Abstract
Chagas disease is caused by Trypanosoma cruzi and is endemic to North, Central and South American countries. Current therapy against this disease is only partially effective and produces adverse side effects. Studies on the metabolic pathways of T. cruzi, in particular those with no equivalent in mammalian cells, might identify targets for the development of new drugs. Ceramide is metabolized to inositolphosphoceramide (IPC) in T. cruzi and other kinetoplastid protists whereas in mammals it is mainly incorporated into sphingomyelin. In T. cruzi, in contrast to Trypanosoma brucei and Leishmania spp., IPC functions as lipid anchor constituent of glycoproteins and free glycosylinositolphospholipids (GIPLs). Inhibition of IPC and GIPLs biosynthesis impairs differentiation of trypomastigotes into the intracellular amastigote forms. The gene encoding IPC synthase in T. cruzi has been identified and the enzyme has been expressed in a cell-free system. The enzyme involved in IPC degradation and the remodelases responsible for the incorporation of ceramide into free GIPLs or into the glycosylphosphatidyl inositols (GPIs) anchoring glycoproteins, and in fatty acid modifications of these molecules of T. cruzi have been understudied. IPC metabolism and remodeling could be exploited as targets for Chagas disease chemotherapy.
Keywords: glycosylphosphatidylinositol, glycosylinositolphospholipids, inositolphosphoceramide, phospholipase C, sphingolipids, Trypanosoma
Trypanosoma cruzi is the etiologic agent of Chagas disease. The disease is endemic to North, Central and South America and is a significant cause of morbidity and mortality. There are no vaccines available against this disease, which has acute, indeterminate and chronic stages. The drugs currently used, nifurtimox and benznidazole, cause adverse reactions and have limited efficacy in the chronic stage (Urbina and Docampo 2003).
T. cruzi has three main developmental stages: two replicative forms, the epimastigote, which replicates in the intestine of the insect vector, and the amastigote which replicates intracellularly in the mammalian host, and one non-replicative form, the trypomastigote, which is the terminal differentiation stage in the vector (metacyclic form) or is found in the bloodstream of the mammalian host (bloodstream forms).
Research on the metabolic pathways of T. cruzi that are different or do not have counterparts in the host might identify targets for the development of new drugs. In this article we propose two potential targets for drug design against T. cruzi involving inositolphosphoceramide (IPC), a phosphosphingolipid that is absent from mammalian cells. These targets are IPC synthesis and degradation, and the remodelases required for the introduction of ceramide into the glycosylphosphatidylinositol (GPI) anchor of T. cruzi glycoproteins and for the modification of its fatty acids. IPC synthesis and degradation are catalyzed by the IPC synthase, and the Inositolphosphosphingolipid phospholipase C (ISC), which replace the sphingomyelin synthase and the sphingomyelinase, respectively, of mammalian cells. In contrast to mammals and the related trypanosomatids, Trypanosoma brucei and Leishmania spp., T. cruzi can utilize IPC instead of glycerolipids as the lipid anchor of many glycoproteins and free glycosylinositolphospholipids (GIPLs) (Lederkremer and Bertello 2001, Previato et al. 2004).
LIPID CONSTITUENTS OF THE GPI ANCHORS OF T. CRUZI GLYCOPROTEINS AND FREE GIPLS
GPI anchoring of glycoproteins is a type of attachment to the cell surface preferred by protist parasites (Ferguson 1999). Besides membrane insertion, GPI anchors have been implicated in increasing lateral mobility of proteins, in targeting of proteins to special microdomains as the lipid rafts, and in mediating the release of proteins by activation of a phospholipase. For instance, the trans-sialidase of T. cruzi (TcTS) is shed into the bloodstream by the action of a phospholipase C (PLC). The soluble trans-sialidase exerts different biological effects on other cells (Buscaglia et al. 2006). Shedding of GPI-anchored proteins by other mechanisms has also been described. For example, a Leishmania donovani lipophosphoglycan, formerly designated as shed membrane antigen 2 (SMA-2) (Kaneshiro et al. 1982), can be released with or without the GPI anchor (Kaneshiro and Wyder 1993), while the Tc85 glycoprotein of T. cruzi is shed with its GPI anchor within vesicles (Abuin et al. 1996).
GPIs share a common structure. A glycan core of Man(α1-2)Man(α1-6)Man(α1-4)GlcNa(α1-6)-D-myoInositol-1PO4-Lipid is recognized in the different GPIs although considerable variability exists in both the glycan and the lipid portions of GPI anchors. For example, other sugars, ethanolamine phosphate or aminoethylphosphonic acid may be branching the main glycan (Ferguson 1999). In addition, the inositol ring can be acylated (generally by palmitate) at position 2. The complex structure of GPI would be expected to be involved in diverse functions. However, definitive experiments that relate GPI structure with function are scarce.
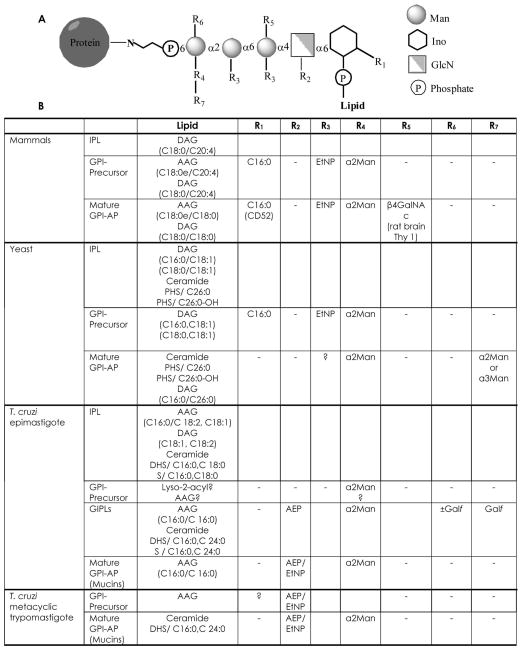
Alkylacylglycerol and ceramide have been detected in the lipid moiety of the GPI of mature glycoproteins in the different stages of T. cruzi (Fig. 1) (Lederkremer and Bertello 2001, Previato et al. 2004). Ceramide is present in free GIPLs of epimastigotes or as anchor of glycoproteins in infective stages of the parasite. These results agree with a recent description of the GPIomics of T. cruzi epimastigotes (Nakayasu et al. 2009). Only hexadecylglycerol with variable size fatty acyl groups at the sn-2 position were identified in protein anchors of the epimastigote stage whereas ceramide was the main constituent of free GIPLs. Unlike T. cruzi (Lederkremer 2009) the lipid moiety diacylglycerol (DAG) was identified in the GPI anchor of the variant surface glycoproteins (VSG) of T. brucei, the first characterized GPI (Ferguson et al. 1985). DAG was also found in GPIs of other parasites, such as Plasmodium falciparum (Gerold et al. 1996) and Toxoplasma gondii (Smith et al. 2007), as well as in some mammalian GPIs (Roberts et al. 1987, Medof et al. 1986, Armesto et al. 1996). Ceramide is present in the anchor of characteristic glycoproteins of T. cruzi, which are actively shed to the medium like the TcTS of trypomastigotes (Agusti et al. 1997) and the specific surface protein Ssp4, a glycoprotein marker of amastigotes (Bertello et al. 1996). Additionally, a free glycoinositolphospholipid (GIPL) with ceramide, originally called lipopeptidophosphoglycan (LPPG), is the major glycoconjugate of T. cruzi epimastigotes. Galactofuranose and aminoethylphosphonic acid (AEP), substituents of the glycan core in the LPPG (Previato et al. 1990, de Lederkremer et al. 1991), are absent in mammalian cells.
