Abstract
Helicobacter pylori infection and peptic ulcer disease are common in developing countries, e.g., Vietnam. An enzyme-linked immunosorbent assay (ELISA) for screening of patients and for seroepidemiology is a useful tool but needs to be validated in the population studied. We used in-house ELISA with sonicated Swedish and Vietnamese strains as antigens to measure immunoglobulin G antibodies after absorption with sonicated Campylobacter jejuni in sera from 270 H. pylori culture-confirmed peptic ulcer patients, 128 Swedish urea-breath test and immunoblot-positive healthy controls, and 432 Vietnamese immunoblot-positive population controls. Sonicated whole-cell antigen based on the local strains showed a significantly better performance. Immunoblot-positive peptic ulcer patients had significantly higher antibody concentrations than immunoblot-positive population controls, necessitating a lower cutoff level if serology is used for screening or epidemiological purposes. The study shows that the parameters of ELISA for H. pylori need to be adjusted for the population being investigated.
Helicobacter pylori infects half the population of the world, causes nonimmune gastritis, peptic ulcer disease, and is associated with gastric cancer (5, 17). H. pylori infection, and hence the diseases caused by the microorganism, is decreasing in younger cohorts in developed countries but remains a major health problem in developing countries, e.g., Vietnam (11). In a large survey conducted at the Hanoi Military Hospital from 1963 to 1983, peptic ulcer was found by endoscopy in 7.8% of 300,000 volunteers investigated (16).
H. pylori infection can be confirmed by endoscopy, followed by culture of H. pylori from biopsies. Noninvasive tests to establish H. pylori infection, such as the urea-breath test and serology, are also widely used in high-income countries (8). These assays have advantages, especially for studies in children and for epidemiological investigations. Serological assays for H. pylori are based either on whole-cell sonicate antigen or on one or several purified components of the bacterium as the antigen. A majority of serological studies are now conducted with commercial kits that have been evaluated in developed countries. These commercial kits are often too expensive for developing countries, and use of a validated in-house enzyme-linked immunosorbent assay (ELISA) based on sonicated antigens would be preferable.
We have previously developed and evaluated an in-house ELISA based on sonicated H. pylori antigen, supplemented with an absorption step with sonicated Campylobacter jejuni antigen to remove cross-reacting antibodies (2, 14, 15). In order to provide serodiagnostic and seroepidemiological tools for H. pylori infection in Vietnam, the present evaluation of the in-house ELISA was initiated in the local population, both in patients with peptic ulcer disease where the infection had been confirmed by culture of H. pylori and in a sample of the general population where immunoblot could be used as reference method.
MATERIALS AND METHODS
Patients with peptic ulcer disease.
Two hundred ninety-six patients with peptic ulcers of at least 5 mm in size, aged 18 to 80 years, and with a positive rapid urease test were included after informed consent was obtained to participate in a treatment trial at Bach Mai Hospital, Hanoi, from May 1999 to June 2001. Included in the present study were 270 patients positive for H. pylori by culture and with a pretreatment blood sample. Blood samples were drawn after endoscopic examination, and sera were separated by centrifugation and stored at −20°C until analyzed.
Population controls.
In Vietnam, healthy individuals 18 to 88 years of age who attended routine medical examinations in Hanoi were asked to volunteer a blood sample for the study. As part of the baseline data collections from each of the 432 volunteers, information on health status was obtained. In addition, information on age, gender, socioeconomic status, smoking, alcohol drinking, history of peptic ulcer disease, and education level were collected. Approximately 5 ml of blood was drawn, and the serum was aliquoted and immediately stored at −20°C until analyzed for antibodies to H. pylori. One of the 432 samples tested by ELISA was insufficient for testing by immunoblot.
In Sweden, 128 healthy individuals aged 19 to 85 years with a positive urea- breath test and a positive immunoblot (12) were tested by ELISA against the two antigens.
ELISA.
