Abstract
Background
We have shown that administration of heparin binding EGF-like growth factor (HB-EGF) protects the intestines from experimental necrotizing enterocolitis (NEC). We have also demonstrated that systemically administered mesenchymal stem cells (MSC) can engraft into injured intestines. The current study investigated the effects of HB-EGF on MSC in vitro, and whether MSC and HB-EGF can act synergistically to prevent NEC in vivo.
Study Design
In vitro, the effect of HB-EGF on MSC proliferation, migration and apoptosis was determined. In vivo, rat pups received MSC either intraperitoneally (IP) or intravenously (IV). Pups were assigned to: (1) breast-feeding, (2) experimental NEC, (3) NEC+HB-EGF, (4) NEC+MSC IP, (5) NEC+HB-EGF+MSC IP, (6) NEC+MSC IV, or (7) NEC+HB-EGF+MSC IV. MSC engraftment, histologic injury, intestinal permeability and mortality were determined.
Results
HB-EGF promoted MSC proliferation and migration, and decreased MSC apoptosis in vitro. In vivo, MSC administered IV had increased engraftment into NEC-injured intestine compared to MSC administered IP (p<0.05). HB-EGF increased engraftment of IP-administered MSC (p<0.01) and IV-administered MSC (p<0.05). Pups in Groups 3-7 had a decreased incidence of NEC compared to non-treated pups (Group 2), with the lowest incidence in pups treated with HB-EGF+MSC IV (p<0.01). Pups in Group 7 had a significantly decreased incidence of intestinal dilation and perforation, and had the lowest intestinal permeability, compared to other treatment groups (p<0.01). Pups in all experimental groups had significantly improved survival compared to pups exposed to NEC, with the best survival in Group 7 (p<0.05).
Conclusions
HB-EGF and MSC act synergistically to reduce injury and improve survival in experimental NEC.
INTRODUCTION
Necrotizing enterocolitis (NEC) is the most common gastrointestinal emergency of newborns, mainly affecting premature newborns with birth weights less than 1500 g.1 Approximately 7% to 10% of premature newborns will develop NEC, with mortality ranging from 20% to 50%.2 Although many studies have been performed during the past several decades, the morbidity and mortality of NEC remain unacceptably high.2
We initially identified heparin binding EGF-like growth factor (HB-EGF) two decades ago,3 and in a series of experiments we have shown that administration of HB-EGF can be used successfully in the treatment of experimental NEC,4-11 intestinal ischemia/reperfusion injury,12-21 and hemorrhagic shock and resuscitation.22-26 HB-EGF protects the intestines from experimental NEC and other forms of intestinal injury, in part, by protecting intestinal stem cells (ISC) from injury,9 by promoting the proliferation and migration of enterocytes,7 by increasing microvascular villous blood flow,8 by increasing gut barrier function,4 and by reducing intestinal apoptosis.6
In recent years, mesenchymal stem cells (MSC) have been shown to protect various tissues including the intestines from injury.27, 28 MSC can engraft into injured tissues where they can differentiate to replace injured cells.29, 30 In addition, MSC may exert paracrine actions by secreting protective factors that then act on injured cells.31, 32
In previous studies of MSC administration in neonatal rats or mice, MSC were administered intraperitoneally33 or via other local routes34-36 due to the small size of the recipient animals. To date, systemic administration of stem cell therapy has not been applied in neonatal models of NEC. We have previously established a successful technique for systemic delivery of MSC in newborn rat pups via umbilical vein injection, with good distribution of MSC in multiple organs including the intestines.37
In the current study, we first examined the effects of HB-EGF on MSC in vitro, and then investigated whether IV- or IP-administered MSC protects the intestines from NEC, whether HB-EGF promotes MSC engraftment into NEC injured intestines, and whether MSC and HB-EGF act synergistically to protect the intestines from NEC.
METHODS
Ethics Statement
All animal procedures (Protocol # 04203AR) were approved by the Institutional Animal Care and Use Committee (IACUC) at the Research Institute at Nationwide Children’s Hospital. Veterinarians skilled in the healthcare and maintenance of rodents supervised all aspects of animal care.
Isolation, Culture and identification of MSC
Murine yellow fluorescence protein (YFP)-labeled bone marrow-derived MSC were kindly provided by Dr. Brett Hall (Research Institute at Nationwide Children’s Hospital, Columbus, OH).38 Briefly, a transgenic construct (pCX::EYFP) containing an enhanced YFP gene under the control of a chicken beta actin promoter coupled with the cytomegalovirus immediate early enhancer, was introduced into (129X1/SvJ × 129S1/Sv) F1-derived R1 mouse embryonic stem cells. The homozygotes (129-Tg (CAG-eYFP) 7AC5Nagy/J, http://jaxmice.jax.org/strain/005483.html) were used as the source of MSC. Bone marrow was harvested from the femurs and tibias of hind limbs and suspended in Dulbecco’s Modified Eagle Medium (D-MEM) Nutrient Mixture F-12/GlutaMAX-ITM medium (GIBCO Invitrogen; Carlsbad, CA). The harvested cell mixtures were pipetted and filtered through a cell strainer with 70μm nylon mesh (Becton Dickinson; Franklin Lakes, NJ). Cells were seeded in D-MEM Nutrient Mixture F-12/GlutaMAX-ITM medium supplemented with 10% MSC-qualified fetal bovine serum (FBS) (GIBCO, Grand Island, NY) and 0.01% gentamicin (GIBCO, Grand Island, NY) at 37°C in 5% CO2.
The isolated MSC were identified by vimentin staining, and adipogenic and osteogenic differentiation assays were used to confirm pleuripotency of the cells, as we have described previously.37
In vitro Studies
Proliferation Assay
MSC proliferation was determined using the CyQUANT Cell Proliferation Assay Kit (Molecular Probes, Eugene, OR). Briefly, 5000 MSCs were plated in 96 well plates in D-MEM Nutrient Mixture F-12/GlutaMAX-ITM medium (GIBCO Invitrogen; Carlsbad, CA) at 37°C in 5% CO2 for 24 h. HB-EGF (0, 25, 50, 100 ng/ml) was added to the culture medium, and 24 h later the culture medium was removed and 100 μl of dye-binding solution was added to each well. The plates were incubated at RT for 1 h. The results were quantified using a fluorescence plate reader and 485/530 nm filter. The relative value of the control groups (0 ng/ml of HB-EGF) was normalized to 100%.
