Abstract
A new type of rodent babesia, which resembled Babesia microti but was phylogenetically placed closest, with the highest level of statistical support, to Babesia canis, a canine babesia, was identified in Thai Bandicota indica in Thai provinces to which malaria is endemic. Close watch should be kept on human babesiosis in Thailand.
Babesiosis is a typical zoonotic disease which is caused by Babesia spp., tick-borne intraerythrocytic protozoan parasites (3, 4). Only a few of the more than 100 Babesia spp. have been known to infect humans. In Europe, a few human babesiosis cases have been attributed to Babesia divergens, the cattle babesia transmitted by Ixodes ricinus. In contrast, hundreds of human babesiosis cases in the northeast and midwest United States have been attributed to Babesia microti, the rodent babesia transmitted by Ixodes scapularis (Ixodes dammini). Newly emerging species, referred to as WA1 and CA1, have been found to cause human infection in the western United States (15, 16).
Cases of human babesiosis have been recently reported consecutively in Taiwan and Japan (19-21). In Taiwan, Manwell and Kuntz identified piroplasms in bandicoot rats, Bandicota indica, and in spiny rats, Rattus coxinga, in 1964 (7), and the first Taiwanese patient was reported in 1997 (21). In Japan, the emergence of human babesiosis has been expected since the early 1980s, when a B. microti-like parasite was found to have infected wild Apodemus speciosus and Apodemus argenteus rodents (24). Saito-Ito et al. reported that the first human case, which occurred in 1999, was caused by a geographical variant of B. microti that was traced back to a blood transfusion (19, 20).
Human babesiosis has sometimes been diagnosed initially as malaria (13, 16, 29; J. B. Bush, M. Isaacson, A. S. Mohamed, F. T. Potgieter, and D. T. de Waal, Letter, S. Afr. Med. J. 78:699, 1990) because of the similarity between the two diseases or the two parasites (4, 27). Thus, it is likely that cases of human babesiosis in countries to which malaria is endemic have been overlooked or misdiagnosed as malaria. In those countries, however, little is known about rodent babesias, which cause most cases of human babesiosis, although Babesia infection is indeed common in other animals (2, 11, 12, 17, 25, 31). Accordingly, this study examined babesial infection in wild rats in areas of Asia to which malaria is endemic to evaluate the possibility of the emergence or latent existence of human babesiosis.
Rats were trapped in the Muang district, San Sai district, and Doi Lo subdistrict of Chiang Mai Province and in the Mae Sariang district of Mae Hong Son province of northwest Thailand from April 2000 to May 2002. The species of captured rats and of ticks infesting them were identified according to the key characteristics described by Lekagul and Jeffrey (5) and by Tanskul and Inlao (26), respectively. Intraerythrocytic parasites were examined under a light microscope with magnification ×1,000 for approximately 100 fields on Giemsa-stained thin blood smears.
The small-subunit ribosomal RNA gene (SSUrDNA) fragments of the parasites, which were amplified from the blood by PCR with a primer set of Anl (5′-AACCTGGTTGATCCTGCCAGT-3′) and Bnl (5′-TGATCCTTCTGCAGGTTCACCTAC-3′) based on the highly conserved regions of SSUrDNA sequences of eukaryotes (9), were ligated into a plasmid and sequenced. The sequences were confirmed not to contain any sequence errors due to Taq polymerase by directly sequencing the PCR products with two pairs of primers: Anl-CR1 and CF1-Bnl. CR1 (5′-TCCTTTAAGTGATAAGGTTCAC-3′) and CF1 (5′-GACGGTAGGGTATTGGCCT-3′) were designed to match the regions of SSUrDNA conserved in various Babesia spp. but not in rodent SSUrDNA sequences.
The phylogenetic analysis was performed by the neighbor-joining and maximum-likelihood methods, using 1,328 unambiguously aligned positions according to the secondary structure information (http://oberon.fvms.ugent.be:8080/rRNA/) (30) for 39 SSUrDNA sequences selected based on the results of a FASTA similarity search (http://www.ddbj.nig.ac.jp/E-mail/homology-j.html) (6, 14).
