Abstract
A general strategy was developed for the diastereo- and enantioselective synthesis of cyclobutanes with four different substituents. It consists of three transition metal-catalyzed reactions — a RhII-catalyzed cyclopropanation, a AgI-catalyzed regioselective and stereospecific ring expansion, and a RhI-catalyzed addition reaction. Structures of pipercyclobutanamide A and piperchabamide G were synthesized and revised.
Keywords: cyclobutane, pipercyclobutanamide, piperchabamide, chabamide, nigramide
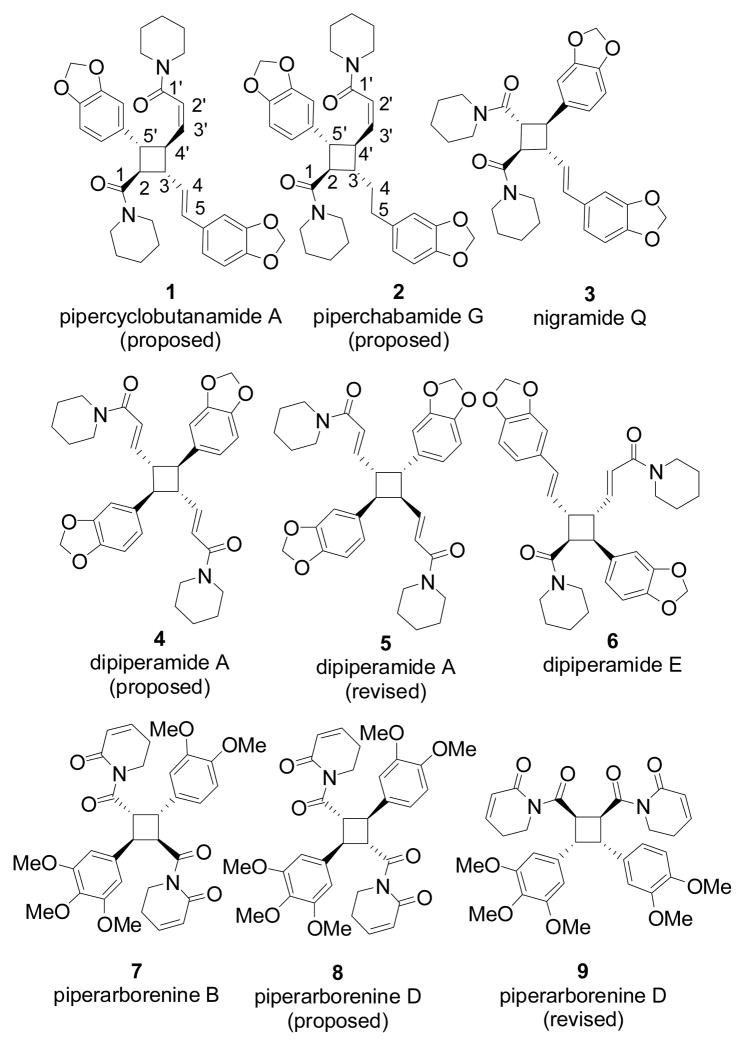
Four-membered rings are important structural motifs frequently present in bioactive natural products and pharmaceutical agents.[1] Pipercyclobutanamides, piperchabamides, nigramides, and dipiperamides (Figure 1) are tetrasubstituted cyclobutanes isolated from Piper nigrum and Piper chaba, the source of white and black pepper and component of traditional medicine.[2–7] Isolation of various polyene precursors and stereoisomeric cyclobutanes from these Pipers suggested that the four-membered rings might be derived from non-selective photolytic [2+2] cycloaddition of alkenes. Cyclobutanes isolated from Piper nigrum and Piper chaba have broad pharmacological activities.[5–7] For example, pipercyclobutanamide A (1) and dipiperamide E (6) are selective inhibitors for CYP2D6 and CYP3A4 respectively, two main P450s responsible for drug metabolism.[4, 7] Piperchabamide G, isolated in 2009, inhibits D-GalN/tumor necrosis factor-α-induced death of hepatocytes and has hepatoprotective effect.[6]
Figure 1.
