Abstract
RamC is required for the formation of spore-forming cells called aerial hyphae by the bacterium Streptomyces coelicolor. This protein is membrane associated and has an amino-terminal protein kinase-like domain, but little is known about its mechanism of action. In this study we found that the presence of multiple copies of a defective allele of ramC inhibits morphogenesis in S. coelicolor, consistent with either titration of a target or formation of inactive RamC multimers. We identified a domain in RamC that is C terminal to the putative kinase domain and forms a dimer with a Kd of ∼0.1 μM. These data suggest that RamC acts as a dimer in vivo.
Germination of Streptomyces coelicolor spores results in the propagation of filamentous substrate hyphae that grow by elongating and branching, which gives rise to a colony referred to as a substrate mycelium. After 24 to 36 h of growth, colonies produce a second filamentous, nonbranching type of cells called aerial hyphae that project from the colony surface. These two cell types have different fates; the substrate hyphae produce secondary metabolites, including many compounds that have antibiotic activity (3), while the aerial hyphae produce spores (5). It has been demonstrated previously that the ramC gene encodes a membrane-associated protein having an amino-terminal serine/threonine kinase-like domain that is required for the production of aerial hyphae (12, 23). RamC is produced in the substrate hyphae but is absent from the aerial hyphae, at least by the time that spore formation has commenced (23), and our current hypothesis is that RamC phosphorylates an unknown target protein and that this helps drive the formation of aerial hyphae. There is a growing body of evidence that intercellular signaling triggers this developmental step in the S. coelicolor life cycle (6, 12, 15, 20-23, 31), and it is possible that RamC is part of this mechanism.
While genetic evidence suggests that RamC is a serine/threonine kinase, it is certainly a very unusual one. Numerous genes encoding this class of kinase have been identified in various bacteria, including, in particular, the myxococci, the mycobacteria, pseudomonads, and Streptomyces (1, 2, 17, 27, 32). The active centers of most of these kinases are highly conserved compared to each other and their eukaryotic counterparts. In contrast, the degree of sequence similarity of the RamC amino-terminal domain to the amino-terminal domains of the other kinases is rather limited, and the similar region includes a ∼120-amino-acid element inserted in the putative nucleotide binding region (12) that has not been found in any other kinase discovered so far. Indeed, at present, the C-terminal boundary of the putative kinase domain has not been defined with certainty, and there has been no convincing demonstration of RamC kinase activity in vitro (unpublished observations).
Aside from information concerning the amino terminus there is little information regarding the structural characteristics or mode of action that can be derived from the primary sequence. There is a notable repeated sequence C terminal to the putative kinase domain consisting of six back-to-back repeats of the consensus sequence VDETTR; however, this does not suggest any known structural motif. Furthermore, there are no RamC homologues with known functions in the genome databases; the only clear homologues are the products of the amfT genes of Streptomyces griseus and Streptomyces avermitilis, and both of these genes lie in gene clusters that are obviously related to the ram genes (13, 26).
We are dissecting RamC to elucidate its mechanism of action during morphogenesis in S. coelicolor. We report here that the presence of a defective allele of ramC on a multicopy plasmid has a partial dominant negative effect on morphogenesis of S. coelicolor, which is consistent with the possibility that RamC might act as a dimer or other higher-order complex in vivo. While the putative kinase domain of RamC did not appear to oligomerize, a short sequence C terminal to it that includes the VDETTR repeat brought about the formation of stable dimers. Our results suggest that RamC acts as a dimer.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Strains used for this work are listed in Table 1. S. coelicolor was grown on R2YE media (16) at 30°C. Escherichia coli was grown on Luria-Bertani medium at 37°C. For two-hybrid analysis, E. coli strain DHP-1 was grown on MacConkey minimal medium (Difco) supplemented with 1% maltose for 12 to 24 h at 30°C (9, 14). Ampicillin and chloramphenicol were used at concentrations of 100 and 25 μg/ml, respectively. Thiostrepton was used at a concentration of 50 μg/ml.
TABLE 1.
Strains used in this study
| Strain | Genotype and/or phenotype | Source or reference |
|---|---|---|
| XL-1 Blue | recA1 endA1 gyrA96 thi-1 glnV44 hsdR17 supE44 relA1 lac [F′ proAB lacIqZΔM15 Tn10(Tetr)] | Stratagene |
| ER2508 | F−ara-14 leuB6 fhuA2 Δ(argF-lac) U169 lacY1 lon::mini-Tn10 (Tetr) glnV44 galK2 rpsL20(Strr) xyl-5 mtl-5 Δ(malB) zjc::Tn5(Kanr) Δ(mcrC-mrr)HB101 | New England Biolabs |
| DHP-1 | F−cya gln44(AS) recA endA1 gyrA96 Nalrthi-l hsdR17 spoT1 rfbD1 | 14 |
| Er2-1 | F′lacI leuB6 thi-1 fbuA31 lacY1 tsx-78 galK2 galT22 supE44 hisG4 rpsL136 (Strr) xyl-5 mtl-1 dam-13::Tn9(Camr) dcm-6 mcrB1 mcrA hsdR2(rK− mK+) | J. McCormick |
| M145 | Prototroph SCP1− SCP2− | Lab stock |
| N373 | ramC::acc(3)IV SCP1− SCP2− | 23 |
Dominant negative mutant.
