Abstract
In order to evaluate the capacity of laser scanning cytometry (LSC) to detect acid-fast bacilli directly on clinical samples, a comparison between Kinyoun-stained smears analyzed under light microscopy and propidium iodide-auramine-stained smears analyzed by LSC was performed. The results were compared with those for culture on BACTEC MGIT 960. LSC is a new, reliable methodology to detect Mycobacteria.
The worldwide incidence of tuberculosis increased dramatically during the last decade, particularly in Southeast Asia and Africa, mainly in association with the progression of infection by human immunodeficiency virus (5, 12). Rapid and accurate detection of Mycobacteria is essential for early treatment and reduction of transmission. In low-resource countries, the detection of acid-fast bacteria relies upon direct microscopy of smears. Classical direct detection methods are easy to perform and inexpensive but present low sensitivity. An additional drawback is the need for a considerable amount of time for the observation of smears. The long delays inherent in cultural methods also contribute directly to the spread of tuberculosis in hospitals and other care facilities and may have a negative impact on the care of individual patients. Flow cytometry shows great potential for application to clinical microbiology (1, 8, 9, 10), but analyzed cells must be suspended in a fluid at a relatively high concentration. Laser scanning cytometry (LSC) is a microscope-based cytofluorometer technology that combines the advantages of both flow and image cytometry (6). It allows multiparametric analysis performed directly on a smear, rapidly measuring the fluorescence of individual cells with an extremely high accuracy (2). This technique may offer increased sensitivity and specificity compared to traditional microscopic techniques. To our knowledge, this paper describes the first use of LSC in clinical microbiology.
Five hundred smears were prepared in duplicate on glass slides from concentrates of N-acetyl-l-cystein-NaOH and specimens (7) of different clinical products (sputum, bronchoalveolar lavage fluid, stool, urine, cerebrospinal fluid, pleural fluid, and gastric lavage fluid) from patients with suspected cases of Mycobacterium infection. The smears were heat fixed, and one set was stained according to the Kinyoun procedure (3). The other set of smears was stained according to our own modification of the classical auramine-staining procedure (11), involving a previous staining with propidium iodide (PI) (1 μg/ml in 30 min; Sigma, St. Louis, Mo.) and rinsing with deionized water followed by staining with auramine (Auramine O and potassium permanganate from Fisher Scientific, Pittsburgh, Pa.) for 30 min (PI-Auramine O). Positive-control smears of acid-fast bacilli (AFB) were used each time a set of smears was stained. The slides stained by the Kinyoun method were analyzed under light bright-field microscopy (×1,000; Leica Laborlux K); three hundred microscope oil fields were observed before a smear was reported as negative for AFB (13). The slides stained with PI-Auramine O were maintained in the dark until LSC analysis. A laser scanning cytometer (LSC 101; Olympus Co., Tokyo, Japan) equipped with a BX50 Olympus microscope and a 488-nm argon laser was used. The scanning was performed under a magnification of ×200, and morphology was confirmed at ×600 without immersion. The cells were randomly selected (gated) based on their position on the slide map with the coordinates x for green fluorescence versus y for the perimeter of the cell. The results were given as positive (Fig. 1) or negative (Fig. 2) for AFB. Before being reported as negative, the entire smear was scanned; one or two AFB were considered negative. Green fluorescent debris was rarely seen and could easily be differentiated from AFB at ×600 magnification by visual inspection.
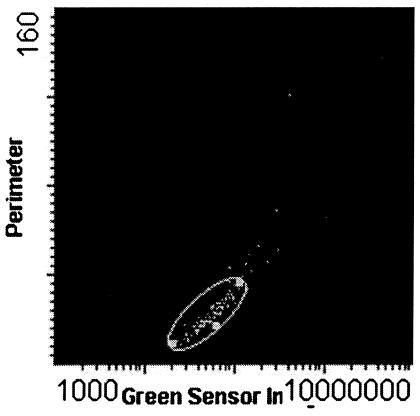
FIG. 1.
Laser-scanned image of a positive smear stained by the PI-Auramine O method. The slide map shows perimeter on the y axis and green fluorescence on the x axis.