Two different antigens were used for ELISA, one based on three clinical isolates from Swedish patients with peptic ulcer and strain NCTC 11638 and another based on five clinical isolates from Vietnamese patients with peptic ulcer diseases and strain NCTC 11638. The antigens were sonicated and used to coat 96-well microplates at a concentration of 5 μg/ml, as previously described (2, 14). Sera were diluted 1:1,000, first 1:100 in phosphate-buffered saline and then 1:10 in phosphate-buffered saline containing 70 mg of Campylobacter jejuni antigen per ml (four clinical isolates) to remove cross-reacting antibodies. Alkaline phosphatase-conjugated anti-human immunoglobulin G (IgG) (Euro-Diagnostica, Malmö, Sweden) was used to detect bound antibodies.
The upper limit of normal values, at an optical density of 0.36 (A405) for the Swedish antigen, had been established in 83 culture-positive adult patients and 43 patients negative by microscopy, culture, rapid urease test, giving a sensitivity of 100% (83 of 83) and specificity of 95.6% (43 of 45) (2, 14, 15).
Immunoblot.
All sera were tested by immunoblot, using the commercially available HelicoBlot 2.1 (Genelabs Diagnostics, Singapore) for detection of antibodies against H. pylori-specific antigens. The kit consists of Western blot membrane strips, made with a surface antigen-enriched preparation of H. pylori including CagA (116 kDa), VacA (89 kDa), and the urease A subunit (30 kDa). All buffers and reagents used were supplied with the kit and used according to the manufacturer's recommendations. The assay was performed with an automated Western blot system (Autoblot system 36; Genelabs Diagnostics). The blots were evaluated as positive or negative according to the criteria supplied by the manufacturer. A positive blot was defined as having a band at 116 kDa (CagA) together with at least one band at 89 kDa (VacA), 37 kDa, 35 kDa, 30 kDa, or 19.5 kDa or at the “current infection marker,” a recombinant antigen supplied by the manufacturer. In addition, a blot was positive if one of the 89-kDa, 37-kDa, or 35-kDa bands was present. The presence of both the 30-kDa and 19.5-kDa bands was the third criterion for a positive blot.
Statistical analyses.
Quantitative data for patients and controls were analyzed by the Mann-Whitney U sum rank test. Differences between age groups at 10-year intervals were compared by the Kruskal-Wallis test. Qualitative data were analyzed by chi-square test. Correlation between titers in the two antigens was analyzed by simple regression analysis.
Ethical clearance.
The project was approved by the Karolinska Hospital ethics committee in Sweden. Approval for the project was also granted in Vietnam. In addition, the study was approved by the Swedish Medical Products Agency.
RESULTS
The 270 peptic ulcer patients with positive cultures for H. pylori had a mean age of 41.4 years (median 40, range 18 to 80), and 97 (35.9%) were female. The 432 healthy controls had a mean age 49.2 years (median 49, range 18 to 88), and 182 (42.1%) were female. The differences were not statistically significant.
In the immunoblot, 269 of 270 peptic ulcer patients were positive according to the manufacturer's criteria. The patient negative in immunoblot also had very low ELISA titers. Of the controls, 376 of 431 (87.2%) were positive by immunoblot (one sample tested by ELISA was insufficient for immunoblot). The 55 blot-negative controls were used to calculate the specificity of the ELISA with the two antigens.
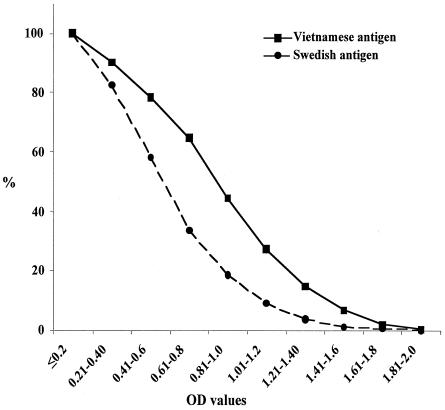
The ELISA results obtained with antigens based on the Swedish and Vietnamese strains showed a good correlation (r = 9.0 for all samples), but titers were significantly higher (P < 0.001) with the Vietnamese antigen when testing Vietnamese samples (Fig. 1). No significant differences by age groups or by gender were found.
FIG. 1.
Reverse cumulative distribution curves showing optical density values of titers in sera from Vietnamese peptic ulcer patients and population controls (n = 701).