Migration Assay
MSC migration was determined using the CHEMICON QCMTM 24-well fluorimetric Cell Migration Assay Kit (Millipore, Billerica, MA). Briefly, 0.3 × 106 MSCs in chemo-attractant free medium were seeded into each insert. In the lower chamber, 500 μl of serum free medium was added in the presence of HB-EGF at various concentrations (0, 25, 50, 100 ng/ml). Plates were incubated at 37°C for 24h in 5% CO2. After the medium and cells in the inserts were removed, inserts were placed into clean wells containing 225 μl of pre-warmed Cell Detachment Solution and incubated at 37°C for 30 minutes. After the cells were dislodged from the bottom of the inserts, 75 μl of CyQUANT GR Dye/Lysis Buffer mixture were added to the detachment solution with the cells that migrated through the membrane. Plates were incubated at RT for 15 min. Migration was quantified using a fluorescent plate reader and 490/520 nm filter set. The relative value of the control group (0 ng/ml of HB-EGF) was normalized to 100%.
Apoptosis Assay
Cleaved caspase-3 immunofluorescent staining was performed to determine MSC apoptosis. MSCs (10 × 103) were seeded on cover slips in 6-well plates in D-MEM Nutrient Mixture F-12/GlutaMAX-ITM medium (GIBCO Invitrogen; Carlsbad, CA) for 24 h. Cells were treated with HB-EGF at various concentrations (0, 25, 50, 100 ng/ml) and exposed to normoxia or to anoxia (95%N2/5%CO2) for 24 h, followed by re-oxygenation at 37°C for an additional 24 h. MSCs were fixed with 4% paraformaldehyde at RT for 30 min. Cells were then blocked with 10% goat serum in PBST (PBS with 0.1% Tween-20), and incubated in a 1:100 dilution of rabbit cleaved-caspase 3 antibody (Cell signaling Technology, Danvers, MA) at 4°C overnight. Cells were then washed with PBST three times, and incubated in Cy3-conjugated Goat anti-Rabbit IgG (H+L) antibody (Jackson Immuno Research, West Grove, PA) at a 1:500 dilution at RT for 1 h. MSCs were washed with PBST three times and mounted with mounting medium containing 4′,6-Diamidino-2-Phenylindole Dihydrochloride (DAPI). Fluorescence was observed using a fluorescent microscope (Axioscope, Carl Zeiss; Jena, Germany) and Cy3 and DAPI channels. Red fluorescence stained apoptotic cells were counted in 5 random fields per well.
In vivo Studies
Cesarean section (C-section) Rat Pup Delivery
Pregnant time-dated Sprague-Dawley rats underwent C-section under CO2 anesthesia on day 21.5 of gestation. Placentas were kept moist and warm. The integrity of the umbilical cords was maintained in pups randomized to intravenous (IV) infusion of MSC. For pups randomized to intraperitoneal (IP) MSC injection, the umbilical cord was removed. Newborn rats were placed in a neonatal incubator at 35°C.
Preparation of MSC for administration
Prior to MSC administration, adherent MSCs were trypsinized (0.25% trypsin, Cellgro, Manassas, VA) for 3 min, and then D-MEM/F12/GlutaMAX-ITM medium supplemented with 10% MSC-qualified FBS was added to neutralize the trypsin. Cells were quantified using a hemacytometer. Supernatants were discarded and MSC pellets were resuspended in sterile saline. Suspended MSC were filtered through a cell strainer with 70 μm nylon mesh. The concentration of MSC was adjusted to 7.5×106 cells/ml, and MSC suspensions were loaded into 0.3 ml low-dose U-100 insulin syringes with 29 gauge needles (Becton Dickinson; Franklin Lakes, NJ). Prior to infusion, syringes were maintained at 4°C with continuous shaking, and MSC gently resuspended to ensure they were not aggregated prior to infusion.
Administration of MSC
MSCs were administered either IP or IV to newborn rat pups immediately following C-section. A total volume of 40 μl containing 300×103 MSCs was injected. For pups undergoing IP injection of MSC, the injection was performed in the right lower quadrant. For pups undergoing IV administration of MSC, the cells were injected through polyethylene-10 (PE-10) tubing (Becton Dickinson, Sparks, MD) that was cannulated into the umbilical vein immediately after C-section, as we have previously described in detail.37
Experimental design and animal model of NEC
Pups were randomly assigned to one of 7 groups: (1) breast-feeding (n=10), (2) NEC (n=38), (3) NEC+HB-EGF (n=43), (4) NEC+MSC IP (n=25), (5) NEC+HB-EGF+MSC IP (n=26), (6) NEC+MSC IV (n=27), or (7) NEC+HB-EGF+MSC IV (n=28). A single injection of MSC (300×103 in 40 μl) was administered to rats in Groups 4-7 either IP or IV. Pups in Groups 1-3 received an IV injection of vehicle (40 μl) only. Pups receiving HB-EGF had the growth factor added to every feed, thus it was given enterally 6 times a day at a dose of 800 μg/kg/dose, a dose and route of administration with proven efficacy in our hands.4
After MSC administration, pups were subjected to experimental NEC using a modification of the methods initially described by Barlow et al.39 Pups were kept in an incubator at 35°C and fed with hypertonic formula containing 15 g of Similac 60/40 (Ross Pediatrics, Columbus, Ohio) in 75 ml of Esbilac (Pet-Ag, New Hampshire, IL), which provided 836.8 kJ/kg per day. Starting at 0.1 ml of formula every 4 h, the volume of formula was advanced to a maximum of 0.4 ml per feed by the fifth day of life. Immediately after feeding, pups were exposed to hypoxia (100% nitrogen for 1 min), followed by hypothermia (4°C for 10 min) twice a day until the end of the experiment. Upon the development of clinical signs of NEC, pups were immediately euthanized by cervical dislocation. All surviving animals were euthanized after 96 hours. Survival of all pups was recorded daily.
Quantification of YFP-MSC engraftment into the intestines
For pups receiving administration of YFP-labeled MSC, the number of YFP-positive cells that had engrafted into the intestines was quantified. The terminal ileum was harvested and fixed in fixation solution containing 1% paraformaldehyde, 15% picric acid, and 0.1 M sodium phosphate buffer (pH 7.0), and shaken gently at 4°C overnight. Frozen sections (10 μm) were mounted in mounting medium containing DAPI (Vector Laboratories, Burlingame, CA). Fluorescence was observed using a fluorescence microscope (Axioscope, Carl Zeiss; Jena, Germany) under GFP and DAPI channels. Quantification of MSC was performed by counting YFP-positive cells per visual field at 100x magnification.
