Abstract
The gamma interferon secretion levels of 52 adults whose T cells had been stimulated with the Mycobacterium tuberculosis antigens CFP-10 and ESAT-6 were measured by enzyme-linked immunospot methods and a whole-blood-based assay. The test results correlated well (r = 0.689, P < 0.0001). Its simple format and use of only small volumes of blood make the whole-blood assay suitable for pediatric practice and field trials.
Mycobacterium tuberculosis is a significant threat to global health (11). The skin test reagent used to aid diagnosis of both active and latent tuberculosis, purified protein derivative (PPD), is impaired in specificity and sensitivity (22), and the skin reaction may cause discomfort. Recent interest in the field of immunodiagnosis has therefore concentrated on antigens encoded by the RD1 genomic segment of M. tuberculosis, which is deleted from all BCG strains (5, 15, 19). In addition, the idea of replacing the tuberculin skin test (TST) by a method that assays the in vitro production of gamma interferon (IFN-γ) in response to M. tuberculosis antigens has gained popularity (2, 6, 9, 17). The most promising approach combines the recognized high sensitivity of ELISPOT analysis with the use of peptides covering the sequence of the RD1-encoded ESAT-6 molecule into a diagnostic test of greater sensitivity and specificity than the TST (4, 13, 18, 21). The enzyme-linked immunospot (ELISPOT) technique requires separation of cells from blood, the use of multiple wells of an expensive precoated plate, and an automated ELISPOT counter. Some have therefore wondered whether and how these advances could be applied to medically underserved environments (3). A kit based on whole-blood production of IFN-γ detected by enzyme-linked immunosorbent assay (ELISA) is available and has the advantage of relative simplicity, but being based on PPD, it does not ameliorate the poor specificity of skin testing (6). In addition, this test requires 10 ml of blood, a large amount if one considers its application in pediatric practice.
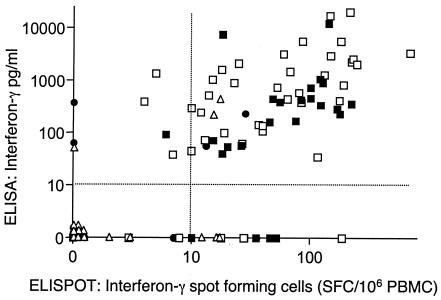
We were interested to determine, by the use of a sensitive IFN-γ ELISA, whether a form of the whole blood stimulation assay that is used by many groups to assay antigen specific responses to RD1 encoded antigens (1, 3, 7, 9, 16) could match the performance of the ELISPOT assay. Ethical permission for this study was provided by the Harrow local research ethics committee (Harrow LREC 1646). To perform the whole-blood assay, heparinized blood was diluted 1:10 in RPMI and 180 μl of the diluted blood was stimulated with 2.5 μg/ml ESAT-6 prepared as described previously (20) or 5 μg/ml CFP-10 (encoded by the gene adjacent to ESAT-6, obtained commercially from Lionex, Braunschweig, Germany) or no stimulus in duplicate on a 96-well plate. Diluted blood was cultured at 37°C in a CO2 incubator for 72 h, at which point the supernatants were aspirated for determination of IFN-γ level by ELISA (Antibody pair from BD Pharmingen catalogue no. 554548 and 554550). This ELISA has a working sensitivity of <10 pg/ml. Initially we determined the kinetics of antigen specific IFN-γ secretion in 4 strongly TST positive (Heaf grade 4) healthy adults (Fig. 1). Responses were heterogenous, but IFN-γ secretion into culture supernatants tended to increase with time up to 144 h. By 72 h all 4 donors had shown an appreciable increment over unstimulated levels in response to one or both antigens to offer the prospect that this assay would be positive in M. tuberculosis sensitized people. We therefore compared the overnight IFN-γ ELISPOT assay as previously described (10) with our 72 h whole-blood assay. IFN-γ spot forming cells and the production of IFN-γ in whole blood was determined for 52 variously sensitized adults (average age 35.8 years, 18 females, 34 males; 13 with culture or biopsy positive active tuberculosis, 23 strongly TST