Fig. 1.
Structure of the lipid in the free GIPLs and GPI anchors of the major glycoproteins of Trypanosoma cruzi. The glycoproteins of T. cruzi may be anchored by a glycerolipid (PI) or by a ceramide lipid (IPC). The PI is constituted by an alkylglycerol, either acylated (AAG) or not (AG). The alkylglycerol is always hexadecylglycerol (C16:0) and may be esterified with fatty acid, mainly palmitic acid (C16: 0) or a C18 fatty acid, which may be unsaturated. No diacylglycerol was found in the mature GPIs. In the IPC anchors only the C18 long chain bases were found, either saturated such as dihydrosphingosine (DHS) or unsaturated in the case of sphingosine (S). The amide fatty acid is mainly palmitic acid, lignoceric acid (C24:0) or stearic acid (C18:0). Abbreviations: ethanolaminephosphate (EtNP), glycan (G), inositol (I), phosphate (P), alkyl substituent (R1), acyl substituent (R2), amide substituent (R3), phosphatidylinositol (PI), inositolphosphoceramide (IPC). Epi and trypo mean epimastigote and trypomastigote forms of the parasite. The (−) symbol indicates that no substituent is present in the 2-position of glycerol.
A common criterion for the identification of a GPI anchor in glycoproteins is the cleavage of the lipid by a bacterial phosphatidylinositol phospholipase C (bPI-PLC) (Low et al. 1988). Designation of the enzyme was based on the release of a glycerolipid from phosphatidylinositol (PI). However, it must be emphasized that the enzyme also releases ceramide from GPI anchors containing IPC instead of PI (de Lederkremer et al. 1990, Kaneshiro and Wyder 1993). This is important because susceptibility to PI-PLC has usually been taken as indicative of the presence of an acyl or alkylglycerolipid anchor, which is not always the case. Ceramide can also be released as ceramide-1-phosphate from GIPLs of T. cruzi by a glycosylphosphatitylinositol phospholipase D (GPI-PLD) from rat serum (de Lederkremer et al. 1996).
With the exception of yeast and T. cruzi, IPC is not a common constituent of eukaryotic GPI anchored glycoproteins. The significance of its presence in glycoproteins and free GIPLs of T. cruzi is not fully understood. In contrast, PI is the lipid constituent in other trypanosomatid pathogens such as T. brucei and Leishmania species. Differences between the structures of the inositol phospholipid (IPL), which is the starting material for GPI, the GPI precursor, and the mature GPI anchoring the protein, indicate remodeling of the lipid during biosynthesis (Fig. 2). The absence of an IPC lipid moiety in mammalian GPI anchored proteins, together with the growth inhibitory properties of ceramide, makes IPC metabolism an attractive target for chemotherapy of Chagas disease.
Fig. 2.
Structures of IPL, GPI-precursor and mature GPI-anchored proteins (GPI-AP) in mammals, yeast and T. cruzi. A. Core structure of the GPI anchor showing substitutions (R1–R7). B. Structure of IPLs and substitutions in the core glycan of GPI precursors and mature GPI-AP. IPL, inositolphospholipid; DAG, diacylglycerol; AAG, alkylacylglycerol; EtNP, ethanolaminephosphate, AEP, aminoethylphosphonic acid; Galf, galactofuranose
SYNTHESIS OF INOSITOLPHOSPHOCERAMIDE IN T. CRUZI AS COMPARED TO OTHER TRYPANOSOMATIDS
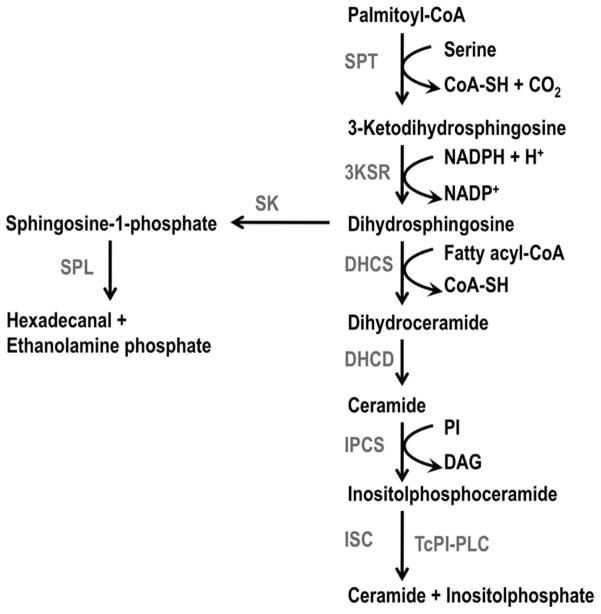
Biosynthesis of ceramide in T. cruzi probably starts as in yeast, Leishmania, and mammalian cells by condensation of serine and pamitoyl-CoA into 3-ketodihydrosphingosine (kDHS) catalyzed by a serine pamitoyl transferase (SPT) (Fig. 3). After this initial step, the kDHS is converted to dihydrosphingosine (DHS) (also known as sphinganine) by the action of a 3-ketosphinganine reductase (3KSR). Amidation of DHS with a fatty acid yields dihydroceramide (or N-acylsphinganine), in a reaction catalyzed by dihydroceramide synthase (DHCS). Desaturation of dihydroceramide catalyzed by dihydroceramide desaturase (DHCD) results in the formation of ceramide, which has different fate in mammals, yeast and trypanosomatids. Mammals mainly transfer phosphocholine from phosphatidylcholine to ceramide to form the major sphingolipid, sphingomyelin, whereas fungi and trypanosomatids add inositol phosphate from PI to ceramide to form IPC. In T. brucei and also in mammals (Sutterwala et al. 2008) transfer of ethanolamine phosphate from phosphatidylethanolamine to ceramide to form ethanolaminephosphoceramide (EPC) can also occur. Gene homologues to all the enzymes in this pathway have been identified in the genome of different trypanosomatids, including T. cruzi (Table 1). Reviews on sphingolipid metabolism in mammals and yeast (Dickson and Lester 1999), T. brucei (Mina et al. 2009, Smith and Butikofer 2010) and Leishmania (Zhang and Beverley 2010) have been published.