NEC evaluation
Immediately upon sacrifice, intestines were carefully removed and visually inspected for typical signs of NEC including intestinal dilation (diameter ≥2.5 mm at the ileum), intestinal narrowing (diameter ≤1.0 mm at the ileum), perforation, intraluminal bleeding, and subserosal collections of gas (pneumatosis), which were recorded. The terminal ileum was then fixed in 10% formalin for 24 h and paraffin-embedded sections made. Hematoxylin and eosin (H&E) staining was performed to evaluate the grade of histologic injury using a standard histological scoring system.40 All H&E stained sections were evaluated blindly by two independent observers, and graded as follows: Grade 0, no damage; Grade 1, epithelial cell lifting or seperation; Grade 2, sloughing of epithelial cells to the mid villus level; Grade 3, necrosis of the entire villus; or Grade 4, transmural necrosis. Grade 2, 3 or 4 injury was defined as being consistent with NEC, with Grade 3 or 4 injury being defined as severe NEC.
Mucosal permeability
Fluorescein isothiocyanate (FITC) labeled dextran molecules (FD 70, molecular weight 73,000) (Sigma-Aldrich Inc, St Louis, Mo) were used as a probe to assess the mucosal permeability of the rat pup intestines. Four hours after gavage of FD70 (750 mg/Kg) via orogastric tube, blood was collected and the plasma levels of FD70 measured with a fluorescent plate reader using a 492/515 nm filter set. The level of FD70 in the plasma was calculated based on standard dilution curves of known dextran concentrations.
Statistical Analyses
Proliferation, migration and apoptosis of cultured YFP-MSC were compared using Student’s t test. The incidence of pathological changes, the incidence of NEC, and 96 h survival rates were compared between groups using Fisher’s exact Test. The numbers of engrafted YFP-MSC and the concentrations of FD70 were compared using analysis of variance. A p value of less than 0.05 was considered statistically significant.
RESULTS
HB-EGF promotes proliferation and migration of YFP-MSC in vitro
Addition of HB-EGF significantly increased proliferation and migration of MSC over the range of HB-EGF doses tested (25-100 ng/ml) (Figure 1A, B). Addition of HB-EGF (100 ng/ml) increased the proliferation of MSC by 44.67% (144.67 ± 12.21% vs. 100.00 ± 6.40%, p<0.01) and promoted MSC migration by 110.25% (210.25 ± 6.93% vs. 100 ± 6.62%, p<0.01) compared to no addition of HB-EGF.
Figure 1.

Effect of HB-EGF on mesenchymal stem cell (MSC) proliferation, migration and apoptosis. (A) MSC proliferation assay. Statistical symbols for Student’s t test are: † ‡p<0.05 vs NA; § p<0.01 vs NA; § p<0.05 vs †. (B) MSC migration assay. Statistical symbols for Student’s t test are: † ‡ §p<0.01 vs NA; § p<0.05 vs †. (C) MSC apoptosis assay. Statistical symbols for Student’s t test are: †p<0.01 vs NA; ∥p<0.05 vs ‡ §; ∥p<0.01 vs †. Values represent mean ± SD. Each experiment was performed 4 times with each sample tested in duplicate. YFP, yellow fluorescent protein; MSC, mesenchymal stem cell; HB-EGF, heparin binding EGF-like growth factor; NA, no addition.
HB-EGF inhibits anoxia/reoxygenation-induced apoptosis of MSC in vitro
MSC exposed to anoxia/reoxygenation had significantly increased apoptosis compared to MSC grown under normoxic conditions (18.8 ± 4.0% vs. 2.9 ± 0.8%, p<0.01) (Figure 1C). Addition of HB-EGF significantly decreased MSC apoptosis at all HB-EGF doses tested (25-100 ng/ml). Addition of HB-EGF (100 ng/ml) most significantly decreased MSC apoptosis compared to no addition of HB-EGF (4.0 ± 1.6% vs. 18.8 ± 4.0%, p<0.01].
HB-EGF promotes engraftment of IP- and IV-administered MSC into NEC-afflicted intestine
IV-administered MSC had increased intestinal engraftment compared to IP-administered MSC (29.1 ± 8.4 cells vs. 21.3 ± 7.1 cells, p<0.05) (Figure 2). HB-EGF increased engraftment of both IP-administered (35.4 ± 9.3 cells vs. 21.3±7.1 cells, p<0.01) and IV-administered MSC (38.3 ± 10.2 cells vs. 29.9 ± 8.4 cells, p<0.05). As we have described previously, the YFP-positive cells had co-localized staining with the mesenchymal cell marker vimentin37 (data not shown).
Figure 2.

Effect of HB-EGF on mesenchymal stem cell (MSC) engraftment into intestines subjected to experimental necrotizing enterocolitis. Shown are representative images from pups subjected to: (A) NEC+MSC IP; (B) NEC+HB-EGF+MSC IP; (C) NEC+MSC IV; or (D) NEC+HB-EGF+MSC IV. Cell nuclei are indicated by blue fluorescent (DAPI) staining and engrafted YFP-positive mesenchymal stem cells are indicated by green fluorescent staining (white arrows). Magnification x100. (E) Quantification of MSC engraftment. Values represent mean ± SD, n=12-16 animals in each group. Statistical symbols for 1-way ANOVA with Turkey test are: †p<0.01 vs *; § p<0.05 vs ‡; ‡p<0.05 vs *. HB-EGF, heparin binding EGF-like growth factor; NEC, necrotizing enterocolitis; YFP, yellow fluorescent protein; MSC, mesenchymal stem cells; IP, intraperitoneal; IV, intravenous.
HB-EGF and MSC decrease intestinal pathology and decrease the incidence of NEC in newborn rats
The typical gross appearance of NEC-injured intestines is shown in Figure 3A, with demonstration of pneumatosis, narrowing, perforation, intraluminal bleeding and intestinal dilation. The incidence of intestinal dilation, perforation, intraluminal bleeding, intestinal narrowing, pneumatosis and cecal swelling is shown in Table 1. Treatment with HB-EGF or MSC significantly decreased the incidences of various pathological changes, with the lowest incidences in pups in the NEC+HB-EGF+MSC IV group (all with p<0.01 compared to untreated NEC). Compared to pups in the NEC+HB-EGF group, pups in the NEC+HB-EGF+MSC IV group had a significantly decreased incidence of intestinal dilation (25% vs. 65.1%, p<0.01) and perforation (17.9% vs. 44.2%, p<0.05).
