Abstract
BRCA1 dysfunction in hereditary breast cancer causes defective homology-directed DNA repair and sensitivity towards DNA damaging agents like the clinically used topoisomerase I inhibitors topotecan and irinotecan. Using our conditional K14cre;Brca1F/F;p53F/F mouse model, we showed previously that BRCA1;p53-deficient mammary tumors initially respond to topotecan, but frequently acquire resistance by overexpression of the efflux transporter ABCG2. Here, we tested the pegylated SN38 compound EZN-2208 as a novel approach to treat BRCA1-mutated tumors that express ABCG2. We found that EZN-2208 therapy resulted in more pronounced and durable responses of ABCG2-positive tumors than topotecan or irinotecan therapy. We also evaluated tumor-specific ABCG2 inhibition by Ko143 in Abcg2−/− host animals that carried tumors with topotecan-induced ABCG2 expression. Addition of Ko143 moderately increased overall survival of these animals, but did not yield tumor responses like those seen after EZN-2208 therapy. Our results suggest that pegylation of Top1 inhibitors may be a useful strategy to circumvent efflux transporter-mediated resistance and to improve their efficacy in the clinic.
Introduction
Poisoning topoisomerase I (Top1) is a useful therapeutic approach to target cancer cells with intrinsic defects in the DNA damage response [1]. In particular the camptothecin derivatives topotecan and irinotecan are frequently used to target Top1 in ovarian, colon, and small cell lung cancer patients. Both topotecan and SN38, the active metabolite of irinotecan, stabilize Top1-DNA cleavage complexes (Top1cc), which are subsequently converted into DNA damage during DNA replication and transcription. The conversion of single stranded breaks (SSB) into double stranded breaks (DSB) during stalling of the replication machinery is the primary cytotoxic effect of Top1 poisons [2]. As a consequence, defects of tumor cells in proper repair of DSB provide an Achilles heel that can be targeted using Top1 inhibitors. An example is the increased topotecan sensitivity of cultured cells that are deficient in BRCA1 function [3], which is critical for error-free repair of DSB by homologous recombination (HR) [4].
We have previously studied topotecan responses in a genetically engineered mouse model for BRCA1-deficient breast cancer [5]. Despite high initial sensitivity, tumors were not eradicated and eventually all tumors acquired resistance to the maximum tolerable dose (MTD) of topotecan. About half of the tumors acquired resistance by overexpression of ABCG2 (or Breast Cancer Resistance Protein/BCRP). ABCG2 is an ATP-binding cassette (ABC) efflux transporter [6]–[8], and its overexpression in cultured cells was found to cause resistance to various clinically used anti-cancer drugs, such as topotecan and irinotecan [2], [9]. In our mouse model, we found that tumor-specific ablation of this efflux transporter substantially increased overall survival of tumor-bearing animals [5]. This observation unambiguously confirmed that induction of ABCG2 expression is an effective mechanism of mammary tumors to evade topotecan-induced DNA damage.
At present, useful in vivo strategies to reverse ABCG2-mediated drug resistance in patients are lacking. ABCB1/P-gp inhibitors like elacridar or tariquidar also inhibit ABCG2 to some extent [10], [11]. However, thus far the clinical benefit of these inhibitors is modest [12]–[14]. The mycotoxin fumitremorgin C (FTC) was identified as a more specific ABCG2 inhibitor [15], [16], but neurotoxicity compromised its clinical potential [17]. Nevertheless, less toxic FTC analogues were explored, and of these Ko143 was found to be the most potent and specific inhibitor, increasing oral topotecan availability in Abcb1a/b knockout mice 4–6-fold [17].
A complication of the systemic application of ABCG2 inhibitors is the fact that ABCG2 is expressed in normal tissues and protects them against xenotoxins [18]. In particular, ABCG2 contributes to the blood-brain barrier [19], and its expression in the liver, gut and kidney results in increased drug clearance. Hence, when combining an ABCG2 inhibitor with topotecan, it may be difficult to distinguish tumor-specific ABCG2 inhibition versus increased drug exposure due to reduced excretion. To be able to make this distinction, the use of ABCG2-deficient mice is helpful. ABCG2-proficient tumors can be grafted into syngeneic mice that lack ABCG2. This allows the analysis of tumor cell-specific effects of the inhibitor. We have shown that the spontaneous mammary tumors of our BRCA1 model can be transplanted orthotopically into syngeneic mice without loss of their genomic profile, morphology, or sensitivity to drug [5], [20], [21]. By transplanting ABCG2-proficient Brca1−/−;p53−/− mammary tumors derived from FVB/N mice into ABCG2-deficient hosts of the same strain, we show here that Ko143 is indeed useful for reversing ABCG2-mediated topotecan resistance in vivo. Nevertheless, the benefit is modest and Ko143 does not result in a clearly detectable increase of intratumoral topotecan concentration. As an alternative approach to overcome ABCG2-mediated resistance, we therefore tested a polyethylene glycol-conjugated (‘pegylated’) Top1 inhibitor EZN-2208, a SN38 conjugate. In xenografts EZN-2208 results in higher and longer-lasting exposure of tumors to the irinotecan metabolite SN38 compared with irinotecan itself [22]–[24]. In our model, we found that EZN-2208 results in durable responses of ABCG2-expressing BRCA1-deficient mammary tumors, in contrast to topotecan or irinotecan treatment.
Materials and Methods
Spontaneous Mammary Tumors, Orthotopic Transplantations, Recipient Animals and Drug Intervention Regimens
All animal experimental procedures have been conducted according to the standard operating procedures of the lab animal facility and were approved by the Animal Ethics Committee of The Netherlands Cancer Institute under references 08.001-B44, 08.001-B45, 11.006-B06 and 11.006-B10.
The generation, genotyping, orthotopic transplantation, daily animal weight and mammary tumor caliper measurements as well as the sampling of Brca1−/−;p53−/− mammary tumors were performed as described previously [5], [25]. Six- to eight-week-old FVB/N recipient animals were purchased from Harlan, while Abcg2−/− host animals of the same age and genetic background were bred within our lab animal facility [26]. The experimental outline of the Ko143 (or vehicle) + topotecan combination therapy interventions in Abcg2−/− tumor-bearing animals is described under the results (Fig. 1A). Intervention with topotecan, irinotecan and EZN-2208 in wildtype FVB/N tumor-bearing animals started when a tumor volume of about 200 mm3 was reached. Animals were either left untreated (control) or received 4 mg topotecan, 40 mg irinotecan or 10 mg EZN-2208 (SN38 equivalents) per kg body weight as a regimen of five consecutive i.v. injections on days 0, 2, 4, 6 and 8. When a tumor volume of about 1500 mm3 was reached, animals were killed by CO2 and tumor samples were harvested for further analyzes.
