Abstract
Objective
Given the importance of ET technique during assisted reproductive technology cycles, we evaluated the effect of embryo afterloading subsequent to placement of the ET catheter on pregnancy rates vs. a standard direct ET.
Design
Retrospective cohort analysis.
Setting
University-based assisted reproductive technology program.
Patient(s)
Patients undergoing a fresh nondonor day 3 ET by a single provider over a 1-year period.
Intervention(s)
None.
Main Outcome Measure(s)
Clinical pregnancy.
Result(s)
One hundred twenty-seven patients met inclusion criteria, and the overall pregnancy rate was 46.5%. There was no difference between the two groups with respect to age, basal FSH, or number of embryos transferred. The ET method used was at the discretion of the provider. There was no difference between the two groups in the presence of blood on the transfer catheter. However, there were significantly more transfer catheters with mucus contamination in the direct transfer group (25.58% vs. 5.95%). The clinical pregnancy rate in the group with ET using the afterloading technique was higher than in the direct ET group (52.4% vs. 34.9%).
Conclusion(s)
There was a trend toward an increase in pregnancy rate when an embryo afterloading technique was used. A prospective randomized trial is needed to examine this issue.
Keywords: Embryo transfer, technique, IVF-ET, pregnancy rate
Over the past 10-15 years there have been increasing success rates with assisted reproductive technologies (ART) in all age groups. The Society for Assisted Reproductive Technology reported an increase in live birth rates from 28% in 1996 to 32% in 2002 (1). This increase has been attributed to multiple factors including improved stimulation protocols (2-4), advances in embryology laboratory techniques (5), and improvement in ET techniques (6, 7).
Embryo transfer is universally accepted as a crucial last step in any ART cycle. The importance of this step has been emphasized by the fact that different providers at the same institution may have disparate pregnancy rates after ET (8, 9). Other variables affecting pregnancy include the ease of ET (7, 10, 11), presence or absence of blood on the transfer catheter (12), type of catheter used (13), technique used to perform the transfer (14-16), and experience of the physician (17).
In the early 1990s, studies were first published on the use of a mock or “dummy” ET before the start of an IVF cycle (11, 18). A mock ET allows the physician to choose the appropriate transfer catheter, measure the depth of the endometrial cavity, and anticipate potential problems at ET. However, a mock transfer remote from the actual ET is done under different circumstances and may not be reflective of actual conditions encountered on the day of ET. Sharif et al. (19) proposed to circumvent this problem by performing a mock ET immediately before the actual ET.
To avoid additional trauma by the passage of two separate catheters, we began transferring embryos by an afterload technique, in which an empty catheter is placed at, or just past, the internal cervical os. The inner sheath is withdrawn, and a second inner sheath containing the embryos is passed. This gives the provider the benefit of an immediate mock transfer while minimizing manipulation of embryos and possibly reducing trauma to the endometrium.
We performed a retrospective analysis of 127 ETs done during a 1-year period of time to determine whether there were differences in pregnancy rates based on the transfer method used.
MATERIALS AND METHODS
Under an approved protocol reviewed by the Department of Clinical Investigation, we performed a retrospective analysis of patients undergoing a day 3 ET at the Walter Reed Army Medical Center ART program from July 2001 to July 2002 by a single provider. Information regarding patient age, day 3 FSH level, number of embryos transferred, method of ET, and clinical pregnancy rates were collected. The ET method used was at the discretion of the provider performing the procedure, and the number of women receiving the afterload technique was proportional throughout the study. Transfers of blastocysts, cryopreserved embryos, and donor oocytes were not included in the analysis. Patients were excluded from analysis if they were greater than 43 years of age or had an FSH level >14 mIU/L on cycle day 3 (or on cycle day 3 or 10 after a clomiphene citrate challenge test). A total of 127 patients met criteria for study.
