Abstract
Among 10 strains of community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) isolated in 2002 from patients with skin infections, seven harbored the Panton-Valentine leukocidin gene, two harbored the exfoliative toxin A gene, and one harbored neither of these genes. CA-MRSA isolates producing a variety of exotoxins are currently spreading in the Swiss community.
Infections due to community-acquired methicillin-resistant Staphylococcus aureus (CA-MRSA) are a recent worldwide phenomenon (1-4, 6-8, 13). Between 13 November 2002 and 5 March 2003, eight reports of CA-MRSA infections or outbreaks were published on the ProMed website of the International Society for Infectious Diseases (http://www.promedmail.org). The cases occurred in North America (in the Los Angeles, Calif., gay community; a jail in Los Angeles; a correctional facility in Ontario, Canada; and a school in Pasadena, Tex.) and in Europe (France, Scotland, and The Netherlands). Most patients had no known risk factors for colonization by health care-associated bacteria, such as recent hospitalization and/or contacts with health care workers. Skin infections were most frequent, but rare cases of frequently fatal severe necrotizing pneumonia also occurred. Most CA-MRSA isolates expressed Panton-Valentine leukocidin (PVL), a highly potent toxin previously implicated in these types of infections (4, 5, 11).
From January to December 2002, we screened all CA-MRSA isolates identified in our laboratory (the main bacteriology laboratory serving private practitioners in the Geneva area of 400,000 inhabitants) for toxin genes (superantigenic toxins, leukocidins, and hemolysins), the accessory gene regulator (agr) allele group (alleles 1 to 4), and the staphylococcal cassette chromosome mec (SCCmec) allele (I to IV). All isolates were subjected to molecular typing with previously described methods (9, 11, 15). Antimicrobial susceptibility testing was based on the microdilution method (Vitek 2 system, AST-P523 cards; bioMérieux, Marcy l'Etoile, France) and the disk diffusion method according to NCCLS recommendations (14). Resistance to methicillin was detected on screening agar medium (Mueller-Hinton oxacillin [bioMérieux] and oxacillin resistance screening agar [Oxoid]) and was confirmed by screening for PBP2a (Slidex MRSA detection; Denka Seiken) and the mecA gene, as described by Murakami et al. (12).
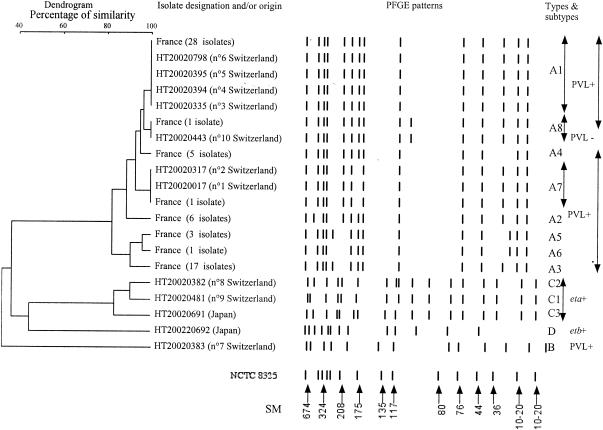
Among the 142 MRSA strains isolated during this period, 10 (7%) were from patients with no risk factors for acquisition of nosocomial bacteria and were thus designated CA-MRSA. The 10 patients, 7 of whom were children, had furunculosis (n = 6), scabby impetigo (n = 3), or folliculitis (n = 1) (Table 1). Methicillin resistance was heterogenous and was not detected on Vitek 2 in two isolates. The 10 CA-MRSA strains were resistant to few other antistaphylococcal antibiotics (Table 1). They all harbored the SCCmec type IV cassette usually found in CA-MRSA (16). Nine strains harbored the accessory gene regulator (agr) 3 allele, while the last strain, isolated from a patient with impetigo, harbored agr2 (Table 1). All six strains isolated from patients with furunculosis harbored the PVL genes and belonged to the French CA-MRSA clone initially reported by Dufour et al. (4), and identified by pulsed-field gel electrophoresis (PFGE) (Fig. 1). This clone probably spread to Geneva from neighboring France. The folliculitis strain also belonged to this clonal group but did not harbor the PVL genes. PVL-positive strains are rarely associated with folliculitis (11), and our strain may have derived from a recent PVL-positive ancestor that had lost the PVL-encoding prophage.
