Abstract
The transforming growth factor beta (TGF-β ) family is comprised of over 30 family members that are structurally related secreted dimeric cytokines, including TGF-β, activins, and bone morphogenetic proteins (BMPs)/growth and differentiation factors (GDFs). TGF-β are pluripotent regulators of cell proliferation, differentiation, apoptosis, migration, and adhesion of many different cell types. TGF-β pathways are highly evolutionarily conserved and control embryogenesis, tissue repair, and tissue homeostasis in invertebrates and vertebrates. Aberrations in TGF-β activity and signaling underlie a broad spectrum of developmental disorders and major pathologies in humans, including cancer, fibrosis and autoimmune diseases. Recent observations indicate an emerging role for TGF-β in regulation of mitochondrial bioenergetics and oxidative stress responses characteristic of chronic degenerative diseases and ageing. Conversely, energy and metabolic sensory pathways cross-regulate mediators of TGF-β signaling. Here we review TGF-β and regulation of bioenergetic and mitochondrial functions, including energy and oxidant metabolism and apoptotic cell death, as well as their emerging relevance in renal biology and disease.
Keywords: Mitochondria, signal transduction, apoptosis, fibrosis, cytokine
Chronic kidney disease (CKD) is estimated to affect 10–15 million Americans 1. The most common causes of CKD include diabetes, hypertension, glomerulonephritis, and polycystic kidney disease 2. In addition to late fibrosis, abnormalities of epithelial and/or endothelial cells, such as atrophy and apoptosis of epithelial and/or endothelial cells and loss of tubular epithelial and postglomerular vascular structures are hallmarks of progressive CKD 3. TGF-β is considered a key mediator of renal cell injury in progressive CKD. TGF-β is induced by angiotensin II 4 and is increased in glomerular diseases 5 and diabetic nephropathy 6. Virtually all human and experimental forms of CKD are characterized by increased expression of TGF-β and TGF-β receptors (reviewed in 7). In addition to the RAS pathway, a broad range of metabolic factors, including glucose, advanced glycation products (AGE), free fatty acids (FFA), reactive oxygen species (ROS), and others (reviewed in 8), mechanical stretch and shear stress can increase TGF-β release and activation in renal and vascular cells 9.
TGF-β are secreted cytokines that signal across the plasma membrane into the cell by inducing heteromeric complexes of TGF-β type I and type II receptors with serine/threonine kinase activity 10. The ligand-induced activation of TGF-β receptor complexes leads to the recruitment and activation of canonical Smad family and non-canonical TGF-β signal transducers. Canonical Smad pathways directly connect TGF-β receptor activation with transcriptional control of TGF-β target genes 11. Non-canonical TGF-β signaling remains incompletely understood and is mediated through mitogen-activated protein kinases (MAPKs), phosphoinositide 3-OH kinases (PI3K), small GTPases, and other mediators 11,12. The conventional paradigm of TGF-β signal transduction proposes a central role for regulation of nuclear target gene expression 13, although direct regulation of microRNA processing 14, and mitochondrial translocation of Smad proteins 15 indicate extranuclear targets and mechanisms in TGF-β signaling.
A large body of evidence demonstrates that TGF-β inhibition improves functional and structural renal defects in experimental renal disease models, confirming a central role for TGF-β in CKD and renal fibrosis (reviewed in 3). Chronically elevated TGF-β activity in transgenic mice overexpressing active TGF-β1 is sufficient to induce progressive renal disease with glomerulosclerosis and tubulointerstitial fibrosis, and knockout of the major signal transducer Smad3, or overexpression of the inhibitory Smad7, prevent defects associated with experimental unilateral ureteral obstruction, diabetic nephropathy, or renal ablation 16–19. Together with numerous additional reports, these results consistently support the paradigm that TGF-β and its signal transducers are central mediators of progression of CKD.
Epithelial cell injury and apoptosis are increasingly considered critical initial responses to various forms of renal injury, and studies of the underlying mechanisms are providing crucial novel insights and therapeutic targets in CKD progression 20–25. TGF-β itself acts on epithelial cells to induce apoptosis in renal tubular epithelial cells and glomerular podocytes and may thereby promote further epithelial injury. Since mitochondria are the main cellular source of energy and reactive oxygen species (ROS) in response to metabolic demands and/or cellular stress, and central regulators of the intrinsic apoptosis pathway, attention is focusing increasingly on the role and mechanisms of bioenergetic defects and mitochondrial dysfunction in renal epithelial and vascular injury.
TGF-β and mitochondrial biogenesis, fission and fusion
Mitochondrial volume density and network properties are regulated by physiological signals as well as environmental stimuli to meet the demand for energy in tissues. In general, mitochondrial mass is elevated in tissues with high ATP consumption, such as cardiac and skeletal muscle, the central nervous system, brown adipose and renal tissue. The peroxisomal proliferator-activated receptor (PPAR) coactivator 1 (PGC-1) family of transcriptional coactivators have emerged as master regulators of mitochondrial biogenesis. PGC-1 activation requires phosphorylation by AMP-activated protein kinase (AMPK) and deacetylation by sirtuins 26,27, constituting an energy sensing network that controls energy expenditure, including mitochondrial energy metabolism. While increasing evidence suggests extensive cross-regulation of TGF-β pathways by energy sensing AMPK and SIRT1, a direct role of TGF-β pathways controlling PGC-1 or energy sensing has not been demonstrated to date.
Mitochondria are organized in a dynamically-regulated filamentous network that extends throughout the cell. The organization of mitochondrial network in the cell result from the balanced activity of two opposite processes – fusion and fission (fragmentation) - regulated by different sets of proteins in response to cellular energetic and metabolic requirements 28. The importance of the correct regulation of the mitochondrial network is highlighted by the findings that two hereditary neurodegenerative disorders, autosomal dominant optic atrophy and Charcot-Marie-Tooth syndrome, are caused by mutations in genes encoding dynamin-related GTPase OPA1 and mitofusin 2 (MFN2), both involved in the mitochondrial fusion regulation 29,30.
In proximal tubular cells, mitochondrial fragmentation driven by the fission-inducer Dynamin-related protein 1 (Drp1) promotes mitochondrial membrane permeabilization, release of apoptogenic factors and apoptosis. Prevention of mitochondrial fragmentation by inhibition of Drp1 activation abrogates mitochondrial damage, tubular cell apoptosis, and renal injury 31. Consistent with its pro-apoptotic activity, TGF-β induces mitochondrial fragmentation in proximal tubular epithelilal cells 32. Wang W. et al. have recently showed ROCK1 as mediator of mitochondrial fission in diabetic nephropathy demonstrating the relevance of mitochondrial modeling in podocytes and endothelial cells function 33. In contrast, mitochondrial fusion and formation of giant mitochondria was associated with TGF-β-induced senescence in epithelial mink lung epithelial cells 34.
