Abstract
Serotype G2 rotavirus strains were isolated in seven countries on the African continent during 1999 and 2000. To investigate the associated DS-1 genogroup characteristics, subgroup (VP6) enzyme-linked immunosorbent assay, polyacrylamide gel electrophoresis, and P genotyping were performed on 10 G2 strains. The antigenic and genetic variation of the gene encoding the major neutralization glycoprotein (VP7) was also investigated by using G2-specific monoclonal antibodies and sequence analysis. Alterations in the characteristic DS-1 genogroup gene constellations were more likely to occur in the VP4 gene, and three genotypes were observed: P[4], P[6], and a dual P[4]-P[6] type. The failure of G2-specific monoclonal antibodies to type African G2 strains was more likely due to improper storage of the original stool, although G2 monotypes were detected. Phylogenetic analyses revealed clusters of serotype G2 strains that were more commonly associated with seasons during which G2 was predominant. No rotavirus vaccine trials have been conducted in an area where G2 strains were the predominant circulating serotype, and the continued surveillance of rotavirus epidemics in Africa will be preparation for future vaccine implementation in an area that clearly needs these preventative medicines.
The chance of a child born in Africa dying before the age of 5 years is one in six (13). Statistics like this highlight the daily struggle to survive on a continent that is rife with wars, corruption, famines, epidemic diseases, and substandard health care. The top three killers of children under five in Africa include lower respiratory infections, diarrheal diseases, and perinatal disorders (28). In First World countries, diarrhea is considered a minor ailment rather than a life-threatening disease. However, early microbial exposure and pediatric malnutrition result in an estimated 2.4 to 3.3 million childhood deaths due to diarrhea per year in developing countries. Rotavirus is responsible for roughly 25% of all diarrheal deaths; i.e., between 1 in 120 and 1 in 150 children will die by age five from rotavirus gastroenteritis in Africa (13).
Rotavirus particles consist of three protein layers, encapsulating 11 distinct segments of double-stranded RNA. Differences in the outer capsid protein allow classification according to two antigenic markers, i.e., VP4 and VP7. The VP7 and VP4 proteins form the smooth outer capsid (G serotype) and short spike (P genotype), respectively, and are the major antigens inducing neutralizing immune responses during rotavirus infections. Although 14 different G serotypes and 21 P genotypes have been detected in humans, serotypes G1P[8], G2P[4], G3P[8], and G4P[8] are thought to be important causes of diarrhea in infants and young children worldwide (16, 22).
Efforts to improve sanitation and provide clean water have not decreased the high mortality due to rotavirus infection in developing countries. Studies of natural rotavirus infections reported that children were protected against developing severe diarrhea upon reinfection (6, 38). This highlighted the need for an effective rotavirus vaccine, preventing infection within the first 2 years of life, when rotavirus disease is most life threatening (7, 9).
Preliminary rotavirus vaccine trials indicated that a vaccine was successful in inducing significant resistance to severe diarrhea in an area where the prevalent G serotype was the same as that of the vaccine strain but was not successful in places where other G serotypes prevailed (10, 26). This indicated the need for a multivalent vaccine able to elicit serotype-specific, homotypic immunity (26). The first licensed human-rhesus reassortant vaccine (RRV-TV) was therefore engineered to contain the most prevalent G antigens found in humans, i.e., G1, G2, G3, and G4. The reported association between rotavirus vaccination and an increased risk of intussusception among vaccine recipients resulted in the withdrawal of RRV-TV (8).
Second-generation bovine rotavirus-based reassortant vaccine candidates are currently under development. Similarly to the reassortant rhesus vaccines, they contain the prevalent VP7 serotypes, and in one case the VP4 serotype, in circulation (25). An alternative candidate is a monovalent human rotavirus bearing the most globally common human rotavirus serotype (G1P[8] (16). The rationale here is that natural human rotavirus infection confers protection after a single episode and that the most common rotavirus strain would induce this effect (5).
To date, no rotavirus vaccine trials have been conducted in an area where G2P[4] strains were the predominant circulating serotype. This raises two important questions: (i) will the G2 strain DS-1, isolated in 1976 and included in bovine-human reassortants, confer enough homotypic protection to protect against the G2 strains currently in circulation and (ii) will a monovalent vaccine elicit enough of a heterotypic response to even protect against G2 strains.
The objective of this study was to investigate the antigenic diversity of circulating serotype G2 strains in Africa. The data will be important to determine whether serotype 2 monotypes may become problematic in successful vaccine development and to ensure the design of an efficacious rotavirus vaccine candidate for use in African children. Serotype G2 strains from various African countries were collected over a 1-year period (1999 to 2000). The genogroup characteristics associated with serotype G2 strains, including RNA profile, subgroup specificity, and VP4 genotype, were investigated. The antigenic and genetic variation in the VP7 gene was also investigated by using G2-specific monoclonal antibodies and sequence analysis.