Selected Four-membered Ring Natural Products
Among dozens of pipercyclobutanamides, piperchabamides, nigramides, and dipiperamides, only the symmetric achiral dipiperamide A (5) has been synthesized.[8, 9] The originally proposed structure 4 for dipiperamide A[3] was revised to 5 after Kibayashi’s synthesis.[9] A solid state [2+2] photolytic homodimerization was employed by Kibayashi to construct the four-membered ring with center-symmetry. Extensive optimization was conducted for the crystallization of ferulic acid derivatives to obtain the α-form crystal,[8] which was required for the regio- and diastereoselective photolytic homodimerization. Research groups of Bergman, Ellman, and Jia used the same protocol to prepare the symmetric cyclobutane core of incarvillateine.[10] The [2+2] cycloaddition has been the main strategy for the synthesis of four-membered ring natural products[11] with a few exceptions.[12] However, it remains a demanding synthetic challenge to prepare unsymmetrical cyclobutanes from heterodimerization of two olefins with high chemo-, regio-, diastereo-, and enantioselectivity.[13]
Recently, an elegant sequential cyclobutane C-H arylation strategy was developed by Baran’s group for the diastereoselective synthesis of pseudosymmetric cyclobutanes such as piperarborenine B (7) and the proposed structure of piperarborenine D (8).[14] The originally proposed structure 8[3] for piperarborenine D was revised to structure 9 after Baran’s synthesis. We herein report our strategy for diastereo- and enantioselective introduction of four different substituents to cyclobutanes in the context of total synthesis of proposed structures of pipercyclobutanamide A (1) and piperchabamide G (2). We also proposed revised structures for these two natural products.[15]
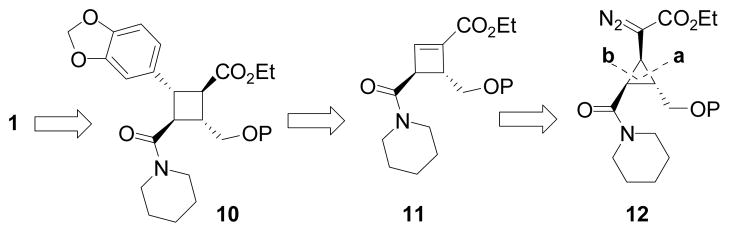
We envisioned that both pipercyclobutanamide A (1) and piperchabamide G (2) could be derived from tetrasubstituted cyclobutane 10 (Scheme 1). The ester and protected primary hydroxyl group in intermediate 10 would serve as aldehyde precursors that could be unmasked at different stages for olefinations. Conjugate addition of an aryl group to cyclobutenoate 11 may provide the tetrasubstituted cyclobutane 10. The aryl group should approach the four-membered ring from the α-face to avoid steric interactions with the adjacent amide substituent. Cyclobutenoate 11 could be prepared from cyclopropane 12 according to a ring expansion method we recently developed.[16, 17] This reaction involved a cyclopropyl metal carbene intermediate derived from transition metal-catalyzed decomposition of diazo compounds. We have demonstrated that the ring expansion was stereospecific and regioselective. The regioselectivity was dependent on the substituents of the cyclopropane ring and the choice of catalysts. The cyclopropane C-C bond that was adjacent to the electron-donating group or away from the electron-withdrawing group could be selectively cleaved when a AgI catalyst was employed.[16] In the case of cyclopropane 12, we expected that bond-a would be selectively cleaved over bond-b. This represents a general and unique strategy for the disastereo- and enantioselective synthesis of unsymmetrical cyclobutanes with four different substituents.
Scheme 1.