The molecular mechanisms mediating mitochondrial fragmentation in response to TGF-β remain unknown. However, because mitochondrial fragmentation enhances pro-apoptotic Bax insertion in the outer mitochondrial membrane 35, unraveling of these mechanisms may advance our understanding of regulation of cell survival and cell death decisions.
TGF-β and bioenergetics
Direct and indirect links between TGF-β and mitochondria energy metabolism and energy sensing pathways are emerging in multiple research areas. The contribution of TGF-β superfamily members in the regulation of adipogenesis is reviewed in 36. For example, the effects of aberrant activin signaling on body fat, adipocytes differentiation, calories consumption, and oxygen consumption were associated with an expression pattern consistent with generalized increased mitochondrial metabolism and with a partial constitutive uncoupling in liver mitochondria 36. Role of TGF-β/Smad3 signaling in the modulation of adipose tissue energy metabolism was confirmed by Yadav H. et al. that proposed protection from obesity and diabetes by blockade of TGF-β/Smad3 signaling 37. In airway smooth muscle (ASM) from patients with asthmatic and chronic obstructive pulmonary disease, TGF-β overexpression deregulates redox state 38. Thus, TGF-β simultaneously stimulates ROS production by Nox4 activation and compromises the antioxidant systems that defend against mitochondrial and peroxisomal derived ROS by inhibiting the expression of MnSOD and catalase, respectively 38.
TGF-β modulates energy metabolism also by controlling mitochondrial metabolism directly. TGF-β-induced reduction of complex IV and mitochondrial respiration leads to increased ROS and decreased mitochondrial membrane potential associated with senescence in mink lung epithelial cells. 34. In breast tumor cells TGF-β/Smad signaling regulates mitochondrial uncoupler UCP2 expression to maintain a well differentiated and low proliferating phenotype associated with a good clinical prognosis 39. In TGF-β-resistant grade 3 tumor cells, elevated expression of UCP2 is associated with increased tumor cell survival and proliferation 39. In preglomerular afferent arteriolar smooth muscle cells TGF-β depresses angiotensin II or endothelin-evoked calcium signaling. The responses of mitochondrial [Ca2+] are particularly depressed and delayed as a consequence of a uncoupling of mitochondria from the ER calcium release 40. On balance, TGF-β signaling appears to inhibit mitochondrial respiration and ATP synthesis (Fig. 1).
Figure 1.
TGF-β targets in mitochondria. TGF-β signaling may act in a direct or indirect manner to control mitochondrial metabolism in different cell types. ⍊ and ↓ indicate inhibition/downregulation by TGF-β pathway. Oxidative phosphorylation system (I-V), membrane potential (ΔΨ), reactive oxygen species (ROS), endoplasmic reticulum (ER). Inhibition of the oxidative phosphorylation system may arise from a direct inhibition of complex IV and/or from increased ROS production due to downregulation of scavenging proteins (MnSOD, Mpv17l). Decrease in membrane potential is associated with cytochrome c release in the cytoplasm with consequent activation of the apoptotic signaling. Apoptosis can be triggered by release of HtrA2 as a consequence of Mpv17l downregulation. Uncoupling of mitochondria from ER calcium release may impair the intracellular calcium sensing responses.
TGF-β and energy sensors AMPK and SIRT1
The interaction between energy metabolism and TGF-β signaling is further demonstrated by cross-regulation between adenosine monophosphate-activated protein kinase (AMPK) pathways and TGF-β. AMPK is a cellular energy sensor activated by increased AMP: ATP ratio, an index of metabolic stress. For example, AMPK activation inhibits TGF-β-induced transcription driven by Smad3-binding cis-elements involved in myofibroblast differentiation in wound healing 41. TGF-β-induced activation and collagen synthesis of hepatic stellate cells was inhibited by AMPK-mediated inhibition of interaction of p300 and Smad3 42. In addition, TGF-β-activated kinase 1 (TAK1) is required for a proper activation of AMPK in cardiac myocytes 43.
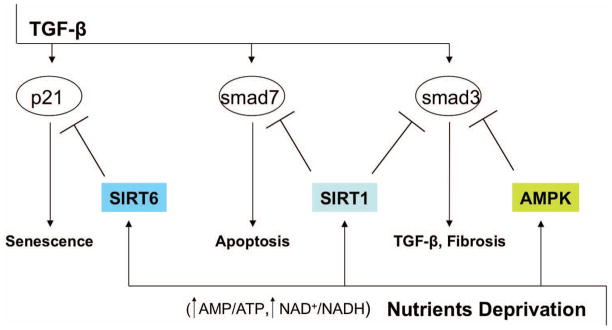
Increasing evidence also suggests extensive interactions between the sirtuin family and TGF-β pathways in metabolic control. Sirtuins are highly conserved NAD(+)-dependent protein deacetylases and/or ADP-ribosyltransferases that regulate and extend the lifespan of lower model organisms, including yeast, worms and flies 44. Mammalian sirtuins, SIRT1 to SIRT7, are critical metabolic sensors, connecting environmental signals to metabolic homeostasis and stress response 44–46. Three sirtuins, SIRT3, SIRT4 and SIRT5, are located in the mitochondria, and play crucial roles in apoptosis and intracellular signaling 46. Mammalian SIRT1 couples protein deacetylation with NAD(+) hydrolysis and links cellular energy and redox state to multiple signaling and survival pathways, including TGF-β pathways. For example, whilst SIRT1 binds and deacetylates Smad7 in mesangial cells, accelerating Smad7 degradation,. loss of SIRT1 stabilizes Smad7 and increases apoptosis 47. Smad3 transcriptional activity is inhibited by SIRT1-mediated deacetylation in renal fibrosis model of unilateral ureteral obstruction 48. Tetrahydroxystilbene glucoside ameliorates diabetic nephropathy in rats by alleviating oxidative stress injury and overexpression of COX-2 and TGF-β 1 via activation of SIRT1 49. TGF-β-induced cellular senescence of primary bronchial epithelial cells is inhibited by SIRT6 via proteasomal degradation of p21 50. In contrast with extramitochondrial SIRT1, interaction of mitochondrial SIRT3, 4, or 5 with TGF-β networks has not been demonstrated to date. The available evidence on energy sensing AMPK and SIRT1 mediators and TGF-β/Smad pathways suggest that activity of AMPK and SIRT1 is inversely correlated with TGF-β/Smad signaling (Fig. 2). This negative cross-regulation may couple information about abundance of nutrients with inhibition of TGF-β/Smad-induced cell death programs.
Figure 2.