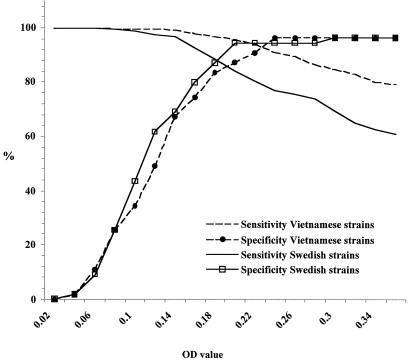
The difference between the antigens based on Swedish and Vietnamese strains was not only a difference in antibody levels, which could have been compensated for by a decrease of the cutoff level. As shown in Fig. 2, the performance of the two antigens differed with respect to sensitivity and specificity. The ELISA based on Vietnamese strains resulted in a higher sensitivity and specificity at any level chosen for these parameters.
FIG. 2.
Receiver operating characteristics curves for sensitivity and specificity obtained with the antigens based on Vietnamese and Swedish strains. The evaluation was made on Vietnamese sera from the normal population (n = 431) and with immunoblot as the reference method.
The difference between the performances of the ELISAs went both ways; i.e., the 128 Swedish population controls had a significantly higher mean optical density value (P < 0.001) when tested with the Swedish antigen than with the Vietnamese antigen.
The intersect of the sensitivity and specificity lines in Fig. 2, indicating the optimal cutoff levels for sensitivity and specificity, was above 90% for the Vietnamese antigen but below 90% for the Swedish antigen with Vietnamese sera. If high specificity were needed in the assay in Vietnam, a specificity of 96.4% with sensitivity at 91% could be obtained with the Vietnamese antigen. The corresponding specificity with the Swedish antigen could only be reached at the price of a low sensitivity at 69.4%.
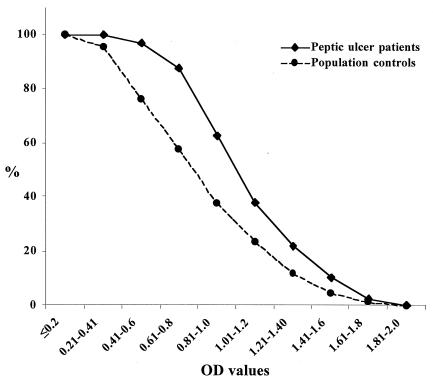
In addition, significantly higher ELISA IgG titers were found in the sera from the immunoblot-positive Vietnamese peptic ulcer patients compared to the immunoblot-positive Vietnamese population controls (P < 0.001 for both antigens). Figure 3 shows the reverse cumulative distribution of titers of immunoblot-positive patients and blot-positive controls for the antigen based on Vietnamese strains. For the antigen based on Swedish strains, the mean optical density value of titers for peptic ulcer patients was 0.605 (95% confidence interval 0.57;0.639) and for the blot-positive normal population controls 0.517 (0.485; 0.549). The corresponding data for the Vietnamese antigen were 0.951 (0.912; 0.989) and 0.72 (0.683; 0.758), respectively.
FIG. 3.
Reverse cumulative distribution graph of the optical density values of antibody titers in ELISA with Vietnamese strains in immunoblot-positive Vietnamese peptic ulcer patients (n = 269) and population controls (n = 376).
If our previously established (2, 13) cutoff level of an optical density of 0.36 in Swedish patients were applied to the two Vietnamese populations studied, the sensitivity of the ELISA based on Vietnamese strains would be 97.4% (262 of 269) and with the Swedish strains 78% (211 of 269) in the culture- and immunoblot-positive Vietnamese peptic ulcer patients. For the immunoblot-positive general population controls, the corresponding data would be 79.3% (298 of 376) and 61% (230 of 376), respectively. The lower titer levels in the general population thus lead to even lower performance of the ELISA with “foreign” strains than in patients with peptic ulcer disease.
DISCUSSION
The present study has shown that ELISA with sonicated H. pylori as the antigen, supplemented with an absorption step with C. jejuni for removal of cross-reacting antibodies, can be a useful assay in a developing country, provided that local strains of H. pylori are used to prepare the antigen. In addition, it was found that patients with peptic ulcer have higher antibody concentrations than infected individuals in the normal population.