Figure 3.

Necrotizing enterocolitis (NEC) evaluation. (A) Gross view of the small intestine subjected to experimental NEC. The proximal small intestine is to the left and the cecum is to the right. pneumatosis; intestinal narrowing; perforation; intraluminal bleeding; intestinal dilation. (B) Histological scoring of intestines from neonatal rats. Magnification x100) (a) Grade 0: uninjured intestines; (b) Grade 1: epithelial cell lifting or separation; (c) Grade 2: epithelial cell sloughing to mid villus level; (d) Grade 3: necrosis of entire villus; (e) Grade 4: transmural necrosis. Grade 2 or higher injury is defined as being consistent with experimental NEC; Grade 3 or 4 injury is defined as being consistent with severe NEC. (C) Incidence of NEC. Statistical symbols for Fisher’s exact test are: †p<0.01 vs BF; § ∥ # p<0.05 vs †; ‡ ¶p<0.01 vs †. (D) Incidence of severe NEC. Statistical symbols for Fisher’s exact test are assigned as follows: †p<0.01 vs BF; ‡ § ∥ ¶ p<0.05 vs †; #p<0.01 vs † ‡. In (C) and (D), the numbers of animals in each group are as follows: BF (n=10), NEC (n=38), NEC+HB-EGF (n=43), NEC+MSC IP (n=25), NEC+HB-EGF+MSC IP (n= 26), NEC+MSC IV (n=27), NEC+HB-EGF+MSC IV (n=28). NEC, necrotizing enterocolitis; BF, breast fed; HB-EGF, heparin binding EGF-like growth factor; MSC, mesenchymal stem cells; IP, intraperitoneal; IV, intravenous.
Table 1.
The Incidence of Pathological Changes in Experimental Necrotizing Enterocolitis
| Groups | ||||||||
|---|---|---|---|---|---|---|---|---|
| BF | NEC | NEC +HB-EGF |
NEC +MSC IP |
NEC +HB-EGF +MSC IP |
NEC +MSC IV |
NEC +HB-EGF +MSC IV |
p Values* | |
|
Total no. of
animals |
10 | 38 | 43 | 25 | 26 | 27 | 28 | N/A |
|
Intestinal
dilation, n (%) |
0 | 33 (86.8) | 28 (65.1) | 14 (56) | 16 (61.5) | 15 (55.6) | 7 (25) | BF vs NEC†; NEC+HB-EGF and NEC+HB-EGF+MSC IP vs NEC‡; NEC+MSC IP and NEC+MSC IV and NEC+HB- EGF+ MSC IV vs NEC†; NEC+HB-EGF+MSC IV vs NEC+HB-EGF and NEC+MSC IP and NEC+HB-EGF+MSC IP and NEC+MSC IV† |
|
Perforation, n
(%) |
0 | 24 (63.2) | 19 (44.2) | 11 (44.0) | 10 (38.5) | 9 (33.3) | 5 (17.9) | BF vs NEC†; NEC+MSC IV vs NEC‡; NEC+HB-EGF+MSC IV vs NEC†; NEC+HB-EGF+MSC IV vs NEC+HB-EGF‡ |
|
Intraluminal
bleeding, n (%) |
0 | 23 (60.5) | 16 (37.2) | 9 (36.0) | 10 (38.5) | 10 (37.0) | 7 (25) | BF vs NEC†; NEC+HB-EGF vs NEC‡; NEC+HB-EGF+MSC IV vs NEC† |
|
Intestinal
narrowing, n (%) |
0 | 18 (47.4) | 5 (11.6) | 3 (12.0) | 4 (15.4) | 4 (14.8) | 3 (10.7) | BF vs NEC†; NEC+HB-EGF and NEC+HB-EGF+MSC IP vs NEC‡; NEC+MSC IP and NEC+MSC IV and NEC+HBEGF+ MSC IV vs NEC† |
|
Pneumatosis,
n (%) |
0 | 17 (44.7) | 9 (20.9) | 6 (24) | 7.7%(2/26) | 5 (18.5) | 2 (7.1) | BF vs NEC†; NEC+HB-EGF and NEC+MSC IV vs NEC‡; NEC+HB-EGF+MSC IP and NEC+HB-EGF+MSC IV vs NEC† |
Fisher’s exact test.
p<0.01.
p<0.05.
BF, breast fed; NEC, necrotizing enterocolitis; HB-EGF, heparin binding EGF-like growth factor; MSC, mesenchymal stem cell; IP, intraperitoneal; IV, intravenous.
Representative images of Grade 0-4 intestinal histologic injury are shown in Figure 3B. None of the breast-fed pups showed abnormalities in intestinal histology (0/10); on the other hand, 68.4% of animals in the NEC group developed histologic evidence of NEC (Figure 3C). Pups treated with MSC or HB-EGF or HB-EGF+MSC (IP or IV) had a significantly decreased incidence of NEC (all with p<0.05) with no significant differences found between these treatment groups. Considering Grade 3 or 4 NEC, pups in all treatment groups had a significantly decreased incidence of severe NEC compared to non-treated pups (all with p<0.05), but pups in the NEC+HB-EGF+MSC IV group had a further decrease in the incidence of severe NEC compared to pups in the other treatment groups (Figure 3D).
HB-EGF and MSC act synergistically to preserve gut barrier function during experimental NEC
In breast fed pups, the serum levels of FD70 were extremely low (0.14 ± 0.08 μg/ml) (Figure 4). After exposure to NEC, serum FD70 levels were significantly elevated compared to breast fed pups (19.97 ± 7.94 μg/ml vs. 0.14 ± 0.08 μg/ml, p<0.01), indicative of increased intestinal permeability (worsened gut barrier function). In all treatment groups, significantly decreased intestinal permeability was noted compared to pups in the NEC group (all with p<0.01). Pups in the NEC+HB-EGF+MSC IV group had the lowest levels of serum FD70. Compared to pups treated with MSC IV alone, pups in the NEC+HB-EGF+MSC IV group had significantly improved gut barrier function (0.87 ± 0.31 vs. 1.31 ± 0.41μg/ml, p<0.01).
Figure 4.
