Abstract
Both Il2−/− mice and Scurfy (Sf) mutant mice that are deficient in FoxP3, develop multi-organ inflammation but only the latter display severe skin and lung inflammation. In contrast, Sf.Il2−/− double mutant mice do not display skin inflammation and markedly reduced lung inflammation. In this review, we summarize our recent findings based on microarray, q-PCR and functional studies of 10 Sf double mutant mice. These studies revealed novel pro-inflammatory functions of IL-2 in regulating inflammation in an organ-specific manner. IL-2 exerts its “organ-specific” pro-inflammatory function by regulating the migration and retention of CD4+ T-cells (both Th1 and Th2) specifically to the skin and lung. In addition, IL-2 is also required for regulating the Th2 cytokine response during T-cell activation. Further studies on these IL-2 –regulated genes will help in identifying novel targets for intervention in inflammatory diseases of skin and lung.
Keywords: Treg, Scurfy mice, autoimmune diseases, inflammation
1. INTRODUCTION
IL-2 is required for the generation and maintenance of T effector and Treg cells and complex mechanisms are operating to keep a delicate balance between these T cell populations [1]. It has been demonstrated that B6.Il2−/− mice develop colitis but not inflammation in the skin and have a significant reduction of inflammation in the lungs in which accumulation was mainly perivascular [2–4]. It is further shown that the generation of Treg in B6.Il2−/− mice is partially compensated by IL-15 and IL-7 [5]. These Treg could have selectively prevented skin and lung inflammation in Il2−/− mice. However, there are no mechanistic reasons or evidence for this type of “organ-specific” control of autoimmunity. These observations created an enigma as to the reason why B6.Il2−/− mice developed weak lung inflammation and no skin inflammation. Our recent studies on Sf mice that are deficient in FoxP3 and its double mutants provide a definitive answer to this enigma.
Because inflammation of skin and lung is often involved in autoimmune disorders such as systemic lupus erythematosus, rheumatoid arthritis, dermatomyositis and systemic sclerosis, our studies provide evidence that IL-2 is crucial in the pathogenesis of such inflammation. The studies over the past several years in our laboratories on B6.Sf and ten Sf double mutants (listed in Table 1) will be reviewed [4, 6, and 7]. In these studies the expression of cytokines, chemokines and trafficking receptor genes in CD4+ T cells were determined. The pertinent data are summarized in tables 1 and 2. Inflammation in the lung and skin was not observed in Sf.Il2−/− mice although systemic inflammation in the liver did not change. This dissociation of skin and lung inflammation from systemic inflammation was due to the lack of expression of Th2 related trafficking receptor genes and some of these receptors expressed by both Th1 and Th2 cells. These observations led us to conclude that IL-2 has new pro-inflammatory functions in vivo. The evidence to support this conclusion will be reviewed. Furthermore, it is our intention that this review will rekindle the interest in targeting IL-2 as a therapeutic approach in skin and lung involvement in autoimmune rheumatological diseases.
Table 1.
Sf double mutant trafficking receptor gene expression and its effect on skin and lung inflammation: Lack of skin and lung inflammation in Sf.II2−/− mice correlates with lack of increased expression of Th2 related trafficking receptor genes. Liver inflammation is representative of systemic inflammation that includes inflammation in the colon, pancreas and salivary glands.*
| Strain | TRG expression | Liver inflammation | Lung inflammation | Skin inflammation | |
|---|---|---|---|---|---|
| Th1-related | Th2-related | ||||
| B6 | 1 | 1 | 0 | 0 | 0 |
| Sf** | 3 | 3 | 3 | 4 | 4 |
| Sf.Il2−/− | 3 | 1 | 3 | 1 | 0 |
| Sf.Il4−/− | 4 | 2 | 4 | 4 | 4 |
| Sf.Ifng−/− (6 wk) | 2 | 3 | 3 | 3 | 3 |
| Sf.Stat6−/− | 4 | 2 | 4 | 4 | 4 |
| Sf.Itgae−/− | 3 | 3 | 3 | 2 | 1 |
| Sf.Ltb4r1−/− | 3 | 3 | 3 | 3 | 2 |
| Sf.Alox5−/− | 3 | 4 | 4 | 4 | 4 |
| Sf.Il10−/− | ND | ND | 4 | 4 | 4 |
| Sf.Cx3cr1gfp/gfp | ND | ND | 3 | 4 | 4 |
| Sf.Fas−/− (12 wk) | ND | ND | 3 | 4 | 4 |
Trafficking receptor gene expression was determined by quantitative PCR and microarray assays [4,6–7]. Inflammation of individual organs was determined by histology and visual examination of the skin. Mice are examined at 3–4 weeks of age. ND: not determined. An arbitrary assignment of “1” for trafficking receptor expression is given to B6 mice as a reference..