Babesia-like intraerythrocytic parasites were found in 17 of the 30 Bandicota indica rats, while no intraerythrocytic parasites were found in any of the 17 Rattus exulans rats (Table 1). All 17 positive Bandicota indica rats were trapped in the three areas in Chiang Mai Province. The percent parasitemia varied from 0.03 to 11%. Another hemoparasite, which resembled Hepatozoon, was found in mononucleated leukocytes of two intraerythrocytic parasite-positive Bandicota indica rats but not in any R. exulans rats. Trypanosoma sp. was found in three R. exulans rats but not in any Bandicota indica rats. Haemaphysalis doenitzi ticks, ranging in number from 1 to 80, infested 12 of the 24 Bandicota indica rats examined for tick infestation. Only intraerythrocytic parasite-positive Bandicota indica was infested with H. doenitzi. No other species of ticks were identified in any of the rats.
TABLE 1.
Summary of epizootiological survey for babesia and other hemoparasite infections in wild rats in Chiang Mai and Mae Hong Son provinces, Thailand, from April 2000 to May 2002
| Host rat | Area | No. of rats trapped | No. smear-positive or tick-positive/no. examined
|
|||
|---|---|---|---|---|---|---|
| Babe- sia | Hepato- zoon | Trypano- soma | Haema- physalis | |||
| B. indica | Doi Lo | 18 | 15/18 | 2/18 | 0/18 | 12/17 |
| Muang | 3 | 1/3 | 0/3 | 0/3 | NDa | |
| San Sai | 2 | 1/2 | 0/2 | 0/2 | ND | |
| Mae Sariang | 7 | 0/7 | 0/7 | 0/7 | 0/7 | |
| Total | 30 | 17/30 | 2/30 | 0/30 | 12/24 | |
| R. exulans | Doi Lo | 0 | ||||
| Muang | 12 | 0/12 | 0/12 | 2/12 | 0/12 | |
| San Sai | 5 | 0/5 | 0/5 | 1/5 | 0/5 | |
| Mae Sariang | 0 | |||||
| Total | 17 | 0/17 | 0/17 | 3/17 | 0/17 | |
ND, not determined.
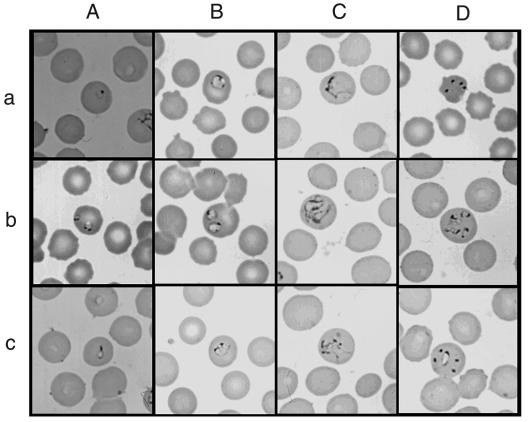
On intraerythrocytic parasite-positive smears, ring-shaped trophozoites (less than 1 to 2 μm in diameter) resembling ring forms of Plasmodium falciparum were often observed (Fig. 1A, panels a and b). Pyriform-shaped trophozoites were occasionally seen (Fig. 1A, panel c). The ring forms seemed to become annular forms (∼3 μm) (Fig. 1B). On smears of higher parasitemia, irregularly large trophozoites were often seen (Fig. 1C). Double infection was frequently observed (Fig. 1A, panel b, B, panel b, and C, panel b). Four trophozoites, so-called “Maltese cross,” were occasionally but not typically seen (Fig. 1D, panel a). However, this configuration could represent multiple infecting trophozoites, because four trophozoites sometimes seemed to be independently developing (Fig. 1D, panel b). Five trophozoites were also observed together in an erythrocyte (Fig. 1D, panel c). Paired pyriforms were seen rarely if at all. In general, the Thai Babesia-like parasites appear morphologically similar to B. microti.
FIG. 1.
Photomicrographs of the Thai babesia on Giemsa-stained thin blood smear of babesia-positive Bandicota indica. (A) Panel a, a ring-shaped trophozoite consisting of a cytoplasmic rim with a chromatin dot; panel b, double infection of ring-shaped trophozoites with double chromatin dots and a vacuolated lesion on the cytoplasmic rim; panel c, a pyriform-shaped trophozoite. (B) Panel a, a large ring or annular form with three chromatin masses, two dot-like and one elongated; panel b, double infection of annular trophozoites; panel c, a trophozoite with bridge-like cytoplasms. (C) Panel a, an irregular large trophozoite; panel b, double infection of irregular large trophozoites; panel c, an irregular form with multiple chromatin dots. (D) Panel a, Maltese-cross-like form; panel b, four trophozoites, presumably developing independently; panel c, five trophozoites observed in one red blood cell.