positive contacts of tuberculosis, 7 weakly TST positive and 9 TST negative). The analysis yielded 104 pairs of data: ESAT-6 and CFP-10 reactivity for each of the 52 subjects. The ELISPOT and ELISA responses correlated well (r = 0.689, 99% confidence limits 0.524-0.804, P < 0.0001, Fig. 2). Using cut-offs of 10 pg/ml for the ELISA and 10 spot forming cells/106 PBMC for the ELISPOT, the observed agreement between the tests was 81% (κ = 0.59). Using the 13 patients with active tuberculosis as a “gold standard” for sensitivity, the CFP-10 or ESAT-6 ELISPOT was positive in 100% and the ELISA in 92.3%. The corresponding percentages for strongly TST positive healthy people were 87 and 91%. Interestingly two TST negative Infectious diseases clinical staff, with known repeated exposure to infectious tuberculosis, had weakly positive reactions in both the ELISPOT and ELISA. Whether these results are true false positives, or indicate that these people had actually become infected without developing skin test positivity, is not clear. In their recent ELISPOT-based analysis of a school outbreak of tuberculosis, Ewer et al. detected similar individuals (13). A large prospective trial in a high incidence environment is required to determine whether the IFN-γ production in response to RD1 encoded antigens better predicts, as suggested (12), the risk of subsequent active tuberculosis than the TST.
FIG. 1.
Kinetics of IFN-γ secretion in the whole blood assay. Heparinised whole blood was diluted 1/10 in RPMI and cultured in the presence or absence of antigen for varying periods. Supernatants were aspirated and the IFN-γ content determined by ELISA. The response of 4 different healthy donors with strong TST reactions is shown. By 72 h all 4 donors had shown an appreciable increment over unstimulated levels in response to one or both antigens. Symbols: ▪, ESAT-6; •, CFP-10; ▵, no stimulus.
FIG. 2.
Correlation between the overnight ELISPOT assay and 72 h whole blood assay. An overnight IFN-γ ELISPOT assay and 72 h whole blood was performed in 52 variously sensitized adults. The horizontal and vertical dotted lines represent the ELISA and ELISPOT thresholds respectively. Symbols: ▪, active tuberculosis; •, Heaf grades 1 to 2; □, Heaf grades 3 to 4; ▵, Heaf grade 0. PBMC, peripheral blood mononuclear cells.
In low incidence environments, the IFN-γ response to RD1 encoded antigens clearly differentiates people sensitized by M. tuberculosis from those who are BCG vaccinated (18). However, the genes for ESAT-6 and CFP-10 are present in M. marinum and M. kansasii and the potential confounding effect of these organisms on specific diagnosis would best be determined prospectively (3, 14). It is interesting to note that while the correlation between the overnight ELISPOT assay and the 72-h whole-blood assay was generally good there were some discordant results (Fig. 2). The fundamental difference between the assays is timing, and it is feasible that the ELISA fails to detect IFN-γ production by short-lived effector cells that are particularly well detected by ELISPOT analysis. Conversely, cells that require to differentiate before producing IFN-γ may not be detected by the overnight ELISPOT method. From a practical perspective, as little as 250 μl of blood used in this whole-blood assay provides ostensibly similar sensitivity to ELISPOT analysis. The requirement for such a small sample may be of particular value when considering the potential to extend RD1 based immunodiagnosis to pediatric practice. In addition the simpler format would be more readily adaptable to medically underserved environments where the research application of such whole-blood assays to populations has been shown to be entirely feasible (8). The difficult clinical scenario presented by HIV coinfection should also be directly addressed, as skin testing performs worst in these people at very high risk of tuberculosis (11).