Wild-type ramC and the inactive mutant ramCD369A gene were excised from plasmids pTO8 and pTO8-D369A by using NheI and HindIII. The fragments were cloned into plasmid pIJ922 (Table 2) at the XbaI and HindIII sites. The resulting constructs were designated pRamC and pRamCD369A. These plasmids were passed through the nonmethylating strain Er2-1 and transformed into S. coelicolor (16).
TABLE 2.
Plasmids used in this study
| Plasmid | Genetic element(s)a | Parent vector | Reference or source |
|---|---|---|---|
| pIJ922 | tsr SCP2* | 16 | |
| pramC | ramC | pIJ922 | This study |
| pramCD369A | ramCD369A | pIJ922 | This study |
| pT18Bam | bla ori colE1 f1 origin T18 MCS with inserted BamHI site | pT18 | M. Eccleston, unpublished data |
| pT25 | cat ori p15A T25 MCS | p15 | 14 |
| pT18-N | ramC N terminus | pT18-Bam | This study |
| pT18-R | ramC repeat | pT18-Bam | This study |
| pT18-C | ramC C terminus | pT18-Bam | This study |
| pT25-N | ramC N terminus | pT25 | This study |
| pT25-R | ramC repeat | pT25 | This study |
| pT25-C | ramC C terminus | pT25 | This study |
| pMAL-c2X | bla ori pMB1 M13 origin lacIqmalE MalE fusion | New England Biolabs | |
| pMAL-rep | MalE-repeat domain fusion | pMAL-c2X | This study |
MCS, multiple cloning site.
Bacterial two-hybrid system.
Oligonucleotides (Table 3) were used to amplify segments of ramC and introduce BamHI restriction sites on either end for cloning into pT18Bam and pT25. The resulting constructs, pT18-N, pT18-R, and pT18-C, fused ramC segments to the 5′ end of the T18 portion of cyaA; constructs pT25-N, pT25-R, and pT25-C fused the same segments to the 3′ end of the T25 portion of cyaA. Combinations of these constructs were introduced into the cya E. coli strain DHP-1 and were analyzed by using the MacConkey indicator medium (as described by Eccleston et al. [9] and Karimova et al. [14]).
TABLE 3.
Oligonucleotides used in this study
| Oligonucleotide | Sequence |
|---|---|
| MBP-rep-top | 5′-GGATTTCAGAATTCGACCGGCACAAGGCGGCA-3′ |
| MBP-rep-bot | 5′-TAGCATGCCTGCAGTCACTGCGCGATGTCACCGGGGAA-3′ |
| RamC-N-Up | 5′-GGACGGATCCTGAACAAGGGCTACGCC-3′ |
| RamC-N-Dn | 5′-CGCCAGCGGGATCCGGTCCACGGCCACCCC-3′ |
| RamC-R-Up | 5′-CTGCGGATCCTCGACCGGCACAAGGC-3′ |
| RamC-R-Dn | 5′-GTCCGGGATCCCATGGACGCGCGTGG-3′ |
| RamC-C-Up | 5′-CACGCGGATCCATGGGGCCCCGGAC-3′ |
| RamC-C-Dn | 5′-TTTTGGATCCGCTCCTGGTGGGGCCGA-3′ |
Repeat domain fusion protein.
The segment of ramC encoding the repeat (rep) domain was amplified by using oligonucleotides MBP-rep-top and MBP-rep-bot (Table 3), which introduced an EcoRI restriction site and a PstI restriction site at the 5′ and 3′ ends of the DNA fragment, respectively. This construct was then introduced into pMAL-c2X to generate pMAL-rep.