Sf, Scurfy and Fox P3 deficient; Ifng, Interferon-γ; Itgae, integrin αE; Ltb4r1, leukotriene B4 receptor 1; Alox5, arachidonate 5-lipoxygenase; Cx3cr1gfp/gfp, Cx3cr1 deficient and expression of EGFP under control of endogenous Cx3cr1 locus.
Table 2.
Traffic and related receptor gene expression that are important for skin and lung inflammation: There is a lack of Th2 related receptor gene expression. Cxcr3 and Cxcr6 expression are not dependent on IL2. Cxcr3 is induced by interferon γ and the expression of Cxcr6 is enhanced by both Th1 and Th2 cytokines. The expression of the adhesion molecule Integrin, alpha E (ITGAE, CD103) is controlled by IL-2.
| Receptor gene | B6 | Sf | Sf.II2−/− | Sf.ll4−/− or Sf. Stat6−/− | Sf.lfng−/− | |
|---|---|---|---|---|---|---|
| Th2-related | Cysltr1* | −** | +++ | − | + | +++ |
| Ltb4r1 | − | +++ | − | + | +++ | |
| II1rl1 | − | +++ | − | + | +++ | |
| Ptgir | − | ++ | − | + | +++ | |
| Th1-related | Cxcr3 | − | +++ | +++ | +++++ | − |
| Th1- and Th2-related | Ccr1 | − | ++ | − | ++ | ++ |
| Ccr8 | − | + | − | + | + | |
| Cxcr6 | − | + | + | ++++ | ++ | |
| Adhesion | Itgae | − | +++ | − | +++ | +++ |
Cysltr1, cysteinyl leukotriene receptor 1; Ltb4r1, leukotriene B4 receptor 1; Ptgir, prostaglandin I2 (prostacyclin) receptor (IP); Itgae, integrin αE.
(−) designates the baseline level in B6 mice.
2. Lack of skin and lung inflammation in Sf.Il2−/− mice suggests Il2 plays a critical role in skin and lung inflammation
Due to a mutation in the transcription factor Foxp3, B6.Sf mice totally lack functional CD4+Foxp3+Treg cells and develop fatal multi-organ inflammation (MOI) within 28 days after birth [8, 9]. The major organs with severe inflammation are skin, lungs and liver. We bred Il2−/− mutant gene into Sf mice (Sf.Il2−/−) to determine how IL-2 controls skin and lung inflammation [4, 6]. The Il2−/− mutant gene effect on skin and lungs is dominant because these mice do not develop inflammation in these organs. In addition, transfer of Sf.Il2−/− lymph node (LN) T-cells also failed to induce inflammation in the skin and lungs of Rag1−/− recipients, although liver inflammation and colitis were elicited, indicating that Sf.Il2−/− LN T-cells do not have the T-cells capable of migration to the skin and lungs to induce inflammation [4, 6].
Sf mice begin to display clinical signs of skin inflammation at 2 weeks old. Lethargy and lack of body weight gain soon follow. The clinical signs associated with skin inflammation were not observed in Sf.Il2−/− mice. The lethargy and lack of body weight gain were still observed but the lifespan was prolonged to 12–17 weeks old [4]. An extensive study on generalized autoimmune disease was reported for Balb.Il2−/− mice that have a 35-day lifespan [3]. The tissue specificity of the autoimmune disease was assessed by histology. However, these tissues were examined at 30 days old at which time more than 80% the mice were dead [3]. In addition, tissue inflammation was largely restricted to the perivascular region. The effect of anti-RBC, which is strongly present in these mice, had not been defined in the inflammation. Most importantly, the skin inflammation and weakened lung inflammation were not addressed [2, 3]. In contrast, both Balb.Sf and B6.Sf mice, which have expanded ability to produce IL-2 due to loss of self-tolerance, develop severe skin and lung inflammation [6, 10].