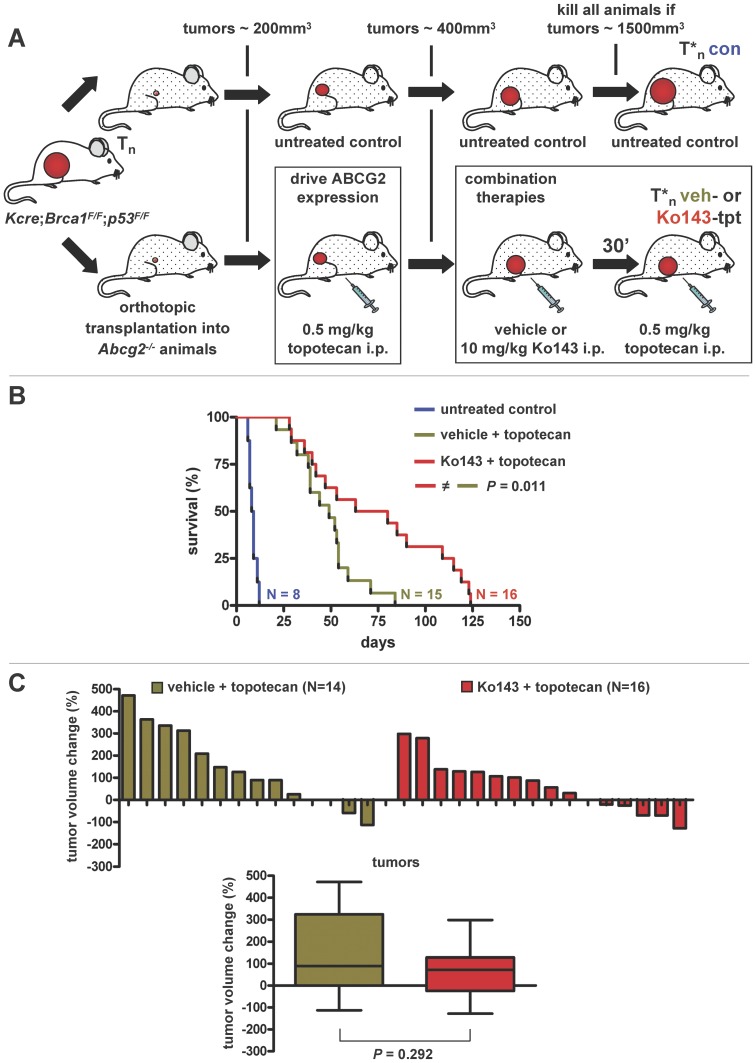
Figure 1. Efficacy of Ko143+ topotecan combination therapy in Brca1−/−;p53−/− mammary tumors.
A, Experimental outline. Spontaneous mammary tumors from K14cre;Brca1F/F;p53F/F females were orthotopically transplanted into six- to eight-week-old Abcg2−/− syngeneic recipients. When tumors reached a volume of about 200 mm3, animals were either left untreated (control) or injected i.p. with 0.5 mg topotecan per kg body weight on days 0–4 and 14–18 resulting in elevated ABCG2 expression. When tumors doubled in size (∼400 mm3), animals were injected i.p. with either vehicle +0.5 mg topotecan or 10 mg Ko143+0.5 mg topotecan combination therapy per kg body weight on days 0–4 and 14–18. If the tumor volume shrank to less than 50% of the start volume, treatments were stopped until tumors relapsed to 100%. The experiment was terminated when tumors reached a volume of about 1500 mm3. B, Kaplan-Meier (K-M) survival curves of untreated and combination therapy-treated tumor-bearing animals. Eight individual tumors were tested, and per tumor one untreated control (blue line), two vehicle + topotecan- (green line) and two Ko143+ topotecan-treated animals (red line) were included. For the vehicle + topotecan group one tumor did not grow out after transplantation. The P value was calculated using the Log-rank test. C, Waterfall plots (upper panel) showing tumor volume change (%) after 5 days of combination therapy per individual mouse, with relative volumes normalized to the treatment start volume (i.e. about 200 mm3). Box and whiskers plots (lower panel) summarizing the waterfall plot data. Lines represent the median response, while the whiskers show the maximum and minimum values. The P values was calculated using the Mann Whitney test.
Drug Injection Solutions
Ko143 was purchased from Tocris Bioscience (Minneapolis, MN, USA) and used by diluting 10 mg/mL DMSO stocks in 15% (w/v) 2-hydroxyl-propyl-β-cyclodextrine/PBS to a final volume of 1 mg/mL and administered at 10 µL/g of body weight by i.p. injection. Topotecan was provided by GlaxoSmithKline PLC (London, UK) and dissolved in 5% (w/v) glucose to yield a solution of 0.4 mg/mL (of active compound) and administered at 10 µL/g of body weight by i.p. injection. EZN-2208 was provided by ENZON pharmaceuticals Inc. (Piscataway, NJ, USA) and dissolved in saline to yield a solution of 1 mg/mL (SN38 equivalents) and administered at 10 µL/g of body weight by i.v. injection. Irinotecan was purchased from Pfizer Inc. (New York, NY, USA) and used by diluting 20 mg/mL stocks in saline to yield a solution of 4 mg/mL and administered at 10 µL/g of body weight by i.v. injection.
Immunohistochemical Analysis of ABCG2
Immunohistochemical stainings were performed as described previously [5]. Briefly, ABCG2 was probed with the rat anti-mouse monoclonal (BXP53) from Abcam (ab24115, 1∶400) and detected with a biotinylated rabbit anti-rat secondary antibody (Dakocytomation, E0468, 1∶100).
Collection of Pharmacokinetics Samples and Quantification of Topotecan Levels
While animals were under isoflurane anesthesia, whole blood was collected by cardiac puncture and transferred into heparinized tubes on ice. Next, the animals were killed by cervical dislocation and their tumors dissected. Blood was centrifuged at 4000 rpm and 4°C for 5 min to separate the plasma fraction. After thawing at 4°C, the mouse tumors were weighed and homogenized in 1% (w/v) BSA in water (equivalent to 500 mg tumor in 2.5 mL volume), using a FastPrep-24 high speed bench top homogenizer (MP-Biomedicals, Santa Ana, CA, USA) at 6.0 M/s for 30 s in 4.5 mL tubes. Homogenized tumor and plasma samples were stored at −20°C until sample pretreatment for reversed-phase high performance liquid chromatography (RP-HPLC) analysis. Total topotecan levels (lactone plus carboxylate form) were determined by using a validated RP-HPLC method, as described previously [27].
Results
Efficacy of Ko143+ Topotecan Combination Therapy in Abcg2−/− Tumor-bearing Animals
To study the effect of ABCG2 inhibition on topotecan efficacy in a tumor-specific fashion, we transplanted spontaneous ABCG2-proficient Brca1−/−;p53−/− mammary carcinomas [25], derived on a FVB/N background, orthotopically into ABCG2-deficient recipients [26] of the same mouse strain (Fig. 1A). Since ABCG2 expression in normal tissues is known to contribute to topotecan clearance [19], we had to lower the topotecan dose in Abcg2−/− mice substantially (Fig. S1A). In fact, the topotecan MTD of these animals was 4-fold lower than in wild-type animals (0.5 mg instead of 2 mg i.p. per kg body weight on days 0–4 and 14–18). The combination of this topotecan dose with 10 mg Ko143 i.p. per kg body weight on days 0–4 and 14–18 was still tolerable in mice that lack ABCG2 (Fig. S1B). This supports the notion that Ko143 is an ABCG2-specific inhibitor which does not cause serious off-target complications.