TABLE 1.
Clinical and microbiological data on CA-MRSA infections
| Clinical presentation | Patient (isolate) no.a | Age (yr)/ sexb | Recurrent infection | Initial treatment | Secondary treatment | Antimicrobial resistanced | mecA | agr allele | Toxin genef
|
PFGE pattern | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukocidin
|
Enterotoxin (sea to see, seh, sek, sel, sem, seo, sep) | Exfoliatin (eta, etb) | tst | |||||||||||
| PVL | lukED | |||||||||||||
| Furunculosis | 1 (HT20020017) | 34/M | No | Fusidic acid | Ciprofloxacin | OX, K, E, TET, FU | +e | 3 | + | + | −g | − | − | A7 |
| 2 (HT20020317) | 21/F | Yes | Surgery, amox-clavc | Ciprofloxacin, rifampin | OX, K, E, TET, FU | + | 3 | + | + | − | − | − | A7 | |
| 3 (HT20020335) | 4/M | Yes | Amox-clav | Co-trimoxazole | OX, K, TET, FU | + | 3 | + | + | − | − | − | A1 | |
| 4 (HT20020394) | 6/F | Yes | Amox-clav | Co-trimoxazole | OX, K, TET, FU | + | 3 | + | + | − | − | − | A1 | |
| 5 (HT20020395) | 9/F | Yes | Amox-clav | Co-trimoxazole | OX, K, TET, FU | + | 3 | + | + | − | − | − | A1 | |
| 6 (HT20020798) | 4/F | Yes | Surgery, amox-clav | Co-trimoxazole | OX, K, TET, FU | + | 3 | + | + | − | − | − | A1 | |
| Impetigo | 7 (HT20020383) | 3/M | No | Amox-clav, fusidic acid | Erythromycin, fusidic acid | OX, TET, SXT | + | 2 | + | + | + (sem, seo) | − | − | B |
| 8 (HT20020382) | 2/M | No | Amox-clav and fusidic acid | Fusidic acid | OX, E, TET, SXT | + | 3 | − | + | − | + (eta) | − | C2 | |
| 9 (HT20020481) | 4/F | No | Amoxicillin | Fusidic acid | OX, TET, SXT | + | 3 | − | + | − | + (eta) | − | C1 | |
| Folliculitis | 10 (HT20020443) | 39/M | Yes | Tetracycline, erythromycin, fusidic acid | Ciprofloxacin | OX, K, E, TET, FU | + | 3 | − | + | − | − | − | A8 |
Patients 1 and 2 were partners, and patient 2 had had furunculosis for 2 years. Patients 3, 4, and 5 were children of a five-member family, all of whom had recurring furunculosis after visiting a swimming pool. Patient 6 was a child with a second episode of furunculosis and a family history of furunculosis in cousins; travel to Algeria was also reported. Patients 7, 8, and 9 were children with impetigo; one had recently traveled to Senegal (patient 7), one had traveled to Angola (patient 8), and one had traveled to The Netherlands (patient 9). Patient 10 was an adult who had had recurrent folliculitis for 1 year; travel in France and Italy was reported.
M, male; F, female.
amox-clav, amoxicillin-clavulanic acid.
OX, oxacillin; K, kanamycin; E, erythromycin; TET, tetracycline; FU, fusidic acid; SXT, co-trimoxazole.
+, present.
lukED, leukocidin lukE-lukD genes; sea to see, seh, sek, sel, sem, seo, and sep, staphylococcal enterotoxin type A to E, H, K, L, M, O, and P genes, respectively; eta and etb, exfoliatin toxin type A and B genes, respectively tst, toxic shock toxin gene.
−, absent.
FIG. 1.
Unweighted pair group method with averages dendrogram of PFGE results based on the Dice distance matrix and schematic representation of the pulsotypes (SmaI restriction enzyme) of PVL-positive and exfoliative toxin-positive CA-MRSA isolates. S. aureus NCTC 8325 is the reference strain for size markers (SM), expressed in kilobases. Strains differing by no more than three fragments were considered to be subtypes of a given clonal type. PVL-positive MRSA strains from Switzerland and France were of clonal type A (subtypes A1 to A8) or B. Exfoliatin A (eta)- or B (etb)-positive strains from Switzerland and Japan were of clonal type C (subtypes C1 to C3) or D.
