Abstract
Objectives
The aim of this study was to compare the efficacy and safety of intravenous iron with oral iron in the treatment of iron deficiency anemia of pregnancy.
Methods
A randomized experimental study was conducted at K. J. Somaiya Hospital involving 200 pregnant women with iron deficiency anemia. In the intravenous group iron dose was calculated from: Total iron dose required (mg) = 2.4 × weight kg × target hemoglobin − actual hemoglobin) g/dl + 500. Target hemoglobin was set at 12 g/dl. In the oral group patients received 200 mg oral ferrous ascorbate daily. Hemoglobin and serum ferritin were reviewed at 2, 4, and 6 weeks. Paired and independent t test was applied.
Results
The change in hemoglobin and ferritin levels from baseline was significantly higher in the intravenous group than the oral group at each measurement (P = 0.000).
Conclusion
Intravenous iron elevates hemoglobin and restores iron stores faster than oral iron, with no severe adverse reactions.
Keywords: Iron deficiency anemia, Hemoglobin, Serum ferritin, Iron sucrose, Oral ferrous ascorbate
Introduction
Anemia is a major public health problem. Anemia (defined by the WHO as hemoglobin of <11 g/dl) is one of the world’s leading cause of disability and thus one of the most serious global public health issues [1].
The prevalence of anemia in pregnancy varies considerably because of the differences in social conditions, lifestyles, and health seeking behaviors across different cultures. Anemia affects all pregnant women in the world—52 % in developing countries compared with 23 % in the developed world. The most common causes of anemia are poor nutrition, deficiencies of iron, micronutrients, malaria, hookworm infestation and schistosomiasis, HIV infection and hemoglobinopathies [1, 2].
Anemia is one of the most prevalent nutritional deficiency problems affecting pregnant females. Iron deficiency is the major cause of anemia followed by folate deficiency. Prevalence of anemia is higher in India as compared to other developing countries. Prevalence of anemia in South Asian countries is the highest among the other countries in the world. WHO estimates that even among the South Asian countries, India has the highest prevalence of anemia. India contributes to about 80 % of the maternal deaths due to anemia. The high prevalence of iron and other micronutrients deficiency among women in developing countries is of concern and maternal anemia is still a cause of considerable perinatal morbidity and mortality [3].
According to the National Family Health Survey (2005–2006) incidence of anemia in pregnant women in India is 54.6 % in urban and 59 % in rural areas [4].
Anemia is responsible for adverse obstetric outcome in a large number of women in developing countries. Almost one thousand severely affected young women are reported to die every week because of inability to cope with the stress of childbirth.
Anemia leads to increased risk of blood transfusion during the peripartum period. Iron therapy before delivery may reduce the transfusion rate for iron deficient women.
The aim of this study was to compare the efficacy and safety of intravenous iron sucrose and oral ferrous ascorbate in the treatment of iron deficiency anemia of pregnancy.
Methods
An experimental randomized study was conducted at K.J. Somaiya Medical College and Research Centre between February 2008 and March 2010. Patients were recruited from the antenatal clinic of the hospital randomly by computer generated randomization. The women were given sequentially numbered sealed opaque envelopes. Eligible participants were pregnant women between 28 and 37 weeks of gestation with established iron deficiency anemia who had hemoglobin levels between 6 and 10 g% and serum ferritin less than 15 ng/ml.
Exclusion Criteria were: Anemia from causes other than iron deficiency, multiple pregnancy, previous blood transfusion, h/o hematological disease, risk of preterm labor, intolerance to iron derivatives, recent administration of iron, and current usage of iron supplements.
A total of 200 patients were studied. All were randomly assigned to either intravenous or oral group. Hundred patients were in intravenous and hundred patients in oral group which was decided by sequentially numbered, sealed opaque envelopes.
In the intravenous group, the total iron dose in mg was calculated from the following formula:
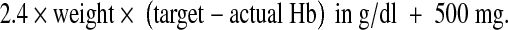 |
Rounded up to nearest multiple of 100.
In the formula since the patient’s pre pregnancy weight was not known, the weight at the time of the first visit was considered in kilograms. Target hemoglobin in g per dl was set at 12 g/dl.
In each infusion the maximum total dose administered was 200 mg elemental iron in 100 ml of normal saline infused over 20–30 min, given on alternate days. Each ampoule was of 2.5 ml containing 50 mg of elemental iron. The ampoules were diluted with normal saline immediately before the infusion. No test dose was given. Treatment was completed after administration of the calculated dose. Additional iron was not administered during the study.