We then investigated the topotecan + Ko143 response of 8 randomly chosen ABCG2-proficient Brca1−/−;p53−/− mammary donor tumors in ABCG2-deficient hosts (Fig. 1A–C). When tumors reached a volume of 150–250 mm3 after transplantation, animals were either left untreated (control) or injected with topotecan until the tumor volume doubled to about 400 mm3 (Fig. 1A). This topotecan selection step was applied to drive ABCG2 expression in these naïve tumors following orthotopic transplantion, as we have reported previously [5]. As expected, there was a clear induction of ABCG2 in the topotecan-resistant tumors also in the donor tumors used for this study (Fig. 2 and Table S2). Once the tumors had doubled in size under topotecan selection, animals were either treated with vehicle + topotecan or Ko143+ topotecan combination therapy. Compared with vehicle + topotecan, Ko143+ topotecan combination therapy significantly (P = 0.022, Log-rank test) increased overall survival of the Abcg2−/− animals that carried Brca1−/−;p53−/− mammary tumors (Fig. 1B). Nevertheless, the median survival increased only from 52 to 71.5 days, showing that the benefit of adding Ko143 to topotecan therapy was rather modest. Indeed, when tumor volume changes after 5 days of therapy were plotted per individual mouse (Fig. 1C), no significant (P = 0.292, Mann Whitney test) increase in tumor shrinkage was found after addition of Ko143.
Figure 2. Topotecan response and ABCG2 immunoreactivity of eight individual Brca1−/−;p53−/− mammary tumors.
A, Relative tumor volume (%) of matched control (blue lines) and topotecan-treated (green lines) tumors over time. Each arrow indicates one dosing regimen of i.p. injections of 0.5 mg topotecan per kg body weight on days 0–4 and 14–18. B, Semi-quantification of ABCG2 immunoreactivity. Representative micrographs of four classes of stained tumor cells (0%, 1–10%, 11–50% and more than 50% of counted cells in 10 independent 400x magnification fields are ABCG2-positive) are shown. C, Table indicating ABCG2 immunoreactivity of the spontaneous (spon), untreated control (control) and topotecan-treated samples per individual tumor. P values of the spon - control (0.865) and spon - topotecan (0.020) comparisons were calculated using the Wilcoxon rank-sum test.
Ko143 and Topotecan Pharmacokinetics
To quantify the effect of tumor-specific ABCG2 inhibition on topotecan accumulation, we developed a reverse phase high-performance liquid chromatography (RP-HPLC) assay to determine Ko143 concentrations in plasma and tumor matrix, using FTC as an internal standard (Zander et al., submitted for publication). Ko143 was administered to Abcg2−/− tumor-bearing animals by i.p. injections of 15% (w/v) 2-hydroxyl-propyl-β-cyclodextrine/PBS solutions of concentrated Ko143 DMSO stocks. To validate that this formulation yields effective Ko143 plasma and tumor levels, we performed a time course experiment. This confirmed that Ko143 reaches transplanted mammary tumors after i.p. injection, as reported elsewhere (Zander et al., submitted for publication). In contrast to Abcb1a/b−/− animals, in which Ko143 treatment resulted in a 4-fold increase in plasma topotecan concentration [17], in our Abcg2−/− tumor-bearing animals topotecan pharmacokinetics (PK) did not significantly (P = 0.870, unpaired t-test) differ between vehicle + topotecan- (Fig. 3A, green line) and Ko143+ topotecan-treated animals (Fig. 3A, red line). This suggests that Ko143 does not block other ABC transporters which may alter topotecan PK. This result is also consistent with the absence of increased toxicity when topotecan is combined with Ko143 (Fig. S1B).
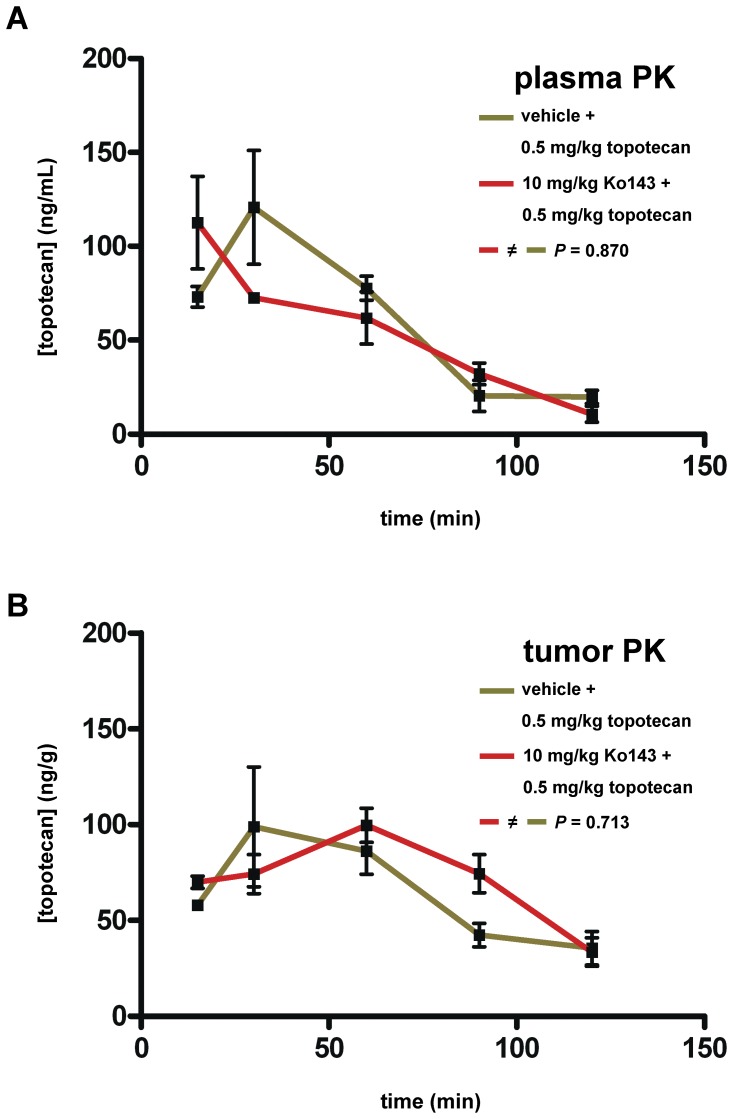
Figure 3. Plasma and tumor topotecan pharmacokinetics (PK).
A, Plasma topotecan PK. Abcg2−/− animals, carrying ABCG2-positive tumor T1 (Fig. 2C), were treated with either vehicle +0.5 mg topotecan (green line) or 10 mg Ko143+0.5 mg topotecan (red line) per kg body weight and plasma was collected at 15, 30, 60, 90 and 120 minutes following i.p. injection. Error bars indicate standard deviations of the mean of at least 3 animals per time point. B, Tumor topotecan PK. The tumors of the animals in A were harvested and homogenized to determine topotecan concentrations at the same time points. Error bars indicate standard deviations of the mean of at least 3 animals per time point. P values were calculated using the unpaired t-test.