TGF-β and AMPK-SIRT crosstalk. The negative cross-regulation between TGF-β signaling and energy sensing mediators AMPK and SIRT has been demonstrated in kidney, liver, and lung. AMPK and SIRTs may downregulate TGF-β messengers either by degradation or inhibition of transcriptional activity. AMPK mediates inhibition of transcription co-activator p300 and Smad3 interaction in hepatic stellate cells. SIRT1-mediated deacetylation of Smad3 and Smad7 results in reduction of apoptosis and fibrosis in kidney cells in vitro and in vivo. SIRT6-mediated proteasomal degradation of p21 prevents TGF-β-induced senescence in primary bronchial epithelial cells.
TGF-β and mitochondrial oxidative stress
Mitochondria are a major source of reactive oxygen species (ROS) production within cells 51. Mitochondrial DNA is directly exposed and particularly susceptible to modification by ROS, and this damage can rapidly lead to functional impairment in the respiratory chain. To prevent oxidative damage, mitochondrial antioxidant systems include the mitochondrial matrix enzyme manganese superoxide dismutase (MnSOD), glutathione peroxidase, and peroxiredoxins 3 and 5 52. Many forms of cellular stress and signaling pathways lead to imbalance of ROS synthesis and antioxidant systems. As a result, persistently increased mitochondrial oxidative stress contributes to a wide range of pathologies, including cardiovascular disease, neurodegeneration, diabetic complications, renal disease, and aging 53–56.
Studies in the liver and pancreas have shown that TGF-β – mediated apoptosis involves activation of caspase proteases, enhanced generation of reactive oxygen species (ROS), loss of glutathione (GSH), loss of mitochondrial membrane potential and alterations in expression of the Bcl-2 family of proteins. The observed changes in mitochondrial membrane potential have been suggested to lead to mitochondrial dysfunction and to occur as a consequence of oxygen radical generation by TGF-β 57.
It is known that at lower doses TGF-β can induce cell-cycle arrest, however, in fetal hepatocytes higher doses of TGF-β induce apoptosis by ROS generation, which is potentially responsible for the decrease in Bcl-xL messanger RNA levels, followed by the loss of membrane potential, the release of cytochrome c, and the activation of caspase 3 58. The same group showed that although inhibition of caspases can block apoptosis associated morphology and nuclear fragmentation, it only delayed cell death, therefore indicating that activation of the apoptotic program by TGF-β in fetal hepatocytes inevitably leads to death, with or without caspases 59. Cell death, however, could be blocked by radical scavengers.
One of the mechanisms proposed for TGF-β-induced ROS production is the activation of NADPH oxidases (Nox), which are multisubunit enzymes that generate superoxide by transferring electrons from NADPH to molecular oxygen. More recently, it has been shown that inhibition of NADPH oxidase activity, blocks all the apoptotic features induced by TGF-β, suggesting that extramitochondrial ROS is critical for initiating TGF-β mediated cell death. Studies have now defined the role of Nox4 in mediating TGF-β apoptosis of hepatocytes 60 and the conversion of cardiac fibroblast into myofibroblasts (important consequence in cardiac fibrosis) by regulating Smad 2/3 activation 61. Hence TGF-β redox-dependent signaling pathways involve mitochondrial independent superoxide by Nox and mitochondrial ROS might increase as a consequence.
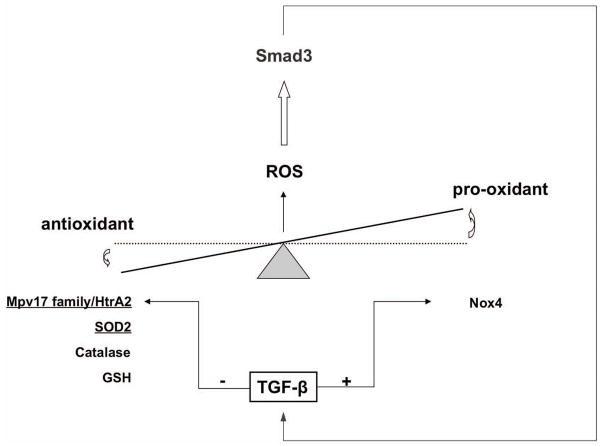
The pro-oxidant effect of TGF-β have been further linked to an inhibition of MnSOD and catalase expression accompanied by upregulation of Nox4 and induction of pro-inflammatory signaling by abnormal airway smooth muscle cells 38. Interestingly the authors report that the early release of ROS in response to TGF-β may activate Smad3 in a positive-feedback manner, thus leading to amplification of oxidative stress and downregulation of antioxidant effects (Fig. 3).
Figure 3.
TGF-β and oxidative stress. TGF-β increases oxidative stress by regulating both antioxidant and pro-oxidant proteins. Downregulation of mitochondrial (underline) Mpv17-HtrA2 system, manganese superoxide-dismutase (SOD2), catalase, and glutathione (GSH) with the concomitant stimulation of pro-oxidant NADPH-oxidase (Nox4) results in increased ROS production that may trigger a positive-feedback response in which TGF-β signaling is amplified by Smad3.
The central role for mitochondrial dysfunction and oxidative stress in cardiovascular and renal disease has been demonstrated by the beneficial effects of mitochondrial targeted antioxidant scavengers in several experimental models. Coenzyme Q (CoQ) redox shuttle is involved in ROS production by complexes I and III. Decreased levels of CoQ are associated with increased ROS production, which can be reduced by delivery of mitochondrial targeted ubiquinone compounds (MitoQ). Oral administration of MitoQ protects against mitochondrial oxidative damage, ameliorates the development of hypertension, improves endothelial dysfunction, and reduces cardiac hypertrophy in the stroke prone spontaneously hypertensive rat model 62. MitoQ treatment also improved tubular and glomerular function in the Ins2+/AkitaJ mouse model of type 1 diabetes. The authors show that MitoQ treatment was associated with decreased nuclear translocation of phospho-Smad2/3 in kidney cells, indicating reduced TGF-β/Smad signaling activity in MitoQ-treated diabetic mice 63.
Mitochondrial targeted antioxidants also prevents tubular cell apoptosis in the UUO model and ameliorates renal fibrosis 64. These observations suggest that TGF-β effects on mitochondria and mitochondrial ROS production can be reversed, however once intrinsic mitochondrial apoptotic pathway is activated cell death will be the consequence.