MATERIALS AND METHODS
Stool selection.
The serotype G2 strains from Africa were obtained during the World Health Organization-sponsored African Rotavirus Network Training Workshop in 1999 and were collected by James Nyangao in Kenya, Lohya Nimzing and Grace Pennap in Nigeria, George Armah in Ghana, Veronique Akran in Ivory Coast, Souleymane Sawadogo in Burkino Faso, and Abdelhalim Trabelsi in Tunisia (33). Rotavirus-positive specimens from Mauritius were collected by N. Pyndiah during 2000 and sent to the MRC Medunsa Diarrhoeal Pathogens Research Unit for further analysis. South African G2 strains were obtained from the historical collection available at the MRC Medunsa Diarrhoeal Pathogens Research Unit and described elsewhere (N. A. Page and A. D. Steele, submitted for publication) and were included for entirety.
Detection of genotype G2 rotaviruses.
RNA was extracted from 500 μl of the stored fecal suspensions. This was followed by a double extraction with phenol-chloroform (1:1) and resuspension in 7 M guanidine isothiocyanate. The RNA was purified by using the RNAid kit (BIO 101), according to the manufacturer's instructions. The G genotype was determined by using the reverse transcription-PCR (RT-PCR) method developed by Gouvea and colleagues (18). Briefly, the full-length VP7 gene was reverse transcribed and amplified by using primers sBeg9 (nucleotides [nt] 1 to 21, 5′-GGCTTTAAAAGAGAGAATTTC-3′) and End9 (nt 1062 to 1036, 5′-GGTCACATCATACAATTCTAATCTAAG-3′), followed by G genotyping with a cocktail of primers specific to the six human rotavirus serotypes (G1 to G4, G8, and G9) (18).
P (VP4) genotyping.
The P genotype was determined by to the method described by Gentsch and colleagues (17), using primers con3 (nt 11 to 32, 5′-TGGCTTCGCCATTTTATAGACA-3′) and con2 (nt 887 to 868, 5′-ATTTCGGACCATTTATAACC-3′) for reverse transcription and amplification of the VP4 gene. The typing primers utilized were specific to the five human VP4 genotypes, P[4], P[6], P[8], P[9], and P[10] (17).
Polyacrylamide gel electrophoresis.
The RNA profile was resolved by extracting the double-stranded RNA, according to previously published methods (35), and running the specimens on a vertical polyacrylamide slab with 3% stacking and 10% resolving gels. The gels were loaded with 30 μl of the extracted RNA and electrophoresed overnight at 100 V at room temperature with a discontinuous buffer system. The gels were stained by using the silver staining technique of Herring and colleagues (21).
Subgroup (VP6) ELISA.
An in-house monoclonal antibody enzyme-linked immunosorbent assay (ELISA), described elsewhere (34), was used to determine subgroup specificities of the serotype G2 strains. The group-specific (4) and subgroup-specific (20) monoclonal antibodies were kind donations from Harry Greenberg and Jon Gentsch.
G (VP7) serotyping ELISA.
VP7 serotyping was completed according to previously published methods (36). The G2-specific capture monoclonal antibodies used included S2-2G10 (36) and IC10 (32). Mab60 (32) was also included as a positive control to detect the presence of the intact double-shelled viral particles. Each plate contained negative controls, and the reactions were read spectrophotometrically at 450 nm.
Cloning and sequencing of the VP7 and VP4 genes of serotype G2 rotavirus strains.
RT-PCR products obtained during first-round reverse transcription and amplification of the VP7 genes of serotype G2 rotaviruses were cloned into the pGEM-T Easy vector (Promega) according to the manufacturer's instructions. Recombinant plasmids were screened for the insert of the correct size by using NotI (Promega). Plasmids were automatically sequenced, using M13 forward and reverse primers, by D. James at the DNA Sequencing Laboratory, University of Cape Town, and by C. van Heerden at the Core DNA Sequencing Laboratory, University of Stellenbosch. A phylogenetic tree was constructed by using genetic distances generated on DNAMan computer software and drawn with the TreeView package (29).
The VP7 sequences (strain DS-1) used for phylogenetic analysis were obtained from GenBank and have accession number AJ540227. The nucleotide sequence for WI61 was obtained from Green and colleagues (19).
Nucleotide sequence accession numbers.
The DNA sequences for the VP7 genes of the African G2 strains were directly submitted to GenBank and were assigned the following GenBank accession numbers: SA514GR/87, AY261338; SA356PT/96, AY261342; SA4476PT/97, AY261343; SA3958GR/97, AY261344; SA4419SB/97, AY261345; SA4372JB/98, AY261346; KY3103/99, AY261349; KY3303/99, AY261350; NG4585/99, AY261351; NG5113/99, AY261352; GH1803/99, AY261353; CI1735/99, AY261354; BF3767/99, AY261355; BF3704/99, AY261356; TN1529/99, AY261357; and MR4717/00, AY261358.
