Capsule Summary
In Kawasaki Disease patients, the authors show associations between high-dose intravenous immunoglobulin (IVIG) response and a polymorphism in the FCγRIIB. This provides basis for defining the IVIG regulatory mechanisms and pharmacogenomic approach to IVIG therapy.
Keywords: Kawasaki disease, IVIG treatment response, FcγR
To the Editor:
Human intravenous immunoglobulin (IVIG) given at high dose suppresses inflammation caused by several autoimmune diseases. Though IVIG is licensed for treatment of patients with Kawasaki Disease (KD), idiopathic thrombocytic purpura (ITP), Guillan-Barre syndrome, and chronic inflammatory demyelinating polyneuropathy (CIDP), the modes of action in these clinical syndromes require elucidation. Proposed mechanisms include F(ab’)2-dependent modulation of pathogenic autoantibody neutralization, cytokine production, complement component neutralization.1 However, studies using clinical and mouse models show that IVIG anti-inflammatory potency depends more on the IgG Fc binding to receptors expressed on the surface of various immune cells. The Fcγ receptor (FcγR) family contains multiple activating receptors, and a single inhibitory, FCγRIIB. Importantly, B cells express only the inhibitory type. Presumably, IVIG modulates the balance between activating and inhibitory receptors.
FCγRIIB play a central role in mediating Fc-dependent anti-inflammatory actions in mice.2 IVIG elicits minimal therapeutic effect in FcγRIIB-deficient mice with autoimmune disease,3 but induces FcγRIIB surface expression on splenic macrophages in wild-type mice,4 thereby preventing antibody induced inflammation. Also, IVIG up-regulates FcγRIIB expression by monocytes and B cells among clinically responding CIDP patients.5 Though these studies in mice and the limited patient data support a model for FcγRIIB involvement in IVIG actions, further clinical confirmation is required.
We thus tested our hypothesis that FcγRIIB participates in IVIG anti-inflammatory action in humans by determining if functionally relevant polymorphisms for this gene influence IVIG response in a KD cohort. This autoimmune syndrome produces acute and diffuse vasculitis. There is predilection for the coronary arteries in which aneurysms occur in 20-25% of untreated patients. IVIG (2 gm/kg) with aspirin represents the only standard therapy recommended for acute KD, but the response is variable. The American Heart Association and American Academy of Pediatrics (AHA/AAP) define failure to respond or IVIG refractoriness to treatment as persistent or recurrent fever (temperature > 38° C) at > 36 hours after completing the initial IVIG infusion.6 The IVIG refractory rate, depending on the clinical series, is reported as between 13 to 30%.6 Coronary artery vasculitis and aneurysms occur at comparatively high rates in refractory patients.7
With institutional review board approval and parental consent, we obtained DNA from KD patients (n = 383) and parents (n = 578) from the three centers in the Pacific Northwest. Qualifying patients met strict AHA/AAP clinical criteria for the KD diagnosis.6 The predominantly male cohort (59%) showed median KD onset age at 34.5 months (IQR: 15-61.5 months). In our IVIG response analysis, we only included the KD patients (231 responders, 76 refractory) if they received standard treatment within 10 days, followed guidelines prescribing IVIG refractoriness,6 and were not participating in other treatment clinical trials. In all KD patients and their parents (for frequency comparisons), we pyrosequenced (see supplement) the DNA regions containing three known functional polymorphisms in the FcγRIIB gene : a SNP in the FcγRIIB region encoding either isoleucine (I) or threonine (T) amino acid at position 232 within the transmembrane (TM) region (nucleotide position +775; IIB+775 (T/c)) known to alter the function of this receptor, and two SNPs at positions −386 (IIB-386 (G/c)) and −120 (IIB-120 (T/a)) upstream from the translation start site known to alter the promoter activity resulting in differing levels of receptor expression.8 We assigned race to individuals from this heterogeneous U.S population using 155 ancestry information markers specific for Asian, African, Native American and European descents (AIMs – see supplement).