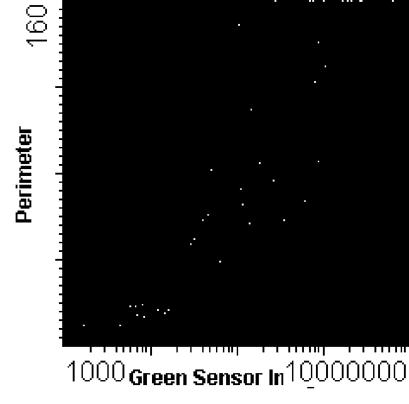
FIG. 2.
Laser-scanned image of a negative smear stained by the PI-Auramine O method. The slide map shows perimeter on the y axis and green fluorescence on the x axis.
The results of both methodologies were afterwards compared with the results of culture performed on a Bactec 960 system (“gold standard”) using χ2 analysis. The isolates were identified with Accuprobe (Gen-Probe Inc.) (4). Culture results yielded M. tuberculosis complex in 95.8% of cases, M. intracellulare in 1% of cases, M. avium complex in 3% of cases, and M. gordonae in 0.2% of cases. The numbers of false-positive and false-negative results, sensitivity, specificity, and the positive and negative predictive values of the newly described method are shown in Table 1. The results by specimen site are detailed in Table 2. For all biological specimens, the new methodology resulted in increased diagnostic sensitivity. LSC was considerably faster in performing the analysis and didn't produce false-negative results. In contrast to other heat-fixed bacterial or host cells, AFB do not stain with PI (unpublished results), probably due to the lipid content of the cell wall, thus staining green with Auramine O. Bacteria other than AFB do not stain with Auramine O because, when this stain is added, the cells are already red-stained by PI. The red PI color vanishes during the washing step of auramine staining. Thus, an increase in specificity in detection of AFB was achieved. This new approach also showed a greater sensitivity than the Kinyoun method, and no false-positive results were registered. Apart from being automated, this method allows the possibility of reanalysis of the smear (also visually), either at the moment of scanning or later on, due to the inherently sophisticated software (Winsyte 3.3). The intensity of fluorescence of the AFB stained by PI-Auramine O remained stable over several days (up to 5 days), providing the stained smears were kept protected from light.
TABLE 1.
Results for 500 clinical specimens either stained by the Kinyoun method and analyzed by light microscopy or stained by the PI-Auramine O method and analyzed by LSC versus results for culture
| Method | Positive (%) | Negative (%) | False- posi- tive (%) | False- nega- tive (%) | % Sensi- tivity | % Speci- ficity | % Posi- tive predic- tive value | % Nega- tive predic- tive value |
|---|---|---|---|---|---|---|---|---|
| Kinyoun/LMa | 198 (39.6) | 302 (60.4) | 0 | 46 (9.2) | 81.1 | 84.7 | ||
| PI-Auramine O/LSCb | 248 (49.6) | 252 (50.4) | 4 (0.8) | 0 | 98.4 | 98.4 | ||
| Culture | 244 (48.8) | 256 (51.2) |
LM, light microscopy.
LSC, laser scanning cytometry.
TABLE 2.
Results by specimen site for the presence of alcohol- and acid-resistant bacilli on Kinyoun-stained smears analyzed by light microscopy or smears stained by PI-Auramine O analyzed by LSC
| Specimen | No. of specimens | No. (%) positive by:
|
|
|---|---|---|---|
| Kinyoun staining | PI-Auramine O staining | ||
| Sputum | 210 | 90 (42.86) | 115 (54.76) |
| Bronchoalveolar lavage fluid | 125 | 67 (53.6) | 75 (60) |
| Stool | 40 | 10 (25) | 10 (25) |
| Urine | 20 | 2 (10) | 5 (25) |
| Cerebrospinal fluid | 20 | 3 (15) | 5 (25) |
| Gastric lavage fluid | 65 | 18 (27.69) | 28 (43.08) |
| Pleural fluid | 20 | 8 (40) | 10 (50) |
Previous studies have shown aspects of the great potentiality of flow cytometry when applied to microbiology (8, 9, 10). In the present paper, we have explored the potential of a different cytometer, developed by Kamentsky and Kamentsky (6). Our results indicate that LSC/PI-Auramine O is an advantageous automated method for evaluating the presence of Mycobacteria in clinical samples. While the analysis of Kinyoun-stained smears is too observer dependent and time-consuming, this new staining method combined with LSC analysis is an observer-independent method that improves diagnostic sensitivity and specificity and saves a considerable amount of time.