One of the three strains associated with scabby impetigo harbored agr2 and, unexpectedly, the PVL genes but not the exfoliative toxin gene (eta or etb) usually associated with bullous impetigo. However, PVL-positive S. aureus strains were recently isolated from patients with nonbullous impetigo by Koning et al. (10). Our agr2-positive, PVL-positive strain had a very different PFGE pattern from those of the other strains (Fig. 1); this was expected from the results of agr grouping, showing the different genetic background of agr3 strains. It is noteworthy that the patient infected by this strain had recently returned from Senegal. The other two impetigo isolates carried the eta gene (encoding exfoliative toxin A) and had a PFGE profile related to that of an eta-positive CA-MRSA strain from Japan (Fig. 1) (17). The two patients had recently visited Angola and The Netherlands, but not Japan. One possible explanation for the similarities between the Japanese clone and our strains is that the eta gene and SSCmecIV may have successfully integrated the genetic background of these peculiar agr3 strains. The genetic event leading to this association may have occurred either independently on the two continents or initially in Japan with subsequent spread to Europe. To our knowledge this is the first report of CA-MRSA carrying exfoliative toxin genes in Europe. The Japanese exfoliative toxin-producing CA-MRSA strains were all associated with bullous impetigo (17), whereas our strains were isolated from patients with scabby impetigo. Similarly, exfoliative toxin-producing strains have been associated with some cases of nonbullous impetigo (10).
Studies of CA-MRSA throughout the world must now take into account the possible presence of exfoliative toxin genes, and no longer only PVL genes. The clinical infections associated with these two types of toxins are usually very different. PVL-producing strains are mainly associated with primary skin infections such as furunculosis, or with pneumonia secondary to influenza, whereas exfoliative toxin-producing strains tend to cause impetigo or scalded-skin syndrome. A limited number of CA-MRSA clones carrying the PVL genes are currently spreading across several continents (16). In Europe, closely related PFGE patterns have been reported for PVL-positive CA-MRSA isolated in Switzerland, France, and The Netherlands, pointing to clonal spread (http://www.eurosurveillance.org/ew/2003/030306.asp). These strains seem to spread by skin-to-skin contact. Our results suggest that exfoliative toxin-positive CA-MRSA strains are also spreading clonally, as two isolates from patients belonging to two distinct families belonged to the same clonal group and were also related to a Japanese exfoliative toxin-positive CA-MRSA strain.
Finally, our results suggest that the prevalence of CA-MRSA in Switzerland may be higher than previously suspected and thus call for closer systematic surveillance. The prevalence of CA-MRSA might be underestimated partly because skin infection samples are not routinely cultured. Moreover, resistance to oxacillin is heterogenous and can easily be missed by suboptimal screening methods. Our findings have led the Geneva city authorities to implement close surveillance of CA-MRSA, notably through family doctors.
Acknowledgments
We thank Motoyuki Sugai for providing the Japanese strains, Françoise Forey for PFGE, Christine Gardon and Christine Courtier for technical assistance, and Luc Perrin for his help and encouragement. We are grateful to David Young for editorial assistance.