Cultures of E. coli strain ER2508 carrying pMAL-rep were grown at 37°C to an optical density at 600 nm of 0.6. Expression of maltose binding protein (MBP)-rep was induced with 1 mM isopropyl-β-d-galactopyranoside at 37°C for 3 h. Cells were harvested by centrifugation, washed in 100 mM Tris (pH 8.0), and resuspended in buffer A (100 mM HEPES [pH 7.4], 200 mM NaCl, 1 mM EDTA, 1 mM dithiothreitol) containing 1 mM phenylmethylsulfonyl fluoride. Cells were lysed by three passes through a French press, and cell debris was removed by centrifugation at 20,000 × g for 30 min. The cleared lysate was applied to a 10-ml amylose resin column (New England Biolabs) by using an Akta Prime fast protein liquid chromatograph (Amersham Biosciences) and a flow rate of 1.0 ml/min. The column was washed with 11 column volumes of buffer A, and bound proteins were eluted by using buffer B (buffer A containing 10 mM maltose) at a flow rate of 1.5 ml/min. The presence of MBP-rep in each fraction was determined by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE). Fractions containing the fusion protein were pooled and concentrated by using Ultrafree-15 centrifugation filters (Millipore). The concentrated protein was applied to a Superdex-200 gel filtration column (Amersham Biosciences) equilibrated with buffer A at a rate of 0.2 ml/min. Fractions containing MBP-rep were pooled, dialyzed against buffer C (25 mM HEPES [pH 7.4], 200 mM NaCl, 1 mM dithiothreitol, 1 mM EDTA) containing 20% glycerol, and stored at −20°C until they were used. Protein concentrations were determined by the Bradford assay (Bio-Rad) by using bovine serum albumin as the standard. MBP was purchased from New England Biolabs. The protein was dialyzed against buffer C with glycerol and stored at −20°C until it was used.
Gel filtration.
A Superdex-200 analytical gel filtration column (Amersham Biosciences) was calibrated with ferritin (440 kDa; Stokes radius [ rS] = 6.1 nm), aldolase (158 kDa; rS = 4.8 nm), albumin (67 kDa; rS = 3.5 nm), ovalbumin (43 kDa; rS = 3.0 nm), chymotrypsinogen A (25 kDa; rS = 2.1 nm), and RNase A (13.7 kDa; rS = 1.6 nm) in buffer A at a flow rate of 0.2 ml/min by using a Beckman high-performance liquid chromatograph. The void volume was determined by using blue dextran 2000. Protein elution was monitored at 280 nm, and a standard curve of the elution volume parameter (Kav) versus rS was determined by using the equation Kav= (Ve − Vo)/(Vt − Vo), where Ve is the elution volume, Vt is the total column volume, and Vo is the void volume. To determine the rS of the fusion protein, 90 μl of a solution containing 0.5 mg of MBP per ml and/or 0.5 mg of MBP-rep per ml was applied to the column and developed as described above. The Kav and apparent rS of MBP and MBP-rep were determined from the standard curve.
Chemical cross-linking.
MBP and MBP-rep were subjected to chemical cross-linking by using the homobifunctional cross-linking agent dimethyl suberimidate (DMS) (Pierce). MBP or MBP-rep at a concentration of 5 μM was mixed with DMS at a concentration of 10 to 1,000 μM in 20-μl reaction mixtures on ice for 30 min. MBP and MBP-rep at a concentration of 5 μM were also reacted with 100 μM DMS on ice for 0 to 120 min. Each reaction was stopped by addition of Tris buffer (pH 8.0) to a concentration of 50 mM, and the mixture was analyzed by SDS-PAGE.
Analytical ultracentrifugation.
Sedimentation equilibrium analysis was performed with a Beckman-Coulter XL-A analytical ultracentrifuge, a four-cell An-60 Ti rotor, and six-channel Epon-charcoal cells with sapphire windows at 4°C; 110-μl samples of MBP-rep in buffer C were analyzed at concentrations corresponding to A280 values of 0.27, 0.2, and 0.1 (which corresponded to 1.45, 1.1, and 0.55 μM) and rotor speeds of 5,000, 10,000 and 15,000 rpm. For reference, 125 μl of buffer C was used in each reference cell. Concentration gradients were observed at 280 nm by using a radial step size of 0.001 and five scan repetitions. Centrifugation was carried out for 16 h, and the equilibrium state was confirmed by comparing absorbance scans obtained at 15 and 16 h. Data for all rotor speeds and protein concentrations were analyzed by using Beckman analysis software based on the Origin 6.0 package (Microcal). To model the experimental data as either a single ideally interacting species or as an equilibrium of a monomer and higher-order complexes, a self-association model was used (29). The protein partial specific volume (0.725 ml/g) and solvent density (1.008 g/ml) were estimated by using the program SEDNTERP.
To determine the molar dissociation constant (Kd) of the MBP-rep complex, the concentration-dependent association constant was derived by using the following equation: Ka(conc) = Ka(abs)(ɛl/2), where Ka(conc) is the per molar association constant, Ka(abs) is the absorbance-based association constant derived by using the Beckman software package, ɛ is the calculated molar extinction coefficient (70,410 cm−1 M−1), and l is the path length of the sample cell (1.2 cm). Kd was calculated by taking the inverse of Ka(conc) (29).