3. IL-2 regulates trafficking receptor genes for skin and lung inflammation
To understand the reasons why Sf.Il2−/− LN lack T-cells that migrate to the skin and lungs, we conducted microarray analyses using B6, Sf and Sf.Il2−/− LN CD4+ T-cells. When compared between Sf and Sf.Il2−/− samples, 346 probes showed significant differences in expression [6]. We eliminated those repetitive for the same gene and those whose function is not known to the immune system. This analysis resulted in 79 IL-2-regulated genes that may have a role in skin and lung inflammation. Among them, 38 are known participants of immune activation and inflammation. Two remarkable and highly relevant differences were observed (table 2). First are the strong expressions in transcriptions noted in comparison with Sf.Il2−/− samples; cysteinyl leukotriene receptor (Cysltr1) with 32-fold increase in transcription, leukotriene B4 receptor (Ltb4r1) with 9-fold increase, IL-1 receptor like-1 (Il1rl1) with 4-fold increase, integrin αE (Itgae) or CD103 with 18-fold increase, and Ccr1 with 8-fold increase. To a lesser extent integrin α6 (Itga6) with 2-fold increase, Ccr2 with 2-fold increase, Ccr8 with 3-fold increase, and Cxcr6 with 3-fold increase were also noted. Many of these genes are trafficking receptor genes that control lymphocyte migration to the non-lymphoid organs. Second is the lack of major differential expression of Th subset cytokine genes between Sf and Sf.Il2−/− samples, although many of these genes were expressed more in both samples as compared with B6 control. Specifically, it is surprising that many of the Th2 cytokine genes were expressed in Sf.Il2−/− samples yet these mice did not develop skin and lung inflammation [6].
The data indicate that IL-2 is required for the expansion of trafficking receptor genes that are often associated with Th response in the skin and lungs. Many of these genes contain STAT-5 and IL-4-controlled STAT-6 responsive elements and they may not be efficiently activated without IL-2. It is important to note that both Th1 and Th2 cells respond to IL-2 and IL-4. Some of the apparent “Th2-associated” trafficking receptor genes are in fact expressed in both subsets [11]. It should also be noted that there were modest decreases in skin and lung inflammation in both Sf.Itgae−/− and Sf.Ltb4r1−/− mice. Thus without the expression of the trafficking receptor gene for skin and lung inflammation in both Th1 and Th2 subsets, a lifelong immunity against skin and lung inflammation in Sf.Il2−/− mice is achieved [4, 6].
4. IL-2 regulates the expression of inflammatory cytokines
Many of the inflammatory cytokine genes are highly up-regulated in the CD4+ T-cells of Sf and Sf.Il2−/− mice when compared with B6 CD4+ T-cells [6]. It is interesting that Th1 and Th2 development based on Th cytokine gene expression in the LN does not require IL-2, although Th cytokine gene expression is always higher in Sf mice than Sf.Il2−/− mice [6]. In addition, Sf.Il2−/− LN T-cells express up-regulated IL-4 and other Th2 cytokine mRNA when compared with B6 control [6]. To resolve the role of IL-2 in cytokine production, we conducted ex vivo stimulation of the CD4+ LN T-cells from B6, Sf, and Sf.Il2−/− mice to determine the levels of Th cytokine-producing cells during T-cell activation by a 4-hour activation with PMA plus ionomycin (6). The results showed that Th1- but not Th2 -cells were activated in Sf.Il2−/− CD4+ T-cells when compared with Sf CD4+ T-cells. Thus, although Th2 cytokine genes were increased in the Sf.Il2−/− LN T-cells, they could not be activated to produce the corresponding cytokines. It has been shown that IL-2 is required for the Th2 expansion in in vitro Th2 induction systems [12]. Our study suggests that IL-2 is not required for the in vivo expression of Th2 cytokine genes but is required for the production of Th2 cytokines upon T-cell activation.