Fig. 3.
Predicted pathway for IPC biosynthesis and degradation in T. cruzi. Abbreviations: SPL, serine palmitoyl transferase; 3KSR, 3 ketodihydrosphingosine reductase; DHCS, dihydroceramide synthase; DHCD, dihydroceramide desaturase; IPCS, inositolphosphoceramide synthase; ISC, inositol sphingolipid phospholipase C; SK, sphingosine kinase; SPL, sphingosine-1-phosphate lyase; TcPI-PLC, T. cruzi phosphoinositide phospholipase C.
Table 1.
Genes encoding predicted enzymes involved in sphingolipid metabolism in Trypanosoma cruzi.
| Enzyme name | TriTrypDB identification | GenBank number |
|---|---|---|
| Serine palmitoyltransferase | Tc00.1047053506405.50 | EAN90888 |
| 3-ketosphinganine reductase | Tc00.1047053510997.10 Tc00.1047053506959.64 |
EAN84677 EAN92292 |
| Dihydroceramide synthase | Tc00.1047053507395.10 Tc00.1047053510087.30 |
EAN84076 EAN89701 |
| Dihydroceramide desaturase | Tc00.1047053510565.20 | EAN94404 |
| Sphingosine kinase | Tc00.1047053508211.30 Tc00.1047053507515.120 |
EAN88819 EAN92586 |
| Sphingosine-1-phosphatelyase | Tc00.1047053506941.150 Tc00.1047053511511.150 |
EAN97232 EAN95730 |
| IPC synthase (TcSLS1.1. and 1.2) | Tc00.1047053506885.124 Tc00.1047053510729.290 |
EAN99655 EAN97033 |
| IPC phospholipase C (TcISC) | Tc00.1047053509777.130 | EAN92382 |
| PI-PLC (TcPI-PLC) | Tc00.1047053504149.160 | EAN96260 (AAD12583) |
Several genes are alleles with minor differences between them and encode the same protein
The presence of an IPC synthase in all stages of T. cruzi was first demonstrated by labeling IPC with [3H]-palmitic acid (Bertello et al. 1995, Uhrig et al. 1996). Release of the ceramide with bPI-PLC and determination of radioactivity incorporated into the long chain base and in the fatty acid demonstrated the presence of the IPC synthesis pathway in T. cruzi. IPC synthase activity in microsomal membranes obtained from different stages of T. cruzi was also studied by transference of inositol phosphate from PI to a fluorescent C6 ceramide derivative (Figueiredo et al. 2005). More recently, an enzyme (LmIPCS) was identified in L. major using bioinformatic and functional genetic approaches and was demonstrated to possess IPC synthase activity after expression of the gene in mammalian cells (Denny et al. 2006). The protein was found to share more similarity with the animal sphingomyelinases than with the fungal IPC synthase (AUR1p) (Sawai et al. 2000). Two orthologues (probably alleles) were found in T. cruzi (Table 1) while 4 orthologs in tandem were found in T. brucei (Denny et al. 2006). The L. major gene (LmIPCS), the four orthologues described in T. brucei (named TbSLS1-4) and one of the genes identified in T. cruzi (named TcSLS1.1) were recently expressed in a cell-free system and the products of the reactions catalyzed by the expressed proteins characterized (Sevova et al. 2010). TbSLS1 produces mainly IPC, TbSLS2 is mainly an EPC synthase and TbSLS3 and TbSLS4 are bifunctional producing mainly SM and EPC (Sevova et al. 2010). Knockdown of all TbSLS by RNAi inhibited growth of T. brucei bloodstream forms demonstrating their essentiality and validating them as drug targets (Sutterwala et al. 2008). LmIPCS was confirmed to produce only IPC and TcSLS1.1 produces IPC (Sevova et al. 2010) and is highly expressed at the mRNA level in epimastigotes and metacyclic trypomastigotes (TriTrypDB.org).
It has been shown that during the differentiation of trypomastigotes to amastigotes inside myoblasts the parasites synthesize IPC using ceramide provided by the parasites and inositol coming from the pool of PI in the myoblasts (Salto et al. 2003). The biosynthesis of IPC also increased during extracellular differentiation of trypomastigotes into amastigotes, at low pH, as shown by labeling with [3H]-inositol (Salto et al. 2003). However, incorporation of [3H]-palmitic acid into IPC greatly decreased with time, remaining at the same level in non-differentiating trypomastigotes. These results suggest that a remodeling step on the ceramide is taking place, with replacement of the N-linked radioactive with a non-labeled fatty acid. The pathway proposed for the remodeling involves the action of an IPC acylhydrolase and an IPC-acyltransferase but neither enzyme has been characterized. Remodeling of the fatty acid was also shown in PIs and lyso PIs. Lyso PIs lack the acyl group at position-1 or 2 of glycerol and are probably formed by the action of two phospholipases named PLA1 or PLA2, respectively. These two enzyme activities have been detected in membranes of all forms of T. cruzi (Salto et al. 2003).
In contrast to T. cruzi, which synthesizes mainly IPC, T. brucei synthesizes SM, EPC or IPC, depending on the life stage of the parasite. IPC was identified only in procyclic forms (Guther et al. 2006, Sutterwala et al. 2008), EPC is restricted to the bloodstream forms while SM was found in both stages (Sutterwala et al. 2008). In Leishmania, IPC is also an abundant membrane component and the primary phosphosphingolipid (Kaneshiro et al. 1986). The other two phosphosphingolipids, EPC and SM were not found in either T. cruzi or Leishmania (Zhang and Beverley 2010). Neutral glycosphingolipids were isolated from amastigote forms of L. amazonensis although their origin is not known (Straus et al. 1993). Although the presence of SM in T. cruzi was reported in early papers (Oliveira et al. 1977, Franco da Silveira and Colli 1981), the authors identified SM only on the basis of its thin layer chromatography mobility but they did not report the presence of IPC, which probably was mistaken for SM.
In summary, IPC synthase activity in T. cruzi was identified by in vivo and in vitro experiments and the gene encoding this enzyme has been identified and expressed in a cell-free system. IPC biosynthesis is increased during differentiation of trypomastigotes into amastigotes, an essential step of its life cycle (Salto et al. 2003). Aureobasidin A (AbA) a potent inhibitor of fungal IPC synthase inhibited trypomastigote differentiation into amastigotes, leading to the increased appearance of intermediate forms called “fat trypomastigotes“ and decreased expression of Ssp4 (Salto et al. 2003). The results suggested that an IPC-synthesizing enzyme was induced upon differentiation and that inhibition of this enzyme led to accumulation of precursors such as ceramide that could be toxic to the cells (Salto et al. 2003). However, recent studies have suggested that IPC synthesis would be an indirect target of AbA in T. cruzi. The IPC synthase activity of T. cruzi amastigotes was inhibited by only 30% by AbA (72 μM) in an in vitro assay monitoring incorporation of a non-natural C-6 ceramide derivative in IPC, and the drug inhibited differentiation of intracellular forms at 22 μM (Figueiredo et al. 2005). In T. brucei, TbSLS1 was inhibited by only 25% in the presence of 500 nM AbA and growth of bloodstream forms was totally inhibited by 2.5 μM AbA (EC50 = 0.8 μM) (Sevova et al. 2010), although these high concentrations of AbA were not tested on the enzyme activity.