Unexpectedly, when we measured topotecan pharmacokinetics in the ABCG2-positive tumor T1 (Fig. 2C) as described previously [27], we were unable to detect significant differences between the vehicle + topotecan-treated (Fig. 3B, green line) and Ko143+ topotecan-treated animals (Fig. 3B, red line) (P = 0.713, unpaired t-test). The short plasma half-life of Ko143 of about 1 hour (Zander et al., submitted for publication) may have contributed to this outcome. Most likely, the increase in topotecan levels within tumors after Ko143 treatment, which must have been responsible for the increased overall survival (Fig. 1B), was rather small. Hence, Ko143 treatment may not be the optimal strategy to increase intratumoral Top1 inhibitor levels.
EZN-2208 as Alternative Strategy to Enhance Tumor-specific Drug Accumulation and Reverse ABCG2-mediated Resistance
Given this limited success of the ABCG2 inhibitor Ko143, we tested EZN-2208, a pegylated form of the active irinotecan metabolite SN38 [28], to treat ABCG2-positive Brca1−/−;p53−/− mammary tumors. Like topotecan, the topoisomerase I inhibitor SN38 is an ABCG2 substrate [29], [30], and therefore drug-naïve tumors with ABCG2-positive nests of tumor cells should quickly acquire resistance during irinotecan treatment. We became interested in EZN-2208, since pegylation promotes tumor-specific drug accumulation by exploiting the phenomenon known as the enhanced permeability and retention effect of solid tumors [31]. Sustained SN38 release from EZN-2208 in the tumor stroma may counteract cellular ABCG2-mediated efflux and maintain a steep diffusion gradient towards the topoisomerase I target within the tumor cell nuclei. Such prolonged tumor-specific drug delivery may help to overcome ABCG2-mediated resistance.
For our analysis, we compared EZN-2208 versus topotecan or irinotecan efficacy in drug-naïve Brca1−/−;p53−/− mammary tumors that showed ABCG2 expression in tumor cell subpopulations. In several spontaneous tumors (T1, T3, T4, T7) we observed varying amounts of ABCG2-positive nests of tumor cells (Fig. 2B+C). Obviously, these cells were selected during topotecan treatment, since there is a clear enrichment of ABCG2-positive cells in the resistant tumors (Fig. 2C). Moreover, these tumors acquire topotecan resistance more rapidly (Fig. 2A). In addition to T1 and T7, we found three other spontaneous tumors (named here T9, T10 and T11) with more than 10% ABCG2-positive tumors cells in our panel of 28 Brca1−/−;p53−/− mammary tumors derived from FVB/N mice (Zander et al., submitted for publication). Importantly, ABCG2-positive cells were still present after orthotopic transplantation of all 5 donor tumors (Table S1).
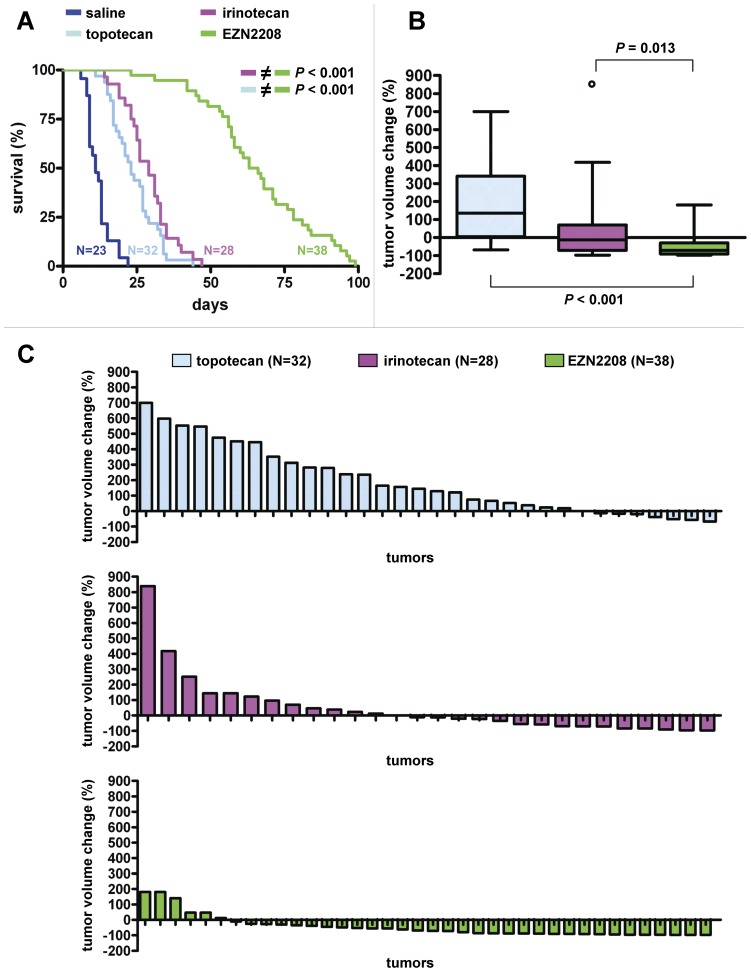
Before drug interventions were started using these tumors, we established the maximum tolerable EZN-2208 dose in our mouse model, guided by earlier studies in xenograft models [23]. When EZN-2208 was injected i.v. at 10 mg (SN38 equivalents) per kg body weight on days 0, 2, 4, 6 and 8, the weight loss was still acceptable (about 10%, Fig. S2A). The MTD of topotecan and irinotecan was 4 mg and 40 mg per kg body weight, respectively, using this regimen of five consecutive i.v. injections (Fig. S2B). These doses were then given to mice carrying the orthotopically transplanted ABCG2-positive Brca1−/−;p53−/− mammary tumors T1, T7, T9, T10 and T11 (Fig. 4). Compared with both the irinotecan- (pink line) and topotecan-treated tumor-bearing animals (light blue line), we found a dramatic increase in survival until a tumor volume of about 1500 mm3 was reached after EZN-2208 treatment (Fig. 4A, green line, P<0.001, Log-rank test). This result was found for all five ABCG2-positive tumors individually (Fig. S3). Moreover, when tumor volume changes after two weeks of treatment were compared (Fig. 4B+C), EZN-2208 outperformed both irinotecan (P = 0.013, Mann Whitney test) and topotecan (P<0.001, Mann Whitney test). This was still the case for topotecan, but not for irinotecan when tumor volume changes were analysed per individual ABCG2-positive tumor (Fig. S4). Together, these data show that the use of EZN-2208 is an effective strategy to target ABCG2-positive Brca1−/−;p53−/− mammary tumors.
Figure 4. Efficacy of topotecan, irinotecan and EZN-2208 in five ABCG2-positive Brca1−/−;p53−/− mammary tumors.