Acknowledgments
Funded by the Wellcome Trust of Great Britain (refs. 064261/Z/01/Z/JW/MW.sf and 059141/Z/99/Z).
REFERENCES
- 1.Aagaard, C., M. Govaerts, L. Meng Okkels, P. Andersen, and J. M. Pollock. 2003. Genomic approach to identification of Mycobacterium bovis diagnostic antigens in cattle. J. Clin. Microbiol. 41:3719-3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arend, S. M., P. Andersen, K. E. van Meijgaarden, R. L. Skjot, Y. W. Subronto, J. T. van Dissel, and T. H. Ottenhoff. 2000. Detection of active tuberculosis infection by T cell responses to early- secreted antigenic target 6-kDa protein and culture filtrate protein 10. J. Infect. Dis. 181:1850-1854. [DOI] [PubMed] [Google Scholar]
- 3.Arend, S. M., K. E. van Meijgaarden, K. de Boer, E. C. de Palou, D. van Soolingen, T. H. Ottenhoff, and J. T. van Dissel. 2002. Tuberculin skin testing and in vitro T cell responses to ESAT-6 and culture filtrate protein 10 after infection with Mycobacterium marinum or M. kansasii. J. Infect. Dis. 186:1797-1807. [DOI] [PubMed] [Google Scholar]
- 4.Barnes, P. F. 2001. Diagnosing latent tuberculosis infection: the 100-year upgrade. Am. J. Respir. Crit. Care Med. 163:807-808. [DOI] [PubMed] [Google Scholar]
- 5.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 6.Bellete, B., J. Coberly, G. L. Barnes, C. Ko, R. E. Chaisson, G. W. Comstock, and W. R. Bishai. 2002. Evaluation of a whole-blood interferon-gamma release assay for the detection of Mycobacterium tuberculosis infection in 2 study populations. Clin. Infect. Dis. 34:1449-1456. [DOI] [PubMed] [Google Scholar]
- 7.Black, G. F., R. E. Weir, S. D. Chaguluka, D. Warndorff, A. C. Crampin, L. Mwaungulu, L. Sichali, S. Floyd, L. Bliss, E. Jarman, L. Donovan, P. Andersen, W. Britton, G. Hewinson, K. Huygen, J. Paulsen, M. Singh, R. Prestidge, P. E. Fine, and H. M. Dockrell. 2003. Gamma interferon responses induced by a panel of recombinant and purified mycobacterial antigens in healthy, non-mycobacterium bovis BCG-vaccinated Malawian young adults. Clin. Diagn. Lab. Immunol. 10:602-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Black, G. F., R. E. Weir, S. Floyd, L. Bliss, D. K. Warndorff, A. C. Crampin, B. Ngwira, L. Sichali, B. Nazareth, J. M. Blackwell, K. Branson, S. D. Chaguluka, L. Donovan, E. Jarman, E. King, P. E. Fine, and H. M. Dockrell. 2002. BCG-induced increase in interferon-gamma response to mycobacterial antigens and efficacy of BCG vaccination in Malawi and the UK: two randomised controlled studies. Lancet 359:1393-1401. [DOI] [PubMed] [Google Scholar]
- 9.Brock, I., M. E. Munk, A. Kok-Jensen, and P. Andersen. 2001. Performance of whole blood IFN-gamma test for tuberculosis diagnosis based on PPD or the specific antigens ESAT-6 and CFP-10. Int. J. Tuberc. Lung Dis. 5:462-467. [PubMed] [Google Scholar]
- 10.Chapman, A. L., M. Munkanta, K. A. Wilkinson, A. A. Pathan, K. Ewer, H. Ayles, W. H. Reece, A. Mwinga, P. Godfrey-Faussett, and A. Lalvani. 2002. Rapid detection of active and latent tuberculosis infection in HIV-positive individuals by enumeration of Mycobacterium tuberculosis-specific T cells. AIDS. 16:2285-2293. [DOI] [PubMed] [Google Scholar]