We also determined the serum levels of these cytokines even though non-CD4+ T cells also produce some of them. No difference in the expression of TNF-α and IFN-γ between Sf and Sf.Il2−/− mice was observed. Serum levels of IL-10 and IL-17 were not significantly different between Sf and Sf.Il2−/− mice. Surprisingly, IL-4, IL-5, and IL-13 were significantly lower in the Sf.Il2−/− sera. IL-3 and M-CSF were also markedly lower in Sf.Il2−/− sera [6]. These observations agree with the interpretation that Th2 cytokine production by Sf.Il2−/− T-cells is dependent on IL-2 in vivo.
The above results suggest that IL-2 regulates skin and lung inflammation at two stages of the inflammation process in Sf mice. The major targets of IL-2 are those receptors required for CD4+ T-cell trafficking. IL-2 also controls the cumulative levels of Th2 cytokines in Sf mice and the frequency of Th2 cells during T-cell activation [6]. The latter observation suggests that each organ of the MOI in Sf.Il2−/−, not restricted to skin and lungs, are all-deficient in Th2 response.
5. IL-2 is the major Th1 cytokine for skin and lung inflammation
Th1 response was originally defined by Coffman and colleagues, based on the ability to produce IL-2 and IFN-γ [13], whereas, the association of IL-4 with Th2 cells was discovered by Howard and Paul [14]. Because IL-2 and IFN-γ control different aspects of the inflammatory responses, we compared the autoimmune response between Sf.Ifng−/−, Sf.Il4−/−, and Sf.Il2−/− mice.
IFN-γ inhibits Th2 response under the in vitro induction condition skewed against Th2 response. It also activates macrophages and neutrophils. It induces CXCL9, CXCL10 and CXCL11 from various cells to attract leukocytes to target organs [15, 16]. It induces strong MHC expression and as such exacerbates ongoing immune response. In this regard, lacking IFN-γ in Sf mice should have a general inhibitory effect or delayed expression on inflammation. Less is known of the effect of IFN-γ on trafficking receptor gene regulation in CD4+ T-cells. Because both Sf.Il2−/− and Sf mice had high serum IFN-γ and IFN-γ+CD4+ T-cells [6] and only the Sf mice developed inflammation in the skin and lungs, it appears that IFN-γ can only delay but cannot stop the inflammation in Sf mice. This issue was further addressed.
In age-matched Sf.Ifng−/− mice, LN CD4+ T-cells produced less IL-2, TNF-α, IL-4, IL-5, and IL-13 as compared with Sf controls [7]. Because IFN-γ is also produced by CD8+ T-cells and because the inflammation-inducing function of IFN-γ is different from IL-2, different manifestations of inflammation occur in Sf.Ifng−/− mice [7]. The clinical signs of inflammation in the skin, eyes, ears and tail were reduced and delayed by 1–3 weeks. Histological analysis of the MOI indicated a significant inhibition of inflammation in the skin and ears, although a slight but non-significant inhibition of inflammation in the lungs and liver was observed [7]. By 3 weeks of age, Sf.Ifng−/− mice had significant reduction of inflammation in ears, skin, lungs and liver as compared with Sf controls. The lifespan of Sf.Ifng−/− mice was prolonged to 6–7 weeks and at that time the MOI was fully developed. The results are in contrast to Sf.Il2−/− mice in which the inflammation in the skin and lungs was inhibited even in the presence of increased T-cells and IFN-γ. Thus, IFN-γ is not the major Th1 cytokine that controls the development of skin and lung inflammation in Sf mice.
6. Sf.II4−/− and Sf.STAT-6−/− mice develop inflammation in the skin and lungs
To further delineate the role of IL-2 on the regulation of trafficking receptor genes and on Th2 development, we generated the Sf.Il4−/− and Sf.STAT-6−/− double mutant mice. In these mice, the clinical signs of skin inflammation and lethargy appeared similar to Sf mice. Histological analysis revealed strong inflammation in the skin, lungs and liver in these mice even though their Th2 response was inhibited. Thus, IL-4/STAT-6-dependent Th2 response is not required for skin and lung inflammation in Sf mice [7].