A, K–M curves showing survival (%) until a tumor volume of about 1500 mm3 was reached after one regimen of five consecutive i.v. injections on days 0, 2, 4, 6 and 8 of saline- (dark blue line, N = 23), topotecan- (light blue line, 4 mg i.v., N = 32), irinotecan- (pink line, 40 mg i.v., N = 28) and EZN-2208-treatments (green line, 10 mg SN38 equivalents i.v., N = 38) per kg body weight. P values were calculated using the Log-rank test. B, Box and whiskers plots indicating per drug the tumor volume change (%) after two weeks of treatment. Lines represent the median response, while the whiskers show the maximum and minimum values; the small circle is an outlier. P values were calculated using the Mann Whitney test. C, Waterfall plots showing tumor volume change (%) after two weeks of treatment per individual mouse, with relative volumes normalized to the treatment start volume.
Discussion
Using a genetically engineered mouse model for BRCA1-deficient breast cancer, we investigated therapeutic strategies to overcome ABCG2-mediated resistance to clinically used topoisomerase I inhibitors. We found that ABCG2-positive Brca1−/−;p53−/− mammary tumors are highly sensitive to EZN-2208, a pegylated SN38 compound. Compared with the MTD of irinotecan or topotecan, ABCG2-positive tumors shrank substantially in size and the time until relapse was greatly increased. Previously, EZN-2208 was also shown to be superior compared with irinotecan in xenograft models of neuroblastoma, B-cell non-Hodgkin’s lymphoma, breast, colon and pancreatic cancer [22]–[24]. Polyethylene glycol (PEG)-conjugation of the active irinotecan metabolite SN38 generates a water soluble camptothecin analogue [28] with enhanced pharmacokinetics profile as shown by Sapra et al. [23] in plasma and tumors of MX-1 breast cancer xenografted nude mice. Pegylation shields a conjugated compound from clearance by the liver reticulo-endothelial system and impairs glomerular filtration [32], [33], resulting in a considerably prolonged plasma half-life compared with irinotecan [23]. Our results suggest that EZN-2208 is capable of overcoming ABCG2-mediated drug resistance in vivo. This may be due to better drug delivery towards tumors by the enhanced permeability and retention (EPR) effect, as suggested previously [31], [34]. The EPR effect is based on the presence of fenestrated blood vessels in tumors, which allow pegylated molecules to enter the tissue. Lack of effective lymphatic drainage within tumors further increases local drug concentration. The poor penetration of pegylated drug into normal tissues may also explain the absence of increased toxicity despite higher and longer-lasting plasma levels [23]. Local esterase activity within the tumor cleaves off the bulky PEG moiety and releases SN38, which passes the plasma membrane and inhibits nuclear topoisomerase I. ABCG2 is apparently not a sufficiently effective defense mechanism against sustained SN38 delivery by EZN-2208. The PEG-liposome encapsulated formulation of the topoisomerase II inhibitor doxorubicin (Doxil or Caelyx) [35] also showed superior efficacy in preclinical mouse models compared with free doxorubicin [36], [37], and was also found to bypass P-glycoprotein-mediated drug efflux [38].
We show here that adding the specific ABCG2 inhibitor Ko143 to topotecan therapy increases the overall survival of animals carrying Brca1−/−;p53−/− mammary tumors. This is not unexpected, given our recent finding that the survival of topotecan-treated animals carrying ABCG2-deficient Brca1−/−;p53−/− mammary tumors is markedly increased over that of animals with ABCG2-proficient tumors. By using ABCG2-proficient tumors in ABCG2-deficient hosts, we ensured that the Ko143 effect was indeed tumor-specific and not due to drug clearance differences. Despite the increase in overall survival, the actual benefit of Ko143 was rather modest. Moreover, we failed to detect increased topotecan accumulation in these tumors. We have shown that dosing animals with 10 mg Ko143 per kg body weight resulted in tumor Ko143 levels of more than 250 ng/g for at least two hours following i.p. injection (Zander et al., submitted for publication). Such levels are well above the EC90 concentration of 25 nM as determined by Allen et al. [17] in tumor cell cultures with Abcg2 amplifications [17], [39], [40]. ABCG2 inhibition by Ko143 in the mouse tumors should therefore be effective during at least this period following administration. However, the differences in tumor topotecan levels were probably small and our RP-HPLC quantification method did not detect them, given the variation between individual animals.
Since the initial report of Ko143 in the literature, several new inhibitors of ABCG2 have been identified [41]. PZ-39, for example, seems to be an attractive candidate, not only inhibiting ABCG2, but also accelerating its lysosome-dependent degradation [42], [43]. This two-pronged action may disrupt tumor ABCG2 function in a more profound and longer-lasting way than treatment with Ko143. However, these newer ABCG2 inhibitors are still at an early stage of development and, in contrast to Ko143, toxicity data are lacking.
Since ABC transporters have been shown to play a critical role in chemoresistance of cultured tumor cells, it has been a long-standing goal to use inhibitors of these transporters as a strategy to overcome resistance in cancer patients [44]. However, successful clinical application of combination therapies has proven to be difficult, often only increasing toxicity of the chemotherapeutic agent without actually improving its efficacy [12], [13], [45]. Since ABC transporters are also involved in the disposition of most of these drugs, pharmacokinetic interactions may considerably complicate successful application of this therapy strategy in the clinic. To avoid intolerable toxicities, dosing of the cytostatic agents has often to be lowered in such combinations. Indeed, we also found that the topotecan MTD was 4-fold lower in Abcg2−/− tumor-bearing animals compared with wild-type animals.
Given these disadvantages, EZN-2208 may be a helpful alternative to the use of ABCG2 inhibitors in combination with topoisomerase inhibitors. Moreover, improved drug delivery to tumors without increased toxicity in other tissues is attractive in combination with other therapeutics that target BRCA-deficient cancer, such as the PARP inhibitor olaparib (AZD2281) [21]. Previously, we treated ABCG2-deficient mammary tumors with topotecan-olaparib combination therapy and observed improved tumor response compared with topotecan monotherapy [5]. However, the topotecan dose had to be lowered 8-fold in this combination, because of the intestinal toxicity. Indeed, dose-limiting haematological toxicity was also reported for patients with advanced solid tumors in phase I clinical trials of the topotecan-olaparib and -ABT-888 combinations [46], [47]. Another reason for dose reduction of irinotecan are glucuronidation-impairing UGT1A1 polymorphisms, such as the UGT1A1*28 genotype, which is known to increase the risk of diarrhea [48]. Mild intestinal toxicity was observed in phase I and II trails with EZN-2208, but this was not dose-limiting [49], [50]. Combination of olaparib with EZN-2208 may therefore be tolerated better, potentially achieving tumor eradication by dose escalation.
In summary, two different therapeutic strategies were tested to overcome ABCG2-mediated topotecan resistance in Brca1−/−;p53−/− mouse mammary tumors. Although tumor-specific ABCG2 inhibition by Ko143 resulted in improved overall survival of tumor-bearing animals, the benefit was rather modest. In contrast, ABCG2-expressing tumors are highly sensitive to EZN-2208, resulting in a substantial increase in survival. Application of this pegylated SN38 formulation may therefore be a useful clinical approach to bypass ABCG2-mediated resistance to conventional camptothecin analogues in cancers with defects in homology-directed DNA repair.