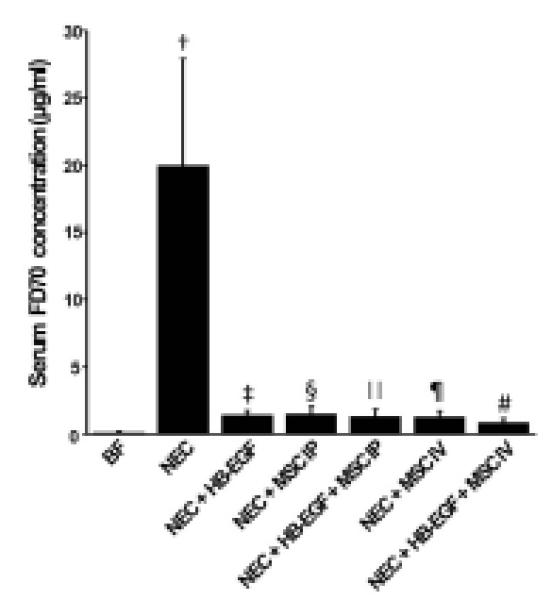
Effect of HB-EGF and mesenchymal stem cells (MSC) on gut barrier function. The concentration of serum FD70 represents intestinal permeability. Higher permeability represents worsened gut barrier function. Values represent mean ± SD; n=9 for each group. Statistical symbols for 1-way ANOVA with Turkey test are: †p<0.01 vs BF; ‡ § ∥ ¶ #p<0.01 vs †; #p<0.05 vs § ∥ ¶; #p<0.01 vs ‡. BF, breast fed; HB-EGF, heparin binding EGF-like growth factor; MSC, mesenchymal stem cells; FD, fluorescein isothiocyanate (FITC)-labeled dextran.
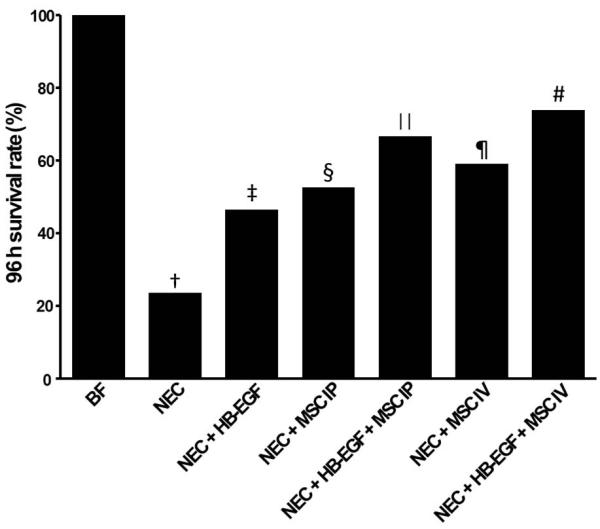
Treatment with HB-EGF plus IV-administered MSC led to the best survival after experimental NEC
At 96 h of life, breast fed pups had 100% percent survival, however, significantly decreased survival was noted in rat pups exposed to experimental NEC (23.7% vs. 100%, p<0.01) (Figure 5). Pups in all treatment groups had improved survival compared to pups in the NEC group (all with p<0.05), with the best survival in pups treated with both HB-EGF and IV-administered MSC (73.9% vs. 23.7%, p<0.01). Compared to pups treated with HB-EGF only, pups in the NEC+HB-EGF+MSC IV group had significantly improved survival (73.9% vs. 52.6%, p<0.05).
Figure 5.
Rat pup 96-hour survival. Statistical symbols for Fisher’s exact test are: †p<0.01 vs BF; ‡ § ∥ ¶ p<0.05 vs †; #p<0.01 vs †; #p<0.05 vs ‡ (Fisher’s exact test). The numbers of animals in each group are as follows: BF (n=10), NEC (n=38), NEC+HB-EGF (n= 43), NEC+MSC IP (n=25), NEC+HB-EGF+MSC IP (n=26), NEC+MSC IV (n=27), and NEC+HB-EGF+MSC IV (n=28). BF, breast fed; NEC, necrotizing enterocolitis; HB-EGF, heparin binding EGF-like growth factor; MSC, mesenchymal stem cells; IP, intraperitoneal; IV, intravenous.
DISCUSSION
During the past 20 years we have demonstrated that HB-EGF can protect the intestines from various forms of intestinal injury including NEC. We have recently shown that HB-EGF protects resident intestinal stem cells (ISC) during experimental NEC.9 This prompted us to further explore the effects of HB-EGF on other types of stem cells. In the current study, we have shown that HB-EGF promotes the migration and proliferation of MSC, and protects MSC from anoxia/reoxygenation-induced apoptosis in vitro. These findings suggest a potential role for HB-EGF in promoting MSC engraftment into injured organs, and in protecting engrafted MSC during tissue injury. Our current study demonstrates that administration of HB-EGF into the gastrointestinal tract promotes the homing and engraftment of MSC delivered via the blood or peritoneal cavity into injured intestine. These findings are consistent with our in vitro studies as well as those of others,41 confirming a chemotactic role of HB-EGF in promoting MSC migration.
As multipotent cells, MSC are able to differentiate into a variety of cell lineages and to secret protective factors.42, 43 It was recently shown that transplanted MSC are versatile in producing cytokines and chemokines under normal physiological conditions, and may exert diverse functions depending on tissue context, changes in physiological conditions and the type of stimuli they have experienced.44 Under physiological conditions, transplanted MSC may suppress proliferation and activity of immune cells, and may regulate cell trafficking including migration of hematopoietic stem cells from the bone marrow to the bloodstream.44 We have recently shown that it is safe and feasible to administer MSC via the umbilical vein to non-injured newborn rat pups,37 suggesting that side-effects of MSC transplantation in premature rat pups, e.g. overwhelmed inflammatory responses, are limited under physiological conditions. However, MSC may be activated under various pathological conditions to act as crucial immunomodulatory factors capable of rescuing injured organs. Regarding HB-EGF, in addition to documenting its ability to act as a potent intestinal cytoprotective agent in several different models of intestinal injury, we have also examined its effects in non-injury situations by examining the GI tract of HB-EGF transgenic mice that constitutively overexpress HB-EGF specifically in the intestinal mucosa under the influence of the villin promoter.45 We found that under basal conditions, the GI tract of these mice is normal at birth and remains normal throughout life. However, the GI tracts of these mice are more resistant to injury than those of their normal counterparts.10,25 Taken together, we believe that administration of MSC or HB-EGF is not deleterious under normal conditions, but has greatest effectiveness under conditions of injury.