The markedly higher performance of the ELISA based on local strains compared to foreign strains should be viewed in the perspective that genotypic and phenotypic differences have been documented in H. pylori isolates from different parts of the world and even in different populations within the same country (1, 4, 18). Whether antigenic differences are important enough to influence diagnostic performance of serological assays has been debated (3, 6, 7, 13). Early studies from Thailand indicated that assays based on local strains performed better than strains of other origin or commercial assays (3). A case-control study of gastric cancer in Venezuela, however, found no differences between four antigens of different origins (10). Other studies indicated that although antigenic variations do occur, they might not represent a major problem in serology (6, 7). In a very recent study from India, however, the same group reported findings similar to ours, i.e., that local strains gave a higher sensitivity in a sonicate whole-cell ELISA (13). Our study thus confirms the observation of that study and expands it by showing that local strains will also result in a higher specificity.
Our finding of significantly higher antibody concentrations by ELISA in immunoblot-positive peptic ulcer patients than in immunoblot-positive population controls was interesting and unexpected. To our knowledge, no previous study has systematically compared antibody levels in individuals from the same population with different manifestations of H. pylori infection. We have previously noted that when using ELISA for screening of Swedish school children at 10 years of age, our cutoff level previously established in culture-confirmed patients within a Swedish population would have resulted in a loss of sensitivity (15). We speculated that the need to lower the cutoff level might have been due to the fact that the children were 10 years of age, while the patients were adults. It was, however, uncertain whether 10 years of age would still necessitate an age-correlated cutoff level, since at that age children have reached adult IgG concentrations (9).
The higher antibody concentrations found in the present study in symptomatic patients than in asymptomatic ones could be explained by the higher degree of inflammation in the symptomatic patients, leading to a greater recruitment of antibody producing cells. It would also explain our previous observation that the cutoff level established for symptomatic patients needed to be lowered for screening purposes for 10- year-old children (15). The problem is of general relevance since cutoff levels in serological assays are established against a “gold standard”, usually culture. For culture of H. pylori, the sample for culture will have to be retrieved by an invasive method from the stomach. Since endoscopy is often perceived as unpleasant, only individuals with some degree of symptoms will undergo the procedure. This selection might, however, entail the risk of setting the cutoff level too high for screening purposes and for seroepidemiological studies in asymptomatic individuals.
The difference in antibody concentration between symptomatic and asymptomatic individuals may explain the controversy on use of ELISAs in Asian populations, found either to be satisfactory or underperforming. An ELISA evaluated in symptomatic individuals with high titers may also show a satisfactory performance with a high cutoff level and when based on foreign strains. The same assay may, however, show an important underperformance in asymptomatic individuals with lower antibody concentrations, leading to an underestimate of H. pylori infection prevalence in seroepidemiological studies.
In conclusion, our study has shown that ELISA for serodiagnosis of H. pylori infection should be based on local strains for optimal sensitivity and specificity. The study also showed an unexpected difference in IgG antibody concentration by ELISA between peptic ulcer patients and population controls when using immunoblot as the reference method. The difference implies that adjusted, lower cutoff levels will have to be used in ELISA when used for screening purposes or for seroepidemiological studies.
Acknowledgments
The study was done within the framework of KIRT, the Karolinska Institute Research Training program, supported by the Swedish International Development Agency, SIDA/SAREC.