A 1,704-bp SSUrDNA sequence of the parasite isolated from a positive bandicoot rat trapped at Doi Lo in 2000 (the BiCM002 isolate) was determined, including sequences of Anl and Bnl primers. All SSUrDNA sequences of the other five Babesia isolates were completely identical to that of the BiCM002 isolate, regardless of the capture site or year.
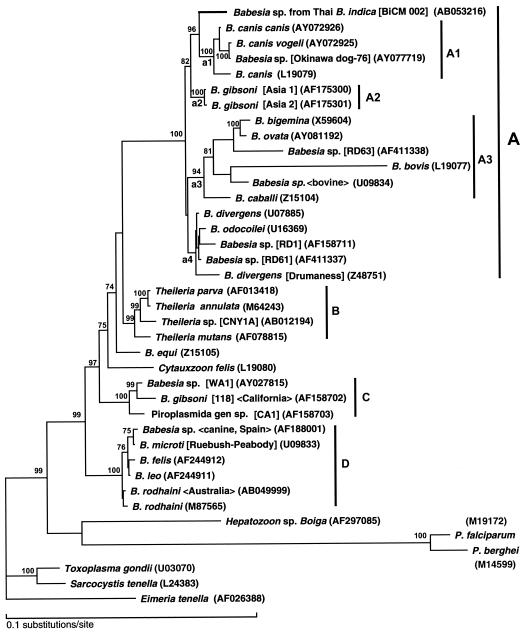
Figure 2 shows the best tree of the maximum-likelihood analysis with the homogeneous model. The tree reconstructed the four monophyletic groups (A to D) with high bootstrap support (more than 90%). Group A contained so-called large babesias or Babesia sensu stricto; group B contained Theileria spp.; group C contained piroplasms isolated from humans (WA1 and CA1) and from canines (Babesia gibsoni [118]) in the western United States; and group D contained B. microti and related so-called small babesias. In group A, three monophyletic subgroups of A1 (B. canis and Babesia sp. from an Okinawa dog), A2 (B. gibsoni [Asia 1 and 2]), and A3 (several ungulate babesias) were reconstructed with high bootstrap supports (more than 90%). The Babesia sp. from Thai Bandicota indica was clustered with subgroup A1, with 96% bootstrap support. The neighbor-joining tree also reconstructed the monophyly of each of the four groups (A to D), positioning the Thai Babesia sp. as the sister clade to subgroup A1 and with subgroup A2 as an outer group (data not shown). To check the robustness of the position of the Thai babesia in the best tree obtained under the assumption of a homogeneous model against a possible long-branch attraction artifact, a rate-across-site model (RAS model) was also applied. Among 105 trees possible for the six lineages (the Thai Babesia sp., a1, a2, a3, a4, and the outgroup), 12 trees unrejected in the analysis with the RAS model (P > 0.05 [approximately unbiased {AU} test]), which approximated the data set better than the homogeneous model did, were selected as possible candidate trees (Table 2). Although 6 of the 12 trees also revealed a close relationship between the Thai babesia and node a1 (group A1), the alternative possibilities still could not be entirely excluded: the close relationship to the common ancestor of nodes a1 and a2 (trees 3 and 4), to that of node a4 (trees 10 and 11), and to the earliest branch of group A (trees 7 and 12).
FIG. 2.
Phylogenetic tree of the SSUrDNA sequences of piroplasms. The best tree finally selected by the maximum-likelihood analysis is shown. Details of the phylogenetic analysis will be described elsewhere (A. Dantrakool, T. Hashimoto, A. Saito et al., unpublished data). Briefly, based on the result obtained by use of FASTA, in total 33 SSUrDNA sequences from Babesia spp., Theileria spp., Cytauxzoon spp., and unclassified piroplasms were included in the data set for the analysis, including the sequence of the Babesia spp. from Thai Bandicota indica. Two SSUrDNA sequences of Plasmodium spp. and the sequence of Hepatozoon sp. Boiga were added to the data set. Three sequences of other apicomplexan parasites, Toxoplasma gondii, Sarcocystis tenella, and Eimeria tenella, were also included as outgroups, and 1,378 unambiguously aligned positions were selected and used. Bootstrap proportions (percent) with more than 70% support are attached to the internal branches. Monophyletic groups and subgroups supported with more than 90% in the analysis are shown by vertical bars. The horizontal length of each branch is proportional to the estimated number of substitutions. GenBank accession numbers are given in parentheses. The name of the isolate, strain, clone or genotype is shown in brackets. The host origin or the isolation site is given within chevrons (<>).