RESULTS
During 1999, serotype G2 strains were detected in various African countries, including Kenya, two provinces in Nigeria, Ghana, Ivory Coast, Burkino Faso, and Tunisia (Table 1). A single G2 strain isolated in Mauritius in 2000 during a season in which G9 was predominant was also analyzed. Serotype G2 strains from South Africa isolated in 1987, when G2 was prevalent, and between 1996 and 1998 were included for a comparison of G2 strains on the African continent from 1995 (Page and Steele, submitted).
TABLE 1.
Subgroup specificities, RNA profiles, P genotypes and VP7 antigenicities of serotype G2 strains isolated in Africa
| Sample | Isolation area | Yr | Predominant serotype | Subgroup specificity | RNA profilee | P genotype | G2 monoclonal antibodies |
|---|---|---|---|---|---|---|---|
| SA514GR/87 | Ga-Rankuwa | 1987 | G2a | I | S | P[4] | Mab60, S2, 1C10 |
| SA356PT/96 | Pretoria | 1996 | UNb | I | S | P[4]-P[6] | NRg |
| SA4476PT/97 | Pretoria | 1997 | UN | I | S | P[4]-P[6] | NR |
| SA3958GR/97 | Ga-Rankuwa | 1997 | G2c | I | S | P[6] | NR |
| SA4419SB/97 | Stellenbosch | 1997 | G2c | I | S | P[4] | NR |
| SA4372JB/98 | Johannesburg | 1998 | G1c | I | S | P[4] | NR |
| KY3103/99 | Kenya | 1999 | G1c | I | S | P[4] | Mab60, 1C10h |
| KY3303/99 | Kenya | 1999 | G1c | I | S | P[4] | Mab60, S2, 1C10 |
| NG4585/99 | Nigeria (Zaria) | 1999 | G9c | I | S | P[6] | NR |
| NG5113/99 | Nigeria (Jos) | 1999 | G8c | I | NTf | P[4]-P[6] | NR |
| GH1803/99 | Ghana | 1999 | G2c | I | S | P[6] | Mab60 |
| CI1735/99 | Ivory Coast | 1999 | G1c | I | S | P[6] | NR |
| BF3767/99 | Burkino Faso | 1999 | G2c | I | S | P[6] | Mab60, S2, 1C10 |
| BF3704/99 | Burkino Faso | 1999 | G2c | I and II | S | P[6] | NR |
| TN1529/99 | Tunisia | 1999 | G1c | I | S | P[4] | NR |
| MR4717/00 | Mauritius | 2000 | G9d | Non-I, non-II | S | P[4] | NDi |
G serotyping was by ELISA and PCR-generated probes.
UN, prevalent serotype unknown.
G serotyping was by ELISA and RT-PCR.
G serotyping was by RT-PCR.
The RNA profile was designated S for short electropherotype.
NT, not typeable.
NR, no reaction with Mab60, S2-2G10, or 1C10.
The isolate reacted with Mab60 and weakly with 1C10.
ND, not done.
Serotype G2 strains display several distinct genogroup features, including subgroup I specificity, short RNA profiles, and a P[4] genotype. Most of the African G2 strains exhibited subgroup I specificity and short RNA profiles but had altered P types (Table 1). Strains from Kenya, Tunisia, and Mauritius displayed the characteristic P[4] genotype, while strains from Nigeria, Ghana, Burkino Faso, and Ivory Coast displayed the neonatal P[6] genotype. An additional G2 strain from Nigeria revealed dual P[4] and P[6] specificity. Altered subgroup specificity was found in a G2 strain from Burkino Faso with subgroup I and II specificity and a G2 strain from Mauritius with non-I, non-II subgroup specificity.
Serotype G2 strains isolated from Africa were screened with the G2-specific monoclonal antibodies 1C10 and S2-2G10 (Table 1). More than half of the African G2 strains did not react with Mab60 or either of the G2-specific monoclonal antibodies used. Only a G2 strain from Kenya and one from Burkino Faso reacted with both monoclonal antibodies, while a further strain from Kenya reacted weakly with 1C10. A G2 strain isolated in Ghana reacted with Mab60, indicating that variants of serotype G2 were not being detected by the two monoclonal antibodies used.
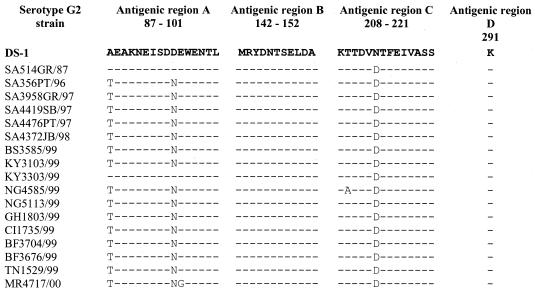
Analysis of antigenic regions A, B, C, and D of the serotype G2 strains revealed amino acid changes at positions 87, 96, and 213 (Fig. 1). No amino acid changes were noted in antigenic region B or D for any of the G2 strains isolated. The Kenyan strain KY3303/99 appeared to be more similar to DS-1 and the South African SA514GR/87 strain in antigenic region A.