Iron sucrose is a complex of poly nuclear iron(III) hydroxide in sucrose for intravenous use. The poly nuclear iron(III) hydroxide cores are superficially surrounded by a large number of non covalently bound sucrose molecules resulting in a complex, with a molecular mass of approximately 60,000 Da, prohibiting renal elimination. The iron in the poly nuclear cores is bound in a similar structure to that of physiologically occurring ferritin. The complex is stable and does not release ionic iron under physiological conditions. Following intra venous administration, iron sucrose is dissociated by the reticuloendothelial system into iron and sucrose. Iron sucrose can be administered as intravenous injection or infusion. Iron sucrose does not contain dextran hence chances of anaphylaxis are negligible. The rate of iron delivery to marrow is a major factor in regulation of marrow proliferation. Iron sucrose has an intermediate stability and strength. It is quickly cleared from the serum with the terminal half life of approximately 5–6 h. It is more rapidly available for erythropoiesis [5–7]. In the oral group, women were instructed to take two tablets (ferrous ascorbate with 100 mg of elemental iron per day with 1.1 mg of folic acid) twice daily throughout the pregnancy. Women were instructed to take the tablets on an empty stomach either 2 h before or after their meals.
Ferrous ascorbate iron salt was chosen because it prevents the formation of insoluble and unabsorbable iron compounds and it causes reduction of ferric to ferrous iron which seems to be a requirement for uptake of iron into duodenal and proximal mucosal cells of the small intestine. The ascorbic acid forms a soluble complex with iron in the stomach and passes into the intestine. The effect of iron absorption inhibitors which would normally bind to iron in the more alkaline pH of upper intestine is limited [8]. Oral ferrous ascorbate has highest bio availability in the range of 26.4–50.4 % and it acts as an antioxidant.
The primary outcome measure was hemoglobin concentration at 2, 4, and 6 weeks. The secondary outcome was serum ferritin levels done at 2, 4, and 6 weeks. All women were followed up every 2 weeks. During each visit all adverse events immediate or delayed were evaluated.
Laboratory evaluation was performed at the time of inclusion in the study and then at 2, 4, and 6 weeks. Initial evaluation included CBC, PCV, MCV, MCH, MCHC, peripheral smear, and iron profile, whenever possible.
Results
No participants were lost to follow up and there were no dropouts. The back count of tablets collected from women in the oral iron group showed that (88 women) 88 % took more than 90 % of their supplements. All women administered intravenous iron dose, which received the calculated total iron dose.
Using SPSS software on computer, paired and independent samples “t” test was applied.
Initial demographic and clinical characteristics were generally similar in the two groups. The mean age of women on inclusion in intravenous group was 24.30 ± 3.98 years with mean gestational age of 32 ± 2.46 weeks, mean hemoglobin of 7.9 ± 0.87 and mean ferritin of 8.44 ± 1.35. In the oral group, mean age was 24.09 ± 3.84 years with gestational age on inclusion 31.93 ± 2.22 weeks, mean hemoglobin 7.925 ± 8.62, and mean serum ferritin was 8.13 ± 1.45.
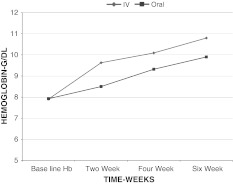
An increase in hemoglobin was observed from baseline to 6 weeks in each group, but the increase in hemoglobin in intravenous iron sucrose group was more than oral ferrous ascorbate group at each point of measurement (P = 0.000) as shown in Table 1 and Graph 1.
Table 1.
Actual hemoglobin levels over 6 weeks
| Group | Hbbaseline (g/dl) | Hb2 weeks (g/dl) | Hb4 weeks (g/dl) | Hb6 weeks (g/dl) |
|---|---|---|---|---|
| Intravenous | 7.9 ± 0.8741 | 9.63 ± 0.885 | 10.09 ± 0.8072 | 10.79 ± 0.8432 |
| Oral | 7.92 ± 0.862 | 8.5 ± 0.862 | 9.32 ± 0.8707 | 9.903 ± 0.8848 |
| P value | 0.884 | 0.000 | 0.000 | 0.000 |
Hb hemoglobin
* P = 0.000-highly significant
Graph 1.
Rise in hemoglobin
The difference in hemoglobin values from baseline in the intravenous group was 1.72 ± 0.484 at 2 weeks, 2.18 ± 0.865 at 4 weeks, 2.89 ± 0.5989 at 6 weeks compared to oral iron, which is 0.5750 ± 0.456 at 2 weeks, 1.39 ± 0.4402 at 4 weeks, and 1.9 ± 0.3020 at 6 weeks. P value was 0.000 which was clinically significant and showed that the hemoglobin levels were increased more in the intravenous group.