In L. major, sphingolipids are essential for differentiation to infective metacyclic parasites (Zhang et al. 2003) and for acidocalcisome biogenesis (Zhang et al. 2005) and even though it was described that LmIPCS is inhibited by AbA (Denny et al. 2006), recent studies suggest that the toxicity of AbA may involve another target (Zhang and Beverley 2010). The simplest explanation for the observed inhibition by AbA of glycerolipid-to-ceramide remodeling in T. cruzi (Salto et al. 2003) is the inhibition of the phosphoryl transfer of a GPI anchored protein or GIPL from the glycerolipid carrier to the ceramide acceptor, a reaction that is essentially identical to sphingolipid synthesis (phosphoryl transfer of a head group from phospholipids to ceramide).
HYDROLYSIS OF INOSITOLPHOSPHOCERAMIDE IN T. CRUZI AS COMPARED TO OTHER TRYPANOSOMATIDS
The enzyme that catalyzes the hydrolysis of inositolphosphoceramide in yeast is called Inositolphosphosphingolipid phospholipase C (ISC) with the reaction generating ceramide and inositol phosphate (Sawai et al. 2000). The ISC1 gene was first identified in Saccharomyces cerevisiae (Sawai et al. 2000) and latter found in other fungi (Shea et al. 2006). An orthologue was recently studied in L. major (LmISC) and the protein product was shown to have a potent sphingomyelinase activity in addition to hydrolyzing IPC (Zhang et al. 2009). The ortologue in T. brucei was found to be a neutral sphingomyelinase involved in the trafficking of the variant surface glycoprotein in the bloodstream forms (Young and Smith 2010). The T. brucei gene is essential and therefore a validated drug target (Young and Smith 2010). A similar orthologue is also present in the T. cruzi genome (Table 1). The predicted protein (TcISC) contains a domain homologous to the P-loop motif of nucleotide binding proteins, termed the P-loop-like (PLL) domain that has been implicated in catalytic activity, and two transmembrane domains near the C terminus as occurs with yeast Isc1p (Clarke et al. 2006). A number of key amino acid residues important for activity are also conserved. Similar to fungal ISC genes, TcISC shows homology to neutral sphingomyelinases from trypanosomatids and mammals, which is expected because of the similarities between sphingomyelin and IPC. In this regard, S. cerevisiae Isc1p also possess sphingomyelinase activity (Sawai et al. 2000).
Although TcISC has not been studied yet, IPC can also be hydrolyzed by a phosphoinositide phospholipase C present in T. cruzi (TcPI-PLC) (Salto et al. 2002). TcPI-PLC is a rather unusual PI-PLC, that unlike other PI-PLCs that catalyze the hydrolysis of phosphatidylinositol 4,5-bisphosphate to generate the second messengers inositol 1,4,5-trisphosphate and diacylglycerol, it can also hydrolyze PI and IPC with similar efficiency (Salto et al. 2002). Unlike other mammalian-type PI-PLCs, TcPI-PLC does not have a pleckstrin homology domain to bind to the plasma membrane, has a highly charged region between the catalytic X and Y domains, and is N-myristoylated and palmitoylated in vivo (Furuya et al. 2000). This lipid modification is important for its plasma membrane localization, and for stimulation of differentiation of the infective trypomastigotes into the extracellular amastigotes (Okura et al. 2005). Recent work has shown that TcPI-PLC localizes to the outer leaflet of the plasma membrane of amastigotes and has therefore access to GPI-anchored glycoproteins (Martins 2010b). TcPI-PLC has two peaks of expression in amastigotes, the first 18–24 h after infection and the second before differentiation of amastigotes into trypomastigotes (Martins 2010a). The first peak of expression coincides with shedding of Ssp4 from the surface of amastigotes and with ceramide production (Bertello et al. 1996) suggesting its involvement in GPI-anchor hydrolysis. TcPI-PLC is apparently an essential gene because numerous attempts to knocking it out have been unsuccessful (Okura et al. 2005). Overexpression of TcPI-PLC led to a faster differentiation rate, while antisense oligonucleotide-treated trypomastigotes showed a reduced rate of differentiation in comparison to controls, as well as accumulation of intermediate forms (Okura et al. 2005).
INCORPORATION OF IPC INTO T. CRUZI GPIS
There is evidence for the remodeling of the lipid constituent of GPI anchors of T. cruzi from a glycerolipid to IPC. Although IPC is a major component of the total inositolphospholipids (IPL) of the three main stages of T. cruzi (Salto et al. 2003, Bertello et al. 1995, Uhrig et al. 1996) it is not found in the complete precursors for the GPI anchors detected in parasites differentiated into metacyclic forms (Heise et al. 1996). In addition, IPC is not a substrate for the incorporation of N-acetyl-D-glucosamine (GlcNAc) (Bertello et al. 2004), the first sugar transferred from the nucleotide to the IPL in the biosynthesis of GPIs (Ferguson 1999) (Fig. 4). Experiments involving incubation of T. cruzi membranes with UDP-[3H]GlcNAc showed that GlcNAc is incorporated into the AAG in the first step and to a minor extent into the DAG containing IPL. The DAG precursor is rapidly deacylated by an endogenous PLA1 to yield GlcN(lyso-2-acyl)PI. Pulse-chase experiments with the nucleotide GDP-Man showed that the GlcN(alkylacyl)PI and the GlcN(lysoacyl)PI are precursors for the biosynthesis of GIPLs (Fig. 4). Also, in the absence of GDP-Man the phosphorylated metabolite, phosphateGlcNH2-(lysoacyl)PI, was identified. However, no lysoacylPI is found in the mature, free or anchored GIPLs in T. cruzi suggesting that a GIPL with a lysoacyl or an alkylacyl PI could be a precursor for the remodeling reaction exchanging the glycerolipid for ceramide (Bertello et al. 2004, Heise et al. 1996). On the other hand, reaction of the GIPL precursors with bPI-PLC indicated the absence of O-2-inositol acylation, a feature observed in GIPLs from mammals (Orlean and Menon 2007), yeast (Fujita and Jigami 2008) and T. brucei (Guther and Ferguson 1995). In T. cruzi, a precursor acylated in position-2 of inositol was described (Heise et al. 1996) although its structure was not conclusively demonstrated.
Fig. 4.