MSC transplantation has been applied broadly for the treatment of various injuries including cardiac ischemia/reperfusion injury46, radiation induced intestinal injury,28 and post-glomerular renal injury.30 In a recent study, IP administration of MSC in newborn rat pups subjected to experimental NEC led to decreased weight loss, decreased clinical illness and reduced bowel damage, but did not improve survival, after experimental NEC.33 In our current study, we used YFP-expressing MSC to examine the therapeutic effect of both IP- and IV-administered MSC in experimental NEC. In addition to decreased histologic injury, decreased incidence of NEC, and improved gut barrier function, administration of MSC either IP or IV both led to improved survival.
We have compared the therapeutic effects of IP- and IV-administered MSC in the treatment of experimental NEC. Although no significant differences were noted between IP- and IV-administered MSC in regards to histologic injury, NEC incidence or gut barrier function, IV-administered MSC had significantly increased engraftment into the intestines, suggesting that IV administration may represent a more efficient delivery mechanism compared to IP administration. Furthermore, since the IV route may be the most convenient for clinical use, this route has been broadly applied in preclinical 47, 48 and clinical trials.49-51 Although both routes of administration may be clinically applicable, IV administration represents an efficient and convenient method for infusing MSC systemically.
Importantly, we have now demonstrated that the combination of enterally-administered HB-EGF and IV-administered MSC leads to the best protection of the intestines from experimental NEC. Pups in the NEC+HB-EGF+MSC IV group had the lowest incidence of overall NEC and severe NEC compared to pups subjected to NEC only. Furthermore, pups in the NEC+HB-EGF+MSC IV group had the lowest intestinal permeability, indicating the best improvement in gut barrier function, and had the best survival, compared to pups in the other treatment groups.
There are several possible explanations for the synergistic protective effects of MSC and HB-EGF in pups subjected to experimental NEC. We have previously shown that HB-EGF improves gut barrier function by enhancing intestinal restitution via PI3K/Akt and MEK/REK activation,18 and that HB-EGF promotes enterocyte migration and proliferation during injury.7 MSC have been shown to increase the viability and proliferative capacity of intestinal epithelial cells via the paracrine release of interleukin-6 (IL-6), hepatocyte growth factor (HGF), and vascular endothelial growth factor (VEGF).32 HB-EGF and MSC may both promote restitution in intestinal epithelial cells, but through different mechanisms, leading to their synergistic effect. In addition, when pups are subjected to experimental NEC, both the enterocytes, and the administered MSC are exposed to hypoxic injury. We have previously demonstrated the anti-apoptotic effects of HB-EGF on intestinal epithelial cells during NEC6 and during other forms of intestinal injury.21, 23, 52 In the current study, HB-EGF significantly decreased apoptosis in MSC exposed to hypoxia in vitro. It is therefore possible that HB-EGF decreases MSC apoptosis in vivo, thus preserving MSC viability and function during intestinal injury.
Future experiments are being designed to investigate the fate of either IV- or IP-injected MSC after engraftment into sites of intestinal injury. This will help to determine whether HB-EGF induces the differentiation of MSC into epithelial cells or other cell types after MSC engraftment, as well as the extent to which MSC-HB-EGF interactions exert protective effects via paracrine pathways during experimental NEC. Based on our current experimental results, combined therapy with the administration of both HB-EGF and MSC transplantation may play a future role in the preventive and/or therapeutic therapy for NEC.
CONCLUSIONS
Our results show that HB-EGF promotes MSC proliferation and migration, and decreases MSC apoptosis in vitro. We have shown that MSC can home and engraft into NEC-injured intestine after either IV or IP administration, and that MSC administered by either route of administration can effectively protect the intestines from NEC. Administration of enteral HB-EGF promotes the engraftment of MSC into NEC-injured intestine. Combined administration of enteral HB-EGF and MSC IV led to maximally synergistic protection of the intestines from NEC, and may represent a novel therapeutic regimen for prevention of NEC in babies at highest risk of developing the disease in the future.
Acknowledgments
This work is supported by NIH R01 DK74611 and NIH R01 GM61193 (GEB).
ABBREVIATIONS
- FD
fluorescein isothiocyanate (FITC)-labeled dextran
- HB-EGF
heparin binding EGF-like growth factor
- IP
intraperitoneally
- IV
intravenously
- BF
breast fed
- MSC
mesenchymal stem cells
- NEC
necrotizing enterocolitis
- YFP
yellow fluorescent protein
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure information: Nothing to disclose.
Presented at the American College of Surgeons 97th Annual Clinical Congress, San Francisco, CA, October 2011.