Supporting Information
Relative animal weights in response to topotecan mono- or Ko143+ topotecan combination therapy. A, Six- to eight-week-old Abcg2−/− females were injected i.p. with either 1 (red line), 0.75 (green line) or 0.5 mg topotecan (blue line) per kg body weight on days 0–4 and 14–18 (arrows) and weighed daily for 28 days. The average relative weight (%) of five animals per treatment is plotted and error bars indicate standard deviations. If weight loss approached 20%, animals were killed by CO2. B, Six- to eight-week-old Abcg2−/− females were injected i.p. with 10 mg Ko143 and 0.5 mg topotecan per kg body weight on days 0–4 and 14–18 (arrows). There was a 30 minute interval between Ko143 and topotecan i.p. injections. The average relative weight (%) of five animals is plotted and error bars indicate standard deviations.
(TIFF)
Relative animal weights in response to EZN-2208, irinotecan and topotecan therapy. A, Six- to eight-week-old FVB/N females were treated with one regimen of five consecutive i.v. injections on days 0, 2, 4, 6 and 8 of either saline (black line) or 15 mg (red line) and 10 mg (SN38 equivalents) EZN-2208 (green line) per kg body weight as indicated by the arrows and weighed daily for 28 days. The average relative weight (%) of five animals per treatment is plotted and error bars indicate standard deviations. If weight loss approached 20%, animals were killed by CO2. B, Six- to eight-week-old FVB/N females were treated with one regimen of five consecutive i.v. injections on days 0, 2, 4, 6 and 8 of either saline (black line) or 4 mg topotecan (light blue line) and 40 mg irinotecan (pink line) per kg body weight as indicated by the arrows. The average relative weight (%) of five animals per treatment is plotted and error bars indicate standard deviations.
(TIFF)
Survival of topotecan-, irinotecan- and EZN-2208-treated animals per individual ABCG2-positive donor tumor. K-M curves showing survival (%) until a tumor volume of about 1500 mm3 was reached after one regimen of five consecutive i.v. injections on days 0, 2, 4, 6 and 8 of saline- (dark blue lines), topotecan- (light blue lines, 4 mg i.v.), irinotecan- (pink line, 40 mg i.v.) and EZN-2208-treatments (green line, 10 mg SN38 equivalents i.v.) per kg body weight. The number of experimental animals per treatment are indicated next to each K-M curve. P values were calculated using the Log-rank test.
(TIFF)
Topotecan, irinotecan and EZN-2208 response per individual ABCG2-positive donor tumor. Waterfall plots showing tumor volume change (%) after two weeks of treatments per individual mouse, with relative volumes normalized to the treatment start volume. Box and whiskers plots summarizing the waterfall plot data. Lines represent the median response, while the whiskers show the maximum and minimum values; the small circles are outliers. P values were calculated using the Mann Whitney test.
(TIFF)
ABCG2 immunoreactivity of EZN-2208 intervention tumors. In addition to the two ABCG2-positive tumors of the Ko143+ topotecan intervention study (Fig. 2C, T1 and T7), three additional spontaneous (spon) Brca1−/−;p53−/− mammary tumors (T9, T10 and T11) were selected with high ABCG2 expression and orthotopically transplanted into wild-type recipients to test EZN-2208 efficacy. Semi-quantified ABCG2 immunoreactivity of the untreated controls is indicated per individual donor tumor.
(TIFF)
ABCG2 immunoreactivity of Ko143 intervention tumors. Semi-quantified ABCG2 immunoreactivity of the untreated controls, vehicle + topotecan- and Ko143+ topotecan-treated animals is indicated per individual donor tumor.
(TIF)
Acknowledgments
We thank Alfred H. Schinkel and Koen van de Wetering for critical reading of the manuscript. We are grateful to Ariena Kersbergen and Guotai Xu for their help with the EZN-2208 injections.
Funding Statement
This work was supported by grants from the Dutch Cancer Society (www.dcs.kwfkankerbestrijding.nl) NKI 2009-4303 to SR, JJ, PB, NKI 2011-5220 to SR, JJ, the European Union FP6 Integrated Project 037665-CHEMORES (www.chemores.org) to PB, SR and TI Center for Translational Molecular Medicine (CTMM Breast Care) (www.ctmm.nl) to JJ, SR. SR is supported by The Netherlands Organisation for Scientific Research NWO VIDI-91711302 (www.nwo.nl). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Pommier Y, Leo E, Zhang H, Marchand C (2010) DNA topoisomerases and their poisoning by anticancer and antibacterial drugs. Chem Biol 17: 421–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pommier Y (2006) Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer 6: 789–802. [DOI] [PubMed] [Google Scholar]
- 3. Fedier A, Steiner RA, Schwarz VA, Lenherr L, Haller U, et al. (2003) The effect of loss of Brca1 on the sensitivity to anticancer agents in p53-deficient cells. Int J Oncol 22: 1169–1173. [PubMed] [Google Scholar]
- 4. Lord CJ, Ashworth A (2012) The DNA damage response and cancer therapy. Nature 481: 287–294. [DOI] [PubMed] [Google Scholar]
- 5. Zander SAL, Kersbergen A, van der Burg E, de Water N, van Tellingen O, et al. (2010) Sensitivity and acquired resistance of BRCA1;p53-deficient mouse mammary tumors to the topoisomerase I inhibitor topotecan. Cancer Res 70: 1700–1710. [DOI] [PubMed] [Google Scholar]
- 6. Allikmets R, Schriml LM, Hutchinson A, Romano-Spica V, Dean M (1998) A human placenta-specific ATP-binding cassette gene (ABCP) on chromosome 4q22 that is involved in multidrug resistance. Cancer Res 58: 5337–5339. [PubMed] [Google Scholar]
- 7. Doyle LA, Yang W, Abruzzo LV, Krogmann T, Gao Y, et al. (1998) A multidrug resistance transporter from human MCF-7 breast cancer cells. Proc Natl Acad Sci U S A 95: 15665–15670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Miyake K, Mickley L, Litman T, Zhan Z, Robey R, et al. (1999) Molecular cloning of cDNAs which are highly overexpressed in mitoxantrone-resistant cells: demonstration of homology to ABC transport genes. Cancer Res 59: 8–13. [PubMed] [Google Scholar]
- 9. Beretta GL, Perego P, Zunino F (2006) Mechanisms of cellular resistance to camptothecins. Curr Med Chem 13: 3291–3305. [DOI] [PubMed] [Google Scholar]
- 10. de Bruin M, Miyake K, Litman T, Robey R, Bates SE (1999) Reversal of resistance by GF120918 in cell lines expressing the ABC half-transporter, MXR. Cancer Lett 146: 117–126. [DOI] [PubMed] [Google Scholar]