Proposed Strategy for Stereoselective Synthesis of Pipercyclobutanamide A and Piperchabamide G
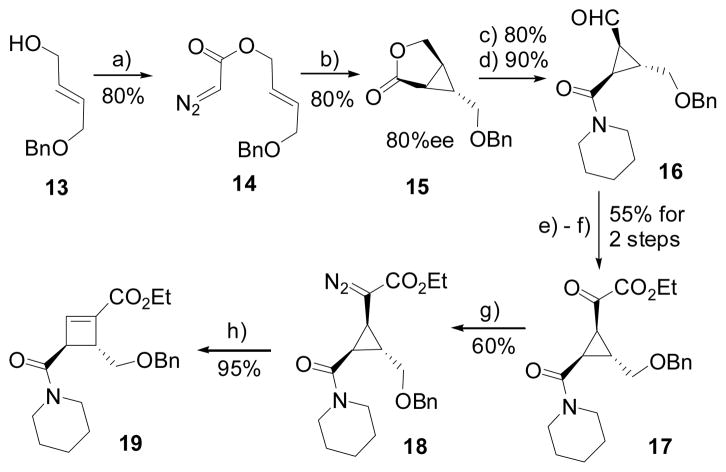
Our synthesis began with the preparation of diazo compound 14 from mono-protected diol 13 (Scheme 2).[18] Bicyclic lactone 15 was obtained via diastereo- and enantioselective intramolecular cyclopropanation of a trans-olefin using chiral Rh2(5S-MEPY)4 catalyst.[19] Opening of the lactone with piperidine followed by oxidation afforded trisubstituted cyclopropane 16, which was then converted to diazo compound 18 through ketoester intermediate 17 in three steps according to procedures we have established previously.[16] Treatment of diazo compound 18 with a catalytic amount of AgOTf indeed yielded cyclobutenoate 19 as a single isomer.
Scheme 2.
Stereoselective Synthesis of Cyclobutenoate
Conditions: a) bromoacetyl bromide then TsNHNHTs, DBU; b) 1.0 mol % Rh2(5S-MEPY)4, CH2Cl2, reflux; c) piperidine, AlMe3, CH2Cl2; d) DMSO, (COCl)2, Et3N; e) NaCN, HOAc, MeOH then EtOH/HCl, 0°C 2 days; f) Dess Martin periodinane; g) TsNHNH2, toluene, 80°C, then DBU, rt; h) 10 mol % AgOTf, CH2Cl2
With cyclobutenoate 19 in hand, we then turned our attention to the conjugate addition of an aryl nucleophile to this enoate. Under various conditions, we were not able to add an aryl cuprate reagent to enoate 19. We were pleased to find that tetrasubstituted cyclobutanes 20 and 21 could be prepared by a RhI-catalyzed addition of arylboronic acid to this enoate (Scheme 3).[20] The aryl group indeed approached the cyclobutene ring selectively from the face away from the amide substituent. The stereoselectivity for the protonation step, however, was low and a diastereomeric ratio of 2:1 favoring isomer 20 was obtained. Simply treating this mixture with NaOEt afforded the thermodynamically more stable product 20 as a single stereoisomer. Removal of the benzyl group followed by oxidation provided aldehyde 22. The stereochemistry of compounds 20 and 22 was confirmed by nOe analysis.[21] For example, nOe was observed between Ha/Ha′ and Hb, between Ha/Ha′ and Hd, between Hb and Hc, and between Hc and Hd of product 22.
Scheme 3.
Stereoselective Synthesis of Proposed Structures of Pipercyclobutanamide A and Piperchabamide G
Conditions: a) 5.0 mol % [Rh(COD)Cl]2, dioxane/H2O (10:1), 3,4-(methylenedioxy) phenylboronic acid; b) EtONa, EtOH; (c) Pd/C, H2, EtOH; d) Dess Martin periodinane; e) KHMDS, reagent A, DMF/HMPA; f) DIBALH, Tol; g) KHMDS, reagent B, THF; h) Pd/C, H2, MeOH.