REFERENCES
- 1.Kliegman RM, Fanaroff AA. Necrotizing enterocolitis. N Engl J Med. 1984;310:1093–1103. doi: 10.1056/NEJM198404263101707. [DOI] [PubMed] [Google Scholar]
- 2.Lee JS, Polin RA. Treatment and prevention of necrotizing enterocolitis. Semin Neonatol. 2003;8:449–459. doi: 10.1016/S1084-2756(03)00123-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Besner G, Higashiyama S, Klagsbrun M. Isolation and characterization of a macrophage-derived heparin-binding growth factor. Cell Regul. 1990;1:811–819. doi: 10.1091/mbc.1.11.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feng J, El-Assal ON, Besner GE. Heparin-binding epidermal growth factor-like growth factor decreases the incidence of necrotizing enterocolitis in neonatal rats. J Pediatr Surg. 2006;41:144–149. doi: 10.1016/j.jpedsurg.2005.10.018. discussion 144-149. [DOI] [PubMed] [Google Scholar]
- 5.Feng J, El-Assal ON, Besner GE. Heparin-binding EGF-like growth factor (HB-EGF) and necrotizing enterocolitis. Semin Pediatr Surg. 2005;14:167–174. doi: 10.1053/j.sempedsurg.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Feng J, El-Assal ON, Besner GE. Heparin-binding epidermal growth factor-like growth factor reduces intestinal apoptosis in neonatal rats with necrotizing enterocolitis. J Pediatr Surg. 2006;41:742–747. doi: 10.1016/j.jpedsurg.2005.12.020. discussion 742-747. [DOI] [PubMed] [Google Scholar]
- 7.Feng J, Besner GE. Heparin-binding epidermal growth factor-like growth factor promotes enterocyte migration and proliferation in neonatal rats with necrotizing enterocolitis. J Pediatr Surg. 2007;42:214–220. doi: 10.1016/j.jpedsurg.2006.09.055. [DOI] [PubMed] [Google Scholar]
- 8.Yu X, Radulescu A, Zorko N, Besner GE. Heparin-binding EGF-like growth factor increases intestinal microvascular blood flow in necrotizing enterocolitis. Gastroenterology. 2009;137:221–230. doi: 10.1053/j.gastro.2009.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen CL, Yu X, James IO, et al. Heparin-binding EGF-like growth factor protects intestinal stem cells from injury in a rat model of necrotizing enterocolitis. Lab Invest. 2011 doi: 10.1038/labinvest.2011.167. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radulescu A, Zhang HY, Yu X, et al. Heparin-binding epidermal growth factor-like growth factor overexpression in transgenic mice increases resistance to necrotizing enterocolitis. J Pediatr Surg. 2010;45:1933–1939. doi: 10.1016/j.jpedsurg.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Radulescu A, Yu X, Orvets ND, et al. Deletion of the heparin-binding epidermal growth factor-like growth factor gene increases susceptibility to necrotizing enterocolitis. J Pediatr Surg. 2010;45:729–734. doi: 10.1016/j.jpedsurg.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pillai SB, Hinman CE, Luquette MH, et al. Heparin-binding epidermal growth factor-like growth factor protects rat intestine from ischemia/reperfusion injury. J Surg Res. 1999;87:225–231. doi: 10.1006/jsre.1999.5764. [DOI] [PubMed] [Google Scholar]
- 13.Xia G, Lara-Marquez M, Luquette MH, et al. Heparin-binding EGF-like growth factor decreases inducible nitric oxide synthase and nitric oxide production after intestinal ischemia/reperfusion injury. Antioxid Redox Signal. 2001;3:919–930. doi: 10.1089/15230860152665073. [DOI] [PubMed] [Google Scholar]
- 14.Xia G, Martin AE, Michalsky MP, Besner GE. Heparin-binding EGF-like growth factor preserves crypt cell proliferation and decreases bacterial translocation after intestinal ischemia/reperfusion injury. J Pediatr Surg. 2002;37:1081–1087. doi: 10.1053/jpsu.2002.33881. discussion 1081-1087. [DOI] [PubMed] [Google Scholar]
- 15.Xia G, Martin AE, Besner GE. Heparin-binding EGF-like growth factor downregulates expression of adhesion molecules and infiltration of inflammatory cells after intestinal ischemia/reperfusion injury. J Pediatr Surg. 2003;38:434–439. doi: 10.1053/jpsu.2003.50075. [DOI] [PubMed] [Google Scholar]
- 16.Xia G, Rachfal AW, Martin AE, Besner GE. Upregulation of endogenous heparin-binding EGF-like growth factor (HB-EGF) expression after intestinal ischemia/reperfusion injury. J Invest Surg. 2003;16:57–63. [PubMed] [Google Scholar]
- 17.El-Assal ON, Besner GE. Heparin-binding epidermal growth factor-like growth factor and intestinal ischemia-reperfusion injury. Semin Pediatr Surg. 2004;13:2–10. doi: 10.1053/j.sempedsurg.2003.09.002. [DOI] [PubMed] [Google Scholar]
- 18.El-Assal ON, Besner GE. HB-EGF enhances restitution after intestinal ischemia/reperfusion via PI3K/Akt and MEK/ERK1/2 activation. Gastroenterology. 2005;129:609–625. doi: 10.1016/j.gastro.2005.05.054. [DOI] [PubMed] [Google Scholar]
- 19.Rocourt DV, Mehta VB, Besner GE. Heparin-binding EGF-like growth factor decreases inflammatory cytokine expression after intestinal ischemia/reperfusion injury. J Surg Res. 2007;139:269–273. doi: 10.1016/j.jss.2006.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Assal ON, Paddock H, Marquez A, Besner GE. Heparin-binding epidermal growth factor-like growth factor gene disruption is associated with delayed intestinal restitution, impaired angiogenesis, and poor survival after intestinal ischemia in mice. J Pediatr Surg. 2008;43:1182–1190. doi: 10.1016/j.jpedsurg.2008.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu X, Radulescu A, Chen CL, et al. Heparin-Binding EGF-Like Growth Factor Protects Pericytes from Injury. J Surg Res. 2012;172:165–176. doi: 10.1016/j.jss.2010.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Assal ON, Radulescu A, Besner GE. Heparin-binding EGF-like growth factor preserves mesenteric microcirculatory blood flow and protects against intestinal injury in rats subjected to hemorrhagic shock and resuscitation. Surgery. 2007;142:234–242. doi: 10.1016/j.surg.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Zhang HY, Radulescu A, Besner GE. Heparin-binding epidermal growth factor-like growth factor is essential for preservation of gut barrier function after hemorrhagic shock and resuscitation in mice. Surgery. 2009;146:334–339. doi: 10.1016/j.surg.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang HY, James I, Chen CL, Besner GE. Heparin-binding epidermal growth factor-like growth factor (HB-EGF) preserves gut barrier function by blocking neutrophil-endothelial cell adhesion after hemorrhagic shock and resuscitation in mice. Surgery. 2011 doi: 10.1016/j.surg.2011.10.001. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang HY, Radulescu A, Chen CL, et al. Mice overexpressing the gene for heparin-binding epidermal growth factor-like growth factor (HB-EGF) have increased resistance to hemorrhagic shock and resuscitation. Surgery. 2011;149:276–283. doi: 10.1016/j.surg.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang HY, Radulescu A, Chen Y, Besner GE. HB-EGF improves intestinal microcirculation after hemorrhagic shock. J Surg Res. 2011;171:218–225. doi: 10.1016/j.jss.2010.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semont A, Mouiseddine M, Francois A, et al. Mesenchymal stem cells improve small intestinal integrity through regulation of endogenous epithelial cell homeostasis. Cell Death Differ. 2010;17:952–961. doi: 10.1038/cdd.2009.187. [DOI] [PubMed] [Google Scholar]