RESULTS AND DISCUSSION
Previously, it was found that the presence of a single copy of a defective allele of ramC in a morphologically wild-type strain of S. coelicolor had no effect on morphogenesis (12), suggesting that balanced levels of active and inactive RamC variants permitted normal function. To determine whether this was true if there was an excess of the inactive polypeptide, we introduced ramC or a ramC allele containing the D369A mutation in the putative kinase domain into a variant of the vector pIJ922 to produce plasmids pramC and pramCD369A. Both alleles were expressed from the ramC promoter. pIJ922 contains the SCP2* origin of replication and has a copy number of approximately five relative to the S. coelicolor chromosome (16). When introduced into the ramC null strain N373 (23), pramC, but not a control plasmid, complemented the developmental defect, as expected (data not shown). In the morphologically wild-type parent strain M145, pIJ922 and pramC had no discernible effect on morphogenesis; however, the presence of pramCD369A caused a reproducible delay in the formation of aerial hyphae (Fig. 1). This could be consistent either with titration of a target protein by inactive RamC or, if RamC formed a dimer or other higher-order complex, with titration of functional RamC into complexes with the defective RamCD369A polypeptide. Any such heteromeric complexes of RamC and RamCD369A must have retained some activity, however, because after incubation for a longer time the cells growing on the plate shown in Fig. 1c formed aerial hyphae.
FIG. 1.
Suppression of morphogenesis by overexpression of a defective ramC allele. S. coelicolor strain M145 containing pIJ922 (a), pramC (b), or pramCD369A (c) was grown on solid medium.
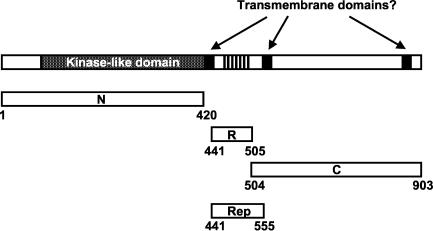
RamC (Fig. 2) can be divided into possible functional domains based on sequence motifs and results of previous work (12). To determine whether any of these domains had the capacity to assemble into a higher-order complex, we fused segments of the ramC gene encoding the 420 amino acid residues at the amino terminus (N fragment), 64 residues containing the VDETTRrepeat sequence (R fragment), and the 399 residues at the carboxy terminus (C fragment) in frame to the two vectors of a two-hybrid system (14). This two-hybrid system was based on the fact that expression of the maltose utilization genes (mal) depends on the presence of cAMP in E. coli and the fact that the Bordetella pertussis cyaA gene can complement an E. coli cya mutant. The B. pertussis adenylate cyclase enzyme can be split into two nonfunctional fragments (T18 and T25) that, when expressed in vivo as fusions to polypeptides that interact with one another, can be brought together to restore enzymatic activity. This can be readily detected by a pink colony phenotype on MacConkey medium containing maltose (14).
FIG. 2.
Schematic representation of RamC. Fragments corresponding to the amino-terminal putative kinase domain (N) (amino acids 1 to 420), a central rep-containing region (R) (amino acids 441 to 505), and a carboxy-terminal region with an unknown function (C) (amino acids 504 to 903) were tested for oligomerization by using a two-hybrid assay. The rep-containing region (residues 441 to 555) was fused to the MBP for in vitro analysis.
When plasmids pT18Bam and pT25 (which encode carboxy- and amino-terminal fragments of the B. pertussis adenylate cyclase) were introduced into the E. coli cya mutant DHP-1 (Table 1) and the resulting strain was plated on MacConkey medium containing maltose, there was no evidence of maltose fermentation, as expected. We introduced all possible combinations of pT18-N, pT18-R, and pT18-C with pT25-N, pT25-R, and pT25-C into DHP-1 and determined the capacity of the resulting strains to metabolize maltose. Combinations of the N and C fragments with each other or themselves did not restore a Mal+ phenotype to DHP-1, suggesting that none of the resulting fusion proteins had the capacity to interact with each other. However, when pT18-R and pT25-R were combined in DHP-1, the resulting colonies exhibited a weak but reproducible Mal+ phenotype. Combinations of either pT18-R or pT25-R with the N or C fusions did not result in a Mal+ phenotype, suggesting that this was a specific property of the R fusions. Finally, combinations of pT18-R and pT25-R with pT18Bam or pT25 did not allow maltose utilization, indicating that the interaction did not involve either fragment of adenylate cyclase but was again specific for the R fusions. These data (summarized in Table 4) suggested that the R fragment of RamC was able to form a higher-order complex with itself.
TABLE 4.
Two-hybrid analysis of fragments of RamCa
| Plasmid | Colony phenotype with:
|
|||
|---|---|---|---|---|
| pT18Bam | pT18N | pT18R | pT18C | |
| pT25 | − | − | − | − |
| pT25N | − | − | − | − |
| pT25R | − | − | + | − |
| pT25C | − | − | − | − |
Colony phenotypes of E. coli strain DHP-1 containing various pairs of plasmids were determined after plating on MacConkey medium containing maltose. −, white colony phenotype; +, pink colony phenotype.