After successfully preparing tetrasubstituted cyclobutane 22 stereoselectively, we then installed the E-olefin in pipercyclobutanamide A by Julia-Kocienski olefination (Scheme 3).[22] Compound 23 was obtained as a single olefin isomer but contaminated with byproduct derived from reagent A. Both polar solvent DMF and HMPA additive were necessary as lower E/Z ratios were observed in THF or in DMF without HMPA.[23] Using Ando’s reagent B, the Z-olefin of pipercyclobutanamide A (1) was prepared from the olefination of an aldehyde intermediate, derived from DIBALH reduction (Z/E>20:1).[24] A Z/E ratio of 2:1 was obtained when the Still-Gennari olefination protocol was employed.[25]
Our spectra (1H and 13C NMR) for product 1, however, did not match the data reported in literature for pipercyclobutanamide A.[2] We then further characterized our synthetic compound 1 by COSY, HMBC, HSQC, ROESY, and HRMS.[21] All of our spectral data were consistent with the proposed structure 1.
One of the most significant discrepancies between our data and that from literature for pipercyclobutanamide A was the chemical shift of the β-styrene hydrogen H4 (Table 1).[2] We did not observe any signal between 5.0 ppm and 5.5 ppm in the 1H NMR of synthetic compound 1. After analyzing similar natural products in the literature, we found that chemical shifts for this type of β-styrene hydrogen ranged from 6.10 to 6.29 ppm.[21] For example, chemical shifts of β-styrene hydrogen in nigramide Q (3)[5] and dipiperamide E (6)[4] are 6.14 and 6.29 ppm, respectively.
Table 1.
Selected 1H NMR and 13C NMR Spectral Data for Compounds 1, 2, 25, and 26[a]

| |||
|---|---|---|---|
| Position | δH/δC Data | ||
| synthetic 1 | 1 (literature)[2] | 25 (literature)[26] | |
|
| |||
| 1 | – / 170.2 | – / 172.2 | – / 172.2 |
| 4 | 6.16 / 128.6 | 5.21 / 128.0 | 5.19 / 128.1 |
| 1′ | – / 165.8 | – / 171.1 | – / 171.1 |
| 2′ | 6.03 / 120.4 | 5.75 / 125.5 | 5.73 / 125.5 |
| 3′ | 5.92 / 140.5 | 5.86 / 130.3 | 5.84 / 130.3 |
|
| |||
| synthetic 2 | 2 (literature)[6] | 26 (literature)[5] | |
|
| |||
| 1 | — / 170.9 | — / 173.2 | — / 173.2 |
| 1′ | — / 165.9 | — / 170.4 | — / 170.4 |
| 2′ | 5.98 / 123.1 | 5.84 / 134.0 | 5.85 / 134.1 |
| 3′ | 5.93 / 142.0 | 5.71 / 123.2 | 5.71 / 123.2 |
See Table S1–S6 in the Supporting Information for all data.
We then prepared piperchabamide G (2),[6] which did not have the styrene olefin. Hydrogenation of intermediate 23 followed by DIBALH reduction and olefination with Ando’s reagent B then afforded product 2 (Z/E>20:1) as shown in Scheme 3. We were surprised that our spectral data (1H NMR and 13C NMR) for product 2 again did not match the data reported in literature for natural product piperchabamide G (Table 1).[6] All of our spectral data (COSY, HMBC, HSQC, ROESY, and HRMS) were consistent with the proposed structure 2.[21]
After failing to find cyclobutane isomers that could match the spectral data of natural products pipercyclobutanamide A and piperchabamide G, we then carefully analyzed all spectroscopic differences between our synthetic compounds and natural products. We found that 13C chemical shifts of 172.2 and 171.1 ppm were assigned to the two carbonyl carbons in natural product pipercyclobutanamide A.[2] We observed 13C chemical shifts of 170.2 and 165.8 ppm for carbonyl carbons 1 and 1′, respectively, in our synthetic compound 1. Our observation is consistent with other closely related natural products.[21] It appeared that the two carbonyl groups in natural product pipercyclobutanamide A were both non-conjugated. Furthermore, the comparison of 13C chemical shifts of the cis-alkene in natural product pipercyclobutanamide A, our synthetic compound 1, and related natural products[21] suggested that the cis-alkene in natural product pipercyclobutanamide A was likely not conjugated to a carbonyl group. Similar conclusions could also be made for natural product piperchabamide G.