TABLE 2.
Alternative trees comparable with the best of 105 trees examined regarding the relationship between group A in the analysis with the RAS model
| Tree no.a | Relationship among species in group A | RAS model
|
Position of Thai isolate | |
|---|---|---|---|---|
| Log-likelihood difference | P valueb | |||
| 1 | (out, (a3, a4), (a2, (Thai, a1))) | Best | 0.967 | Close to al |
| 2 | (out, (Thai, a1), (a2, (a3, a4))) | −5.5 | 0.273 | Close to al |
| 3 | (out, (a3, a4), (Thai, (a1, a2))) | −6.3 | 0.269 | Sister clade position to the common ancestor of a1 and a2 |
| 4 | (out, a4, (a3, (Thai, (a1, a2)))) | −7.6 | 0.053 | Sister clade position to the common ancestor of a1 and a2 |
| 5 | (out, (Thai, a1), (a3, (a4, a2))) | −9.0 | 0.206 | Close to a1 |
| 6 | (out, a3, (a2, (a4, (Thai, a1)))) | −9.6 | 0.094 | Close to a1 |
| 7 | (out, Thai, (a1, (a2, (a3, a4)))) | −9.6 | 0.184 | Earliest branch of group A |
| 8 | (out, a2, (a3, (a4, (Thai, a1)))) | −9.8 | 0.052 | Close to a1 |
| 9 | (out, a2, (a4, (a3, (Thai, a1)))) | −9.8 | 0.052 | Close to a1 |
| 10 | (out, a1, (a3, (a2, (Thai, a4)))) | −16.9 | 0.151 | Close to a4 |
| 11 | (out, (a3, a1), (a2, (Thai, a4))) | −16.9 | 0.151 | Close to a4 |
| 12 | (out, Thai, (a1, (a3, (a4, a2)))) | −17.1 | 0.073 | Earliest branch of group A |
Babesia spp. are known to show pyriform shapes with the unique appearance of paired trophozoites for the large babesias and the so-called Maltese-cross arrangement of four trophozoites for the small babesias (10). However, only ring-shaped trophozoites that are quite similar to ring forms of P. falciparum are seen often on smears with low parasitemia. Although trophozoites of Babesia spp. always lack the pigment that is believed to distinguish Babesia spp. from Plasmodium spp. (4, 27), the latter species do not show any malaria pigment at the ring form stage. It must also be noted that most antimalarial drugs, such as chloroquine, mefloquine, and artemisinine, have no effect on babesiosis (8). Quinine and clindamycin, the former of which is often used for treatment of drug-resistant malaria, are the first-choice drugs against babesiosis (1, 18). Therefore, babesiosis in areas to which malaria is endemic might be misdiagnosed as drug-resistant malaria. From this standpoint, the identification of babesial parasites in wild rats in Chiang Mai, Thailand, where malaria is endemic, seems to deserve attention, considering that recent Asian cases of human babesiosis emerged in Taiwan and Japan, where B. microti-like parasites had earlier been identified in rodents (19-21).
van Peenen et al. detailed the morphological features and host ranges of the babesial parasites identified, respectively, in Taiwanese Bandicota indica and R. coxinga and concluded that the parasites in both species of rats seemed to represent a cospecific geographical strain of B. microti (28). The reservoir host for the etiological Babesia sp. (TW1) of the first Taiwanese case of human babesiosis was suspected to be R. coxinga (21). Although the morphological features of the Thai and Taiwanese rodent babesias appear to be similar to each other, the relationships among the Thai and Taiwanese rodent babesias, at present, remain unknown, because the sequences of SSUrDNA are lacking for the Taiwanese rodent babesias.
Because the babesia in Thai bandicoot rats was morphologically pleomorphic, it might not be a single species. However, the possibility of multiple infection by different species seems quite low, because there were no ambiguities in any of the results of direct sequencing of the PCR products. It can be concluded that the B. microti-like parasite in Thai Bandicota indica is a new type of rodent babesia that seems to be phylogenetically closest to B. canis, a canine babesia, which belongs to the group Babesia sensu stricto.