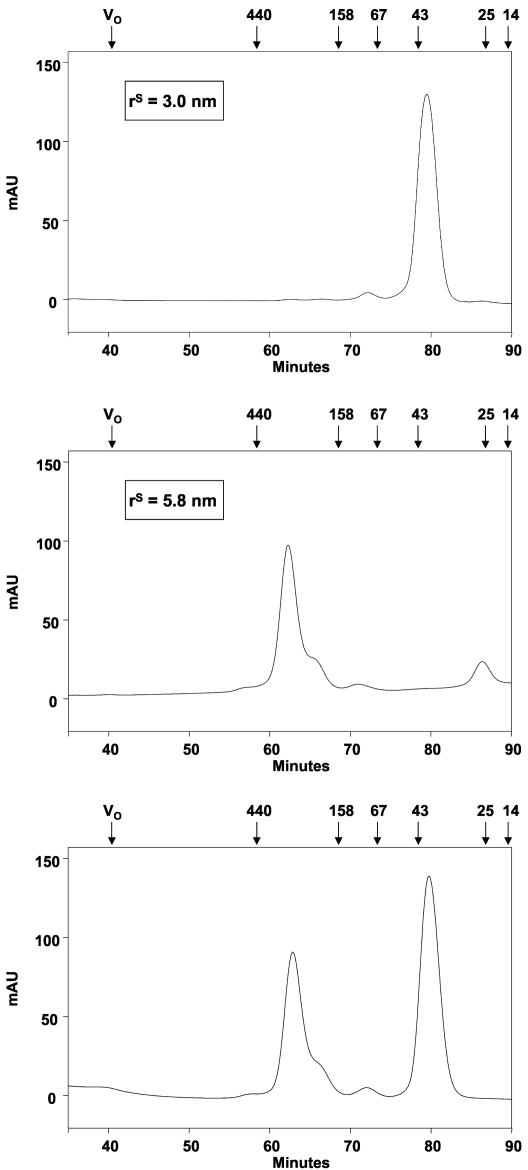
To examine this effect in vitro, we created a gene fusion of a ramC segment encoding residues 441 to 555 (the rep fragment, which was larger than the R fragment used in the two-hybrid analysis [see Fig. 2 and 6]) to the E. coli gene malE in the context of plasmid pMAL-c2X to create an expression vector for the fusion protein MBP-rep (Fig. 2). The product of malE, MBP, is a monomer in solution, so we determined whether the rep fragment caused it to form a higher-order complex. We therefore determined the rS values of purified MBP and MBP-rep by gel filtration chromatography. As shown in Fig. 3 (upper panel), MBP eluted from a Superdex-200 column at ∼80 min, at a position between ovalbumin (43 kDa; rS = 3.0) and chymotrypsinogen (25 kDa; rS = 2.1), which is consistent with its known molecular mass (43 kDa), and gave a calculated rS of 3.0 nm. These data are consistent with the fact that MBP is a monomer. In contrast, the MBP-rep fusion, which has a calculated molecular mass of 55 kDa, eluted from a Superdex-200 column at ∼62 min, between ferritin (440 kDa; rS = 6.1 nm) and aldolase (158 kDa; rS = 4.8 nm) (Fig. 3, middle panel). The derived rS value, 5.8 nm, is much larger than the value expected for a monomeric 55-kDa protein, suggesting either that the fusion protein had formed a nonspecific aggregate, that it was unfolded, or that it had formed a specific higher-order complex. We believe that the shoulder on the MBP-rep peak (Fig. 3, middle and lower panels) contained partially degraded protein, which could not be completely eliminated during purification.
FIG. 3.
Gel filtration analysis of MBP and MBP-rep. (Upper and middle panels) The mobility of MBP (upper panel) and MBP-rep (middle panel) during gel filtration yielded rS values for each protein. (Lower panel) Migration of the two proteins when they were subjected to gel filtration as a 1:1 mixture. The molecular masses (in kilodaltons) and elution times of the standard proteins are indicated at the top in each panel. mAU, milliabsorbance units.
The apparent oligomerization of MBP-rep could have been due either to a specific interaction of rep with itself or to a nonspecific interaction with MBP. To distinguish between these possibilities, we carried out an experiment in which a 1:1 mixture of MBP and MBP-rep was analyzed by gel filtration. The two polypeptides eluted from the column in discrete peaks at 80 and 62 min, respectively (Fig. 3, lower panel); no intermediate peak was observed, and SDS-PAGE analysis confirmed that each peak contained exclusively MBP or MBP-rep (data not shown), suggesting that no heterooligomers of MBP and MBP-rep had formed. Therefore, the apparent oligomerization of MBP-rep was likely due to specific interactions of the RamC rep fragment with itself. The behavior of proteins and protein complexes during gel filtration chromatography is sensitive to the shape of the protein or complex; hence, the results shown in Fig. 3 did not accurately reveal either the molecular weight or stoichiometry of the apparent MBP-rep complexes. Indeed, while the data were consistent with the formation of a higher-order complex by MBP-rep, they could also have suggested that the fusion was simply a very asymmetric molecule, a property that would result in excessively large rS values (4).