All patients had undergone controlled ovarian stimulation by using a combination of long-term gonadotropin-releasing hormone (GnRH) agonist (Lupron, 1.0 mg/day; TAP Pharmaceuticals, Deerfield, IL) or microdose flare GnRH agonist (40 μg twice daily and gonadotropins Gonal-F (Serono, Rockland, MA) or a combination of Gonal-F and Repronex (Ferring Pharmaceuticals, Suffern, NY) as described elsewhere (20). The dose of gonadotropins was individualized based on the patient’s age, history, and response to medication. Cycles were monitored using serial transvaginal ultrasounds to chronicle follicular growth and the measurement of serum E2 levels. Administration of hCG occurred when follicular size and E2 levels were appropriate. Transvaginal oocyte retrieval was performed approximately 36 hours later (20). Patients received IM progesterone in oil (50 mg/d) beginning the night of the retrieval until 8 weeks’ gestation.
Embryo Transfer Techniques
A mock ET and a saline sonohysterogram were performed in all patients before enrollment in the ART cycle. All ETs were performed with a full bladder under ultrasound guidance (Ultramark 4 with a 5-MHz transducer; ATL, Bothell, WA) using the Edward-Wallace catheter (Simcare, West Sussex, UK). The difficulty of the ET was determined by the performing physician and scored as easy, moderate, or difficult.
Direct Embryo Transfer
The patient was placed in the lithotomy position. A sterile bivalve speculum was placed in the patient’s vagina, and the cervix was exposed. Excess mucus and debris were cleared from the ectocervix using sterile cotton swabs dampened with phosphate-buffered saline. The embryos were loaded into the transfer catheter by the embryologist as described elsewhere, and the catheter was passed to the transfer physician (4). The embryos were then deposited approximately 1.5 cm from the uterine fundus under ultrasound guidance. After approximately 5 seconds the catheter was gently rotated 180° and removed, with care being taken to keep the plunger of the catheter depressed until it had been completely removed from the cervix, usually 5-10 seconds. The embryologist immediately flushed the catheter with media to check for retained embryos, blood, or mucus. Patients remained supine for 20 minutes after the procedure.
Afterloaded Embryo Transfer
The patients were placed in the lithotomy position, and the cervix was exposed and prepped in the same manner as the direct technique. An empty Wallace catheter was passed to the level of the lower uterine segment under ultrasound guidance to a point where the inner catheter entered the endometrial cavity, typically about 5 cm. The inner sheath was slowly removed, leaving the outer sheath just beyond the internal os. A second inner sheath was loaded by an embryologist who then assisted the transfer physician in threading the inner sheath into the catheter. The inner catheter was slowly advanced by the physician, and the embryos were deposited 1.5 cm from the fundus. After approximately 5-10 seconds, the catheter was gently rotated and removed, with care being taken to keep the plunger of the catheter depressed until it had been completely removed from the cervix over 15 seconds. The embryologist flushed the catheter with media to check for retained embryos. Patients remained supine for 20 minutes after the transfer.
All patients from both groups who had appropriately rising quantitative hCG values underwent transvaginal ultrasound at 6-8 weeks’ gestation to look for gestational sacs.
Statistical Analysis
Since these data were retrospectively analyzed, sample size was determined by the study time interval and not a power analysis. The method of transfer was recorded in our ART database along with other cycle parameters/outcomes kept for the purpose of Society for Assisted Reproductive Technology reporting. The primary outcome analyzed was clinical pregnancy rate per transfer as defined by the presence of a gestational sac on ultrasound at 6-8 weeks of gestation. Nonsustained rises in hCG levels and ectopic pregnancies were not scored as clinical pregnancies. Implantation rates were defined as number of sacs on ultrasound per number of embryos transferred. Statistical differences were determined using Student’s t-test, Fisher’s exact, and χ2 analysis where appropriate. A Mann-Whitney U-test was used to determine the statistical difference between nonparametric variables. An alpha error of <.05 was considered significant.
RESULTS
One hundred twenty-seven day 3 ETs were performed by a single provider over a 1-year period of time. The average age of all the patients was 33.6 ± 4.38 years, with a range of 20-42 years. The overall pregnancy rate was 46.5%. Eighty-four ETs were performed by the afterload technique, and 43 as direct ETs.
As depicted in Table 1, there was no difference between the two groups with respect to age (33.4 vs. 33.9), basal FSH levels (6.98 vs. 6.45), or number of embryos transferred (2.87 vs. 2.95).