REFERENCES
- 1.Alm, R. A., L.-S. L. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 2.Befrits, R., M. Granström, M. Rylander, and C. Rubio. 1993. Helicobacter pylori in 205 consecutive endoscopy patients. Scand. J. Infect. Dis. 25:185-191. [DOI] [PubMed] [Google Scholar]
- 3.Bodhidatta, L., C. W. Hoge, S. Churnratanakul, W. Nirdnoy, P. Sampathanakul, C. Tungtaem, S. Raktham, C. D. Smith, and P. Echeverria. 1993. Diagnosis of Helicobacter pylori infection in a developing country: comparison of two ELISA and a seroprevalence study. J. Infect. Dis. 168:1549-1553. [DOI] [PubMed] [Google Scholar]
- 4.Campbell, S., A. Fraser, B. Holliss, J. Schmid, and P. W. O'Toole. 1997. Evidence for ethnic tropism of Helicobacter pylori. Infect. Immun. 65:3708-3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dixon, M., and G. Sobala. 1992. Gastritis and duodenitis: the histopathological spectrum. Eur. J. Gastroenterol. Hepatol. 4(Suppl.):17-23. [Google Scholar]
- 6.Groves, F. D., L. Zhang, J. Li, W. C. You, Y. S. Chang, L. Zhao, W.-D. Liu, C. S. Rabkin, G. I. Pérez-Pérez, M. J. Blaser, and M. H. Gail. 1997. Comparison of two enzyme-linked immunosorbent assay tests for diagnosis of Helicobacter pylori infection in China. Cancer Epidemiol. Biomark. Prev. 6:551-552. [PubMed] [Google Scholar]
- 7.Höök-Nikanne, J., G. I. Perez-Perez, and M. J. Blaser. 1997. Antigenic characterization of Helicobacter pylori strains from different parts of the world. Clin. Diagn. Lab. Immunol. 4:592-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Monteiro, L., A. de Mascarel, A. M. Sarrasqueta, B. Bergey, C. Barberis, P. Talby, D. Roux, L. Shouler, D. Goldfain, H. Lamouliatte, and F. Megraud. 2001. Diagnosis of Helicobacter pylori infection: noninvasive methods compared to invasive methods and evaluation of two new tests. Am. J. Gastroenterol. 96:353-358. [DOI] [PubMed] [Google Scholar]
- 9.Morell, A., F. Skvaril, W. H. Hitzig, and S. Barandun. 1972. IgG subclasses: development of the serum concentration in “normal” infants and children. J. Pediatr. 80:960-964. [DOI] [PubMed] [Google Scholar]
- 10.Plummer, M., J. Vivas, J. L. Fauchère, G. Del Giudice, S. A. Peña, A. Ponzetto, G. Lopez, K. Miki, W. Oliver, and N. Muñoz. 2000. Helicobacter pylori and stomach cancer: a case control study in Venezuela. Cancer Epidemiol. Biomark. Prev. 9:961-965. [PubMed] [Google Scholar]
- 11.Pounder, R. E., and D. Ng. 1995. The prevalence of Helicobacter pylori infection in different countries. Alimen. Pharmacol. Ther. 9(Suppl. 2):33-39. [PubMed] [Google Scholar]
- 12.Rehnberg A.-S., C. Bengtsson, R. Befritz, M. Granström, and P. M. Hellström. 2001. Refinement of the 14C-urea breath test for detection of Helicobacter pylori. Scand. J. Gastroenterol. 36:822-826. [DOI] [PubMed] [Google Scholar]
- 13.Romero-Gallo, J., G. I. Pérez-Pérez, R. P. Novick, P. Kamath, T. Norbu, and M. J. Blaser. 2002. Responses of endoscopy patient in Ladakh, India, to Helicobacter pylori whole-cell and CagA antigens. Clin. Diagn. Lab. Immunol. 9:1313-1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sörberg, M., L. Engstrand, M. Ström, K.-Å. Jönsson, H. Jörbeck, and M. Granström. 1997. The diagnostic value of enzyme immunoassay and immunoblot for monitoring eradication of Helicobacter pylori. Scand. J. Infect. Dis. 29:147-151. [DOI] [PubMed] [Google Scholar]
- 15.Tindberg, Y., C. Bengtsson, M. Bergström, and M. Granström. 2000. The accuracy of serologic diagnosis of Helicobacter pylori in school-aged children of mixed ethnicity. Helicobacter 6:24-30. [DOI] [PubMed] [Google Scholar]
- 16.Tran, V., L. Ta, L. Bui, and M. Ha. 1993. H. pylori and gastritis and peptic ulcers (a histology study). Vietnam Military Med. 7:23-24. [Google Scholar]
- 17.Uemura, N., S. Okamoto, S. Yamamoto, N. Matsumura, S. Yamaguchi, M. Yamakido, K. Taniyama, N. Sasaki, and R. J. Schlemper. 2001. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 345:784-789. [DOI] [PubMed] [Google Scholar]
- 18.Wang, G., M. Z. Humayun, and D. E. Taylor. 1999. Mutation as an origin of genetic variability in Helicobacter pylori. Trends Microbiol. 7:488-493. [DOI] [PubMed] [Google Scholar]