TGF-β and mitochondrial DNA depletion
Mitochondrial DNA (mtDNA) mutations and deletions have been the subject of numerous studies and have been implicated in age and disease-associated mitochondrial dysfunctions. The mtDNA encodes for 13 structural genes of OXPHOS enzymes, two ribosomal RNAs, and 22 transfer RNAs. The mtDNA is prone to oxidative stress, since it lacks histone-like coverage and is localized closely to the inner mitochondrial membrane, a major site of ROS in cells. Several reports demonstrate focal segmental glomerulosclerosis (FSGS) and proteinuria in patients with an A-to-G transition at mtDNA position 3243 in the gene for tRNALeu(UUR) 65, 66. Mice carrying mutant mtDNA with a 4696-bp deletion develop FSGS and die within 6 months due to renal failure. In this mouse model, the kidney carried the greatest accumulation of the mtDNA deletion of any organ 67. TGF-β relevance in the pathogenesis of glomerulosclerosis is well known. Puromycin-induced FSGS in rats was associated with a marked reduction in mtDNA copy number and reduced levels of cytochrome oxidase I in glomeruli, suggesting that mitochondrial dysfunction by mtDNA depletion potentially plays a key role in the pathogenesis of FSGS in puromycin aminonucleoside nephropathy 68.
Recently, mutations in the protein kinase, DNA-activated, catalytic polypeptide (Prkdc) gene, which encodes a critical nuclear DNA double-stranded break repair protein, were associated with impaired mtDNA maintenance and underlie susceptibility to adriamycin (ADR) nephropathy in mice 69. Adriamycin (ADR) nephropathy is a classic experimental model of podocytopathy, resulting from selective injury to glomerular podocytes 70. Interestingly, genetic interaction of Prkdc mutant and Mpv17 mutant alleles was required to manifest the mtDNA maintenance defects 69.
The MPV17 gene encodes a mitochondrial inner membrane protein that is implicated in the preservation of mitochondrial homeostasis by controlling mitochondrial DNA (mtDNA) maintenance and oxidative phosphorylation (OXPHOS) activity 71. Mice carrying Mpv17 null alleles developed FSGS-like glomerular pathology and proteinuria 72. Increased ROS production in isolated glomeruli and renal damage is prevented by oxygen radical scavengers 73. TGF-β treatment of podocytes is associated with rapid and profound downregulation of Mpv17, increased mitochondrial ROS production and apoptosis (Krick S and Bottinger E, unpublished observations). The Mpv17 family member Mpv17l is also localized in mitochondria and downregulated by TGF-β, albeit in proximal tubular epithelial cells 32. Loss of the Mpv17l/HtrA2 complex in mitochondria in response to TGF-β causes mitochondrial dysfunction and apoptosis 32. Thus, TGF-β may suppress the function of Mpv17/HtrA2 mitochondrial complexes in podocytes and proximal tubular epithelial cells to induce mitochondrial dysfunction and apoptosis associated with mitochondrial DNA depletion.
Mitochondrial localization/translocation of TGF-β and Smad proteins
Mitochondria represent one locus of intracrine action of growth factors, including TGF-β. TGF-β is localized in the mitochondria of cardiac myocytes and hepatic cells and is also seen in association with the contractile filaments of cardiac myocytes 74. The functional role of mitochondrial TGF-β that was first documented over 20 years ago, still remain poorly understood.
Recent results suggest that Smad4 translocates to mitochondria in TGF-β or UV damage induced apoptotic cells 15. Transfection of cells with mitochondrial-targeted Smad4 inhibited oxidative respiratory chain through direct interaction of mitochondrial Smad4 with cytochrome c oxidase subunit II (COXII), a subunit of cytochrome oxidase c. Targeting Smad4 to mitochondria enhanced TGF-β-induced apoptosis, suggesting that the interaction between the Smad4 and COXII in mitochondria may be an important mechanism for TGF-β-induced apoptosis 15.
Smad5, a receptor-regulated Smad in the bone morphogenetic proteins (BMP) pathway, manifested mitochondrial localization in sub-confluent chondrogenic progenitor cells 75. Interestingly, mitochondrial abnormalities, including swelling and decreased mitochondrial membrane potential, and apoptosis were characteristic of cardiomyocytes derived from Smad5-deficient embryos or in vitro-differentiated ES cells 76. Together these isolated observations point to direct, extranuclear roles of TGF-β and Smad signals in mitochondrial function, integrity, and apoptosis. Further studies will be required to expand our understanding of the role and regulation of mitochondrial TGF-β/Smad signaling, in particular its potential relevance to TGF-β induced apoptosis in renal cells.
microRNA and TGF-β in control of mitochondrial function and energy metabolism
microRNAs are a group of small non-coding RNAs capable of regulating expression of hundreds of target genes simultaneously by interacting with target sequences in their 3′-untranslated regions, resulting in transcript degradation or repression of translation 77. MicroRNA deregulation contributes to the pathogenesis of a large number of human diseases, including kidney disease 78,79. Cre/loxP-mediated deletion of the microRNA processing enzyme dicer selectively in glomerular podocytes of mice resulted in progressive proteinuria and glomerulosclerosis associated with progressive podocyte apoptosis and podocyte depletion, suggesting that microRNA are required for podocyte homeostasis 80–82.
Mitochondrial and metabolic regulation by microRNA has recently been demonstrated. For example, the miR-30 family of microRNA has recently been shown to target p53 directly in human cardiomyocytes, resulting in inhibition of Drp1-mediated mitochondrial fission and apoptosis in response to oxidative stress stimulus 83. Interestingly, TGF-β downregulates all five miR-30s in podocytes and overexpression of miR-30s prevents TGF-β-induced podocyte apoptosis (Shi S and Bottinger E; unpublished data). MicroRNA profiling of Parkinson’s disease brains identified downregulation of miR-34b/c associated with alterations in mitochondrial protein expression that underlie neuronal mitochondrial dysfunction and impaired cell viability 84. Moreover, miR-34 is downregulated in nasopharyngeal carcinoma and miR-34 target gene analysis indicate that miR-34 represses TGF-β pathway signaling 85. Hypoxia induced miR-210 via HIF-1alpha resulting in miR-210-mediated repression of iron-sulfate cluster assembly proteins 1/2 (ISCU1/2) that are critical for electron transport and mitochondrial oxidation-reduction reactions 86. In addition to ISCU proteins, hypoxia-induced miR-210 also represses cytochrome c oxidase assembly protein COX10 associated with increase ROS and mitochondrial dysfunction in cancer cell lines 87. Interestingly, miR-210 represses activin A receptor type 1B (AcvR1b) to promote osteoblast differentiation 88. Because activin signaling controls genes for mitochondrial biogenesis and mitochondrial function 36, one can reason that miR-210 may control mitochondrial function and energy homeostasis via regulation of activin signaling.