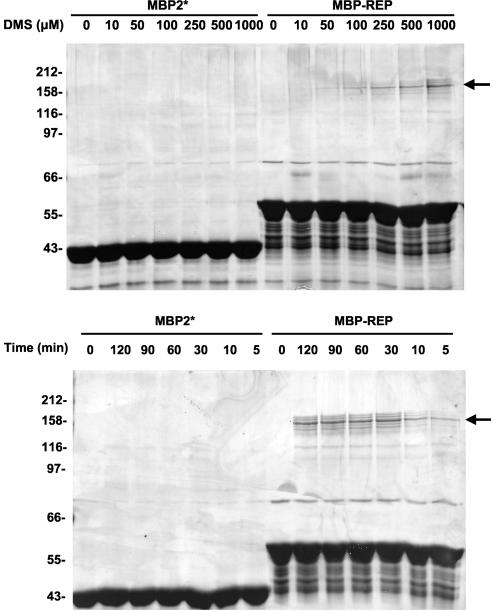
To determine whether a complex was formed, we carried out a cross-linking experiment with the reagent DMS. Various amounts of DMS and 5 μM MBP were mixed together and allowed to react. After 30 min the products were electrophoresed on an SDS-PAGE gel, and the gel was stained with silver. As shown in Fig. 4 (upper panel), addition of DMS to MBP had little or no effect on its subsequent migration on an SDS-PAGE gel even at a molar ratio of DMS to protein of 200:1, which is consistent with the monomeric nature of MBP. In contrast, when DMS was added to MBP-rep, a relatively modest molar ratio of DMS to the polypeptide (2:1 to 5:1) induced the formation of a covalent complex that migrated more slowly on SDS-PAGE gels. The formation of this cross-linked species was relatively inefficient; increasing the amount of DMS resulted in proportionate increases in the cross-linked product, but the preparation never reached saturation even with a vast molar excess of DMS compared to the amount of MBP-rep. We also examined time course variation in this experiment (Fig. 4, lower panel) and observed the same cross-linked species. We presumed that the inefficiency of cross-linking reflected the scarcity or orientation of DMS-reactive residues in the rep region of the fusion protein; DMS reacts with primary amines, and there is only one of these in the rep fragment. The five to seven minor cross-linked species surrounding the major band may have represented cross-links between full-length MBP-rep and partial proteolyzed protein. We do not believe that any of the bands reflected trimers, tetramers, or higher-order complexes because if this were the case, we would have expected a progression from lower-molecular-weight species to higher-molecular-weight species as cross-linking proceeded. We suspect, therefore, that all of the cross-linked species shown in Fig. 4 are dimers of MBP-rep.
FIG. 4.
Chemical cross-linking of MBP and MBP-rep: SDS-PAGE analysis of DMS cross-linking reactions of MBP and MBP-rep. (Upper panel) Reaction mixtures containing 5 μM MBP (MBP2*) either alone or with various concentrations of DMS. (Lower panel) Reaction mixtures containing 5 μM MBP and 1,000 μM DMS incubated for up to 120 min. Arrows indicate products of cross-linking reactions.
Finally, to determine the stoichiometry of the MBP-rep complex, we carried out an equilibrium sedimentation experiment with purified MBP-rep. This technique yields a precise mass measurement that is independent of a protein's or protein complex's shape and therefore allows precise assignment of stoichiometry (7). Figure 5 shows sample data for an experiment carried out at 15,000 rpm in which MBP-rep at concentrations of 0.55, 1.1, and 1.45 μM were used. The data for MBP-rep were fitted to the expected curves for a monomer, a dimer, a trimer, a tetramer, and a pentamer of a 55-kDa protein. MBP-rep's behavior was an excellent match for the behavior predicted for a dimer in all three curves. The residual plot described above showed the position of points relative to the origin, corresponding to the positions relative to the curve predicting the behavior of a dimer of 55-kDa proteins. The random distributions of points above and below the origin reflected the strong correlation of these data with the dimeric state. The results of experiments performed by using 5,000 and 10,000 rpm (data not shown) were virtually identical to those shown in Fig. 5, demonstrating that there was a rep fragment-induced dimer rather than any other oligomeric state or a monomer.
FIG. 5.
Analytical ultracentrifugation of MBP-rep. Equilibrium centrifugation data for 1.45, 1.1,and 0.55 μM RamC (◊) were fitted to curves predicted for a monomer, a dimer, a trimer, a tetramer,and a pentamer of a 55-kDa protein. The curves at the top show the residuals representing the accuracy of the dimeric protein model when they were compared to the actual data. AU, absorbance units.