FIG. 1.
Deduced amino acid sequences of antigenic regions A (positions 87 to 101), B (positions 142 to 152), C (positions 208 to 211), and D (position 291) of the VP7 genes of African G2 strains. DS-1 is included as the G2 standard.
The rotavirus-specific glycosylation sites at amino acid residue 69 (N-S-T) and 238 (N-I-S) were conserved in all samples except KY3303/99 (data not shown). The Kenyan isolate displayed an S→L amino acid change at position 70 and an I→V change at position 239. The amino acid changes, however, did not alter the glycosylation potential at these sites. The G2-specific glycosylation site at amino acid residue 146 (N-T-S) was conserved in all serotype G2 strains isolated in Africa (Fig. 1).
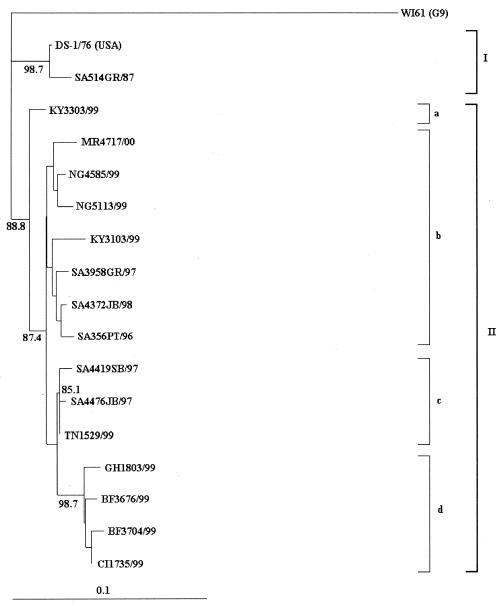
A phylogenetic tree was constructed by using the deduced amino acid sequences of the VP7 genes of the G2 strains isolated from various African countries in 1999 (Fig. 2). Two major lineages were noted, the first (I) containing the older G2 strains DS-1 and SA514GR/87 and the second (II) containing all of the G2 strains isolated in Africa after 1995.
FIG. 2.
Phylogenetic analysis of the VP7 deduced amino acid sequences of African serotype G2 strains. DS-1 is included as the G2 standard, and WI61 is included as the outgroup strain. The phylogenetic tree was constructed by using the TreeView program (29), and genetic distances were generated with DNAMan computer software. The length of the abscissa to the connecting node is proportional to the genetic distance between sequences and is indicated by the scale bar. Bootstrap values of above 85% are indicated at the appropriate nodes.
Within lineage II, the “new” G2 strains isolated after 1995 could be divided into four sublineages. The first (IIa) contained a single isolate from Kenya, KY3303/99, that appeared to be antigenically similar to DS-1 but grouped with the new G2 lineage (Fig. 1 and 2). The second (IIb) contained G2 strains from Mauritius (MR4717/00), Nigeria (NG4585/99 and NG5113/99), Kenya (KY3103/99), and the Johannesburg-Pretoria-Ga-Rankuwa area in South African isolated between 1996 and 1998 (SA3958GR/97, SA4372JB/98, and SA356PT/96). Sublineage IIc contained G2 strains isolated from two regions (Ga-Rankuwa and Stellenbosch) in South Africa in 1997 and a strain isolated in Tunisia in 1999. In sublineage IId, serotype G2 strains were isolated from three West African countries, including Ghana (GH1803/99), Burkino Faso (BF3676/99 and BF3704/99), and Ivory Coast (CI1735/99).
DISCUSSION
Serotype G2 strains were isolated from eight African countries between 1996 and 2000. During this time, G2 was the predominant serotype in 1997 in the South African areas of Ga-Rankuwa and Stellenbosch and in 1999 in the West African countries of Ghana and Burkino Faso. In the remaining five African countries, serotype G1 strains were the predominant circulating strains.
The subgroup specificities and RNA profiles of most of the African G2 strains were consistent with the subgroup I, short-RNA DS-1 characteristics. Two exceptions were noted; i.e., a strain from Burkino Faso displayed subgroup I and II specificity, and a strain from Mauritius displayed non-subgroup I or II specificity. Iturriza-Gómara and colleagues (24) similarly detected a limited number of strains displaying altered subgroup specificity and suggested that the mechanisms involved in generating diversity were not solely confined to the VP7 and VP4 genes.