Declining oxygen tension in cardiac myocytes causes downregulation of miR-199a, resulting in derepression of miR-199a targets HIF-1alpha and SIRT1 that is associated with p53-mediated apoptosis 89. Since SIRT1 negatively cross-modulates TGF-β/Smad3 signaling, it is possible that miR-199a functions as an upstream regulator of TGF-β/Smad signaling in mitochondrial and metabolic control. Although there is only limited evidence linking microRNA and TGF-β pathways to date, it is likely that microRNA and TGF-β coordinately regulate mitochondrial dysfunction, oxidative stress, and energy metabolism in oxidative stress-associated renal injury.
TGF-β and Bcl2 family proteins
Bcl2 family proteins are key regulators of apoptosis by controlling the integrity of the outer mitochondrial membrane (OMM). During apoptosis, permeabilization of the OMM leads to release of cytochrome c and other proapoptotic factors that can initiate formation of the apoptosome 90. Insertion of anti-apoptotic Bcl2 proteins, such as Bcl2 and Bcl-xl, reduces OMM permeability, while insertion of pro-apoptotic Bcl2 protein, such as Bax and Bak, promotes OMM permeability and apoptosis. Perhaps not surprisingly, overexpression of Bax sensitizes prostate cancer cells to TGF-β-induced apoptosis 91. Conversely, Bcl-xl expression prevents TGF-β-induced cytochrome c release and apoptosis 92. The role and regulation of Bcl-2 family members in mitochondrial apoptosis signaling in lower organisms and mammals has been reviewed extensively 90. Whereas Bcl2 proteins exclusively promote mitochondrial fission in apoptotic cells, they may also regulate mitochondrial fusion in non-apoptotic cells. For example, Bcl2 proteins regulate the mitochondrial fusion machinery by directly interacting with its components including mitofusins 93. The functional role of regulation of mitochondrial dynamics by Bcl2 proteins in non-apoptotic cells remains poorly understood, but will likely be a productive area of investigation.
Summary and future perspectives
Historically the focus of investigation of TGF-β ’s expansive involvement in the pathogenesis of progressive renal disease and fibrosis has been on its nuclear transcriptional targets. As we discuss in this review, increasing evidence points to a critical role for TGF-β pathways in controlling extranuclear targets involved in energy balance, metabolism, and oxidative species, in particular mitochondria. On balance, the available data suggest that increasing TGF-β activity is associated with mitochondrial dysfunction and increasing mitochondrial ROS synthesis with detrimental consequences, in particular mitochondrial apoptosis. Conversely, energy and metabolic sensory pathways including AMPK and SIRT1 may downmodulate TGF-β/Smad pathways to promote cell viability.
The clinical relevance of mitochondrial dysfunction and oxidative stress in progressive renal disease is highlighted by recent studies demonstrating that new antioxidant therapeutics targeting specifically mitochondrial ROS ameliorate diabetic nephropathy and tubulointerstitial fibrosis lesions in experimental models. Renal cell apoptosis, in particular affecting podocytes and tubular epithelial cells, is a driving mechanism in progression of glomerulosclerosis, nephron loss, and subsequent fibrosis. Future progress in our understanding how mitochondria control and respond to TGF-β pathway activation in response to metabolic and environmental stress factors associated with renal disease progression is expected to lead to novel treatments in progressive CKD.
Acknowledgments
Supported in part by National Institutes of Health grants 5R01DK060043, 5R01DK073960, 5U01DK060995 and 5R01DK056077 to E.P.B..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xue JL, Ma JZ, Louis TA, Collins AJ. Forecast of the number of patients with end-stage renal disease in the United States to the year 2010. J Am Soc Nephrol. 2001;12:2753–8. doi: 10.1681/ASN.V12122753. [DOI] [PubMed] [Google Scholar]
- 2.Collins AJ, Foley RN, Herzog C, et al. Excerpts from the US Renal Data System 2009 Annual Data Report. Am J Kidney Dis. 55:S1–420. A6–7. doi: 10.1053/j.ajkd.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bottinger EP. TGF-beta in renal injury and disease 4. Semin Nephrol. 2007;27:309–20. doi: 10.1016/j.semnephrol.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 4.Kagami S, Border WA, Miller DE, Noble NA. Angiotensin II stimulates extracellular matrix protein synthesis through induction of transforming growth factor-beta expression in rat glomerular mesangial cells. J Clin Invest. 1994;93:2431–7. doi: 10.1172/JCI117251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okuda S, Languino LR, Ruoslahti E, Border WA. Elevated expression of transforming growth factor-beta and proteoglycan production in experimental glomerulonephritis. Possible role in expansion of the mesangial extracellular matrix [published erratum appears in J Clin Invest 1990 Dec;86(6):2175] J Clin Invest. 1990;86:453–62. doi: 10.1172/JCI114731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma K, Ziyadeh FN. Renal hypertrophy is associated with upregulation of TGF-beta 1 gene expression in diabetic BB rat and NOD mouse. Am J Physiol. 1994;267:F1094–F01. doi: 10.1152/ajprenal.1994.267.6.F1094. [DOI] [PubMed] [Google Scholar]
- 7.Border WA, Noble NA. Transforming growth factor beta in tissue fibrosis. N Engl J Med. 1994;331:1286–92. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- 8.Ziyadeh FN. Mediators of diabetic renal disease: the case for tgf-Beta as the major mediator. J Am Soc Nephrol. 2004;15 (Suppl 1):S55–S7. doi: 10.1097/01.asn.0000093460.24823.5b. [DOI] [PubMed] [Google Scholar]
- 9.Cortes P, Riser B, Narins RG. Glomerular hypertension and progressive renal disease: the interplay of mesangial cell stretch, cytokine formation and extracellular matrix synthesis. [Review] [14 refs] Contributions to Nephrology. 1996;118:229–33. doi: 10.1159/000425098. [DOI] [PubMed] [Google Scholar]