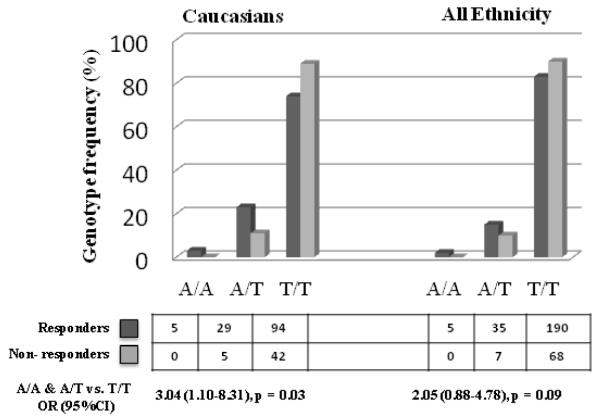
The allele frequency for all three polymorphisms however differed substantially among the major racial and ethnic groups. Most striking, the A allele at FcγRIIB -120T/a was totally absent in Asian patients (n = 48) and their unaffected parents (n = 86), African Americans patients (n = 10) and their parents (n = 16); it was rare in Hispanic patients (n = 40) and their parents (n = 67). Thus, we analyzed the impact of the polymorphisms on IVIG treatment response for each race as well as the heterogeneous Hispanic population, separately. In Caucasians, the minor allele, A at FcγRIIB -120T/a, occurred more frequently among the responders (15%) than non-responders (5%) with an OR = 3.23 [1.22-8.33]; p-value = 0.01 (Table 1). The overall genotypic distribution was different in responders and non-responders (p-value = 0.03). Further, no IVIG non-responders was AA homozygous, while 5/128 (4%) responders were homozygotes and similar trend was observed with heterozygotes, occurring more frequently (23% vs. 11%) among the responders (Figure 1). Armitage trend test suggested a higher likelihood of being a responder with increase number of A allele (p-value =0.02). Only two Hispanic KD patients were heterozygotes (AT); both were also IVIG responders. Consistent with previous report on IIB-386 (G/c) and IIB-120 (T/a) haplotypes,9 the frequencies of G-T, T-A and T-T haplotypes in Caucasian unaffected parents were 91%, 8% and 1% respectively. In patients, the distributions of these haplotypes differed significantly when comparing responders with non-responders − 84.5% vs. 92%, 15% vs. 6% and 0.5% vs. 2% (overall p-value = 0.02), respectively, with the elevated T-A haplotype in the responders tagging IIB-120A. In Asians, FcγRIIB+775T/c, encoding the transmembrane region, showed trends related to IVIG treatment response, but the power was limited by small sample size.
Table 1.
Polymorphisms in FcγRIIB gene and response to IVIG treatment among Kawasaki disease patients in all ethnic groups and only Caucasians
| Polymorphisms | Minor Allele Freq (%) |
Allelic Odds Ratios (95% CI) Rp vs. NRp |
p-value |
||||
|---|---|---|---|---|---|---|---|
| Parents |
Responders (Rp) |
Non-Responders (NRp) |
Allelica | Genotypeb | Trendc | ||
| All Ethnic groups | n = 578 | n=231 | n=76 | ||||
| FcγRIIB+775T/c | 15 | 18 | 14 | 1.35 (0.0.79-2.22) | 0.28 | 0.42 | 0.34 |
| FcγRIIB -386G/c | 9 | 10 | 7 | 1.61 (0.79-3.23) | 0.18 | 0.32 | 0.12 |
| FcγRIIB -120T/a | 9 | 10 | 5 | 2.22 (0.98-5.02) | 0.05 | 0.17 | 0.06 |
| Caucasians | n = 369 | n=129 | n=48 | ||||
| FcγRIIB+775T/c | 13 | 12 | 13 | 0.97 (0.47-2.01) | 0.94 | 0.97 | 0.95 |
| FcγRIIB −386G/c | 13 | 16 | 8 | 2.04 (0.91-4.55) | 0.08 | 0.21 | 0.10 |
| FcγRIIB −120T/a | 12 | 15 | 5 | 3.23 (1.22-8.33) | 0.01 | 0.03 | 0.02 |
| Asians | n = 86 | n=40 | n=8 | ||||
| FcγRIIB+775T/c | 27 | 40 | 19 | 1.54 (0.39-6.25) | 0.11 | 0.15 | 0.14 |
| FcγRIIB −386G/c | 1 | 0 | 0 | - | - | - | - |
| FcγRIIB -120T/a | 0 | 0 | 0 | - | - | - | - |
| Hispanic | n =67 | n=29 | n=11 | ||||
| FcγRIIB+775T/c | 11 | 20 | 14 | - | 0.49 | 0.65 | 0.51 |
| FcγRIIB −386G/c | 3 | 4 | 0 | - | - | - | - |
| FcγRIIB -120T/a | 4 | 3 | 0 | - | - | - | - |
χ2 (d.f.=1);
χ2 (d.f.=2);
Cochran-Armitage test
Figure 1.
Genotype distribution (counts) of FcγRIIB -120T/a and association with IVIG response in Caucasian and in the mixed population.