Only H. doenitzi ticks, which sometimes infest humans, were identified exclusively on Babesia-positive Bandicota indica, so this species is most probably the vector tick of the Thai babesia in Bandicota indica. Although at present it is unknown whether the Thai babesia is infectious in humans, a close watch should be kept on human babesiosis in Thailand.
Nucleotide sequence accession number.
The sequence of SSUrDNA for Babesia sp. from Thai Bandicota indica has been submitted to DDBJ under accession no. AB053216.
Acknowledgments
We thank A. Kawai for excellent technical assistance.
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (13670244 and 15590367) and for International Medical Cooperation from the Ministry of Health, Welfare and Labor of Japan.
REFERENCES
- 1.Dammin, G. S., A. Spielman, E. B. Mahoney, E. F. Bracker, and K. Kaplan. 1983. Clindamycin and quinine treatment for Babesia microti infections. Morb. Mortal. Wkly. Rep. 32:65-66, 72. [PubMed] [Google Scholar]
- 2.Friedhoff, K. T. 1997. Tick-borne diseases of sheep and goats caused by Babesia, Theileria or Anaplasma spp. Parassitologia 39:99-109. [PubMed] [Google Scholar]
- 3.Gorenflot, A., K. Moubri, E. Precigout, B. Carcy, and T. P. Schetters. 1998. Human babesiosis. Ann. Trop. Med. Parasitol. 92:489-501. [DOI] [PubMed] [Google Scholar]
- 4.Homer, M. J., D. I. Aguilar, S. R. I. Telford, P. J. Krause, and D. H. Persing. 2000. Babesiosis. Clin. Microbiol. Rev. 13:451-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lekagul, B., and A. M. Jeffrey. 1988. Mammals of Thailand, 2nd ed., p. 758. Damsutha Press, Bangkok, Thailand.
- 6.Lipman, D. J., and W. R. Pearson. 1985. Rapid and sensitive protein similarity searches. Science 227:1435-1441. [DOI] [PubMed] [Google Scholar]
- 7.Manwell, D. R., and E. R. Kuntz. 1964. A new Babesia from the Indian Bandicoot. J. Parasitol. 50:390-393. [PubMed] [Google Scholar]
- 8.Marley, S. E., M. L. Eberhard, F. J. Steurer, W. L. Ellis, P. B. McGreevy, and T. K. I. Ruebush. 1997. Evaluation of selected antiprotozoal drugs in the Babesia microti-hamster model. Antimicrob. Agents Chemother. 41:91-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Medlin, L., H. J. Elwood, S. Stickel, and M. L. Sogin. 1988. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 71:491-499. [DOI] [PubMed] [Google Scholar]
- 10.Mehlhorn, H., and E. Schein. 1984. The piroplasms: life cycle and sexual stages. Adv. Parasitol. 23:37-103. [DOI] [PubMed] [Google Scholar]
- 11.Miranpuri, G. S. 1988. Ticks parasitising the Indian buffalo (Bubalus bubalis) and their possible role in disease transmission. Vet. Parasitol. 27:357-362. [DOI] [PubMed] [Google Scholar]
- 12.Niak, A., M. Anwar, and S. Khatibi. 1973. Canine babesiosis in Iran. Trop. Anim. Health Prod. 5:200-201. [DOI] [PubMed] [Google Scholar]
- 13.Olmeda, A. S., P. M. Armstrong, B. M. Rosenthal, B. Valladares, A. del Castillo, F. de Armas, M. Miguelez, A. Gonzalez, R. J. A. Rodriguez, A. Spielman, and S. R. I. Telford. 1997. A subtropical case of human babesiosis. Acta Trop. 67:229-234. [DOI] [PubMed] [Google Scholar]
- 14.Pearson, W. R., and D. J. Lipman. 1988. Improved tools for biological sequence comparison. Proc. Natl. Acad. Sci. USA 85:2444-2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persing, D. H., B. L. Herwaldt, C. Glaser, R. S. Lane, J. W. Thomford, D. Mathiesen, P. J. Krause, D. F. Phillip, and P. A. Conrad. 1995. Infection with a babesia-like organism in northern California. N. Engl. J. Med. 332:298-303. [DOI] [PubMed] [Google Scholar]