TABLE 1. Clinical characteristics of the two comparison groups.
| Group 1, afterloaded ET (n = 84) |
Group 2, direct ET (n = 43) |
P | |||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | Range | Mean (SD) | Median | Range | ||
| Age (y) | 33.45 ± 4.09 | 33 | 24–41 | 33.98 ± 4.90 | 35 | 20–42 | .52a |
| Basal FSH (mIU/L) | 6.98 ± 1.98 | 6.7 | 2.1–13 | 6.45 ± 1.87 | 6.6 | 2.5–10.5 | .24b |
| No. of ETs | 2.87 ± 0.90 | 3.0 | 1–6 | 2.95 ± 0.69 | 3 | 2–5 | .35b |
Two-tailed Student’s t-test.
Mann-Whitney U-test.
Neithardt. Embryo afterloading. Fertil Steril 2005.
Table 2 describes the features of the ET for both groups. No transfers in either group were characterized as difficult; 7.1% of transfers in the afterload group and 9.3% in the direct group were characterized as moderately difficult. This difference did not reach statistical significance (P=.73). There was no difference between the two groups in the presence of blood on the transfer catheter, with approximately 9% in each group. However, there were significantly more transfer catheters with mucus in the catheter in the direct transfer group (25.58% vs. 5.95%, P=.002).
TABLE 2. Features of the ET.
| Afterload (n = 84) |
Direct (n = 43) |
P | |
|---|---|---|---|
| Difficulty, hard | 0 | 0 | 1.00a |
| Difficulty, moderate | 6(7.10) | 4 (9.30) | .73a |
| Presence of blood | 8 (9.5) | 4 (9.30) | 1.00a |
| Presence of mucus | 5 (5.95) | 11 (25.58) | .002b |
Note: Data in parentheses are percents.
By a two-tailed Fischer’s exact test.
By χ2 analysis.
Neithardt. Embryo afterloading. Fertil Steril 2005.
Cycle outcomes for the two groups are shown in Figure 1. The implantation rate per embryo was higher, although not significantly, in the afterload group, with a rate of 24.7% vs. 20.5%. The clinical pregnancy rate in the afterload group was also higher than in the direct transfer group, at 52.4% vs. 34.9% (P=.06).
FIGURE 1.
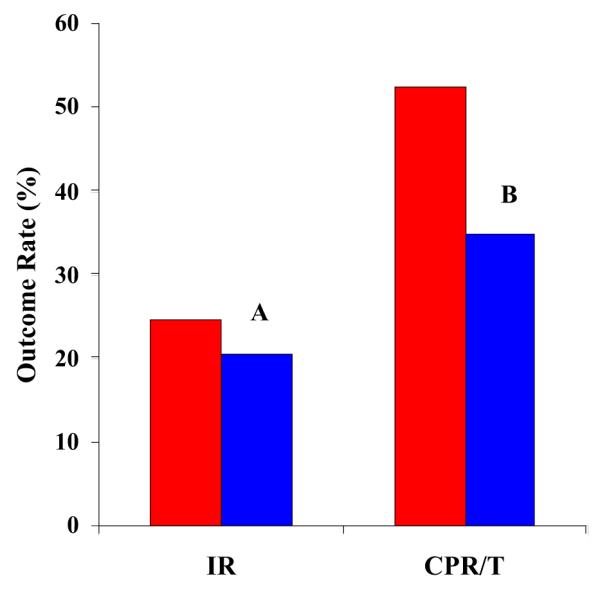
Comparison of pregnancy outcomes with direct vs. afterloaded ET methods. IR = implantation rate, defined as the number of embryos transferred divided by number of gestational sacs on ultrasound. CPT/T = clinical pregnancy per ET cycle based on ultrasound evidence of fetal cardiac activity at 6-8 weeks of gestation. A, P = NS; B, P=.06 by χ2 analysis. Red, afterloaded ET; blue, direct ET.
Neithardt. Embryo afterloading. Fertil Steril 2005.