The equilibrium sedimentation data were used to derive an absorbance-based association constant [Ka(abs)] for the MBP-rep dimer of 206 and therefore an association constant (Ka) of 8.7 × 106 M−1 and a dissociation constant (Kd) of 115 nM (see Materials and Methods). We noted that these values are consistent with our observation that all of the MBP-rep behaved as a dimer during the gel filtration experiment (Fig. 3) as the protein was applied to the gel filtration column at a concentration of ∼9 μM.
RamC overexpressed in E. coli was irreversibly insoluble in our hands, and this prevented us from testing the full-length protein for dimer formation. Nevertheless, taken together, our data demonstrate that the rep fragment of RamC is an efficient dimerization motif and therefore suggest that full-length RamC is also dimeric in nature. This in turn is consistent with a model in which the delay in morphogenesis induced by the presence of multiple copies of the ramCD369A allele is caused by the presence of heterodimers of wild-type RamC with RamCD369A and homodimers of RamCD369A. We concluded, therefore, that ideal RamC function requires assembly of homodimers of the active protein.
Dimer formation and autophosphorylation are common themes in the biochemistry of protein kinases (8, 10, 11, 18, 19, 24, 25, 28, 30). At present, we do not know the role of RamC dimerization; however, it is possible that the in vivo activity of RamC involves autophosphorylation or phosphorylation of a dimeric target. A most intriguing question concerns the role of the C-terminal half of the protein. We presume that the activity of this portion also depends on dimer formation.
Acknowledgments
We thank Tamara O'Connor for critical reading of the manuscript and Huy Nguyen for technical assistance.
This work was supported by an Ontario graduate scholarship to M.H. J.N. was supported by a new investigator award and by operating grant MT-15108 from the Canadian Institutes for Health Research.
REFERENCES
- 1.Av-Gay, Y., and M. Everett. 2000. The eukaryotic-like Ser/Thr kinases of Mycobacterium tuberculosis. Trends Microbiol. 8:238-244. [DOI] [PubMed] [Google Scholar]
- 2.Av-Gay, Y., S. Jamil, and S. J. Drews. 1999. Expression and characterization of the Mycobacterium tuberculosis serine/threonine protein kinase PknB. Infect. Immun. 67:5676-5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bibb, M. 1995. 1995 Colworth Prize Lecture. The regulation of antibiotic production in Streptomyces coelicolor A3(2). Microbiology 142:1335-1344. [DOI] [PubMed] [Google Scholar]
- 4.Cantor, C., and P. Schimmel. 1980. Biophysical chemistry, part II: techniques for the study of biological structure and function. W. H. Freeman and Company, San Francisco, Calif.
- 5.Chater, K. F. 2001. Regulation of sporulation in Streptomyces coelicolor A3(2): a checkpoint multiplex? Curr. Opin. Microbiol. 4:667-673. [DOI] [PubMed] [Google Scholar]
- 6.Chater, K. F., and S. Horinouchi. 2003. Signaling early developmental events in two highly diverged Streptomyces species. Mol. Microbiol. 48:9-15. [DOI] [PubMed] [Google Scholar]
- 7.Cole, J. L., and J. C. Hansen. 1999. Analytical ultracentrifugation as a contemporary biomolecular research tool. J. Biomol. Tech. 10:163-176. [PMC free article] [PubMed] [Google Scholar]
- 8.Dutta, R., L. Qin, and M. Inouye. 1999. Histidine kinases: diversity of domain organization. Mol. Microbiol. 34:633-640. [DOI] [PubMed] [Google Scholar]
- 9.Eccleston, M., R. Ahmed Ali, R. Seyler, J. Westpheling, and J. R. Nodwell. 2002. Structural and genetic analysis of the BldB protein of Streptomyces coelicolor. J. Bacteriol. 184:4270-4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanlon, W. A., M. Inouye, and S. Inouye. 1997. Pkn9, a Ser/Thr protein kinase involved in the development of Myxococcus xanthus. Mol. Microbiol. 23:459-471. [DOI] [PubMed] [Google Scholar]
- 11.Heldin, C. H. 1995. Dimerization of cell surface receptors in signal transduction. Cell 80:213-223. [DOI] [PubMed] [Google Scholar]
- 12.Hudson, M. E., D. Zhang, and J. R. Nodwell. 2002. Membrane association and kinase-like motifs of the RamC protein of Streptomyces coelicolor. J. Bacteriol. 184:4920-4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikeda, H., J. Ishikawa, A. Hanamoto, M. Shinose, H. Kikuchi, T. Shiba, Y. Sakaki, M. Hattori, and S. Omura. 2003. Complete genome sequence and comparative analysis of the industrial microorganism Streptomyces avermitilis. Nat. Biotechnol. 21:526-531. [DOI] [PubMed] [Google Scholar]
- 14.Karimova, G., J. Pidoux, A. Ullmann, and D. Ladant. 1998. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc. Natl. Acad. Sci. USA 95:5752-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelemen, G. H., and M. J. Buttner. 1998. Initiation of aerial mycelium formation in Streptomyces. Curr. Opin. Microbiol. 1:656-662. [DOI] [PubMed] [Google Scholar]
- 16.Kieser, T., M. Bibb, M. Buttner, K. Chater, and D. Hopwood. 2000. Practical Streptomyces genetics. John Innes Foundation, Norwich, England.