- 16.Quick, R. E., B. L. Herwaldt, J. W. Thomford, M. E. Garnett, M. L. Eberhard, M. Wilson, D. H. Spach, J. W. Dickerson, S. R. I. Telford, K. R. Steingart, R. Pollock, D. H. Persing, J. M. Kobayashi, D. D. Juranek, and C. P. A. 1993. Babesiosis in Washington State: a new species of Babesia? Ann. Intern. Med. 119:284-290. [DOI] [PubMed] [Google Scholar]
- 17.Rajamanickam, C., E. Wiesenhutter, F. M. Zin, and J. Hamid. 1985. The incidence of canine haematozoa in Peninsular Malaysia. Vet. Parasitol. 17:151-157. [DOI] [PubMed] [Google Scholar]
- 18.Rowin, K. S., H. B. Tanowitz, and M. Wittner. 1982. Therapy of experimental babesiosis. Ann. Intern. Med. 97:556-558. [DOI] [PubMed] [Google Scholar]
- 19.Saito-Ito, A., S. K. Rai, S. He, M. Kohsaki, M. Tsuji, and C. Ishihara. 1999. First demonstration of Babesia parasitizing in human in Japan (in Japanese). J. Jpn. Assoc. Infect. Dis. 11:1163-1164. [DOI] [PubMed] [Google Scholar]
- 20.Saito-Ito, A., M. Tsuji, Q. Wei, S. He, T. Matsui, M. Kohsaki, S. Arai, T. Kamiyama, K. Hioki, and C. Ishihara. 2000. Transfusion-acquired, autochthonous human babesiosis in Japan: isolation of Babesia microti-like parasites with hu-RBC-SCID mice. J. Clin. Microbiol. 38:4511-4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shih, C. M., L. P. Liu, W. C. Chung, S. J. Ong, and C. C. Wang. 1997. Human babesiosis in Taiwan: asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J. Clin. Microbiol. 35:450-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shimodaira, H. 2002. An approximately unbiased test of phylogenetic tree selection. Syst. Biol. 51:492-508. [DOI] [PubMed] [Google Scholar]
- 23.Shimodaira, H., and M. Hasegawa. 2001. CONSEL: a program for assessing the confidence of phylogenetic tree selection. Bioinformatics 17:1246-1247. [DOI] [PubMed] [Google Scholar]
- 24.Shiota, T., H. Kurimoto, N. Haguma, and Y. Yoshida. 1984. Studies on babesia first found in murine in Japan: epidemiology, morphology and experimental infection. Zentbl. Bakteriol. Mikrobiol. Hyg. 256:347-355. [PubMed] [Google Scholar]
- 25.Subharngkasen, S. 1970. Diseases transmitted by ticks in Thailand. Bull. Off. Int. Epizoot. 73:109-113. [PubMed] [Google Scholar]
- 26.Tanskul, P., and I. Inlao. 1989. Keys to the adult ticks of Haemaphysalis Koch, 1844, in Thailand with notes on changes in taxonomy (Acari: Ixodoidea: Ixodidae). J. Med. Entomol. 26:573-601. [DOI] [PubMed] [Google Scholar]
- 27.Telford, S. R. I., and A. Spielman. 1998. Babesiosis of humans, p. 349-359. In L. Collier, A. Balows, and M. Sussman (ed.), Topley & Wilson's microbiology and microbial infection, 9th ed., vol. 5. Arnold, London, United Kingdom.
- 28.van Peenen, P. F., S. J. Chang, A. R. Banknieder, and F. J. Santana. 1977. Piroplasms from Taiwanese rodents. J. Protozool. 24:310-312. [DOI] [PubMed] [Google Scholar]
- 29.Western, K. A., G. D. Benson, N. N. Gleason, G. R. Healy, and M. G. Schultz. 1970. Babesiosis in a Massachusetts resident. N. Engl. J. Med. 283:854-856. [DOI] [PubMed] [Google Scholar]
- 30.Wuyts, J., Y. Van de Peer, T. Winkelmans, and R. De Wachter. 2002. The European database on small subunit ribosomal RNA. Nucleic Acids Res. 30:183-185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamane, I., P. A. Conrad, and I. A. Gardener. 1993. Babesia gibsoni infections in dogs. J. Protozool. Res. 3:111-125. [Google Scholar]