DISCUSSION
Embryo transfer, the final step in most ART cycles, is intuitively a simple procedure. However, it has been demonstrated by multiple investigators that great care and patience should be observed when performing an ET because even small differences in methods may affect pregnancy rates (7, 13-17). Given the importance of technique, we report a refinement of the ET technique, embryo afterloading. This is a method by which embryos are loaded into the inner sheath of a soft-tipped catheter and threaded through the remaining outer sheath of another catheter that has already passed through the endocervix. This may improve clinical pregnancy rates by facilitating the ease of ET. In addition, since the uterus often changes position during stimulation a previous mock transfer may not be relevant on the day of ET (21).
It has been postulated that “endometrial movements” are present during the natural menstrual cycle (22, 23) and that they may impact fecundity (24). Thus, it is not a far stretch to imagine that uterine contractility and subsequent endometrial movements could impact ET. In fact, this is highly suggested by the observation that factors that facilitate smooth ET and minimize inadvertent stimulation of the fundus, such as the use of soft-tipped catheters, ultrasound, or fixed distance transfers, have been linked to improved pregnancy rates with IVF-ET (1, 3, 14, 16).
Lesny et al. (10) nicely demonstrated that light stimulation of the fundus with a soft-tipped catheter was enough to produce fundocervical waves that displaced a small bolus of echovist, placed in the endometrial cavity to mimic ET, towards the cervix.
For these reasons, a mock ET done before the treatment cycle is commonly performed. This ensures that entry into the uterine cavity is possible and enables the measurement of the uterocervical angle and depth of the endometrial cavity. Thereby difficulties, which may lead to a traumatic ET or even failure of ET, can be anticipated and avoided. Mansour et al. (11) demonstrated the effectiveness of this practice in a randomized study of 335 patients. Pregnancy rates were significantly higher, and the incidence of difficult transfers were lower in the group of patients who had a dummy ET performed.
Other investigators have proposed performing a mock ET immediately before the actual ET. In a descriptive study by Sharif et al. (19), this was associated with a good pregnancy rate of 45%. This saves the patient and the physician the time and expense of another office procedure and ensures that both transfers are being done under the same conditions. However, this does not allow advanced planning in the case of the complete inability to enter the cavity, and a separate mock on the day of ET may be needlessly traumatic.
There is a moderate amount of evidence to suggest that minimal uterine manipulation and avoidance of contact with the fundus leads to better results (6, 7, 14). In addition, Lesny et al. (10) demonstrated that uterine contractions may last up to 45 minutes after stimulation of the fundus. In consequence, the performance of a full mock transfer, in which the catheter is advanced to the fundus immediately before the actual ET, may be detrimental to pregnancy rates.
Although our study was limited by a small sample size and its retrospective design, it suggested a trend towards improved clinical pregnancy rates with ET performed by the afterloading method. Indeed there are multiple benefits to this method. It allows the transferring physician to take the time needed to slowly and gently pass a catheter through the endocervix while the embryos remain in the incubator, thus facilitating an atraumatic transfer while limiting the time the embryos are exposed. In addition, the embryos will not pass through the initial inner sheath that was placed through the cervix. In theory, this would decrease mucus contamination of the catheter, which has been proposed to adversely affect implantation either by contamination of the cavity or by causing retention or displacement of the embryos (7, 25, 26). Consistent with this idea, we demonstrated a statistically significant lower number of transfer catheters contaminated with mucus in the afterload group.
In conclusion, embryo afterloading is a refinement of standard ET and may improve clinical pregnancy rates. This method may be especially useful in centers that are training physicians to perform ET. A randomized clinical trial would be required to confirm an increase in clinical pregnancy rates using this method.
Acknowledgments
The authors thank the ART Institute of Washington, D.C., including the embryology laboratory, Kerry Polsen, M.S., Donna Materia, R.N., Darshana Niak, R.N., and Stephanie Hosid, R.N., without whom this study would not be possible. They also acknowledge Dr. Fred Larsen (Walter Reed Army Medical Center) for his contributions to the editing of the manuscript.