- 10.Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 11.Derynck R, Zhang YE. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature. 2003;425:577–84. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 12.Xavier S, Niranjan T, Krick S, et al. TbetaRI independently activates Smad- and CD2AP-dependent pathways in podocytes. J Am Soc Nephrol. 2009;20:2127–37. doi: 10.1681/ASN.2008070806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.ten Dijke P, Hill CS. New insights into TGF-beta-Smad signalling. Trends Biochem Sci. 2004;29:265–73. doi: 10.1016/j.tibs.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Davis BN, Hilyard AC, Lagna G, Hata A. SMAD proteins control DROSHA-mediated microRNA maturation. Nature. 2008;454:56–61. doi: 10.1038/nature07086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pang L, Qiu T, Cao X, Wan M. Apoptotic role of TGF-beta mediated by Smad4 mitochondria translocation and cytochrome c oxidase subunit II interaction. Exp Cell Res. 2011;317:1608–20. doi: 10.1016/j.yexcr.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 16.Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of TGF-beta1/Smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003;112:1486–94. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Terada Y, Hanada S, Nakao A, Kuwahara M, Sasaki S, Marumo F. Gene transfer of Smad7 using electroporation of adenovirus prevents renal fibrosis in post-obstructed kidney 7. Kidney Int. 2002;61:94–8. doi: 10.1046/j.1523-1755.2002.0610s1094.x. [DOI] [PubMed] [Google Scholar]
- 18.Fujimoto M, Maezawa Y, Yokote K, et al. Mice lacking Smad3 are protected against streptozotocin-induced diabetic glomerulopathy 11. Biochem Biophys Res Commun. 2003;305:1002–7. doi: 10.1016/s0006-291x(03)00885-4. [DOI] [PubMed] [Google Scholar]
- 19.Hou CC, Wang W, Huang XR, et al. Ultrasound-Microbubble-Mediated Gene Transfer of Inducible Smad7 Blocks Transforming Growth Factor-{beta} Signaling and Fibrosis in Rat Remnant Kidney. Am J Pathol. 2005;166:761–71. doi: 10.1016/s0002-9440(10)62297-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiffer M, Bitzer M, Roberts IS, et al. Apoptosis in podocytes induced by TGF-beta and Smad7. J Clin Invest. 2001;108:807–16. doi: 10.1172/JCI12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Savill J, Mooney A, Hughes J. What role does apoptosis play in progression of renal disease? 13. Curr Opin Nephrol Hypertens. 1996;5:369–74. doi: 10.1097/00041552-199607000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Kumar D, Zimpelmann J, Robertson S, Burns KD. Tubular and interstitial cell apoptosis in the streptozotocin-diabetic rat kidney. Nephron Exp Nephrol. 2004;96:E77–E88. doi: 10.1159/000076749. [DOI] [PubMed] [Google Scholar]
- 23.Susztak K, Ciccone E, McCue P, Sharma K, Bottinger EP. Multiple metabolic hits converge on CD36 as novel mediator of tubular epithelial apoptosis in diabetic nephropathy. PLoS Med. 2005;2:e45. doi: 10.1371/journal.pmed.0020045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schiffer M, Mundel P, Shaw AS, Bottinger EP. A novel role for the adaptor molecule CD2-associated protein in TGF-beta-induced apoptosis. J Biol Chem. 2004:37004–12. doi: 10.1074/jbc.M403534200. [DOI] [PubMed] [Google Scholar]
- 25.Mundel P, Shankland SJ. Podocyte biology and response to injury. J Am Soc Nephrol. 2002;13:3005–15. doi: 10.1097/01.asn.0000039661.06947.fd. [DOI] [PubMed] [Google Scholar]
- 26.Lin JD. Minireview: the PGC-1 coactivator networks: chromatin-remodeling and mitochondrial energy metabolism. Mol Endocrinol. 2009;23:2–10. doi: 10.1210/me.2008-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canto C, Auwerx J. PGC-1alpha, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr Opin Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otera H, Mihara K. Molecular mechanisms and physiologic functions of mitochondrial dynamics. J Biochem. 2011;149:241–51. doi: 10.1093/jb/mvr002. [DOI] [PubMed] [Google Scholar]
- 29.Verhoeven K, Claeys KG, Zuchner S, et al. MFN2 mutation distribution and genotype/phenotype correlation in Charcot-Marie-Tooth type 2. Brain. 2006;129:2093–102. doi: 10.1093/brain/awl126. [DOI] [PubMed] [Google Scholar]
- 30.Olichon A, Landes T, Arnaune-Pelloquin L, et al. Effects of OPA1 mutations on mitochondrial morphology and apoptosis: relevance to ADOA pathogenesis. J Cell Physiol. 2007;211:423–30. doi: 10.1002/jcp.20950. [DOI] [PubMed] [Google Scholar]
- 31.Brooks C, Wei Q, Cho SG, Dong Z. Regulation of mitochondrial dynamics in acute kidney injury in cell culture and rodent models. J Clin Invest. 2009;119:1275–85. doi: 10.1172/JCI37829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Krick S, Shi S, Ju W, et al. Mpv17l protects against mitochondrial oxidative stress and apoptosis by activation of Omi/HtrA2 protease. Proc Natl Acad Sci USA. 2008;105:14106–11. doi: 10.1073/pnas.0801146105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang, et al. Mitochondrial Fission Triggered by Hyperglycemia Is Mediated by ROCK1 Activation in Podocytes and Endothelial Cells. Cell Metabolism. 2012 doi: 10.1016/j.cmet.2012.01.009. IN PRESS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yoon YS, Lee JH, Hwang SC, Choi KS, Yoon G. TGF beta1 induces prolonged mitochondrial ROS generation through decreased complex IV activity with senescent arrest in Mv1Lu cells. Oncogene. 2005;24:1895–903. doi: 10.1038/sj.onc.1208262. [DOI] [PubMed] [Google Scholar]
- 35.Brooks C, Cho SG, Wang CY, Yang T, Dong Z. Fragmented mitochondria are sensitized to Bax insertion and activation during apoptosis. Am J Physiol Cell Physiol. 2011;300:C447–55. doi: 10.1152/ajpcell.00402.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zamani N, Brown CW. Emerging Roles for the Transforming Growth Factor-{beta} Superfamily in Regulating Adiposity and Energy Expenditure. Endocr Rev. 2011;32:387–403. doi: 10.1210/er.2010-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yadav H, Quijano C, Kamaraju AK, et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab. 2011;14:67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michaeloudes C, Sukkar MB, Khorasani NM, Bhavsar PK, Chung KF. TGF-beta regulates Nox4, MnSOD and catalase expression, and IL-6 release in airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L295–304. doi: 10.1152/ajplung.00134.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sayeed A, Meng Z, Luciani G, Chen LC, Bennington JL, Dairkee SH. Negative regulation of UCP2 by TGFbeta signaling characterizes low and intermediate-grade primary breast cancer. Cell Death Dis. 2010;1:e53. doi: 10.1038/cddis.2010.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pacher P, Sharma K, Csordas G, Zhu Y, Hajnoczky G. Uncoupling of ER-mitochondrial calcium communication by transforming growth factor-beta. Am J Physiol Renal Physiol. 2008;295:F1303–12. doi: 10.1152/ajprenal.90343.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mishra R, Cool BL, Laderoute KR, Foretz M, Viollet B, Simonson MS. AMP-activated protein kinase inhibits transforming growth factor-beta-induced Smad3-dependent transcription and myofibroblast transdifferentiation. J Biol Chem. 2008;283:10461–9. doi: 10.1074/jbc.M800902200. [DOI] [PubMed] [Google Scholar]