REFERENCES
- 1.Adhikari, R. P., G. M. Cook, I. Lamont, S. Lang, H. Heffernan, and J. M. Smith. 2002. Phenotypic and molecular characterization of community occurring, Western Samoan phage pattern methicillin-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 50:825-831. [DOI] [PubMed] [Google Scholar]
- 2.Collignon, P., I. Gosbell, A. Vickery, G. Nimmo, T. Stylianopoulos, T. Gottlieb, et al. 1998. Community-acquired methicillin-resistant Staphylococcus aureus in Australia. Lancet 352:145-146. [DOI] [PubMed] [Google Scholar]
- 3.Daum, R. S. 1998. Community-acquired methicillin-resistant Staphylococcus aureus infections. Pediatr. Infect. Dis. J. 17:745-746. [DOI] [PubMed] [Google Scholar]
- 4.Dufour, P., Y. Gillet, M. Bes, G. Lina, F. Vandenesch, D. Floret, J. Etienne, and H. Richet. 2002. Community-acquired methicillin-resistant Staphylococcus aureus infections in France: emergence of a single clone that produces Panton-Valentine leukocidin. Clin. Infect. Dis. 35:819-824. [DOI] [PubMed] [Google Scholar]
- 5.Gillet, Y., B. Issartel, P. Vanhems, J. C. Fournet, G. Lina, M. Bes, F. Vandenesch, Y. Piemont, N. Brousse, D. Floret, and J. Etienne. 2002. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet 359:753-759. [DOI] [PubMed] [Google Scholar]
- 6.Gosbell, I. B., J. L. Mercer, S. A. Neville, K. G. Chant, and R. Munro. 2001. Community-acquired, non-multiresistant oxacillin-resistant Staphylococcus aureus (NORSA) in South Western Sydney. Pathology 33:206-210. [PubMed] [Google Scholar]
- 7.Groom, A. V., D. H. Wolsey, T. S. Naimi, K. Smith, S. Johnson, D. Boxrud, K. A. Moore, and J. E. Cheek. 2001. Community-acquired methicillin-resistant Staphylococcus aureus in a rural American Indian community. JAMA 286:1201-1205. [DOI] [PubMed] [Google Scholar]
- 8.Herold, B. C., L. C. Immergluck, M. C. Maranan, D. S. Lauderdale, R. E. Gaskin, S. Boyle-Vavra, C. D. Leitch, and R. S. Daum. 1998. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA 279:593-598. [DOI] [PubMed] [Google Scholar]
- 9.Jarraud, S., M. A. Peyrat, A. Lim, A. Tristan, M. Bes, C. Mougel, J. Etienne, F. Vandenesch, M. Bonneville, and G. Lina. 2001. egc, a highly prevalent operon of enterotoxin gene, forms a putative nursery of superantigens in Staphylococcus aureus. J. Immunol. 166:669-677. [DOI] [PubMed] [Google Scholar]
- 10.Koning, S., A. van Belkum, S. Snijders, W. van Leeuwen, H. Verbrugh, J. Nouwen, M. Op 't Veld, L. W. van Suijlekom-Smit, J. C. van der Wouden, and C. Verduin. 2003. Severity of nonbullous Staphylococcus aureus impetigo in children is associated with strains harboring genetic markers for exfoliative toxin B, Panton-Valentine leukocidin, and the multidrug resistance plasmid pSK41. J. Clin. Microbiol. 41:3017-3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lina, G., Y. Piemont, F. Godail-Gamot, M. Bes, M. O. Peter, V. Gauduchon, F. Vandenesch, and J. Etienne. 1999. Involvement of Panton-Valentine leukocidin-producing Staphylococcus aureus in primary skin infections and pneumonia. Clin. Infect. Dis. 29:1128-1132. [DOI] [PubMed] [Google Scholar]
- 12.Murakami, K., W. Minamide, K. Wada, E. Nakamura, and H. Teraoka. 1991. Identification of methicillin-resistant strains of staphylococci by polymerase chain reaction. J. Clin. Microbiol. 29:2240-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naimi, T. S., K. H. LeDell, D. J. Boxrud, A. V. Groom, C. D. Steward, S. K. Johnson, J. M. Besser, C. O'Boyle, R. N. Danila, J. E. Cheek, M. T. Osterholm, K. A. Moore, and K. E. Smith. 2001. Epidemiology and clonality of community-acquired methicillin-resistant Staphylococcus aureus in Minnesota, 1996-1998. Clin. Infect. Dis. 33:990-996. [DOI] [PubMed] [Google Scholar]
- 14.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility testing for bacteria that grow aerobically. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 15.Oliveira, D., and H. de Lencastre. 2002. Multiplex PCR strategy for rapid identification of structural types and variants of the mec element in methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 46:2155-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vandenesch, F., T. Naimi, M. C. Enright, G. Lina, G. Nimmo, H. Hefferman, N. Liassine, B. Bes, T. Greenland, M. E. Reverdy, and J. Etienne. 2003. Community-acquired methicillin-resistant Staphylococus aureus carrying the Panton-Valentine leukocidin genes: worldwide emergence. Emerg. Infect. Dis. 9:978-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi, T., Y. Yokota, J. Terajima, T. Hayashi, M. Aepfelbacher, M. Ohara, H. Komatsuzawa, H. Watanabe, and M. Sugai. 2002. Clonal association of Staphylococcus aureus causing bullous impetigo and the emergence of new methicillin-resistant clonal groups in Kansai district in Japan. J. Infect. Dis. 185:1511-1516. [DOI] [PubMed] [Google Scholar]