7. IL-2, IL-4, and IFN-γ are the major cytokines that regulate trafficking receptor gene expression in Th subsets in Sf mice
To further clarify the role of various cytokines in the inflammation of skin and lungs, the CD4+ T-cells that produced IL-2, IFN-γ, TNF-α, IL-10, IL-4, IL-5, IL-13, and IL-17 in the LN cells upon activation were compared among the 3 weeks old B6, Sf. Sf.Il2−/−, and Sf.Il4−/− mice [7]. B6 mice expressed little IFN-γ+CD4+ T-cells, which were increased significantly in Sf mice. In Sf.Il2−/− mice, IL-2+CD4+ T-cells were absent but the frequency of IFN-γ+CD4+ T-cells was similar to that in Sf mice, indicating that IL-2 deficiency did not affect IFN-γ production in Th1 cells.
B6 mice had few IL-4+CD4+, IL-5+CD4+, and IL-13+CD4+ T-cells, which were significantly increased in Sf mice but strongly inhibited in Sf.Il2−/− mice [6]. When compared with Sf mice, Sf.Il4−/− mice had no IL-4+CD4+ T-cells. Their IL-5+CD4+ T-cells were strongly inhibited but IL-13+CD4+ T-cells were observed albeit reduced [7]. The IFN-γ+CD4+ T-cells (~60%) were significantly higher than Sf and Sf.Il2−/− samples. In addition, this value was higher than the 30% of IL-2+CD4+ T-cells in the same samples. These observations agree with the reports that IL-4 and STAT-6 are negative regulators for IFN-γ production. By contrast, a significant expression of IL-10+CD4+ T-cells was observed in Sf.Il4−/− and Sf.Il2−/− mice, suggesting IL-10 expression in Sf CD4+ T-cells is not controlled by IL-2 or IL-4. Similarly, TNF-α+CD4+ T-cells were also increased in Sf mice as compared to B6 and the strong expression was not diminished in Sf.Il2−/− and Sf.Il4−/− mice [7].
Sf.Il4−/− mice do not express IL-4+Th2 cells. In Sf.STAT-6−/− mice, IL-4+ Th2 cells were significantly reduced but they were significantly more than that in B6 mice, suggesting that IL-2 is more critical than STAT-6 for the Th2 response but STAT-6 is still needed for the optimal expansion of the IL-4+CD4+ T-cells in Sf mice [7]. Th17 cells remain low as compared with Th1 and Th2 cells. This low value was observed in all Sf double mutant mice examined.
These observations strongly suggest that trafficking receptor gene expression following T-cell activation does not depend on the TNF-α, IL-10, IL-5, IL-13, and IL-17. Although other Th cytokines were not determined, it appears that IL-2, IL-4, and IFN-γ are the major cytokines that regulate trafficking receptor gene expression in Th subsets in Sf mice.
8. IL-2 regulates the expression of trafficking receptor genes that are crucial for homing of T cells to the skin and the lung
We determined the expression by real-time PCR of Cysltr1, Ltb4r1, Ptgir (prostacyclin receptor), Il1rl1, Ccr1, Ccr3, Ccr4, Ccr8, Cxcr3 and Cxcr6 trafficking receptor genes(7). They were chosen because some of them were selectively increased in Sf LN CD4+ T-cells when compared with Sf.Il2−/− samples whereas others were enhanced in both when compared with B6 mice [6]. The expression of Cysltr1, Ltb4r1, and Ptgir were significantly inhibited in Sf.STAT-6−/− CD4+ T-cells when compared with Sf mice. The expression of Il1rl1 was also reduced, but was not statistically significant. The results were confirmed with Sf.Il4−/− LN T-cells. In contrast, the expression of Ccr1, Ccr3, Ccr4, Ccr8, Cxcr3 and Cxcr6 remained high in Sf.STAT-6−/− mice [7].