REFERENCES
- 1.National Summary and Fertility Clinic Reports Assisted Reproductive Technology Success Rates. 2001 doi: 10.1089/jwh.1998.7.301. Accessed at www.cdc.gov/reproductivehealth/art.htm. [DOI] [PubMed]
- 2.Bergh C, Howles CM, Borg K, Hamberger L, Josefsson B, Nilsson L, et al. Recombinant human follicle stimulating hormone (r-hFSH; Gonal-F®) versus highly purified urinary FSH (Metrodin HP®): results of a randomized comparative study in women undergoing assisted reproductive techniques. Hum Reprod. 1997;12:2133–9. doi: 10.1093/humrep/12.10.2133. [DOI] [PubMed] [Google Scholar]
- 3.Schats R, De Sutter P, Bassil S, Kremer JAM, Tournaye H, Donnez J. Ovarian stimulation during assisted reproduction treatment: a comparison of recombinant and highly purified urinary human FSH. Hum Reprod. 2000;15:1691–7. doi: 10.1093/humrep/15.8.1691. [DOI] [PubMed] [Google Scholar]
- 4.Hughes EG, Fedorkow DM, Daya S, Sagle MA, Van de Koppel P, Collins JA. The routine use of gonadotropin-releasing hormone agonists prior to in vitro fertilization and gamete intrafallopian transfer: a meta-analysis of randomized controlled trials. Fertil Steril. 1992;58:888–96. doi: 10.1016/s0015-0282(16)55430-2. [DOI] [PubMed] [Google Scholar]
- 5.Scott L. Embryological strategies for overcoming recurrent assisted reproductive technology treatment failure. Hum Fertil. 2002;5:206–14. doi: 10.1080/1464727022000199142. [DOI] [PubMed] [Google Scholar]
- 6.Choe JK, Nazari A, Check JH, Summers-Chase D, Swenson K. Marked improvement in clinical pregnancy rates following in vitro fertilization–embryo transfer seen when transfer technique and catheter were changed. Clin Exper Obstet Gynecol. 2001;28:223–4. [PubMed] [Google Scholar]
- 7.Mansour RT, Aboulghar MAA. Optimizing the embryo transfer technique. Hum Reprod. 2002;17:1149–53. doi: 10.1093/humrep/17.5.1149. [DOI] [PubMed] [Google Scholar]
- 8.Hearns-Stokes RM, Miller BT, Scott L, Creuss D, Chakraborty PK, Segars JH. Pregnancy rates after embryo transfer depend on the provider at embryo transfer. Fertil Steril. 2000;74:80–6. doi: 10.1016/s0015-0282(00)00582-3. [DOI] [PubMed] [Google Scholar]
- 9.Karande VC, Morris R, Chapman C, Rinehart J, Gleicher N. Impact of the “physician factor” on pregnancy rates in a large assisted reproductive technology program: do too many cooks spoil the broth? Fertil Steril. 1999;71:1001–10. doi: 10.1016/s0015-0282(99)00139-9. [DOI] [PubMed] [Google Scholar]
- 10.Lesny P, Killick SR, Tetlow RL, Robinson J, Maguiness SD. Embryo transfer—can we learn anything new from the observation of junctional zone contractions? Hum Reprod. 1998;13:1540–6. doi: 10.1093/humrep/13.6.1540. [DOI] [PubMed] [Google Scholar]
- 11.Mansour RT, Aboulghar M, Serour G. Dummy embryo transfer: a technique that minimizes the problems of embryo transfer and improves the pregnancy rate in human in vitro fertilization. Fertil Steril. 1990;54:678–81. doi: 10.1016/s0015-0282(16)53829-1. [DOI] [PubMed] [Google Scholar]
- 12.Alvero R, Hearns-Stokes RM, Catherino WH, Leondires MP, Segars JH. The presence of blood in the transfer catheter negatively influences outcome at embryo transfer. Hum Reprod. 2003;18:1848–52. doi: 10.1093/humrep/deg359. [DOI] [PubMed] [Google Scholar]
- 13.van Weering H, Schats R, McDonnell J, Vink JM, Vermeiden J, Hompes P. The impact of the embryo transfer catheter on the pregnancy rate in IVF. Hum Reprod. 2002;17:666–70. doi: 10.1093/humrep/17.3.666. [DOI] [PubMed] [Google Scholar]