While the subgroup I, short-RNA DS-1 characteristics appeared to be more constant, the VP4 genotype of African G2 strains varied according to year of isolation and geographical location (Table 1). West African isolates were more commonly associated with the P[6] genotype (3). The association of serotype G2 strains with P[6] was not unexpected, as this P type is becoming more prevalent worldwide; e.g., P[6] was predominant in Guinea-Bissau during 1996 and 1997 (15), in Nigeria during 1999 and 2000 (1), in India during 1993 (31), and in Malawi during 1997 and 1998 (12). The P[6] genotype has emerged in association with G9 and G1 strains in the United States in the 1996 to 1997 rotavirus season (30).
Rotavirus isolates with single G serotypes and mixed P genotypes have previously been detected in various African countries, including Malawi (11), Ivory Coast (33), Ghana (33), Nigeria (33), Burkino Faso (33), Zimbabwe (33), Guinea-Bissau (14), and South Africa (28a). Therefore, the isolation of a Nigerian G2 strain displaying dual P[4] and P[6] specificity was not unexpected. While the packaging of rotaviruses (39) is reportedly strictly equimolar for all RNA segments of the genome, there is room for the packaging of extra genetic material (2), and a single G2 strain carrying both forms of the VP4 gene cannot be discounted. The inclusion of alternative P types in serotype G2 strain gene constellations may provide a means of escaping protective immunity and generating increased rotavirus diversity.
Iturriza-Gómara and colleagues (23) linked the failure of G2-specific monoclonal antibodies to react to an amino acid substitution (D→N) at position 96 in antigenic region A. However, a G2 strain from Burkino Faso (BF3767/99) displaying the same amino acid changes as the British 1996 isolates reacted with both monoclonal antibodies used. In addition, an isolate from Kenya, KY3103/99, displaying a similar amino acid change in antigenic region A and an additional change at position 213 in antigenic region C reacted with Mab60 and weakly with 1C10 (Fig. 1 and Table 1). Furthermore, an isolate from Ghana with the same amino acid substitutions as the Kenyan isolate reacted only with Mab60 and not with either of the G2-specific monoclonal antibodies used. The nonreactivity of the G2-specific monoclonal antibodies could therefore not be linked to specific amino acid substitutions in antigenic regions A, B, and C, and the binding of monoclonal antibodies to G2 strains may be more complex than previously thought. These results highlight the antigenic diversity of rotavirus strains and prompt investigation into the role that monotypes play in rotavirus epidemiology.
Additional factors resulting in VP7 serotype failure, including the destruction of the outer capsid by proteolytic stool enzymes or improper storage conditions (37), may be largely responsible for the nonreactivity of the African G2 isolates with the monoclonal antibodies used. However, the Ghanaian G2 strain reacted with Mab60, indicating the presence of an intact VP7 capsid, and failed to react with either 1C10 or S2-2G10, illustrating the need for additional monoclonal antibodies for the antigenic analysis of rotavirus strains.
Phylogenetic analysis of G2 strains from Africa revealed two lineages, i.e., the pre-1995 “old” G2 strains and post-1995 “new” strains. These results were similar to those obtained during the analyses of South African G2 strains (28a). The old G2 strains were derived from a common ancestor with DS-1, while the new G2 strains seem to have emerged from a common ancestor around 1995 or 1996.
The new G2 strains could be further split into four lineages (Fig. 2). Serotype G2 strains in sublineages IIa and IIb were isolated in countries where G2 strains were not the predominant circulating serotype. The exception was strain SA3958GR/97, isolated in Ga-Rankuwa during the 1997 rotavirus season when G2 was predominant. Within the Ga-Rankuwa area, serotype G2 strains circulated at levels comparable to those of G1 strains in 1984 (29 versus 22%), 1990 (30 versus 25%), and 1993 (30 versus 25%) and to those of G4 strains in 1987 (22 versus 33%) (A. Geyer, I. Peenze, M. C. de Beer, P. Bos, and A. D. Steele, Abstr. 12th Int. Cong. Virol., abstr.V-1419, 2002). The 1997 season when G2 was predominant therefore appears to be due to the cyclic nature of serotype G2P[4] rotavirus infections observed within this area rather than to G2 strains with an increased ability to cause an epidemic outbreak of diarrhea.
Conversely, G2 strains clustering in sublineages IIc and IId were isolated in countries where G2 was the predominant serotype, with the exception of two strains. In sublineage IIc, the 1997 South African G2 strains were isolated in areas where serotype G2 predominated, while the 1999 G2 strain from Tunisia (TN1529/99) was isolated during a G1 season. Subsequent analysis of rotavirus strains from Tunisia in 2000, however, revealed that 11 of 20 (55%) were G2P[4] (N. A. Page, A. Trabelsi, and A. D. Steele, unpublished data) and that serotype G2 strains had replaced G1 as the predominant circulating type.