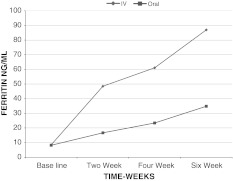
There was a significant rise in serum ferritin levels from baseline to 6 weeks in both groups, but the increase in intravenous group was more than oral group at each point of measurement (P = 0.000) as shown in Tables 2, 3 and Graph 2.
Table 2.
Actual ferritin levels over 6 weeks
| Group | Fe baseline (ng/ml) | Fe 2 weeks (ng/ml) | Fe 4 weeks (ng/ml) | Fe 6 weeks (ng/ml) |
|---|---|---|---|---|
| Intravenous | 8.44 ± 1.355 | 48.46 ± 16.66 | 61.05 ± 19.662 | 86.98 ± 19.939 |
| Oral | 8.13 ± 1.457 | 16.65 ± 4.87 | 23.36 ± 8.570 | 34.78 ± 8.793 |
| P value | 0.126 | 0.000** | 0.000** | 0.000** |
Fe serum ferritin levels
** P value = 0.000-highly significant
Table 3.
Serum ferritin levels difference from baseline
| Serum ferritin levels difference from baseline | Intravenous (ng/ml) | Oral (ng/ml) | P value |
|---|---|---|---|
| Fe2 weeks − Fe baseline | 40.020 ± 17.02 | 8.5 ± 4.5 | 0.000** |
| Fe4 weeks − Fe baseline | 52.612 ± 19.88 | 15.23 ± 8.09 | 0.000** |
| Fe6 weeks − Fe baseline | 78.53 ± 19.82 | 26.6 ± 8.56 | 0.000** |
Fe serum ferritin levels
** P value = 0.000-highly significant
Graph 2.
Rise in ferritin
There were no serious adverse drug reactions recorded. There were no episodes of anaphylaxis or hypotensive shock. There were no patient withdrawals and no drug discontinuation caused by drug related adverse events in the intravenous group. Adverse events in the intravenous group were metallic taste in (five) patients, hot flushes (two), arthralgia (one), dizziness (one), and nausea (four).
In the oral group gastrointestinal symptoms were experienced by 27 women. Twenty-two women had upper gastrointestinal symptoms including epigastric discomfort, nausea and vomiting and five women suffered from diarrhea which was managed by symptomatic treatment. No women discontinued the drug because of gastrointestinal symptoms.
Discussion
The study confirmed that parenterally administered iron sucrose elevated hemoglobin and restored iron stores better than oral ferrous ascorbate.
Al Momen et al. [6], in their study compared 52 women treated with intravenous iron sucrose and 59 women treated with 300 mg oral iron sulfate. Intravenous iron sucrose complex group achieved significantly higher hemoglobin levels 128.5 ± 6.6 versus 111.4 ± 12.4 g/l in the oral iron group (P < 0.001) in a shorter period 6.9 ± 1.8 versus 14.9 ± 3.1 weeks in control group (P ≤ 0.001). Iron sucrose complex group showed no major side effects while 4 (6 %) of the control group could not tolerate ferrous sulfate, 18 (30 %) complained of disturbing gastrointestinal symptoms, and 18 (30 %) had poor compliance. The authors concluded that iron sucrose was a safe and effective alternative in the treatment of iron deficiency anemia during pregnancy [9]. This study is comparable to our study in that hemoglobin concentration was higher in the intravenous group in a shorter period of time.
In the study done by Bayoumeu et al. [10], involving 50 women intravenous iron sucrose was compared with oral ferrous sulfate. In the intravenous group an increase in hemoglobin was observed rising from 9.6 ± 0.79 to 11.11 ± 1.3 g/dl on day 30 and from 9.7 ± 0.5 to 11 ± 1.25 g/dl on day 30 in the oral group which was not significant. Ferritin values were higher in intravenous group, on day 30 (P < 0.0001) and at delivery P = 0.01 which was significant. This study slightly deviates from our study because sample size was small and iron sucrose was given over 21 days [10].
In a study by Al RA et al. [11] compared intravenous iron sucrose with oral iron polymaltose complex (300 mg elemental iron per day). The change in hemoglobin from baseline was significantly higher in the intravenous group than the oral group at each measurement; the changes with respect to subsequent hemoglobin were significantly higher on day 14th (P = 0.004) and 28th (P = 0.031). Serum ferritin levels were changed significantly across time with both the oral (P < 0.05) and intravenous group (P < 0.5). Serum ferritin levels were higher in the intravenous group, than in the oral group at each point of measurement. In the oral group it was 11 ± 11 μg/l compared to 28 ± 26 μg/l in the intravenous group (P < 0.001) at the fourth week and 18.1 ± 11 μg/l, compared with 23.7 ± 13.8 μg/l (P = 0.04) at birth in oral and intravenous group, respectively. This study is comparable to our study because there was a significant rise in hemoglobin and ferritin levels in intravenous group compared to oral group [11].