Proposed biosynthesis of GIPLs in T. cruzi. Inositolphosphoceramide is not an acceptor for the first sugar in the biosynthesis of GIPLs. The glycerolipid in the PI acceptor has AAG or DAG. DAG is not present in mature GIPLs that may contain ceramide. Thus, a remodeling step must introduce a ceramide. The remodeling enzymes were not characterized in T. cruzi. Abbreviations: phosphatidylinositol (PI), alkylacylglycerol (AAG), diacylglycerol (DAG), inositolphosphoceramide (IPC), ceramide (Cer).
In summary, the results suggest that remodeling of the lipid must occur in T. cruzi for the introduction of ceramide into GIPLs. This interesting process was well studied in S. cerevisiae mainly by Conzelmann’s group (Ghugtyal et al. 2007). In S. cerevisiae, the ceramide replaces the DAG in the GPI lipid moiety after it is transferred to the protein in the endoplasmic reticulum (Sipos et al. 1997). This would not be a requisite for T. cruzi as ceramide-containing GIPLs not linked to protein, like the lipopeptidophosphoglycan (LPPG) (Fig. 4) (Lederkremer 2009), are abundant on the surface of epimastigotes. The relative sequence and the enzymes implicated in the introduction of galactofuranose, aminoethylphosphonic acid and ceramide in the GIPLs of T. cruzi (Fig. 3) are not known and constitute attractive subjects for investigation.
IS IPC THE DONOR FOR THE CERAMIDE IN T. CRUZI GPIS?
In S. cerevisiae the ceramide used for remodeling of GPI anchors is newly synthesized and not derived from IPC (Reggiori and Conzelmann 1998). This was demonstrated because AbA does not inhibit remodeling of the glycerolipid in the GPI to a ceramide (Reggiori and Conzelmann 1998). It was suggested that remodeling occurs through a transesterification reaction performed by a phosphodiesterase exchanging either DAG with the ceramide or phosphatidic acid by ceramide phosphate. The protein CWH43 was found to be responsible for replacement of DAG by ceramide in the GPI of S. cerevisiae (Ghugtyal et al. 2007, Umemura et al. 2007). In contrast to S. cerevisiae, during differentiation of trypomastigotes to amastigotes, Aureobasidin A (AbA) (at 5.5 μM) inhibited IPC biosynthesis in vivo, and almost completely the biosynthesis of ceramide-containing GIPLs (Salto et al. 2003). According to the results from other laboratories obtained using a cell free system and discussed in a previous section, the drug minght be inhibiting the remodelases. However, even considering that AbA is a poor inhibitor of IPC in T. cruzi as compared to yeast, the ratio of glycerolipid to IPC would be altered and affect the parasite. Development of novel drugs for the inhibition of IPC synthase and GPI remodeling may shed light on the importance of IPC metabolism in trypanosomatid pathogenesis.
LIPID REMODELING IN T. CRUZI GIPLS
In addition to the remodeling of glycerolipid to ceramide, there is also evidence for remodeling of the fatty acid in ceramide-based GPI anchored proteins and GIPLs. Lignoceric acid (C24:0) is the major fatty acid in LPPG but no lignoceric acid is found in IPCs or free ceramides. Thus, it must be introduced by a remodeling reaction on the GIPLs (Lederkremer 2009).
On incubation of radioactive IPC or GIPLs with membranes of the parasite, free fatty acid and ceramide are detected and their amounts increase with time (Salto et al. 2003, Bertello et al. 2000). In contrast, after incubation, the radioactivity ratio of [3H]-long chain base to [3H]-fatty acid in the ceramide released by the endogenous PI-PLC is four times higher than in the original IPC. The lower amount of radioactivity found in the fatty acid of the free ceramide suggests that IPC-acylhydrolase and IPC acyltransferase activities should be involved in replacing the radioactive fatty acid by a non-labeled fatty acid.
In T. cruzi GIPLs, the lipid changes from an alkylacylglycerol at the logarithmic phase of growth to a ceramide at the stationary phase (de Lederkremer et al. 1993). The same remodeling takes place in the GPI that anchors the abundant mucins to the membrane when the parasite differentiates from epimastigotes to metacyclic trypomastigotes (Serrano et al. 1995). In both cases the glycan remains unchanged during transformation. AAG, but not DAG, was also detected in mature GIPLs or GPI anchors. However DAG, together with AAG, was identified in the first precursor of the biosynthesis. Deacylation of the DAG by a PLA1 was very fast and monoacyl glycerol (lyso-2-AG) was the major lipid in GlcNH2PI, together with AAG (Lederkremer 2009).
In summary, since lysoAG was not found in mature GIPLs from T. cruzi a remodeling reaction replacing AAG or lysoAG with ceramide in the GIPLs and GPIs, must take place. The structural and enzymatic requirements for the remodeling process have not been elucidated.
CONCLUSIONS
In contrast to mammals and other trypanosomatids, T. cruzi utilizes IPC rather than glycerolipids as the lipid anchor of GIPLs and several glycoproteins. In addition, there are two remodeling phenomena occurring in GPI anchored proteins and GIPLs: glycerolipid to ceramide conversion and fatty acid remodeling. Remodeling of the lipid with introduction of ceramide into T. cruzi GPIs has no mammalian counterpart. The available results point to IPC as the donor for ceramide but the acceptor substrate has not been fully identified. GIPL precursors with lyso-acylglycerol or AAG were identified and could be the substrates for the remodeling reaction because no lysoAG or DAG was found in T. cruzi GPI anchors. In addition, the pathway involved in the introduction of the amide linked lignoceric acid (C24:0) into the ceramide of LPPG has not been elucidated. The identification and subcellular localization of the enzymes exchanging the glycerolipid by ceramide and the fatty acid in the ceramide may provide valuable targets. The biological significance of ceramide remodeling is still unknown but the presence of ceramide in GPI anchors could be related to their active shedding to the medium as is the case of the trans-sialidase and the Ssp4 glycoproteins.
Aureobasidin A does not appear to inhibit T. cruzi IPC synthase at similar concentrations to those that inhibit yeast IPC synthase although it inhibits the formation of IPC in vivo and growth and differentiation of trypomastigotes into amastigotes at higher concentrations, suggesting that it inhibits another enzyme of this pathway. Inhibition of this pathway, which is absent in mammalian cells could be useful for the development of new chemotherapeutic drugs against Chagas disease.
Acknowledgments
This work was supported by grants from Agencia Nacional de Promoción Científica y Tecnológica and Universidad de Buenos Aires to R.M.L. R.M.L and R.A. are research members from the National Research Council (CONICET), Argentina. R.D. was supported by a grant from the U.S. National Institutes of Health (AI-058297).