Sublineage IId comprised strains from the 1999 seasons in Ghana and Burkino Faso when G2 was predominant and an additional G2 strain from Ivory Coast (CI1735/99) isolated during a G1 season. Analysis of rotavirus strains from Ivory Coast in 2000 and 2002 revealed only 17 of 101 (17%) G2P[6] strains in 2000 and 5 of 47 (11%) G2P[6] strains in 2002 (6; N. A. Page, V. Akran, and A. D. Steele, unpublished data). Unlike the trend in Tunisia, where the sublineage IIc G2 strain became the predominant serotype in the following season, the failure of the sublineage IId G2 strain to become the predominant serotype in Ivory Coast was unexpected. However, further investigation revealed that a military coup and political violence erupted in Ivory Coast in December 1999 and continued during 2000, which could have resulted in an altered movement of people and disrupted the expected season of G2 predominance in 2000 or 2001.
The phylogeny of serotype G2 strains from the African continent revealed two sublineages (IIc and IId), the strains of which were more likely to be isolated in seasons when G2 was predominant than strains clustering in the remaining two sublineages (IIa and IIb). Hybridization analysis of these serotype G2 strains will be conducted to determine whether these strains may be related to the Taiwanese G2 isolates responsible for the epidemic outbreak of rotavirus-associated diarrhea in 1993 (27, 40).
Furthermore, the West African serotype G2 strains in sublineage IId cluster with a 98.7% bootstrap value, suggesting that the epidemiology and transmission of rotavirus strains in this area are geographically limited and more distinct than those of G2 strains in the remaining African countries.
In summary, serotype G2 strains were isolated in eight countries between 1996 and 2000 on the African continent. Alterations in the characteristic DS-1 genogroup gene constellations were more likely to occur in the VP4 gene, and three genotypes were observed, including a dual P[4]-P[6] type. The poor reaction of G2-specific monoclonal antibodies with the African G2 strains was probably due to improper storage, although G2 monotypes were detected. Phylogenetic analyses potentially identified serotype G2 strains that were more commonly associated with seasons when G2 was predominant. Continuous surveillance of rotavirus epidemics in Africa will be preparation for future vaccine implementation in an area that clearly needs these preventative medicines. In addition, further research will aid in the establishment of an accurate model of serotype epidemiology and transmission dynamics of rotavirus strains on the African continent.
Acknowledgments
This study was supported by a grant from the South African Medical Research Council, Tygerberg, and the Poliomyelitis Research Foundation, Sandringham. N. A. Page is the recipient of the Paul and Stella Loewenstein Doctoral Scholarship.
We thank James Nyangao, Lohya Nimzing, Grace Pennap, George Armah, Veronique Akran, Souleymane Sawadogo, Abdelhalim Trabelsi, and N. Pyndiah for providing the clinical material used in this study. We also thank Ruth Bishop, Harry Greenberg, and Jon Gentsch for providing the monoclonal antibodies used in the study.
REFERENCES
- 1.Adah, M. I., A. Wade, and K. Taniguchi. 2001. Molecular epidemiology of rotaviruses in Nigeria: detection of unusual strains with G2P[6] and G8P[1] specificities. J. Clin. Microbiol. 39:3969-3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen, A. M., and U. Desselberger. 1985. Reassortment of human rotaviruses carrying rearranged genomes with bovine rotavirus. J. Gen. Virol. 66:2703-2714. [DOI] [PubMed] [Google Scholar]
- 3.Armah, G. E., C. T. Pager, R. H. Asmah, F. R. Anto, A. R. Oduro, F. Binka, and D. Steele. 2001. Prevalence of unusual human rotavirus strains in Ghanaian children. J. Med. Virol. 63:67-71. [PubMed] [Google Scholar]
- 4.Beards, G. M., A. D. Campbell, R. Cottrell, S. M. Peiris, N. Rees, R. C. Sanders, J. A. Shirley, H. C. Woods, and T. H. Flewett. 1984. Enzyme-linked immunosorbent assay based on polyclonal and monoclonal antibodies for rotavirus detection. J. Clin. Microbiol. 19:248-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein, D. I., D. A. Sack, E. Rothstein, K. Reisinger, V. E. Smith, D. O'Sullivan, D. R. Spriggs, and R. L. Ward. 1999. Efficacy of live, attenuated, human rotavirus vaccine 89-12 in infants: a randomized placebo-controlled trial. Lancet 354:287-290. [DOI] [PubMed] [Google Scholar]