- 28.Kudo K, Liu Y, Takahashi K, et al. Transplantation of mesenchymal stem cells to prevent radiation-induced intestinal injury in mice. J Radiat Res (Tokyo) 2010;51:73–79. doi: 10.1269/jrr.09091. [DOI] [PubMed] [Google Scholar]
- 29.Yan Q, Ruan JW, Ding Y, et al. Electro-acupuncture promotes differentiation of mesenchymal stem cells, regeneration of nerve fibers and partial functional recovery after spinal cord injury. Exp Toxicol Pathol. 2011;63:151–156. doi: 10.1016/j.etp.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 30.Wong CY, Cheong SK, Mok PL, Leong CF. Differentiation of human mesenchymal stem cells into mesangial cells in post-glomerular injury murine model. Pathology. 2008;40:52–57. doi: 10.1080/00313020701716367. [DOI] [PubMed] [Google Scholar]
- 31.Zarjou A, Kim J, Traylor AM, et al. Paracrine effects of mesenchymal stem cells in cisplatin-induced renal injury require heme oxygenase-1. Am J Physiol Renal Physiol. 2011;300:F254–262. doi: 10.1152/ajprenal.00594.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weil BR, Markel TA, Herrmann JL, et al. Mesenchymal stem cells enhance the viability and proliferation of human fetal intestinal epithelial cells following hypoxic injury via paracrine mechanisms. Surgery. 2009;146:190–197. doi: 10.1016/j.surg.2009.03.031. [DOI] [PubMed] [Google Scholar]
- 33.Tayman C, Uckan D, Kilic E, et al. Mesenchymal stem cell therapy in necrotizing enterocolitis: a rat study. Pediatr Res. 2011;70:489–494. doi: 10.1203/PDR.0b013e31822d7ef2. [DOI] [PubMed] [Google Scholar]
- 34.Phinney DG, Baddoo M, Dutreil M, et al. Murine mesenchymal stem cells transplanted to the central nervous system of neonatal versus adult mice exhibit distinct engraftment kinetics and express receptors that guide neuronal cell migration. Stem Cells Dev. 2006;15:437–447. doi: 10.1089/scd.2006.15.437. [DOI] [PubMed] [Google Scholar]
- 35.Moreno R, Martinez-Gonzalez I, Rosal M, et al. Fetal liver-derived mesenchymal stem cell engraftment after allogeneic in utero transplantation into rabbits. Stem Cells Dev. 2012;21:284–295. doi: 10.1089/scd.2010.0483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu H, Zhang J, Liu CY, et al. Cell therapy of congenital corneal diseases with umbilical mesenchymal stem cells: lumican null mice. PLoS One. 2010;5:e10707. doi: 10.1371/journal.pone.0010707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang J, Watkins D, Chen CL, et al. A technique for systemic mesenchymal stem cell transplantation in newborn rat pups. J Invest Surg. 2012 doi: 10.3109/08941939.2012.661519. In press. [DOI] [PubMed] [Google Scholar]
- 38.Hall B, Andreeff M, Marini F. The participation of mesenchymal stem cells in tumor stroma formation and their application as targeted-gene delivery vehicles. Handb Exp Pharmacol. 2007:263–283. doi: 10.1007/978-3-540-68976-8_12. [DOI] [PubMed] [Google Scholar]
- 39.Barlow B, Santulli TV, Heird WC, et al. An experimental study of acute neonatal enterocolitis--the importance of breast milk. J Pediatr Surg. 1974;9:587–595. doi: 10.1016/0022-3468(74)90093-1. [DOI] [PubMed] [Google Scholar]
- 40.Caplan MS, Hedlund E, Adler L, Hsueh W. Role of asphyxia and feeding in a neonatal rat model of necrotizing enterocolitis. Pediatr Pathol. 1994;14:1017–1028. doi: 10.3109/15513819409037698. [DOI] [PubMed] [Google Scholar]
- 41.Ozaki Y, Nishimura M, Sekiya K, et al. Comprehensive analysis of chemotactic factors for bone marrow mesenchymal stem cells. Stem Cells Dev. 2007;16:119–129. doi: 10.1089/scd.2006.0032. [DOI] [PubMed] [Google Scholar]
- 42.Satija NK, Singh VK, Verma YK, et al. Mesenchymal stem cell-based therapy: a new paradigm in regenerative medicine. J Cell Mol Med. 2009;13:4385–4402. doi: 10.1111/j.1582-4934.2009.00857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hwang NS, Zhang C, Hwang YS, Varghese S. Mesenchymal stem cell differentiation and roles in regenerative medicine. Wiley Interdiscip Rev Syst Biol Med. 2009;1:97–106. doi: 10.1002/wsbm.26. [DOI] [PubMed] [Google Scholar]
- 44.Shi C. Recent progress toward understanding the physiological function of bone marrow mesenchymal stem cells. Immunology. 2012;136:133–138. doi: 10.1111/j.1365-2567.2012.03567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen CL, Metha VB, Zhang HY, et al. Intestinal phenotype in mice overexpressing a heparin-binding EGF-like growth factor transgene in enterocytes. Growth factors. 2010;28:82–97. doi: 10.3109/08977190903407365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Poynter JA, Herrmann JL, Manukyan MC, et al. Intracoronary mesenchymal stem cells promote postischemic myocardial functional recovery, decrease inflammation, and reduce apoptosis via a signal transducer and activator of transcription 3 mechanism. J Am Coll Surg. 2011;213:253–260. doi: 10.1016/j.jamcollsurg.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 47.Abdel-Mageed AS, Senagore AJ, Pietryga DW, et al. Intravenous administration of mesenchymal stem cells genetically modified with extracellular superoxide dismutase improves survival in irradiated mice. Blood. 2009;113:1201–1203. doi: 10.1182/blood-2008-07-170936. [DOI] [PubMed] [Google Scholar]
- 48.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lazarus HM, Haynesworth SE, Gerson SL, et al. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): implications for therapeutic use. Bone Marrow Transplant. 1995;16:557–564. [PubMed] [Google Scholar]
- 50.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 51.Wu KH, Chan CK, Tsai C, et al. Effective treatment of severe steroid-resistant acute graft-versus-host disease with umbilical cord-derived mesenchymal stem cells. Transplantation. 2011;91:1412–1416. doi: 10.1097/TP.0b013e31821aba18. [DOI] [PubMed] [Google Scholar]
- 52.Mehta VB, Besner GE. Inhibition of NF-kappa B activation and its target genes by heparin-binding epidermal growth factor-like growth factor. J Immunol. 2003;171:6014–6022. doi: 10.4049/jimmunol.171.11.6014. [DOI] [PubMed] [Google Scholar]