FcγRIIB inhibits immune response through co-ligation with either activating FcγRs or with B cell receptors bound to immune complexes.2 This co-ligation leads to phosphorylation of the cytoplasmic immunoreceptor tyrosine-based inhibitory motif (ITIM) within the transmembrane FcγRIIB portion. ITIM phosphorylation leads to downstream target dephosphorylation and inhibition of the activating signaling cascade. Inhibition increases with elevated FcγRIIB surface expression, controlled in part at the transcriptional level. Transfection of the IIB-386/C and IIB-120/A promoter haplotype into BJAB or U397 cells increases constitutive and cAMP stimulated FCγRIIB promoter activity through enhanced binding to GATA4 and Yin-Yang1 (YY1) transcription factors.9 Accordingly, our data obtained in humans and linking increased FcγRIIB promoter activity with improved clinical response, confirm the importance of FcγRIIB in mediating high-dose IVIG anti-inflammatory action.
The prevailing models derived primarily from murine experiments suggest that IVIG sensing occurs by one or multiple candidates such as FCGRIII or DC sign related -1.2 FCGRIIB is downstream within a signaling cascade, but is required to trigger IVIG mediated inflammatory inhibition. Thus, functional polymorphisms or other influences in one or multiple candidate genes along this cascade could also affect the IVIG response. The specificity of this promoter polymorphism for the Caucasian population suggests that these candidates or others, possibly involving the activating FCγRs, could be responsible for the IVIG response variation in other races. This study provides the basis for defining the IVIG regulatory mechanisms in patients and for using a clinical pharmacogenomic approach to IVIG therapy.
Supplementary Material
Acknowledgements
We thank the participating patients and their parents. We also thank investigators, pediatricians and staffs of the participating clinics. We thank Norman Buroker for handling of the biospecimens and for DNA extraction, and Aditi Shendre and Deborah S McDuffie for genotyping. This study was supported by grant NHLBI-R21-HL90558, the University of Washington Clinical and Translational Science Award Grant 1ULI RR025014-01, and a grant from the Thrasher Foundation Research Fund.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Negi VS, Elluru S, Siberil S, Graff-Dubois S, Mouthon L, Kazatchkine MD, et al. Intravenous immunoglobulin: an update on the clinical use and mechanisms of action. J Clin Immunol. 2007;27:233–45. doi: 10.1007/s10875-007-9088-9. [DOI] [PubMed] [Google Scholar]
- 2.Smith KG, Clatworthy MR. FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol. 2010;10:328–43. doi: 10.1038/nri2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Samuelsson A, Towers TL, Ravetch JV. Anti-inflammatory activity of IVIG mediated through the inhibitory Fc receptor. Science. 2001;291:484–6. doi: 10.1126/science.291.5503.484. [DOI] [PubMed] [Google Scholar]
- 4.Kaneko Y, Nimmerjahn F, Madaio MP, Ravetch JV. Pathology and protection in nephrotoxic nephritis is determined by selective engagement of specific Fc receptors. J Exp Med. 2006;203:789–97. doi: 10.1084/jem.20051900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tackenberg B, Jelcic I, Baerenwaldt A, Oertel WH, Sommer N, Nimmerjahn F, et al. Impaired inhibitory Fcgamma receptor IIB expression on B cells in chronic inflammatory demyelinating polyneuropathy. Proc Natl Acad Sci U S A. 2009;106:4788–92. doi: 10.1073/pnas.0807319106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Newburger JW, Takahashi M, Gerber MA, Gewitz MH, Tani LY, Burns JC, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Pediatrics. 2004;114:1708–33. doi: 10.1542/peds.2004-2182. [DOI] [PubMed] [Google Scholar]
- 7.Tremoulet AH, Best BM, Song S, Wang S, Corinaldesi E, Eichenfield JR, et al. Resistance to intravenous immunoglobulin in children with Kawasaki disease. J Pediatr. 2008;153:117–21. doi: 10.1016/j.jpeds.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li S, Ptacek TS, Brown EE, Edberg JC. FcG Receptors: Structure, Function and Role as Genetic Risk Factors in SLE. Genes and Immunity. 2009;10:380–9. doi: 10.1038/gene.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su K, Li X, Edberg JC, Wu J, Ferguson P, Kimberly RP. A promoter haplotype of the immunoreceptor tyrosine-based inhibitory motif-bearing FcgammaRIIb alters receptor expression and associates with autoimmunity. II. Differential binding of GATA4 and Yin-Yang1 transcription factors and correlated receptor expression and function. J Immunol. 2004;172:7192–9. doi: 10.4049/jimmunol.172.11.7192. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.