- 6.Bhan, M. K., J. F. Lew, S. Sazawal, B. K. Das, J. R. Gentsch, and R. I. Glass. 1993. Protection conferred by neonatal rotavirus infection against subsequent diarrhea. J. Infect. Dis. 168:282-287. [DOI] [PubMed] [Google Scholar]
- 7.Bresee, J. S., R. I. Glass, B. Ivanoff, and J. R. Gentsch. 1999. Current status and future priorities for rotavirus vaccine development, evaluation and implementation in developing countries. Vaccine 17:2207-2222. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention. 1999. Intussusception among recipients of rotavirus vaccine—United States, 1998-1999. Morb. Mortal. Wkly. Rep. 48:577-581. [PubMed] [Google Scholar]
- 9.Clemens, J., N. Keckich, A. Naficy, R. Glass, and M. Rao. 1999. Public health considerations for the introduction of new rotavirus vaccines for infants: a case study of tetravalent rhesus rotavirus-based reassortant vaccine. Epidemiol. Rev. 21:24-42. [DOI] [PubMed] [Google Scholar]
- 10.Conner, M. E., D. O. Matson, and M. K. Estes. 1994. Curr. Top. Microbiol. Immunol. 185:285-337. [DOI] [PubMed] [Google Scholar]
- 11.Cunliffe, N. A., J. S. Gondwe, S. M. Graham, B. D. M. Thindwa, W. Dove, R. L. Broadhead, M. E. Molyneux, and C. A. Hart. 2001. Rotavirus strain diversity in Blantyre, Malawi, from 1997 to 1999. J. Clin. Microbiol. 39:836-843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cunliffe, N. A., J. S. Gondwe, R. L. Broadhead, M. E. Molyneux, P. A. Woods, J. S. Bresee, R. I. Glass, J. R. Gentsch, and C. A. Hart. 1999. Rotavirus G and P types in children with acute diarrhoea in Blantyre, Malawi, from 1997 to 1998: predominance of novel P[6]G8 strains. J. Med. Virol. 57:308-312. [PubMed] [Google Scholar]
- 13.Cunliffe, N. A., P. E. Kilgore, J. S. Breese, A. D. Steele, N. K. Luo, C. A. Hart, and R. I. Glass. 1998. Epidemiology of rotavirus diarrhoea in Africa. A review of studies to anticipate rotavirus immunization. Bull. W. H. O. 76:525-537. [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer, T. K., N. A. Page, D. D. Griffin, J. Eugen-Olsen, A. G. Pedersen, P. Valentiner-Branth, K. Mølbak, H. Sommerfelt, and N. Munk-Nielsen. 2003. Characterization of incompletely typed rotavirus strains from Guinea-Bissau: identification of G8 and G9 types and a high frequency of mixed infections. Virology 311:125-133. [DOI] [PubMed] [Google Scholar]
- 15.Fischer, T. K., H. Steinsland, K. Mølbak, R. Ca, J. R. Gentsch, P. Valentiner-Branth, P. Aaby, and H. Sommerfelt. 2000. Genotype profiles of rotavirus strains from children in a suburban community in Guinea-Bissau, Western Africa. J. Clin. Microbiol. 38:264-267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gentsch, J. R., P. A. Woods, M. Ramachandran, B. K. Das, J. P. Leite, A. Alfieri, R. Kumar, M. K. Bhan, and R. I. Glass. 1996. Review of G and P typing results from a global collection of rotavirus strains; implications for vaccine development. J. Infect. Dis. 174:530-536. [DOI] [PubMed] [Google Scholar]
- 17.Gentsch, J. R., R. I. Glass, P. Woods, V. Gouvea, M. Gorziglia, J. Flores, B. K. Das, and M. K. Bhan. 1992. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J. Clin. Microbiol. 30:1365-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gouvea, V., R. I. Glass, P. Woods, K. Taniguchi, H. F. Clark, B. Forrester, and Z. Fang. 1990. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J. Clin. Microbiol. 28:276-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Green, K. Y., Y. Hoshino, and N. Ikegami. 1989. Sequence analysis of the gene encoding the serotype-specific glycoprotein (VP7) of two new human rotavirus serotypes. Virology 168:429-433. [DOI] [PubMed] [Google Scholar]
- 20.Greenberg, H. B., V. McAuliffe, J. Valdesuso, R. G. Wyatt, J. Flores, A. R. Kalica, Y. Hoshino, and N. H. Singh. 1983. Serological analysis of the subgroup protein of rotavirus, using monoclonal antibodies. Infect. Immun. 39:91-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herring, A. J., N. F. Inglis, C. K. Ojeh, D. R. Snodgrass, and J. D. Menzies. 1982. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J. Clin. Microbiol. 16:473-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoshino, Y., and A. Z. Kapikian. 1994. Rotavirus antigens. Curr. Top. Microbiol. Immunol. 185:179-227. [DOI] [PubMed] [Google Scholar]