- 42.Frye RE, Rossignol DA. Mitochondrial dysfunction can connect the diverse medical symptoms associated with autism spectrum disorders. Pediatr Res. 2011 doi: 10.1203/PDR.0b013e318212f16b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calvo S, Jain M, Xie X, et al. Systematic identification of human mitochondrial disease genes through integrative genomics. Nat Genet. 2006;38:576–82. doi: 10.1038/ng1776. [DOI] [PubMed] [Google Scholar]
- 44.Hao CM, Haase VH. Sirtuins and their relevance to the kidney. J Am Soc Nephrol. 2010;21:1620–7. doi: 10.1681/ASN.2010010046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunet A, Sweeney LB, Sturgill JF, et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 2004;303:2011–5. doi: 10.1126/science.1094637. [DOI] [PubMed] [Google Scholar]
- 46.Verdin E, Hirschey MD, Finley LW, Haigis MC. Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem Sci. 2010;35:669–75. doi: 10.1016/j.tibs.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kume S, Haneda M, Kanasaki K, et al. SIRT1 inhibits transforming growth factor beta-induced apoptosis in glomerular mesangial cells via Smad7 deacetylation. J Biol Chem. 2007;282:151–8. doi: 10.1074/jbc.M605904200. [DOI] [PubMed] [Google Scholar]
- 48.Li J, Qu X, Ricardo SD, Bertram JF, Nikolic-Paterson DJ. Resveratrol inhibits renal fibrosis in the obstructed kidney: potential role in deacetylation of Smad3. Am J Pathol. 2010;177:1065–71. doi: 10.2353/ajpath.2010.090923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li C, Cai F, Yang Y, et al. Tetrahydroxystilbene glucoside ameliorates diabetic nephropathy in rats: involvement of SIRT1 and TGF-beta1 pathway. Eur J Pharmacol. 2010;649:382–9. doi: 10.1016/j.ejphar.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 50.Minagawa S, Araya J, Numata T, et al. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-beta-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;300:L391–401. doi: 10.1152/ajplung.00097.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murphy MP. How mitochondria produce reactive oxygen species. Biochem J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andreyev AY, Kushnareva YE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Mosc) 2005;70:200–14. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- 53.Kwong JQ, Beal MF, Manfredi G. The role of mitochondria in inherited neurodegenerative diseases. J Neurochem. 2006;97:1659–75. doi: 10.1111/j.1471-4159.2006.03990.x. [DOI] [PubMed] [Google Scholar]
- 54.Sugamura K, Keaney JF., Jr Reactive oxygen species in cardiovascular disease. Free Radic Biol Med. 2011 doi: 10.1016/j.freeradbiomed.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perico N, Remuzzi G, Benigni A. Aging and the kidney. Curr Opin Nephrol Hypertens. 2011;20:312–7. doi: 10.1097/MNH.0b013e328344c327. [DOI] [PubMed] [Google Scholar]
- 56.Singh DK, Winocour P, Farrington K. Oxidative stress in early diabetic nephropathy: fueling the fire. Nat Rev Endocrinol. 2011;7:176–84. doi: 10.1038/nrendo.2010.212. [DOI] [PubMed] [Google Scholar]
- 57.Ribeiro A, Bronk SF, Roberts PJ, Urrutia R, Gores GJ. The transforming growth factor beta(1)-inducible transcription factor TIEG1, mediates apoptosis through oxidative stress. Hepatology. 1999;30:1490–7. doi: 10.1002/hep.510300620. [DOI] [PubMed] [Google Scholar]
- 58.Herrera B, Alvarez AM, Sanchez A, et al. Reactive oxygen species (ROS) mediates the mitochondrial-dependent apoptosis induced by transforming growth factor (beta) in fetal hepatocytes. FASEB J. 2001;15:741–51. doi: 10.1096/fj.00-0267com. [DOI] [PubMed] [Google Scholar]
- 59.Herrera B, Fernandez M, Alvarez AM, et al. Activation of caspases occurs downstream from radical oxygen species production, Bcl-xL down-regulation, and early cytochrome C release in apoptosis induced by transforming growth factor beta in rat fetal hepatocytes. Hepatology. 2001;34:548–56. doi: 10.1053/jhep.2001.27447. [DOI] [PubMed] [Google Scholar]
- 60.Carmona-Cuenca I, Roncero C, Sancho P, et al. Upregulation of the NADPH oxidase NOX4 by TGF-beta in hepatocytes is required for its pro-apoptotic activity. J Hepatol. 2008;49:965–76. doi: 10.1016/j.jhep.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 61.Cucoranu I, Clempus R, Dikalova A, et al. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–7. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 62.Graham D, Huynh NN, Hamilton CA, et al. Mitochondria-targeted antioxidant MitoQ10 improves endothelial function and attenuates cardiac hypertrophy. Hypertension. 2009;54:322–8. doi: 10.1161/HYPERTENSIONAHA.109.130351. [DOI] [PubMed] [Google Scholar]
- 63.Chacko BK, Reily C, Srivastava A, et al. Prevention of diabetic nephropathy in Ins2(+/)(AkitaJ) mice by the mitochondria-targeted therapy MitoQ. Biochem J. 2010;432:9–19. doi: 10.1042/BJ20100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mizuguchi Y, Chen J, Seshan SV, Poppas DP, Szeto HH, Felsen D. A novel cell-permeable antioxidant peptide decreases renal tubular apoptosis and damage in unilateral ureteral obstruction. Am J Physiol Renal Physiol. 2008;295:F1545–53. doi: 10.1152/ajprenal.00395.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Doleris LM, Hill GS, Chedin P, et al. Focal segmental glomerulosclerosis associated with mitochondrial cytopathy. Kidney Int. 2000;58:1851–8. doi: 10.1111/j.1523-1755.2000.00356.x. [DOI] [PubMed] [Google Scholar]
- 66.Hotta O, Inoue CN, Miyabayashi S, Furuta T, Takeuchi A, Taguma Y. Clinical and pathologic features of focal segmental glomerulosclerosis with mitochondrial tRNALeu(UUR) gene mutation. Kidney Int. 2001;59:1236–43. doi: 10.1046/j.1523-1755.2001.0590041236.x. [DOI] [PubMed] [Google Scholar]