- 14.van de Pas MMC, Weima S, Looman CWN, Broekmans FJM. The use of fixed distance embryo transfer after IVF/ICSI equalizes the success rates among physicians. Hum Reprod. 2003;18:774–80. doi: 10.1093/humrep/deg175. [DOI] [PubMed] [Google Scholar]
- 15.Prapas Y, Prapas N, Hatziparasidou A, Vanderzwalmen P, Nihs M, Prapa S, et al. Ultrasound-guided embryo transfer maximizes the IVF results on day 3 and day 4 embryo transfer but has no impact on day 5. Hum Reprod. 2001;16:1904–8. doi: 10.1093/humrep/16.9.1904. [DOI] [PubMed] [Google Scholar]
- 16.Coroleu B, Carreras O, Veija A, Martell A, Martinez F, Belil I, et al. Embryo transfer under ultrasound guidance improves pregnancy rates after in vitro fertilization. Hum Reprod. 2000;15:616–20. doi: 10.1093/humrep/15.3.616. [DOI] [PubMed] [Google Scholar]
- 17.Papageorgiou T, Hearns-Stokes RM, Leondires MP, Miller BT, Chakrabarty P, Cruess D, et al. Training of providers in embryo transfer: what is the minimum number of transfers required for proficiency? Hum Reprod. 2001;16:1415–9. doi: 10.1093/humrep/16.7.1415. [DOI] [PubMed] [Google Scholar]
- 18.Knutzen V, Stratton CJ, Sher G, McNamee PI, Huang TT, Soto-Albors C. Mock embryo transfer in early luteal phase, the cycle before in vitro fertilization and embryo transfer: a descriptive study. Fertil Steril. 1992;57:156–62. [PubMed] [Google Scholar]
- 19.Sharif K, Afnan M, Lenton W. Mock embryo transfer with a full bladder immediately before the real transfer for in-vitro fertilization treatment: the Birmingham experience of 113 cases. Hum Reprod. 1995;10:1715–8. doi: 10.1093/oxfordjournals.humrep.a136161. [DOI] [PubMed] [Google Scholar]
- 20.Leondires M, Escalpes M, Segars J, Scott RT, Miller BT. Microdose follicular phase GnRH-a is a suitable alternative to luteal phase GnRH-a for ovarian stimulation at IVF. Fertil Steril. 1999;72:1018–23. doi: 10.1016/s0015-0282(99)00423-9. [DOI] [PubMed] [Google Scholar]
- 21.Henne MB, Milki AA. Uterine position at real embryo transfer compared with mock embryo transfer. Hum Reprod. 2004;19:570–2. doi: 10.1093/humrep/deh116. [DOI] [PubMed] [Google Scholar]
- 22.Ijland MM, Evers JL, Dunselman GA, Van Katwijk C, Lo CR, Hoogland HJ. Endometrial wavelike movements during the menstrual cycle. Fertil Steril. 1996;65:746–9. doi: 10.1016/s0015-0282(16)58207-7. [DOI] [PubMed] [Google Scholar]
- 23.Kunz G, Leyendecker G. Uterine peristalsis throughout the menstrual cycle: physiological and pathophysiological aspects. Hum Reprod Update. 1996;2(4) doi: 10.1093/humupd/4.5.647. item 16, video, CD-ROM. [DOI] [PubMed] [Google Scholar]
- 24.Ijlnad MM, Evers JLH, Hoogland HJ. Relation between endometrial wavelike activity and fecundability in spontaneous cycles. Fertil Steril. 1997;68:72–5. doi: 10.1016/s0015-0282(97)80075-1. [DOI] [PubMed] [Google Scholar]
- 25.Mansour RT, Aboulghar MA, Serour GI, Amin YM. Dummy embryo transfer using methylene blue dye. Hum Reprod. 1994;9:1257–9. doi: 10.1093/oxfordjournals.humrep.a138690. [DOI] [PubMed] [Google Scholar]
- 26.Nabi A, Awonuga A, Birch H, Barlow S, Stewart B. Multiple attempts at embryo transfer: does this affect in-vitro fertilization treatment outcome? Hum Reprod. 1997;12:1188–90. doi: 10.1093/humrep/12.6.1188. [DOI] [PubMed] [Google Scholar]