REFERENCES
- 1.Ávarez-Barrientos, J. A., R. Cantón, C. Nombela, and M. Sánchez-Pérez. 2000. Applications of flow cytometry to clinical microbiology. Clin. Microbiol. Rev. 13:167-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bedner, E., M. R. Melamed, and Z. Darzynkiewicz. 1998. Enzyme kinetic reactions and fluorochrome uptake rates measured in individual cells by laser scanning cytometer. Cytometry 33:1-9. [DOI] [PubMed] [Google Scholar]
- 3.Bishop, P. J., and G. Neumann. 1970. The history of the Ziehl-Neelsen stain. Tubercle 51:196-206. [DOI] [PubMed] [Google Scholar]
- 4.Drobniewski, F. A., M. Caws, A. Gibson, and D. Young. 2003. Modern laboratory diagnosis of tuberculosis. Lancet Infect. Dis. 3:141-147. [DOI] [PubMed] [Google Scholar]
- 5.Hayward, A. C., T. Darton, J. N. Van-Tam, J. M. Watson, R. Coker, and V. Schwoebel. 2003. Epidemiology and control of tuberculosis in Western European cities. Int. J. Tuberc. Lung Dis. 7:751-757. [PubMed] [Google Scholar]
- 6.Kamentsky, L. A., and L. D. Kamentsky. 1991. Microscope-based multiparameter laser scanning cytometer yielding data comparable in flow cytometry. Cytometry 12:381-387. [DOI] [PubMed] [Google Scholar]
- 7.Pfyffer, G. E., B. A. Brown-Elliot, and R. J. Wallace, Jr. 2003. Mycobacterium: general characteristics, isolation, and staining procedures, p. 532-559. In P. A. Murray (ed.), Manual of clinical microbiology, 8th ed. American Society for Microbiology, Washington, D.C.
- 8.Pina-Vaz, C., F. Sansonetty, A. G. Rodrigues, S. Costa-de-Oliveira, J. Martinez-de-Oliveira, and A. F. Fonseca. 2001. Cytometric approach for a rapid evaluation of susceptibility of Candida strains to antifungals. J. Clin. Microbiol. Infect. 7:609-618. [DOI] [PubMed] [Google Scholar]
- 9.Pina-Vaz, C., F. Sansonetty, A. G. Rodrigues, J. Martinez-de-Oliveira, A. F. Fonseca, and P.-A. Mårdh. 2000. Antifungal activity of ibuprofen alone andin combination with fluconazole against Candida species. J. Med. Microbiol. 49:831-840. [DOI] [PubMed] [Google Scholar]
- 10.Pina-Vaz, C., A. G. Rodrigues, F. Sansonetty, J. Martinez-de-Oliveira, A. F. Fonseca, and P.-A. Mårdh. 2000. Antifungal activity of local anesthetics against Candida species. Infect. Dis. Obstet. Gynecol. 8:124-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Richards, O. W., E. W. Kline, and R. E. Leach. 1941. Demonstration of tubercle bacilli by fluorescence microscopy. Am. Rev. Tuberc. 44:255-266. [Google Scholar]
- 12.Schurmann, D., S. D. Nightingale, F. Bergmann, and B. Ruf. 1997. Tuberculosis and HIV infection: a review. Infection 25:274-280. [DOI] [PubMed] [Google Scholar]
- 13.Somoskovi, Á., E. Jacquelin, B. S. Hotaling, M. Fitzgerald, D. O'Donnell, L. M. Parsons, and M. Salfinger. 2001. Lessons from a proficiency testing event for acid-fast microscopy. Chest 120:250-257. [DOI] [PMC free article] [PubMed] [Google Scholar]