- 11.Corbett, E. L., C. J. Watt, N. Walker, D. Maher, B. G. Williams, M. C. Raviglione, and C. Dye. 2003. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch. Intern. Med. 163:1009-1021. [DOI] [PubMed] [Google Scholar]
- 12.Doherty, T. M., A. Demissie, J. Olobo, D. Wolday, S. Britton, T. Eguale, P. Ravn, and P. Andersen. 2002. Immune responses to the Mycobacterium tuberculosis-specific antigen ESAT-6 signal subclinical infection among contacts of tuberculosis patients. J. Clin. Microbiol. 40:704-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ewer, K., J. Deeks, L. Alvarez, G. Bryant, S. Waller, P. Andersen, P. Monk, and A. Lalvani. 2003. Comparison of T-cell-based assay with tuberculin skin test for diagnosis of Mycobacterium tuberculosis infection in a school tuberculosis outbreak. Lancet 361:1168-1173. [DOI] [PubMed] [Google Scholar]
- 14.Gey van Pittius, N. C., R. M. Warren, and P. D. van Helden. 2002. ESAT-6 and CFP-10: what is the diagnosis? Infect. Immun. 70:6509-6510, 6511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gordon, S. V., R. Brosch, A. Billault, T. Garnier, K. Eiglmeier, and S. T. Cole. 1999. Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Mol. Microbiol. 32:643-655. [DOI] [PubMed] [Google Scholar]
- 16.Johnson, P. D., R. L. Stuart, M. L. Grayson, D. Olden, A. Clancy, P. Ravn, P. Andersen, W. J. Britton, and J. S. Rothel. 1999. Tuberculin-purified protein derivative-, MPT-64-, and ESAT-6-stimulated gamma interferon responses in medical students before and after Mycobacterium bovis BCG vaccination and in patients with tuberculosis. Clin. Diagn. Lab. Immunol. 6:934-937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jurcevic, S., A. Hills, G. Pasvol, R. N. Davidson, J. Ivanyi, and R. J. Wilkinson. 1996. T cell responses to a mixture of Mycobacterium tuberculosis peptides with complementary HLA-DR binding profiles. Clin. Exp. Immunol. 105:416-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lalvani, A., A. A. Pathan, H. McShane, R. J. Wilkinson, M. Latif, C. P. Conlon, G. Pasvol, and A. V. Hill. 2001. Rapid detection of M. tuberculosis infection by enumeration of antigen-specific T cells. Am. J. Resp. Crit. Care Med. 15:824-828. [DOI] [PubMed] [Google Scholar]
- 19.Mahairas, G. G., P. J. Sabo, M. J. Hickey, D. C. Singh, and C. K. Stover. 1996. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J. Bacteriol. 178:1274-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhodes, S. G., D. Gavier-Widen, B. M. Buddle, A. O. Whelan, M. Singh, R. G. Hewinson, and H. M. Vordermeier. 2000. Antigen specificity in experimental bovine tuberculosis. Infect. Immun. 68:2573-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Surcel, H.-M., M. Troye-Blomberg, S. Paulie, G. Andersson, C. Moreno, G. Pasvol, and J. Ivanyi. 1994. Th1/Th2 profiles in tuberculosis based on proliferation and cytokine response of blood lymphocytes to mycobacterial antigens. Immunology 81:171-176. [PMC free article] [PubMed] [Google Scholar]
- 22.Wilkinson, R. J., K. Hasløv, R. Rappuoli, F. Giovannoni, P. R. Narayanan, C. R. Desai, M. Vordermeier, J. Paulsen, G. Pasvol, J. Ivanyi, and M. Singh. 1997. Evaluation of the recombinant 38-kilodalton antigen of Mycobacterium tuberculosis as a potential immunodiagnostic reagent. J. Clin. Microbiol. 35:553-557. [DOI] [PMC free article] [PubMed] [Google Scholar]