When compared with B6 CD4+ T-cells, Ccr1 and Ccr8 were selectively enhanced in Sf but not Sf.Il2−/− samples whereas Ccr3, Ccr4, Cxcr3 and Cxcr6 were up-regulated in both samples, confirming the microarray analysis [6]. The data obtained with Sf.Ifng−/− and Sf.STAT-6−/− mice demonstrated that Cxcr3 expression was critically dependent on IFN-γ [7]. The Sf.STAT-6−/− mice had the highest IFN-γ and this correlated with the strongest expression of Cxcr3 and Cxcr6 among all samples examined. Overall, these data demonstrate that inhibiting the trafficking receptor genes specifically regulated by IL-4, STAT-6 or IFN-γ is not sufficient to prevent inflammation in the skin and lungs as in Sf.Il2−/− mice [7].
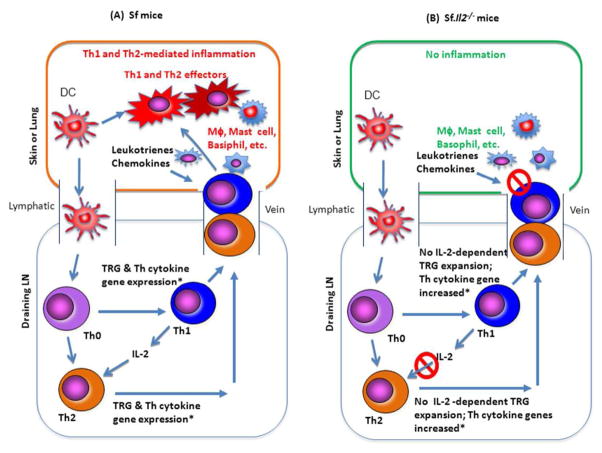
A large number of trafficking receptor genes such as Cysltr1, Ltβ4r, CD103, CCR8, and others were differentially expressed in LN Sf CD4+ T-cells when compared with Sf.Il2−/− mice [6]. These observations suggest that T-cells entering skin and lung is a critical step preceding the T-cell activation in these organs and the subsequent inflammatory response. Consequently, even a strong expansion of potential inflammation-inducing Th subsets in the LN cannot induce skin inflammation when the expression of the trafficking receptor genes is inhibited. In a recent study, it was reported that Sf.MyD88−/− mutant (in B6 background) also failed to develop skin and lung inflammation by not being able to induce TLR-dependent induction of chemokines around the environmental interfaces in the mice [17]. The chemotactic factors for T-cell entrance to skin and lungs are likely produced by mast cells, basophils and dermal micro-vessels, melanocytes, and Langerhans cells [18, 19]. IL-2, by regulating the receptors for these ligands, enables T-cell infiltration into skin and lungs to induce clinical symptoms in an apparently “organ-specific” manner (Figure 1).
Figure 1.
A schematic of Il2−/− mutant gene effect on skin and lung inflammation in Sf mice: (A) The dendritic cells (DC) that travel from skin and lungs and carry Ag will go to the draining lymph nodes where they will activate in an unabated manner the Ag-specific Th-cells in the absence of Treg. IL-4-producing Th2 response is also expanded based on Th-subset cytokine mRNA responses. In addition, a large panel of trafficking receptor genes for skin and lungs is also induced, allowing both Th1 and Th2 cells to enter the skin and lungs upon receiving chemokines and chemo-attractants from macrophages, mast cells, etc. Once entering the organs, they will be activated by local APC bearing the specific Ag. Again, the inflammation will be unabated in the absence of Treg. (B) In Sf.Il2−/− mice, although the reactions in the draining LN and their Th-subset cytokine mRNA response seem similar to or just slightly lower than that in Sf mice, the trafficking receptor genes for skin and lungs are not expanded without IL-2. Th1 and Th2 cells could not enter the skin and lungs to induce inflammation. It is important to note that the development of Th1 and Th2 subsets do not depend on IL-2. However, our ex vivo analysis of the subsets indicates that Th2 cells of Sf.Il2−/− LN T-cells could not be effectively activated whereas Th1 cells were strongly stimulated based on their Th cytokine production. It is also noted that strong inflammation was observed in Sf.Il4−/− and Sf.STAT-6−/− mice, indicating that IL-4+ Th2 cells were not required for Sf mice to develop skin and lung inflammation.