Bencaivo et al. [12] assessed and compared the efficacy and safety of intravenous iron sucrose to oral ferrous sulfate. There was a non significant increase in hemoglobin in the intravenous group but the repleted iron stores were significantly higher than in the oral group. This study deviates from our study as only ferritin levels were significantly raised, whereas difference in hemoglobin level was not significant [12].
Conclusion
Iron sucrose is an effective alternative to oral ferrous ascorbate in the treatment of iron deficiency anemia of pregnancy. Intravenous iron sucrose produces a more rapid increase in hemoglobin concentration and serum ferritin levels than oral ferrous ascorbate.
Intravenous iron therapy is a safe alternative for the treatment of anemia, being able to reduce the need for blood transfusion and its concomitant side effect such as anaphylactic shock, febrile and hemolytic reactions, infections (hepatitis B, C, HIV, protozoan and bacterial) alloimmunization and graft versus host disease. During pregnancy and puerperium it helps to rebuild iron stores, helping the symptoms of anemia to subside at a faster rate and reduces the risk of developing anemia in subsequent pregnancies. Major advantages are safety, efficacy, compliance, simple mode of administration in an outpatient setting and cost effectiveness because admission is not needed in all cases.
Normally blood transfusion is an option in the cases of moderate and severe anemia in the third trimester of pregnancy. The given Iron sucrose intravenously may reduce the need for blood transfusion because of its faster action. Therefore, it can be considered as an alternative to blood transfusion in the treatment of pregnant women with moderate iron deficiency anemia during the third trimester.
References
- 1.Candio F, Hofmeyr GJ. Treatments for iron deficiency anemia in pregnancy. RHL commentary. The WHO Reproductive Health Library. Geneva: World Health Organisation; 2007.
- 2.UNICEF/UNO/WHO. Iron deficiency anemia: assessment, prevention and control. Geneva: World Health Organisation; 2001.
- 3.Kalaivani K. Prevalence and consequences of anemia in pregnancy. Indian J Med Res. 2009;130:627–633. [PubMed] [Google Scholar]
- 4.National Family Health Survey (NFHS-3), India; 2005–2006.
- 5.Danielson BG, Salmonson T, Derendorf H, et al. Pharmacokinetics of iron(III) hydroxide sucrose complex after a single intravenous dose in healthy volunteers. Arzneimittelforschung. 1996;46:615–621. [PubMed] [Google Scholar]
- 6.Beshara S, Lundqvist H, Sundin J, et al. Pharmacokinetics and red cell utilization of iron(III) hydroxide–sucrose complex in anaemic patients: a study using positron emission tomography. Br J Haematol. 1999;104:296–302. doi: 10.1046/j.1365-2141.1999.01179.x. [DOI] [PubMed] [Google Scholar]
- 7.Yee J, Besarab A. Iron sucrose: the oldest iron therapy becomes new. Am J Kidney Dis. 2002;40:1111–1121. doi: 10.1053/ajkd.2002.36853. [DOI] [PubMed] [Google Scholar]
- 8.Teucher B, Olivares M, Cori H. Enhancers of iron absorption: ascorbic acid and other organic acids. Int J Vitam Nutr Res. 2004;74:403–419. doi: 10.1024/0300-9831.74.6.403. [DOI] [PubMed] [Google Scholar]
- 9.al-Momen AK, al-Meshari A, al-Nuaim L, et al. Intravenous iron sucrose complex in the treatment of iron deficiency anemia during pregnancy. Eur J Obstet Gynecol Reprod Biol. 1996;69:121–124. doi: 10.1016/0301-2115(95)02538-3. [DOI] [PubMed] [Google Scholar]
- 10.Bayoumeu F, Subiran-Buisset C, Baka NE, et al. Iron therapy in iron deficiency anemia in pregnancy: intravenous route versus oral route. Am J Obstet Gynecol. 2002;186:518–522. doi: 10.1067/mob.2002.121894. [DOI] [PubMed] [Google Scholar]
- 11.Al RA, Unlubilgin E, Kandemir O, et al. Intravenous versus oral iron for treatment of anemia in pregnancy: a randomized trial. Obstet Gynecol. 2005;106:1335–1340. doi: 10.1097/01.AOG.0000185260.82466.b4. [DOI] [PubMed] [Google Scholar]
- 12.Bencaiova G, Mandach U, Zimmermann R. Iron prophylaxis in pregnancy: intravenous route versus oral route. Eur J Obstet Gynecol Reprod Biol. 2009;144:135–139. doi: 10.1016/j.ejogrb.2009.03.006. [DOI] [PubMed] [Google Scholar]