- 11. Robey RW, Steadman K, Polgar O, Morisaki K, Blayney M, et al. (2004) Pheophorbide a is a specific probe for ABCG2 function and inhibition. Cancer Res 64: 1242–1246. [DOI] [PubMed] [Google Scholar]
- 12. Robey RW, Massey PR, Amiri-Kordestani L, Bates SE (2010) ABC transporters: unvalidated therapeutic targets in cancer and the CNS. Anticancer Agents Med Chem 10: 625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Robey RW, Ierano C, Zhan Z, Bates SE (2011) The challenge of exploiting ABCG2 in the clinic. Curr Pharm Biotechnol 12: 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rottenberg S, Borst P (2012) Drug resistance in the mouse cancer clinic. Drug Resist Updat 15: 81–89. [DOI] [PubMed] [Google Scholar]
- 15. Rabindran SK, He H, Singh M, Brown E, Collins KI, et al. (1998) Reversal of a novel multidrug resistance mechanism in human colon carcinoma cells by fumitremorgin C. Cancer Res. 58: 5850–5858. [PubMed] [Google Scholar]
- 16. Rabindran SK, Ross DD, Doyle LA, Yang W, Greenberger LM (2000) Fumitremorgin C reverses multidrug resistance in cells transfected with the breast cancer resistance protein. Cancer Res 60: 47–50. [PubMed] [Google Scholar]
- 17. Allen JD, van Loevezijn A, Lakhai JM, van der Valk M, van Tellingen O, et al. (2002) Potent and specific inhibition of the breast cancer resistance protein multidrug transporter in vitro and in mouse intestine by a novel analogue of fumitremorgin C. Mol Cancer Ther. 1: 417–425. [PubMed] [Google Scholar]
- 18. Vlaming ML, Lagas JS, Schinkel AH (2009) Physiological and pharmacological roles of ABCG2 (BCRP): recent findings in Abcg2 knockout mice. Adv Drug Deliv Rev 61: 14–25. [DOI] [PubMed] [Google Scholar]
- 19. de Vries NA, Zhao J, Kroon E, Buckle T, Beijnen JH, et al. (2007) P-glycoprotein and breast cancer resistance protein: two dominant transporters working together in limiting the brain penetration of topotecan. Clin Cancer Res 13: 6440–6449. [DOI] [PubMed] [Google Scholar]
- 20. Rottenberg S, Nygren AO, Pajic M, van Leeuwen FW, van der Heijden I, et al. (2007) Selective induction of chemotherapy resistance of mammary tumors in a conditional mouse model for hereditary breast cancer. Proc Natl Acad Sci U S A 104: 12117–12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rottenberg S, Jaspers JE, Kersbergen A, van der Burg E, Nygren AOH, et al. (2008) High sensitivity of BRCA1-deficient mammary tumors to the PARP inhibitor AZD2281 alone and in combination with platinum drugs. Proc Natl Acad Sci U S A 105: 17079–17084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pastorino F, Loi M, Sapra P, Becherini P, Cilli M, et al. (2010) Tumor regression and curability of preclinical neuroblastoma models by PEGylated SN38 (EZN-2208), a novel topoisomerase I inhibitor. Clin Cancer Res 16: 4809–4821. [DOI] [PubMed] [Google Scholar]
- 23. Sapra P, Zhao H, Mehlig M, Malaby J, Kraft P, et al. (2008) Novel delivery of SN38 markedly inhibits tumor growth in xenografts, including a camptothecin-11-refractory model. Clin Cancer Res 14: 1888–1896. [DOI] [PubMed] [Google Scholar]
- 24. Sapra P, Kraft P, Mehlig M, Malaby J, Zhao H, et al. (2009) Marked therapeutic efficacy of a novel polyethylene glycol-SN38 conjugate, EZN-2208, in xenograft models of B-cell non-Hodgkin’s lymphoma. Haematologica 94: 1456–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu X, Holstege H, van der Gulden H, Treur-Mulder M, Zevenhoven J, et al. (2007) Somatic loss of BRCA1 and p53 in mice induces mammary tumors with features of human BRCA1-mutated basal-like breast cancer. Proc Natl Acad Sci U S A 104: 12111–12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jonker JW, Buitelaar M, Wagenaar E, van der Valk MA, Scheffer GL, et al. (2002) The breast cancer resistance protein protects against a major chlorophyll-derived dietary phototoxin and protoporphyria. Proc Natl Acad Sci U S A 99: 15649–15654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Vries NA, Ouwehand M, Buckle T, Beijnen JH, van Tellingen O (2007) Determination of topotecan in human and mouse plasma and in mouse tissue homogenates by reversed-phase high-performance liquid chromatography. Biomed Chromatogr 21: 1191–1200. [DOI] [PubMed] [Google Scholar]
- 28. Zhao H, Rubio B, Sapra P, Wu D, Reddy P, et al. (2008) Novel prodrugs of SN38 using multiarm poly(ethylene glycol) linkers. Bioconjug Chem 19: 849–859. [DOI] [PubMed] [Google Scholar]
- 29. Kawabata S, Oka M, Shiozawa K, Tsukamoto K, Nakatomi K, et al. (2001) Breast cancer resistance protein directly confers SN-38 resistance of lung cancer cells. Biochem Biophys Res Commun 280: 1216–1223. [DOI] [PubMed] [Google Scholar]
- 30. Kawabata S, Oka M, Soda H, Shiozawa K, Nakatomi K, et al. (2003) Expression and functional analyses of breast cancer resistance protein in lung cancer. Clin Cancer Res 9: 3052–3057. [PubMed] [Google Scholar]
- 31. Fang J, Nakamura H, Maeda H (2011) The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev 63: 136–151. [DOI] [PubMed] [Google Scholar]
- 32. Caliceti P, Veronese FM (2003) Pharmacokinetic and biodistribution properties of poly(ethylene glycol)-protein conjugates. Adv Drug Deliv Rev 55: 1261–1277. [DOI] [PubMed] [Google Scholar]
- 33. Harris JM, Chess RB (2003) Effect of pegylation on pharmaceuticals. Nat Rev Drug Discov 2: 214–221. [DOI] [PubMed] [Google Scholar]
- 34. Maeda H, Wu J, Sawa T, Matsumura Y, Hori K (2000) Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release 65: 271–284. [DOI] [PubMed] [Google Scholar]
- 35. Gabizon A, Shmeeda H, Grenader T (2012) Pharmacological basis of pegylated liposomal doxorubicin: impact on cancer therapy. Eur J Pharm Sci 45: 388–398. [DOI] [PubMed] [Google Scholar]
- 36. Gabizon A, Tzemach D, Mak L, Bronstein M, Horowitz AT (2002) Dose dependency of pharmacokinetics and therapeutic efficacy of pegylated liposomal doxorubicin (DOXIL) in murine models. J Drug Target 10: 539–548. [DOI] [PubMed] [Google Scholar]
- 37. Gabizon A, Tzemach D, Gorin J, Mak L, Amitay Y, et al. (2010) Improved therapeutic activity of folate-targeted liposomal doxorubicin in folate receptor-expressing tumor models. Cancer Chemother Pharmacol 66: 43–52. [DOI] [PubMed] [Google Scholar]
- 38. Goren D, Horowitz AT, Tzemach D, Tarshish M, Zalipsky S, et al. (2000) Nuclear delivery of doxorubicin via folate-targeted liposomes with bypass of multidrug-resistance efflux pump. Clin Cancer Res 6: 1949–1957. [PubMed] [Google Scholar]