The ring expansion of vinylcyclobutane 1 through the cleavage of C4′-C5′ bond would produce a hypothetical cyclohexene isomer, which contains a non-conjugated cis-alkene and a non-conjugated carbonyl group. Numerous six-membered cyclohexenes have been isolated from Piper nigrum and Piper Chaba.[5, 26] It was proposed that they were derived from Diels-Alder cycloaddition of their polyene precursors.
Based on the above analysis, we turned our attention to six-membered ring isomers of structures 1 and 2. After examining all known six-membered ring natural products isolated from Piper nigrum and Piper Chaba,[5, 26] we were pleased to find that spectral data (1H NMR and 13C NMR) of natural product chabamide (25) was identical to that of natural product pipercyclobutanamide A, within experimental error.[26, 27] The unusual 5.2 ppm chemical shift of β-styrene hydrogen (H4) in compound 25 was presumably due to the shielding effect of the adjacent cis-aryl group. We also found that spectral data (1H NMR and 13C NMR) of natural product nigramide F (26) was identical to that of natural product piperchabamide G, within experimental error.[21]
In summary, we have developed a new general strategy for the diastereo- and enantioselective introduction of four different substituents to a cyclobutane ring. The ring expansion of a cyclopropyl AgI carbene derived from diazo compound 18 occurred regioselectively and stereospecifically to afford cyclobutenoate 19. A RhI-catalyzed conjugate addition of aryl boronic acid to this cyclobutenoate provided the fourth substituent diastereoselectively. Stereoselective synthesis of the trans- and cis-alkenes in the proposed structure of pipercyclobutanamide A was realized by judicious selection of reaction conditions. After the proposed structures for pipercyclobutanamide A (1) and piperchabamide G (2) were synthesized, these two natural products were revised to their six-membered ring isomers chabamide (25) and nigramide F (26), respectively. These structural revisions are important for further studies of biological activities of ingredients in white and black peppers and drug-herb interactions.
Supplementary Material
Acknowledgments
We thank the NIH (R01GM088285) and the UW-Madison for funding. W.T. is grateful for a Young Investigator Award from Amgen. We thank the Analytical Instrumentation Center at the UW-Madison for the facilities to acquire spectroscopic data. This study made use of the National Magnetic Resonance Facility at Madison, which is supported by NIH grants P41RR02301 (BRTP/ NCRR) and P41GM66326 (NIGMS); additional equipment was purchased with funds from the UW-Madison, the NIH (RR02781, RR08438), the NSF (DMB-8415048, OIA-9977486, BIR-9214394), and the USDA.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.For selected reviews on four-membered ring containing natural products, see: Hansen TV, Stenstrom Y. Organic Synthesis: Theory and Applications. Vol. 5. Elsevier Science Ltd; 2001. Naturally Occurring Cyclobutanes; p. 1.Dembitsky VM. J Nat Med. 2008;62:1. doi: 10.1007/s11418-007-0166-3.