Abbreviations used
- AAG
alkylacyl glycerol
- AbA
aureobasidin A
- AEP
aminoethylphosphonic acid
- bPI-PLC
bacterial phosphatidylinositol phospholipase C
- DAG
diacylglycerol
- DHCD
dihydroceramide desaturase
- DHCS
dihydroceramide synthase
- EPC
ethanolaminephosphoceramide
- GIPL
glycoinositolphospholipid
- GPI
glycosylphosphatidylinositol
- GPI-AP
GPI-anchored proteins
- IPC
inositolphosphoceramide
- IPCS
inositolphosphoceramide synthase
- IPL
inositolphospholipids
- ISC
inositolphosphosphingolipid phospholipase C
- LPPG
lipopeptidophosphoglycan
- MAG
monoacylglycerol
- PI
phosphatidylinositol
- PLC
phospholipase C
- SM
sphingomyelin
- SK
sphingosine kinase
- SPL
sphingosine-1-phosphate lyase
- SPT
serine palmitoyl transferase
- TcPI-PLC
T. cruzi phosphoinositide phospholipase C
References
- Abuin G, Couto AS, de Lederkremer RM, Casal OL, Galli C, Colli W, Alves MJ. Trypanosoma cruzi: the Tc-85 surface glycoprotein shed by trypomastigotes bears a modified glycosylphosphatidylinositol anchor. Exp Parasitol. 1996;82:290–297. doi: 10.1006/expr.1996.0036. [DOI] [PubMed] [Google Scholar]
- Agusti R, Couto AS, Campetella OE, Frasch AC, de Lederkremer RM. The trans-sialidase of Trypanosoma cruzi is anchored by two different lipids. Glycobiology. 1997;7:731–735. doi: 10.1093/glycob/7.6.731. [DOI] [PubMed] [Google Scholar]
- Armesto J, Hannappel E, Leopold K, Fischer W, Bublitz R, Langer L, Cumme GA, Horn A. Microheterogeneity of the hydrophobic and hydrophilic part of the glycosylphosphatidylinositol anchor of alkaline phosphatase from calf intestine. Eur J Biochem. 1996;238:259–269. doi: 10.1111/j.1432-1033.1996.0259q.x. [DOI] [PubMed] [Google Scholar]
- Bertello LE, Andrews NW, de Lederkremer RM. Developmentally regulated expression of ceramide in Trypanosoma cruzi. Mol Biochem Parasitol. 1996;79:143–151. doi: 10.1016/0166-6851(96)02645-x. [DOI] [PubMed] [Google Scholar]
- Bertello LE, Alves MJ, Colli W, de Lederkremer RM. Inositolphosphoceramide is not a substrate for the first steps in the biosynthesis of glycoinositolphospholipids in Trypanosoma cruzi. Mol Biochem Parasitol. 2004;133:71–80. doi: 10.1016/j.molbiopara.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Bertello LE, Goncalvez MF, Colli W, de Lederkremer RM. Structural analysis of inositol phospholipids from Trypanosoma cruzi epimastigote forms. Biochem J. 1995;310:255–261. doi: 10.1042/bj3100255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertello LE, Alves MJ, Colli W, de Lederkremer RM. Evidence for phospholipases from Trypanosoma cruzi active on phosphatidylinositol and inositolphosphoceramide. Biochem J. 2000;345:77–84. [PMC free article] [PubMed] [Google Scholar]
- Buscaglia CA, Campo VA, Frasch AC, Di Noia JM. Trypanosoma cruzi surface mucins: host-dependent coat diversity. Nat Rev Microbiol. 2006;4:229–236. doi: 10.1038/nrmicro1351. [DOI] [PubMed] [Google Scholar]
- Clarke CJ, Snook CF, Tani M, Matmati N, Marchesini N, Hannun YA. The extended family of neutral sphingomyelinases. Biochemistry. 2006;45:11247–11256. doi: 10.1021/bi061307z. [DOI] [PubMed] [Google Scholar]
- de Lederkremer RM, Lima C, del CVM. Ceramide 1-phosphate is released from a glycoinositolphosphoceramide of Trypanosoma cruzi by rat blood plasma. Mol Biochem Parasitol. 1996;79:219–223. doi: 10.1016/0166-6851(96)02644-8. [DOI] [PubMed] [Google Scholar]
- de Lederkremer RM, Lima C, Ramirez MI, Casal OL. Structural features of the lipopeptidophosphoglycan from Trypanosoma cruzi common with the glycophosphatidylinositol anchors. Eur J Biochem. 1990;192:337–345. doi: 10.1111/j.1432-1033.1990.tb19232.x. [DOI] [PubMed] [Google Scholar]
- de Lederkremer RM, Lima CE, Ramirez MI, Goncalvez MF, Colli W. Hexadecylpalmitoylglycerol or ceramide is linked to similar glycophosphoinositol anchor-like structures in Trypanosoma cruzi. Eur J Biochem. 1993;218:929–936. doi: 10.1111/j.1432-1033.1993.tb18449.x. [DOI] [PubMed] [Google Scholar]
- de Lederkremer RM, Lima C, Ramirez MI, Ferguson MA, Homans SW, Thomas-Oates J. Complete structure of the glycan of lipopeptidophosphoglycan from Trypanosoma cruzi epimastigotes. J Biol Chem. 1991;266:23670–23675. [PubMed] [Google Scholar]
- Denny PW, Shams-Eldin H, Price HP, Smith DF, Schwarz RT. The protozoan inositol phosphorylceramide synthase: a novel drug target that defines a new class of sphingolipid synthase. J Biol Chem. 2006;281:28200–28209. doi: 10.1074/jbc.M600796200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson RC, Lester RL. Metabolism and selected functions of sphingolipids in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 1999;1438:305–321. doi: 10.1016/s1388-1981(99)00068-2. [DOI] [PubMed] [Google Scholar]
- Ferguson MA. The structure, biosynthesis and functions of glycosylphosphatidylinositol anchors, and the contributions of trypanosome research. J Cell Sci. 1999;112:2799–2809. doi: 10.1242/jcs.112.17.2799. [DOI] [PubMed] [Google Scholar]
- Ferguson MA, Low MG, Cross GA. Glycosyl-sn-1,2-dimyristylphosphatidylinositol is covalently linked to Trypanosoma brucei variant surface glycoprotein. J Biol Chem. 1985;260:14547–14555. [PubMed] [Google Scholar]
- Figueiredo JM, Dias WB, Mendonca-Previato L, Previato JO, Heise N. Characterization of the inositol phosphorylceramide synthase activity from Trypanosoma cruzi. Biochem J. 2005;387:519–529. doi: 10.1042/BJ20041842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco da Silveira J, Colli W. Chemical composition of the plasma membrane from epimastigote forms of Trypanosoma cruzi. Biochim Biophys Acta. 1981;644:341–350. doi: 10.1016/0005-2736(81)90392-8. [DOI] [PubMed] [Google Scholar]
- Fujita M, Jigami Y. Lipid remodeling of GPI-anchored proteins and its function. Biochim Biophys Acta. 2008;1780:410–420. doi: 10.1016/j.bbagen.2007.08.009. [DOI] [PubMed] [Google Scholar]