9. CONCLUSIONS
In this review, we have summarized the results of studies on Sf double mutant mice to address how IL-2 controls the apparent “organ-specific” inflammation in the skin and lungs. Sf.Il2−/− T-cells failed to express a large panel of trafficking receptor genes implicated for skin and lung inflammation. In addition, although Th2 cytokine genes were elevated in Sf.Il2−/− mice, in vitro activation of LN T-cells failed to optimally activate them to the level of Sf LN CD4+ T-cells [6]. The latter observation also suggests that Th2 responses are not participating in the inflammation in other organs of Sf.Il2−/− mice. These studies demonstrate that IL-2 controls trafficking receptor gene expression involved in skin and lung inflammation and Th2 responses in all inflamed organs. Indeed, inflammation in skin and lungs was not inhibited in the Th2-deficient Sf.Il4−/− and Sf.STAT-6−/− mice. We also demonstrated that Sf.Il2−/− but not Sf.Ifng−/− CD4+ T-cells lacked trafficking receptor gene over-expansion for the skin and lung inflammation. Our studies, therefore, have identified a new function of IL-2 that induces trafficking receptor genes in CD4+ T-cells necessary for skin and lung inflammation in Sf mice and as such results in an apparent “organ-specific” manifestation of the spontaneous inflammatory response.
We have recently proposed that IL-2 is a “two-faced master regulator of autoimmunity”. IL-2 is a powerful pro-inflammatory cytokine that activates and induces proliferation of various T-cell subsets [1]. However, IL-2 also has an anti-inflammatory property by being a critical component in the development and maintenance of Treg cells [8, 9]. In addition, IL-2 also has an anti-inflammatory effect by activating Fas/FasL pathway that serves to down-regulate the size of the expanded repertoire [4]. Here, we demonstrate that IL-2 has a novel pro-inflammatory function for its ability to induce a large panel of trafficking receptor genes for skin and lung inflammation and for Th2 cytokine production. Many T-cell subsets such as CD8+ T-cells have IL-2R and IL-4R. To date, few studies of CD8+ T-cells in skin and lung inflammation in Il2−/− mice have been reported. Whether IL-2 is necessary for the induction of those trafficking receptor genes required for infiltrating skin and lungs will be of interest and should be determined in the near future. The Sf.Il2−/− mice are unique in which the effect of lacking IL-2 begins at the start of immune development. To what extent does IL-2 affects skin and lung inflammation under non-knockout condition and whether IL-2-deficiency (but not knockout) also displays such regulated functions also remain to be determined. Finally, the observations summarized here suggest that IL-2 should be seriously considered as a target for therapy for skin and lung autoimmune disorders.
HIGHLIGHTS.
IL2 is an inflammatory cytokine for skin and lung as well as T cell growth
Th1 cytokine and related TRGs insufficient to induce skin and lung inflammation
IL2 is required for the expression of many of the Th2 related traffic genes
IL1 controls the expression of the adhesion molecule ITGAE, CD103
Acknowledgments
Funding: This work was supported by National Institutes of Health [R01 AR051203 and R01 DE017579 to STJ], and [R01 AR047988 and R01 AR049449 to SMF], and an Alliance for Lupus Research Target Identification in Lupus [Award #187966 to SMF].
