the IMPer/Duox system, by silencing either gene, results in very high levels of NOS expression and a dramatic decrease in Plasmodium survival (12). The hypothesis that increased NO levels in IMPer-silenced females accelerate HPX2/NOX5-mediated nitration was investigated. Midgut nitration was significantly higher when IMPer expression was silenced (Fig. 4B). However, although HPX2 or NOX5 silencing did not affect NOS or TEP1 expression (Fig. S5), co-silencing IMPer/HPX2 or IMPer/NOX5 decreased nitration to control levels (Fig. 4B), indicating that the HPX2/NOX5 system is required for efficient nitration. Moreover, the antiparasitic effect of IMPer silencing was completely reversed when either HPX2 or NOX5 was co-silenced (Fig. 4C).
We also explored the participation of TEP1 in the antiplasmodial response in IMPer-silenced mosquitoes. Co-silencing TEP1 did not affect midgut nitration in IMPer-silenced midguts, (Fig. 4B) but completely reverted the antiparasitic effect (Fig. 4C); indicating that HPX2/NOX5-nitration does not directly kill Plasmodium, but requires TEP1-activation. TEP1-mediated lysis also did not take place when either HPX2 or NOX5 were silenced (Fig. 4C). We performed immunofluorescence analysis to directly assess whether HPX2-mediated nitration affected TEP1 binding to Plasmodium (Fig. 4D). HPX-2 silencing significantly reduced the proportion of parasites labeled with TEP1 (P = 0.003) (Fig. 4E). Furthermore, Plasmodium-induced TEP1 binding to the mosquito midgut was also significantly reduced (P < 0.001) (Fig. 4F).
All ookinetes that traverse the midgut epithelium come in contact with the complement system, so it has been difficult to understand why TEP1 lyses some ookinetes while others are spared. Our findings indicate that the nitration response mediated by HPX2/NOX5 is required for effective TEP1-mediated lysis. We propose that nitration modifies ookinetes during their transit through the midgut and that—although TEP1 is the final effector of antiplasmodial immunity—the outcome of infection depends, to a large extent, on the interaction of a given ookinete with the cell(s) it traverses. We have shown that the rate of nitration mediated by HPX2/NOX5 is a major determinant of Plasmodium survival. This predicts that physiologic conditions that accelerate nitration, such as faster or higher expression of these enzymes, or that increased the availability of substrates, such as arginine, would be detrimental to the parasite. This is in agreement with previous studies indicating that a higher rate of hydrogen peroxide generation by mitochondria (15) or a reduced rate of detoxification (16, 17) promotes ookinete lysis. This model also predicts that ookinetes that migrate faster through invaded cells would be more likely to survive. If ookinetes are not detected by the mosquito complement system, they recover from exposure to toxic nitrogen-reactive species and proceed to transform into oocysts. Our studies revealed that NOS induction was not sufficient to achieve an effective antiplasmodial response and identified HPX2 and NOX5 as strong potentiators of NO toxicity. In humans, increased NOX5 activity has been associated with coronary artery disease (18). Our findings indicate the possibility of a human myeloperoxidase/NOX5 system that would potentiate NO toxicity in endothelial cells and promote complement activation.
Supplementary Material
Acknowledgments
This work was supported by the Division of Intramural Research, NIAID, National Institutes of Health. We thank André Laughinghouse and Kevin Lee for insectary support; José Ribeiro and Jesus Valenzuela for their comments and insight; and Lindsey Garver and Brenda Marshall for editorial assistance.
Footnotes
References
- 1.Baxter R, et al. Proc Natl Acad Sci U S A. 2007;104:28. doi: 10.1073/pnas.0704967104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blandin S, et al. Cell. 2004;116:661. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- 3.Povelones M, et al. Science. 2009;324:258. doi: 10.1126/science.1171400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Povelones M, et al. PLoS Pathog. 2011;7:4. doi: 10.1371/journal.ppat.1002023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fraiture M, et al. Cell Host Microbe. 2009;5:273. doi: 10.1016/j.chom.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 6.Han YS, et al. EMBO J. 2000;19:6030. doi: 10.1093/emboj/19.22.6030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar S, et al. J Biol Chem. 2004;279:51. doi: 10.1074/jbc.M409905200. [DOI] [PubMed] [Google Scholar]
- 8.Pfeiffer S, et al. FASEB J. 2001;15:2355. doi: 10.1096/fj.01-0295com. [DOI] [PubMed] [Google Scholar]
- 9.Byun J, et al. Biochemistry. 1999;38:8. doi: 10.1021/bi9822980. [DOI] [PubMed] [Google Scholar]
- 10.Shiao S, et al. PLoS Pathog. 2006;2:12. doi: 10.1371/journal.ppat.0020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waterhouse R, et al. Science. 2007;316:5832. [Google Scholar]
- 12.Kumar S, et al. Science. 2010;327:1644. doi: 10.1126/science.1184008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Materials and Methods are available as supporting material on Science Online.
- 14.Kawahara T, et al. BMC Evol Biol. 2007;7:109. doi: 10.1186/1471-2148-7-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliveira J, et al. Insect Biochem Mol Biol. 2011;41:6. doi: 10.1016/j.ibmb.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molina-Cruz A, et al. J Biol Chem. 2008;283:6. doi: 10.1074/jbc.M705873200. [DOI] [PubMed] [Google Scholar]
- 17.Jaramillo-Gutierrez G, et al. PLoS One. 2010;5:6. doi: 10.1371/journal.pone.0011168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guzik T, et al. J Am Coll Cardiol. 2008;52:22. doi: 10.1016/j.jacc.2008.07.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.