- Furuya T, Kashuba C, Docampo R, Moreno SN. A novel phosphatidylinositol-phospholipase C of Trypanosoma cruzi that is lipid modified and activated during trypomastigote to amastigote differentiation. J Biol Chem. 2000;275:6428–6438. doi: 10.1074/jbc.275.9.6428. [DOI] [PubMed] [Google Scholar]
- Gerold P, Schofield L, Blackman MJ, Holder AA, Schwarz RT. Structural analysis of the glycosyl-phosphatidylinositol membrane anchor of the merozoite surface proteins-1 and -2 of Plasmodium falciparum. Mol Biochem Parasitol. 1996;75:131–143. doi: 10.1016/0166-6851(95)02518-9. [DOI] [PubMed] [Google Scholar]
- Ghugtyal V, Vionnet C, Roubaty C, Conzelmann A. CWH43 is required for the introduction of ceramides into GPI anchors in Saccharomyces cerevisiae. Mol Microbiol. 2007;65:1493–1502. doi: 10.1111/j.1365-2958.2007.05883.x. [DOI] [PubMed] [Google Scholar]
- Guther ML, Ferguson MA. The role of inositol acylation and inositol deacylation in GPI biosynthesis in Trypanosoma brucei. EMBO J. 1995;14:3080–3093. doi: 10.1002/j.1460-2075.1995.tb07311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guther ML, Lee S, Tetley L, Acosta-Serrano A, Ferguson MA. GPI-anchored proteins and free GPI glycolipids of procyclic form Trypanosoma brucei are nonessential for growth, are required for colonization of the tsetse fly, and are not the only components of the surface coat. Mol Biol Cell. 2006;17:5265–5274. doi: 10.1091/mbc.E06-08-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise N, Raper J, Buxbaum LU, Peranovich TM, de Almeida ML. Identification of complete precursors for the glycosylphosphatidylinositol protein anchors of Trypanosoma cruzi. J Biol Chem. 1996;271:16877–16887. doi: 10.1074/jbc.271.28.16877. [DOI] [PubMed] [Google Scholar]
- Kaneshiro ES, Wyder MA. Lipophosphoglycan antigen shedding by Leishmania donovani. J Eukaryot Microbiol. 1993;40:336–340. doi: 10.1111/j.1550-7408.1993.tb04925.x. [DOI] [PubMed] [Google Scholar]
- Kaneshiro ES, Gottlieb M, Dwyer DM. Cell surface origin of antigens shed by Leishmania donovani during growth in axenic culture. Infect Immun. 1982;37:558–567. doi: 10.1128/iai.37.2.558-567.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneshiro ES, Jayasimhulu K, Lester RL. Characterization of inositol lipids from Leishmania donovani promastigotes: identification of an inositol sphingophospholipid. J Lipid Res. 1986;27:1294–1303. [PubMed] [Google Scholar]
- Lederkremer RM, Bertello LE. Glycoinositolphospholipids, free and as anchors of proteins, in Trypanosoma cruzi. Curr Pharm Des. 2001;7:1165–1179. doi: 10.2174/1381612013397519. [DOI] [PubMed] [Google Scholar]
- Lederkremer RMd, Agusti R. Glycobiology of Trypanosoma cruzi. Adv Carbohydr Chem Biochem. 2009;62:311–366. doi: 10.1016/S0065-2318(09)00007-9. [DOI] [PubMed] [Google Scholar]
- Low MG, Stiernberg J, Waneck GL, Flavell RA, Kincade PW. Cell-specific heterogeneity in sensitivity of phosphatidylinositol-anchored membrane antigens to release by phospholipase C. J Immunol Methods. 1988;113:101–111. doi: 10.1016/0022-1759(88)90386-9. [DOI] [PubMed] [Google Scholar]
- Martins VP, Galizzi M, Salto ML, Docampo R, Moreno SNJ. Developmental expression of a Trypanosoma cruzi phosphoinositide-specific phospholipase C in amastigotes and stimulation of host phosphoinositide hydrolysis. Infect Immun. 2010a;78:4206–4212. doi: 10.1128/IAI.00473-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins VP, Okura M, Maric D, Engman DM, Vieira M, Docampo R, Moreno SNJ. Acylation-dependent export of Trypanosoma cruzi phosphoinositide-specific phospholipase C to the outer surface of amastigotes. J Biol Chem. 2010b;285:30906–30917. doi: 10.1074/jbc.M110.142190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medof ME, Walter EI, Roberts WL, Haas R, Rosenberry TL. Decay accelerating factor of complement is anchored to cells by a C-terminal glycolipid. Biochemistry. 1986;25:6740–6747. doi: 10.1021/bi00370a003. [DOI] [PubMed] [Google Scholar]
- Mina JG, Pan SY, Wansadhipathi NK, Bruce CR, Shams-Eldin H, Schwarz RT, Steel PG, Denny PW. The Trypanosoma brucei sphingolipid synthase, an essential enzyme and drug target. Mol Biochem Parasitol. 2009;168:16–23. doi: 10.1016/j.molbiopara.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Nakayasu ES, Yashunsky DV, Nohara LL, Torrecilhas AC, Nikolaev AV, Almeida IC. GPIomics: global analysis of glycosylphosphatidylinositol-anchored molecules of Trypanosoma cruzi. Mo l Syst Biol. 2009;5:261, 1–17. doi: 10.1038/msb.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okura M, Fang J, Salto ML, Singer RS, Docampo R, Moreno SN. A lipid-modified phosphoinositide-specific phospholipase C (TcPI-PLC) is involved in differentiation of trypomastigotes to amastigotes of Trypanosoma cruzi. J Biol Chem. 2005;280:16235–16243. doi: 10.1074/jbc.M414535200. [DOI] [PubMed] [Google Scholar]
- Oliveira MM, Timm SL, Costa SC. Lipid composition of Trypanosoma cruzi. Comp Biochem Physiol B. 1977;58:195–199. doi: 10.1016/0305-0491(77)90109-2. [DOI] [PubMed] [Google Scholar]
- Orlean P, Menon AK. Thematic review series: lipid posttranslational modifications. GPI anchoring of protein in yeast and mammalian cells, or: how we learned to stop worrying and love glycophospholipids. J Lipid Res. 2007;48:993–1011. doi: 10.1194/jlr.R700002-JLR200. [DOI] [PubMed] [Google Scholar]
- Previato JO, Gorin PA, Mazurek M, Xavier MT, Fournet B, Wieruszesk JM, Mendonca-Previato L. Primary structure of the oligosaccharide chain of lipopeptidophosphoglycan of epimastigote forms of Trypanosoma cruzi. J Biol Chem. 1990;265:2518–2526. [PubMed] [Google Scholar]
- Previato JO, Wait R, Jones C, DosReis GA, Todeschini AR, Heise N, Previato LM. Glycoinositolphospholipid from Trypanosoma cruzi: structure, biosynthesis and immunobiology. Adv Parasitol. 2004;56:1–41. doi: 10.1016/s0065-308x(03)56001-8. [DOI] [PubMed] [Google Scholar]