- 67.Inoue K, Nakada K, Ogura A, et al. Generation of mice with mitochondrial dysfunction by introducing mouse mtDNA carrying a deletion into zygotes. Nat Genet. 2000;26:176–81. doi: 10.1038/82826. [DOI] [PubMed] [Google Scholar]
- 68.Hagiwara M, Yamagata K, Capaldi RA, Koyama A. Mitochondrial dysfunction in focal segmental glomerulosclerosis of puromycin aminonucleoside nephrosis. Kidney Int. 2006;69:1146–52. doi: 10.1038/sj.ki.5000207. [DOI] [PubMed] [Google Scholar]
- 69.Papeta N, Zheng Z, Schon EA, et al. Prkdc participates in mitochondrial genome maintenance and prevents Adriamycin-induced nephropathy in mice. J Clin Invest. 2010;120:4055–64. doi: 10.1172/JCI43721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang Y, Wang YP, Tay YC, Harris DC. Progressive adriamycin nephropathy in mice: sequence of histologic and immunohistochemical events. Kidney Int. 2000;58:1797–804. doi: 10.1046/j.1523-1755.2000.00342.x. [DOI] [PubMed] [Google Scholar]
- 71.Spinazzola A, Viscomi C, Fernandez-Vizarra E, et al. MPV17 encodes an inner mitochondrial membrane protein and is mutated in infantile hepatic mitochondrial DNA depletion. Nat Genet. 2006;38:570–5. doi: 10.1038/ng1765. [DOI] [PubMed] [Google Scholar]
- 72.Weiher H, Noda T, Gray DA, Sharpe AH, Jaenisch R. Transgenic mouse model of kidney disease: insertional inactivation of ubiquitously expressed gene leads to nephrotic syndrome. Cell. 1990;62:425–34. doi: 10.1016/0092-8674(90)90008-3. [DOI] [PubMed] [Google Scholar]
- 73.Binder CJ, Weiher H, Exner M, Kerjaschki D. Glomerular overproduction of oxygen radicals in Mpv17 gene-inactivated mice causes podocyte foot process flattening and proteinuria: A model of steroid-resistant nephrosis sensitive to radical scavenger therapy. Am J Pathol. 1999;154:1067–75. doi: 10.1016/S0002-9440(10)65359-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heine UI, Burmester JK, Flanders KC, et al. Localization of transforming growth factor-á1 in mitochondria of murine heart and liver. Cell Regulation. 1991;2:467–77. doi: 10.1091/mbc.2.6.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jullig M, Stott NS. Mitochondrial localization of Smad5 in a human chondrogenic cell line. Biochem Biophys Res Commun. 2003;307:108–13. doi: 10.1016/s0006-291x(03)01139-2. [DOI] [PubMed] [Google Scholar]
- 76.Sun Y, Zhou J, Liao X, et al. Disruption of Smad5 gene induces mitochondria-dependent apoptosis in cardiomyocytes. Exp Cell Res. 2005;306:85–93. doi: 10.1016/j.yexcr.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 77.John B, Enright AJ, Aravin A, Tuschl T, Sander C, Marks DS. Human MicroRNA targets. PLoS Biol. 2004;2:e363. doi: 10.1371/journal.pbio.0020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang Q, Wang Y, Hao Y, et al. miR2Disease: a manually curated database for microRNA deregulation in human disease. Nucleic Acids Res. 2009;37:D98–104. doi: 10.1093/nar/gkn714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kato M, Arce L, Natarajan R. MicroRNAs and their role in progressive kidney diseases. Clin J Am Soc Nephrol. 2009;4:1255–66. doi: 10.2215/CJN.00520109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harvey SJ, Jarad G, Cunningham J, et al. Podocyte-specific deletion of dicer alters cytoskeletal dynamics and causes glomerular disease. J Am Soc Nephrol. 2008;19:2150–8. doi: 10.1681/ASN.2008020233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ho J, Ng KH, Rosen S, Dostal A, Gregory RI, Kreidberg JA. Podocyte-specific loss of functional microRNAs leads to rapid glomerular and tubular injury. J Am Soc Nephrol. 2008;19:2069–75. doi: 10.1681/ASN.2008020162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shi S, Yu L, Chiu C, et al. Podocyte-Selective Deletion of Dicer Induces Proteinuria and Glomerulosclerosis. J Am Soc Nephrol. 2008 doi: 10.1681/ASN.2008030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li J, Donath S, Li Y, Qin D, Prabhakar BS, Li P. miR-30 regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. PLoS Genet. 2010;6:e1000795. doi: 10.1371/journal.pgen.1000795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Minones-Moyano E, Porta S, Escaramis G, et al. MicroRNA profiling of Parkinson’s disease brains identifies early downregulation of miR-34b/c which modulate mitochondrial function. Hum Mol Genet. 2011 doi: 10.1093/hmg/ddr210. [DOI] [PubMed] [Google Scholar]
- 85.Chen HC, Chen GH, Chen YH, et al. MicroRNA deregulation and pathway alterations in nasopharyngeal carcinoma. Br J Cancer. 2009;100:1002–11. doi: 10.1038/sj.bjc.6604948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10:273–84. doi: 10.1016/j.cmet.2009.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chen Z, Li Y, Zhang H, Huang P, Luthra R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene. 2010;29:4362–8. doi: 10.1038/onc.2010.193. [DOI] [PubMed] [Google Scholar]
- 88.Mizuno Y, Tokuzawa Y, Ninomiya Y, et al. miR-210 promotes osteoblastic differentiation through inhibition of AcvR1b. FEBS Lett. 2009;583:2263–8. doi: 10.1016/j.febslet.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 89.Rane S, He M, Sayed D, et al. Downregulation of miR-199a derepresses hypoxia-inducible factor-1alpha and Sirtuin 1 and recapitulates hypoxia preconditioning in cardiac myocytes. Circ Res. 2009;104:879–86. doi: 10.1161/CIRCRESAHA.108.193102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang C, Youle RJ. The role of mitochondria in apoptosis*. Annu Rev Genet. 2009;43:95–118. doi: 10.1146/annurev-genet-102108-134850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lin PH, Pan Z, Zheng L, Li N, Danielpour D, Ma JJ. Overexpression of Bax sensitizes prostate cancer cells to TGF-beta induced apoptosis. Cell Res. 2005;15:160–6. doi: 10.1038/sj.cr.7290281. [DOI] [PubMed] [Google Scholar]
- 92.Chipuk JE, Bhat M, Hsing AY, Ma J, Danielpour D. Bcl-xL blocks transforming growth factor-beta 1-induced apoptosis by inhibiting cytochrome c release and not by directly antagonizing Apaf-1-dependent caspase activation in prostate epithelial cells. J Biol Chem. 2001;276:26614–21. doi: 10.1074/jbc.M100913200. [DOI] [PubMed] [Google Scholar]
- 93.Cleland MM, Norris KL, Karbowski M, Wang C, Suen DF, Jiao S, George NM, Luo X, Li Z, Youle RJ. Bcl-2 family interaction with the mitochondrial morphogenesis machinery. Cell Death Differ. 2011 Feb;18(2):235–47. doi: 10.1038/cdd.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]