- 17.Mukhopadhyay, S., V. Kapatral, W. Xu, and A. M. Chakrabarty. 1999. Characterization of a Hank's type serine/threonine kinase and serine/threonine phosphoprotein phosphatase in Pseudomonas aeruginosa. J. Bacteriol. 181:6615-6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munoz-Dorado, J., S. Inouye, and M. Inouye. 1991. A gene encoding a protein serine/threonine kinase is required for normal development of M. xanthus, a gram-negative bacterium. Cell 67:995-1006. [DOI] [PubMed] [Google Scholar]
- 19.Neu, J. M., S. V. MacMillan, J. R. Nodwell, and G. D. Wright. 2002. StoPK-1, a serine/threonine protein kinase from the glycopeptide antibiotic producer Streptomyces toyocaensis NRRL 15009, affects oxidative stress response. Mol. Microbiol. 44:417-430. [DOI] [PubMed] [Google Scholar]
- 20.Nodwell, J. R., M. Yang, D. Kuo, and R. Losick. 1999. Extracellular complementation and the identification of additional genes involved in aerial mycelium formation in Streptomyces coelicolor. Genetics 151:569-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nodwell, J. R., and R. Losick. 1998. Purification of an extracellular signaling molecule involved in production of aerial mycelium by Streptomyces coelicolor. J. Bacteriol. 180:1334-1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nodwell, J. R., K. McGovern, and R. Losick. 1996. An oligopeptide permease responsible for the import of an extracellular signal governing aerial mycelium formation in Streptomyces coelicolor. Mol. Microbiol. 22:881-893. [DOI] [PubMed] [Google Scholar]
- 23.O'Connor, T. J., P. Kanellis, and J. R. Nodwell. 2002. Cell type specific expression and requirement for morphogenesis of the ramC gene of Streptomyces coelicolor. Mol. Microbiol. 45:45-57. [DOI] [PubMed] [Google Scholar]
- 24.Schlessinger, J. 2002. Ligand-induced, receptor mediated dimerization and activation of EGF receptor. Cell 110:669-672. [DOI] [PubMed] [Google Scholar]
- 25.Udo, H., M. Inouye, and S. Inouye. 1997. Biochemical characterization of Pkn2, a protein Ser/Thr kinase from Myxococcus xanthus, a Gram-negative developmental bacterium. FEBS Lett. 400:188-192. [DOI] [PubMed] [Google Scholar]
- 26.Ueda, K., S. Miyake, S. Horinouchi, and T. Beppu. 1993. A gene cluster involved in aerial mycelium formation in Streptomyces griseus encodes proteins similar to the response regulator and membrane translocator. J. Bacteriol. 175:2006-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Umeyama, T., P. C. Lee, and S. Horinouchi. 2002. Protein serine/threonine kinases in signal transduction for secondary metabolism and morphogenesis in Streptomyces. Appl. Microbiol. Biotechnol. 59:419-425. [DOI] [PubMed] [Google Scholar]
- 28.Urabe, H., and H. Ogawara. 1995. Cloning, sequencing and expression of serine/threonine kinase-encoding genes from Streptomyces coelicolor A3(2). Gene 153:99-104. [DOI] [PubMed] [Google Scholar]
- 29.Voelker, P., and D. McRorie. 1994. α-Chymotrypsin: characterization of a self-associating system in the analytical ultracentrifuge. Beckman Instruments technical bulletin T-1782A. Beckman Instruments, Inc., Fullerton, Calif.
- 30.Vomastek, T., R. Nadvornik, J. Janecek, Z. Technikova, J. Weiser, and P. Branny. 1998. Characterisation of two putative protein Ser/Thr kinases from actinomycete Streptomyces granaticolor both endowed with different properties. Eur. J. Biochem. 257:55-61. [DOI] [PubMed] [Google Scholar]
- 31.Willey, J., J. Schwedock, and R. Losick. 1993. Multiple extracellular signals govern the production of a morphogenetic protein involved in aerial mycelium formation by Streptomyces coelicolor. Genes Dev. 7:895-903. [DOI] [PubMed] [Google Scholar]
- 32.Zhang, C. C. 1996. Bacterial signaling involving eukaryotic-type protein kinases. Mol. Microbiol. 20:9-15. [DOI] [PubMed] [Google Scholar]