- 2.Fujiwara Y, Naithou K, Miyazaki T, Hashimoto K, Mori K, Yamamoto Y. Tetrahedron Lett. 2001;42:2497. [Google Scholar]
- 3.Tsukamoto S, Cha BC, Ohta T. Tetrahedron. 2002;58:1667. [Google Scholar]
- 4.Tsukamoto S, Tomise K, Miyakawa K, Cha BC, Abe T, Hamada T, Hirota H, Ohta T. Bioorg Med Chem. 2002;10:2981. doi: 10.1016/s0968-0896(02)00130-x. [DOI] [PubMed] [Google Scholar]
- 5.Wei K, Li W, Koike K, Chen YJ, Nikaido T. J Org Chem. 2005;70:1164. doi: 10.1021/jo040272a. [DOI] [PubMed] [Google Scholar]
- 6.Matsuda H, Ninomiya K, Morikawa T, Yasuda D, Yamaguchi I, Yoshikawa M. Bioorg Med Chem. 2009;17:7313. doi: 10.1016/j.bmc.2009.08.050. [DOI] [PubMed] [Google Scholar]
- 7.a) Subehan, Usia T, Kadota S, Tezuka Y. Planta Med. 2006;72:527. doi: 10.1055/s-2006-931558. [DOI] [PubMed] [Google Scholar]; b) Subehan, Usia T, Kadota S, Tezuka Y. Nat Prod Commun. 2006;1:1. [PubMed] [Google Scholar]
- 8.Ichikawa M, Takahashi M, Aoyagi S, Kibayashi C. J Am Chem Soc. 2004;126:16553. doi: 10.1021/ja0401702. [DOI] [PubMed] [Google Scholar]
- 9.Takahashi M, Ichikawa M, Aoyagi S, Kibayashi C. Tetrahedron Lett. 2005;46:57. [Google Scholar]
- 10.a) Tsai AS, Bergman RG, Ellman JA. J Am Chem Soc. 2008;130:6316. doi: 10.1021/ja8012159. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Zhang F, Jia Y. Tetrahedron. 2009;65:6840. [Google Scholar]
- 11.For selected syntheses of four-membered ring natural products via [2+2] cycloaddition, see: Mascitti V, Corey EJ. J Am Chem Soc. 2004;126:15664. doi: 10.1021/ja044089a.Birman VB, Jiang XT. Org Lett. 2004;6:2369. doi: 10.1021/ol049283g.Mascitti V, Corey EJ. J Am Chem Soc. 2006;128:3118. doi: 10.1021/ja058370g.Reisman SE, Ready JM, Hasuoka A, Smith CJ, Wood JL. J Am Chem Soc. 2006;128:1448. doi: 10.1021/ja057640s.Reisman SE, Ready JM, Weiss MM, Hasuoka A, Hirata M, Tamaki K, Ovaska TV, Smith CJ, Wood JL. J Am Chem Soc. 2008;130:2087. doi: 10.1021/ja076663z.Zhang P, Wang Y, Bao R, Luo T, Yang Z, Tang Y. Org Lett. 2012;14:162. doi: 10.1021/ol2029433.Lu P, Bach T. Angew Chem. 2012;124:1287. doi: 10.1002/anie.201106889.Angew Chem Int Ed. 2012;51:1261.
- 12.For selected examples, see: Baran PS, Zografos AL, O’Malley DP. J Am Chem Soc. 2004;126:3726. doi: 10.1021/ja049648s.Baran PS, Richter JM. J Am Chem Soc. 2005;127:15394. doi: 10.1021/ja056171r.O’Malley DP, Li K, Maue M, Zografos AL, Baran PS. J Am Chem Soc. 2007;129:4762. doi: 10.1021/ja069035a.
- 13.For selected examples of heterodimerization of alkenes or alkynes, see: Inanaga K, Takasu K, Ihara M. J Am Chem Soc. 2005;127:3668. doi: 10.1021/ja042661s.Liu YH, Liu MN, Song ZQ. J Am Chem Soc. 2005;127:3662. doi: 10.1021/ja042636m.Canales E, Corey EJ. J Am Chem Soc. 2007;129:12686. doi: 10.1021/ja0765262.Ischay MA, Anzovino ME, Du J, Yoon TP. J Am Chem Soc. 2008;130:12886. doi: 10.1021/ja805387f.Du J, Yoon TP. J Am Chem Soc. 2009;131:14604. doi: 10.1021/ja903732v.Ischay MA, Lu Z, Yoon TP. J Am Chem Soc. 2010;132:8572. doi: 10.1021/ja103934y.
- 14.Gutekunst WR, Baran PS. J Am Chem Soc. 2011;133:19076. doi: 10.1021/ja209205x.For a highlight of this work, see: Frebault F, Maulide N. Angew Chem. 2012;124:2869. doi: 10.1002/anie.201108592.Angew Chem Int Ed. 2012;51:2815.