- 23.Iturriza-Gómara, M., D. Cubitt, U. Desselberger, and J. Gray. 2001. Amino acid substitution within the VP7 protein of G2 rotavirus strains associated with failure to serotype. J. Clin. Microbiol. 39:3796-3798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iturriza-Gómara, M., B. Isherwood, U. Desselberger, and J. Gray. 2001. Reassortment in vivo: driving force for diversity of human rotavirus strains isolated in the United Kingdom between 1995 and 1999. J. Virol. 75:3696-3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kapikian, A. Z. 2001. A rotavirus vaccine for prevention of severe diarrhoea of infants and young children: development, utilization and withdrawal, p. 153-179. In D. Chadwick and J. A. Goode (ed.), Gastroenteritis viruses. Novartis Foundation, West Sussex, England. [DOI] [PubMed]
- 26.Kapikian, A. Z., J. Flores, K. Midthun, Y. Hoshino, K. Y. Green, M. Gorziglia, K. Nishikawa, R. M. Chanock, L. Potash, and I. Perez-Schael. 1990. Strategies for the development of a rotavirus vaccine against infantile diarrhoea with an update on clinical trials of rotavirus vaccines. Adv. Exp. Med. Biol. 257:67-90. [DOI] [PubMed] [Google Scholar]
- 27.Masendycz, P. J., and E. A. Palombo. 2001. Genetic relatedness of VP1 genes of Australian and Taiwanese rotavirus isolates. FEMS Microbiol. Lett. 198:147-150. [DOI] [PubMed] [Google Scholar]
- 28.Murray, C. J. L., and A. D. Lopez. 1997. Global mortality, disability, and the contribution of risk factors: global burden of disease study. Lancet 349:1436-1442. [DOI] [PubMed] [Google Scholar]
- 28a.Page, N. A., and A. D. Steele. 2004. Antigenetic and genetic characterization of serotype G2 rotavirus strains from South Africa from 1984 to 1998. J. Med. Virol. 72:320-327. [DOI] [PubMed] [Google Scholar]
- 29.Page, R. M. D. 1996. TREEVIEW: an application to display phylogenetic trees on personal computers. Comput. Appl. Biosci. 12:357-358. [DOI] [PubMed] [Google Scholar]
- 30.Ramachandran, M., J. R. Gentsch, U. D. Parashar, S. Jin, P. A. Woods, J. L. Holmes, C. D. Kirkwood, R. F. Bishop, H. B. Greenberg, S. Urasawa, G. Gerna, B. S. Coulson, K. Taniguchi, J. S. Bresee, R. I. Glass, et al. 1998. Detection and characterization of novel rotavirus strains in the United States. J. Clin. Microbiol. 36:3223-3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramachandran, M., B. K. Das, A. Vij, R. Kumar, S. S. Bhambal, N. Kesari, H. Rawat, L. Bahl, S. Thukar, P. A. Woods, R. I. Glass, M. K. Bhan, and J. R. Gentsch. 1996. Unusual diversity of human G and P genotypes in India. J. Clin. Microbiol. 34:436-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shaw, R. D., D. L. Stoner-Ma, M. K. Estes, and H. B. Greenberg. 1985. Specific enzyme-linked immunoassay for rotavirus serotypes 1 and 3. J. Clin. Microbiol. 22:286-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steele, A. D., B. Ivanoff, et al. 2003. Rotavirus strains circulating in Africa during 1996-1999: emergence of G9 and P[6] strains. Vaccine 21:361-367. [DOI] [PubMed] [Google Scholar]
- 34.Steele, A. D., and J. J. Alexander. 1988. The relative frequency of subgroup I and II rotaviruses in black infants in South Africa. J. Med. Virol. 24:321-327. [DOI] [PubMed] [Google Scholar]
- 35.Steele, A. D., and J. J. Alexander. 1987. Molecular epidemiology of rotavirus in black infants in South Africa. J. Clin. Microbiol. 25:2384-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taniguchi, K., T. Urasawa, Y. Morita, H. B. Greenberg, and S. Urasawa. 1987. Direct serotyping of human rotavirus in stools by enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J. Infect. Dis. 155:1159-1166. [DOI] [PubMed] [Google Scholar]
- 37.Unicomb, L. E., B. S. Coulson, and R. F. Bishop. 1989. Experience with an enzyme immunoassay for serotyping human group A rotaviruses. J. Clin. Microbiol. 27:586-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward, R. L., D. I. Berstein, et al. 1994. Protection against rotavirus disease after natural rotavirus infection. J. Infect. Dis. 174(Suppl.):S51-S58. [DOI] [PubMed] [Google Scholar]
- 39.Whitton, J. L., F. Hundley, B. O'Donnell, and U. Desselberger. 1983. Silver staining of nucleic acids. Applications in virus research and in diagnostic virology. J. Virol. Methods 7:185-198. [DOI] [PubMed] [Google Scholar]
- 40.Zao, C.-L., W.-N. Yu, C.-L. Kao, K. Taniguchi, C.-Y. Lee, and C.-N. Lee. 1999. Sequence analysis of VP1 and VP7 genes suggests occurrence of a reassortant of G2 rotavirus responsible for an epidemic of gastroenteritis. J. Gen. Virol. 80:1407-1415. [DOI] [PubMed] [Google Scholar]