- 39. Allen JD, Brinkhuis RF, Wijnholds J, Schinkel AH (1999) The mouse Bcrp1/Mxr/Abcp gene: amplification and overexpression in cell lines selected for resistance to topotecan, mitoxantrone, or doxorubicin. Cancer Res 59: 4237–4241. [PubMed] [Google Scholar]
- 40. Maliepaard M, van Gastelen MA, de Jong LA, Pluim D, van Waardenburg RC, et al. (1999) Overexpression of the BCRP/MXR/ABCP gene in a topotecan-selected ovarian tumor cell line. Cancer Res 59: 4559–4563. [PubMed] [Google Scholar]
- 41. Henrich CJ, Robey RW, Bokesch HR, Bates SE, Shukla S, et al. (2007) New inhibitors of ABCG2 identified by high-throughput screening. Mol Cancer Ther 6: 3271–3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Peng H, Dong Z, Qi J, Yang Y, Liu Y, et al. (2009) A novel two mode-acting inhibitor of ABCG2-mediated multidrug transport and resistance in cancer chemotherapy. PLoS One 4: e5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Peng H, Qi J, Dong Z, Zhang JT (2010) Dynamic vs static ABCG2 inhibitors to sensitize drug resistant cancer cells. PLoS One 5: e15276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Amiri-Kordestani L, Basseville A, Kurdziel K, Fojo AT, Bates SE (2012) Targeting MDR in breast and lung cancer: Discriminating its potential importance from the failure of drug resistance reversal studies. Drug Resist Updat 15: 50–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Shukla S, Wu CP, Ambudkar SV (2008) Development of inhibitors of ATP-binding cassette drug transporters: present status and challenges. Expert Opin Drug Metab Toxicol 4: 205–223. [DOI] [PubMed] [Google Scholar]
- 46. Kummar S, Chen A, Ji J, Zhang Y, Reid JM, et al. (2011) Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas. Cancer Res 71: 5626–5634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samol J, Ranson M, Scott E, Macpherson E, Carmichael J, et al. (2011) Safety and tolerability of the poly(ADP-ribose) polymerase (PARP) inhibitor, olaparib (AZD2281) in combination with topotecan for the treatment of patients with advanced solid tumors: a phase I study. Invest New Drugs. [DOI] [PubMed]
- 48. Ando Y, Saka H, Ando M, Sawa T, Muro K, et al. (2000) Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res 60: 6921–6926. [PubMed] [Google Scholar]
- 49.Kurzrock R, Wheler J, Hong DS, Guo Z, Mulcahy MF, et al. (2009) Phase 1, first-in-human, dose-escalation study of EZN-2208, a novel anticancer agent, in patients with advanced malignancies. Mol Cancer Ther 8(12 Suppl): Abstract C216.
- 50.Di Fiore F, Van Cutsem E, Laurent-Puig P, Siena S, Frattini F, et al. (2008) Role of K-RAS mutation in predicting response, progression-free survival, and overall survival in irinotecan-refractory patients treated with cetuximab plus irinotecan for a metastatic colon cancer: analysis of 281 individual data from published series. J Clin Oncol (ASCO Annual Meeting Abstracts) 26(May 20 suppl): Abstract 4035.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Relative animal weights in response to topotecan mono- or Ko143+ topotecan combination therapy. A, Six- to eight-week-old Abcg2−/− females were injected i.p. with either 1 (red line), 0.75 (green line) or 0.5 mg topotecan (blue line) per kg body weight on days 0–4 and 14–18 (arrows) and weighed daily for 28 days. The average relative weight (%) of five animals per treatment is plotted and error bars indicate standard deviations. If weight loss approached 20%, animals were killed by CO2. B, Six- to eight-week-old Abcg2−/− females were injected i.p. with 10 mg Ko143 and 0.5 mg topotecan per kg body weight on days 0–4 and 14–18 (arrows). There was a 30 minute interval between Ko143 and topotecan i.p. injections. The average relative weight (%) of five animals is plotted and error bars indicate standard deviations.
(TIFF)
Relative animal weights in response to EZN-2208, irinotecan and topotecan therapy. A, Six- to eight-week-old FVB/N females were treated with one regimen of five consecutive i.v. injections on days 0, 2, 4, 6 and 8 of either saline (black line) or 15 mg (red line) and 10 mg (SN38 equivalents) EZN-2208 (green line) per kg body weight as indicated by the arrows and weighed daily for 28 days. The average relative weight (%) of five animals per treatment is plotted and error bars indicate standard deviations. If weight loss approached 20%, animals were killed by CO2. B, Six- to eight-week-old FVB/N females were treated with one regimen of five consecutive i.v. injections on days 0, 2, 4, 6 and 8 of either saline (black line) or 4 mg topotecan (light blue line) and 40 mg irinotecan (pink line) per kg body weight as indicated by the arrows. The average relative weight (%) of five animals per treatment is plotted and error bars indicate standard deviations.
(TIFF)
Survival of topotecan-, irinotecan- and EZN-2208-treated animals per individual ABCG2-positive donor tumor. K-M curves showing survival (%) until a tumor volume of about 1500 mm3 was reached after one regimen of five consecutive i.v. injections on days 0, 2, 4, 6 and 8 of saline- (dark blue lines), topotecan- (light blue lines, 4 mg i.v.), irinotecan- (pink line, 40 mg i.v.) and EZN-2208-treatments (green line, 10 mg SN38 equivalents i.v.) per kg body weight. The number of experimental animals per treatment are indicated next to each K-M curve. P values were calculated using the Log-rank test.
(TIFF)
Topotecan, irinotecan and EZN-2208 response per individual ABCG2-positive donor tumor. Waterfall plots showing tumor volume change (%) after two weeks of treatments per individual mouse, with relative volumes normalized to the treatment start volume. Box and whiskers plots summarizing the waterfall plot data. Lines represent the median response, while the whiskers show the maximum and minimum values; the small circles are outliers. P values were calculated using the Mann Whitney test.
(TIFF)
ABCG2 immunoreactivity of EZN-2208 intervention tumors. In addition to the two ABCG2-positive tumors of the Ko143+ topotecan intervention study (Fig. 2C, T1 and T7), three additional spontaneous (spon) Brca1−/−;p53−/− mammary tumors (T9, T10 and T11) were selected with high ABCG2 expression and orthotopically transplanted into wild-type recipients to test EZN-2208 efficacy. Semi-quantified ABCG2 immunoreactivity of the untreated controls is indicated per individual donor tumor.
(TIFF)
ABCG2 immunoreactivity of Ko143 intervention tumors. Semi-quantified ABCG2 immunoreactivity of the untreated controls, vehicle + topotecan- and Ko143+ topotecan-treated animals is indicated per individual donor tumor.
(TIF)