- 15.For selected reviews on misassigned natural products, see: Nicolaou KC, Snyder SA. Angew Chem. 2005;117:2086. doi: 10.1002/anie.200460864.Angew Chem Int Ed. 2005;44:1012.Maier ME. Nat Prod Rep. 2009;26:1105. doi: 10.1039/b809658a.Suyama TL, Gerwick WH, McPhail KL. Bioorg Med Chem. 2011;19:6675. doi: 10.1016/j.bmc.2011.06.011.
- 16.Xu H, Zhang W, Shu D, Werness JB, Tang W. Angew Chem. 2008;120:9065. doi: 10.1002/anie.200803910.Angew Chem Int Ed. 2008;47:8933.For related synthesis of cyclobutenes, see: Barluenga J, Riesgo L, Lopez LA, Rubio E, Tomas M. Angew Chem. 2009;121:7705. doi: 10.1002/anie.200903902.Angew Chem Int Ed. 2009;48:7569.Li C-W, Pati K, Lin G-Y, Abu Sohel SM, Hung H-H, Liu R-S. Angew Chem. 2010;122:10087. doi: 10.1002/anie.201004647.Angew Chem Int Ed. 2010;49:9891.For a recent review on cyclobutene synthesis, see: Luparia M, Audisio D, Maulide N. Synlett. 2011:735.
- 17.Um JM, Xu H, Houk KN, Tang W. J Am Chem Soc. 2009;131:6664. doi: 10.1021/ja9016446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Toma T, Shimokawa J, Fukuyama T. Org Lett. 2007;9:3195. doi: 10.1021/ol701432k. [DOI] [PubMed] [Google Scholar]
- 19.a) Doyle MP, Pieters RJ, Martin SF, Austin RE, Oalmann CJ, Muller P. J Am Chem Soc. 1991;113:1423. [Google Scholar]; b) Doyle MP, Austin RE, Bailey AS, Dwyer MP, Dyatkin AB, Kalinin AV, Kwan MMY, Liras S, Oalmann CJ, Pieters RJ, Protopopova MN, Raab CE, Roos GHP, Zhou QL, Martin SF. J Am Chem Soc. 1995;117:5763. [Google Scholar]
- 20.Sakai M, Hayashi H, Miyaura N. Organometallics. 1997;16:4229. [Google Scholar]
- 21.See Supporting Information for details.
- 22.Blakemore PR, Cole WJ, Kocienski PJ, Morley A. Synlett. 1998:26. [Google Scholar]
- 23.a) Liu P, Jacobsen EN. J Am Chem Soc. 2001;123:10772. doi: 10.1021/ja016893s. [DOI] [PubMed] [Google Scholar]; b) Nicolaou KC, Lister T, Denton RM, Gelin CF. Tetrahedron. 2008;64:4736. doi: 10.1016/j.tet.2008.02.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ando K, Nagaya S, Tarumi Y. Tetrahedron Lett. 2009;50:5689. [Google Scholar]
- 25.Still WC, Gennari C. Tetrahedron Lett. 1983;24:4405. [Google Scholar]
- 26.Rukachaisirikul T, Prabpai S, Champung P, Suksamrarn A. Planta Med. 2002;68:853. doi: 10.1055/s-2002-34410.Chabamide and related analogues were also isolated and characterized by other groups. See reference 5 and the following: Rao VRS, Kumar GS, Sarma VUM, Raju SS, Babu KH, Babu KS, Babu TH, Rekha K, Rao JM. Tetrahedron Lett. 2009;50:2774.Rao VRS, Suresh G, Babu KS, Raju SS, Vardhan MVPSV, Ramakrishna S, Rao JM. Tetrahedron. 2011;67:1885.
- 27.Wei K, Li W, Koike K, Nikaido T. Org Lett. 2005;7:2833. doi: 10.1021/ol050689i. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.