- Reggiori F, Conzelmann A. Biosynthesis of inositol phosphoceramides and remodeling of glycosylphosphatidylinositol anchors in Saccharomyces cerevisiae are mediated by different enzymes. J Biol Chem. 1998;273:30550–30559. doi: 10.1074/jbc.273.46.30550. [DOI] [PubMed] [Google Scholar]
- Roberts WL, Kim BH, Rosenberry TL. Differences in the glycolipid membrane anchors of bovine and human erythrocyte acetylcholinesterases. Proc Natl Acad Sci U S A. 1987;84:7817–7821. doi: 10.1073/pnas.84.22.7817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salto ML, Furuya T, Moreno SN, Docampo R, de Lederkremer RM. The phosphatidylinositol-phospholipase C from Trypanosoma cruzi is active on inositolphosphoceramide. Mol Biochem Parasitol. 2002;119:131–133. doi: 10.1016/s0166-6851(01)00392-9. [DOI] [PubMed] [Google Scholar]
- Salto ML, Bertello LE, Vieira M, Docampo R, Moreno SN, de Lederkremer RM. Formation and remodeling of inositolphosphoceramide during differentiation of Trypanosoma cruzi from trypomastigote to amastigote. Eukaryot Cell. 2003;2:756–768. doi: 10.1128/EC.2.4.756-768.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai H, Okamoto Y, Luberto C, Mao C, Bielawska A, Domae N, Hannun YA. Identification of ISC1 (YER019w) as inositol phosphosphingolipid phospholipase C in Saccharomyces cerevisiae. J Biol Chem. 2000;275:39793–39798. doi: 10.1074/jbc.M007721200. [DOI] [PubMed] [Google Scholar]
- Serrano AA, Schenkman S, Yoshida N, Mehlert A, Richardson JM, Ferguson MA. The lipid structure of the glycosylphosphatidylinositol-anchored mucin-like sialic acid acceptors of Trypanosoma cruzi changes during parasite differentiation from epimastigotes to infective metacyclic trypomastigote forms. J Biol Chem. 1995;270:27244–27253. doi: 10.1074/jbc.270.45.27244. [DOI] [PubMed] [Google Scholar]
- Sevova ES, Goren MA, Schwartz KJ, Hsu FF, Turk J, Fox BG, Bangs JD. Cell-free synthesis and functional characterization of sphingolipid synthases from parasitic trypanosomatid protozoa. J Biol Chem. 2010;285:20580–20587. doi: 10.1074/jbc.M110.127662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea JM, Kechichian TB, Luberto C, Del Poeta M. The cryptococcal enzyme inositol phosphosphingolipid-phospholipase C confers resistance to the antifungal effects of macrophages and promotes fungal dissemination to the central nervous system. Infect Immun. 2006;74:5977–5988. doi: 10.1128/IAI.00768-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos G, Reggiori F, Vionnet C, Conzelmann A. Alternative lipid remodelling pathways for glycosylphosphatidylinositol membrane anchors in Saccharomyces cerevisiae. EMBO J. 1997;16:3494–3505. doi: 10.1093/emboj/16.12.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Butikofer P. Lipid metabolism in Trypanosoma brucei. Mol Biochem Parasitol. 2010;172:66–79. doi: 10.1016/j.molbiopara.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TK, Kimmel J, Azzouz N, Shams-Eldin H, Schwarz RT. The role of inositol acylation and inositol deacylation in the Toxoplasma gondii glycosylphosphatidylinositol biosynthetic pathway. J Biol Chem. 2007;282:32032–32042. doi: 10.1074/jbc.M703784200. [DOI] [PubMed] [Google Scholar]
- Straus AH, Levery SB, Jasiulionis MG, Salyan ME, Steele SJ, Travassos LR, Hakomori S, Takahashi HK. Stage-specific glycosphingolipids from amastigote forms of Leishmania (L.) amazonensis. Immunogenicity and role in parasite binding and invasion of macrophages. J Biol Chem. 1993;268:13723–13730. [PubMed] [Google Scholar]
- Sutterwala SS, Hsu FF, Sevova ES, Schwartz KJ, Zhang K, Key P, Turk J, Beverley SM, Bangs JD. Developmentally regulated sphingolipid synthesis in African trypanosomes. Mol Microbiol. 2008;70:281–296. doi: 10.1111/j.1365-2958.2008.06393.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhrig ML, Couto AS, Colli W, de Lederkremer RM. Characterization of inositolphospholipids in Trypanosoma cruzi trypomastigote forms. Biochim Biophys Acta. 1996;1300:233–239. doi: 10.1016/0005-2760(96)00021-5. [DOI] [PubMed] [Google Scholar]
- Umemura M, Fujita M, Yoko OT, Fukamizu A, Jigami Y. Saccharomyces cerevisiae CWH43 is involved in the remodeling of the lipid moiety of GPI anchors to ceramides. Mol Biol Cell. 2007;18:4304–4316. doi: 10.1091/mbc.E07-05-0482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbina JA, Docampo R. Specific chemotherapy of Chagas disease: controversies and advances. Trends Parasitol. 2003;19:495–501. doi: 10.1016/j.pt.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Young SA, Smith TK. The essential neutral sphingomyelinase is involved in the trafficking of the variant surface glycoprotein in the bloodstream form of Trypanosoma brucei. Mol Microbiol. 2010;76:1461–1482. doi: 10.1111/j.1365-2958.2010.07151.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Beverley SM. Phospholipid and sphingolipid metabolism in Leishmania. Mol Biochem Parasitol. 2010;170:55–64. doi: 10.1016/j.molbiopara.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Hsu FF, Scott DA, Docampo R, Turk J, Beverley SM. Leishmania salvage and remodelling of host sphingolipids in amastigote survival and acidocalcisome biogenesis. Mol Microbiol. 2005;55:1566–1578. doi: 10.1111/j.1365-2958.2005.04493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Showalter M, Revollo J, Hsu FF, Turk J, Beverley SM. Sphingolipids are essential for differentiation but not growth in Leishmania. EMBO J. 2003;22:6016–6026. doi: 10.1093/emboj/cdg584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang O, Wilson MC, Xu W, Hsu FF, Turk J, Kuhlmann FM, Wang Y, Soong L, Key P, Beverley SM, Zhang K. Degradation of host sphingomyelin is essential for Leishmania virulence. PLoS Pathog. 2009;5:e1000692. doi: 10.1371/journal.ppat.1000692. [DOI] [PMC free article] [PubMed] [Google Scholar]