Abbreviations
- MOI
Multi-organ Inflammation
Footnotes
Conflict of Interests: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sharma R, Fu SM, Ju ST. IL-2: a two-faced master regulator of autoimmunity. J Autoimmun. 2011;36:91–97. doi: 10.1016/j.jaut.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sadlack B, Merz H, Schorle H, Schimpl A, Feller AC, Horak I. Ulcerative colitis-like disease in mice with a disrupted interleukin-2 gene. Cell. 1993;75:253–261. doi: 10.1016/0092-8674(93)80067-o. [DOI] [PubMed] [Google Scholar]
- 3.Sadlack B, Löhler J, Schorle H, Klebb G, Haber H, Sickel E, Noelle RJ, Horak I. Generalized autoimmune disease in interleukin-2-deficient mice is triggered by an uncontrolled activation and proliferation of CD4+ T cells. Eur J Immunol. 1995;25:3053–3059. doi: 10.1002/eji.1830251111. [DOI] [PubMed] [Google Scholar]
- 4.Zheng L, Sharma R, Gaskin F, Fu SM, Ju ST. A novel role of IL-2 in organ-specific autoimmune inflammation beyond regulatory T cell checkpoint: Both IL-2 Knockout and Fas mutation prolong lifespan of Scurfy mice but by different mechanisms. J Immunol. 2007;179:8035–8041. doi: 10.4049/jimmunol.179.12.8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vang KB, Yang J, Mahmud SA, Burchill MA, Vegoe AL, Farrar MA. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J Immunol. 2008;181:3285–3290. doi: 10.4049/jimmunol.181.5.3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sharma R, Sharma PR, Kim Y-C, Leitinger N, Lee JK, Fu SM, S-T IL-2-controlled expression of multiple T cell trafficking genes and Th2 cytokines in the regulatory T cell-deficient Scurfy mice: Implication to multiorgan inflammation and control of skin and lung inflammation. J Immunol. 2011;186:1268–1278. doi: 10.4049/jimmunol.1002677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharma R, Sung SSJ, Gaskin F, Fu SM, Ju ST. A novel function of IL-2: Chemokine and chemoattractant receptor genes induction in Th subsets for skin and lung inflammation. J Autoimmun. 2013;38:322–331. doi: 10.1016/j.jaut.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 9.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 10.Lin W, Truong N, Grossman WJ, Haribhai D, Williams CB, Wang J, Martin MG, Chatila TA. Allergic dysregulation and hyperimmunoglobulinemia E in Foxp3 mutant mice. J Allergy Clin Immunol. 2005;116:1106–1115. doi: 10.1016/j.jaci.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 11.Nanki T, Lipsky PE. Lack of correlation between chemokine receptor and T(h)1/T(h)2 cytokine expression by individual memory T cells. Int Immunol. 2000;12:1659–1667. doi: 10.1093/intimm/12.12.1659. [DOI] [PubMed] [Google Scholar]
- 12.Cote-Sierra J, Foucras G, Guo L, Chiodetti L, Young HA, Hu-Li J, Zhu J, Paul WE. Interleukin 2 plays a central role in Th2 differentiation. Proc Natl Acad Sci USA. 2004;101:3880–3885. doi: 10.1073/pnas.0400339101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–2357. [PubMed] [Google Scholar]
- 14.Howard M, Paul WE. Interleukins for B lymphocytes. Lymphokine Res. 1982;1:1–4. [PubMed] [Google Scholar]
- 15.Mach F, Sauty A, Iarossi AS, Sukhova GK, Neote K, Libby P, Luster AD. Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells. J Clin Invest. 1999;104:1041–1050. doi: 10.1172/JCI6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cole KE, Strick CA, Paradis TJ, Ogborne KT, Loetscher M, Gladue RP, Lin W, Boyd JG, Moser B, Wood DE, Sahagan BG, Neote K. Interferon-inducible T cell alpha chemoattractant (I-TAC): a novel non-ELR CXC chemokine with potent activity on activated T cells through selective high affinity binding to CXCR3. J Exp Med. 1998;187:2009–2021. doi: 10.1084/jem.187.12.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivas MN, Koh YT, Chen A, Nguyen A, Lee YH, Lawson G, Chatila TA. MyD88 is critically involved in immune tolerance breakdown at environmental interfaces of Foxp3-deficient mice. J Clin Invest. 2012;122:1933–1947. doi: 10.1172/JCI40591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duroudier NP, Tulah AS, Sayers I. Leukotriene pathway genetics and pharmacogenetics in allergy. Allergy. 2009;64:823–839. doi: 10.1111/j.1398-9995.2009.02015.x. [DOI] [PubMed] [Google Scholar]
- 19.Imai T, Hieshima K, Haskell C, Baba M, Nagira M, Nishimura M, Kakizaki M, Takagi S, Nomiyama H, Schall TJ, Yoshie O. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91:521–530. doi: 10.1016/s0092-8674(00)80438-9. [DOI] [PubMed] [Google Scholar]