Abstract
Aggressive fibromatosis is a rare neoplasm arising from musculoaponeurotic structures. Our aim is to share our experience with this rare tumor in our institute and to discuss the more perplexing recurrence patterns and the management options. This is a retrospective study of the disease, treated in our institute for the past fourteen years. A total of 36 patients were analyzed. The demographic pattern of the disease, various treatment modalities offered and their outcome along with patterns of recurrence were studied. Our study showed a demographic pattern mostly similar to the rest of the world. But the pattern of recurrence and the multicentric and the non-random pattern of presentation observed in our study showed some difference from the other studies. We suggest surgery as the primary modality with radiation reserved for select patients with margin positivity, inoperable tumors, and multiple tumors. Since the disease has a long natural history a wait and watch policy can be observed for giving adjuvant RT. There is need for prospective multi-institutional RCTs to shed light on the unknown facts about this disease.
Keywords: Fibromatosis, Recurrence patterns, Surgical excision, Adjuvant, Radiotherapy, Tamoxifen
Introduction
Aggressive Fibromatosis also known as “Desmoids” is a group of clinically diverse deep-seated fibrous neoplasms [1]. It is a relatively rare neoplasm constituting less than 3 % of all soft tissue tumors with an incidence of 2–4 per 100000 population [1–5]. It is a locally aggressive tumor with a high rate of recurrence but with no tendency to metastasize [6]. Factors influencing such a high rate of recurrence and the pattern in which it recurs are not similar in various studies. Surgery is the primary modality of treatment. But, due to their enigmatic recurrence pattern, adjuvant and salvage modalities of treatment for this disease are not optimized. In this article, we will be discussing our 14 year experience with this relatively rare tumor and the recurrence patterns and treatment strategies we offered.
Patients and Methods
We made a retrospective analysis of 36 patients treated over the past 14 years (1998–2011). This included patients with extra-abdominal, abdominal and intra-abdominal fibromatoses. By analyzing the various data, we obtained information regarding demographic patterns, various surgical therapies offered. Special attention was paid while collecting data regarding the recurrence patterns, salvage modalities, and adjuvant offered, as they are the most intriguing aspect of this disease.
Findings
There was a slight female preponderance in the ratio of 1.25:1 (20 female patients vs. 16 male patients). In children, the disease is very rare and has a male preponderance. We had 5 patients within the age group of 12 and below (13.8 %) and 4 of them were male. Mean age at presentation was 27 years (range 7 to 64). The number of tumors at presentation in a single patient ranged from 1 to a maximum of 6 with a tumor size ranging from 3 cm to 20 cm, with an average size of 8.8 cm. 23 were primary tumors and 13 were recurrent. The duration of presenting symptoms was more with recurrent tumors since late recurrence is a common phenomenon in this disease.
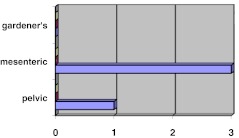
There were 31 cases of extra-abdominal, 4 cases of intra abdominal and a case of abdominal fibromatosis (based on Enzeinger & Weiss Classification), in our study. Within the intraabdominal group, we have not encountered any case of mesenteric fibromatosis associated with Gardener’s syndrome as shown in the Fig. 4.
Fig. 4.
Incidence of intraabdominal fibromatoses in our study
Most of the cases of extra-abdominal fibromatosis occurred at sites in and around the shoulder and pelvic girdle, as shown in the Table 1.
Table 1.
Site distribution of Extra-abdominal fibromatosis
| SITE | Number of cases |
|---|---|
| Shoulder | 4 |
| Arm | 9 |
| Forearm | 2 |
| Loin | 1 |
| Gluteal | 3 |
| Leg & foot | 3 |
| Head & neck | 2 |
| Lower limb | 2 |
| Thigh | 5 |
Management
After discussion in multidisciplinary tumor board, patients were offered surgical excision as primary modality for tumors that were operable (31 patients) and radiotherapy or systemic therapy for inoperable tumors, those with margin positivity and recurrent tumors on an individual basis after discussion in tumor board. Tumors with inconclusive biopsy results were operated on after considering clinical and radiological features. Surgery was done following adequate oncological principles but consideration was given for preservation of function (Figs. 1, 2 and 3).
Fig. 1.

Pre operative photo
Fig. 2.

Intraoperative defect
Fig. 3.
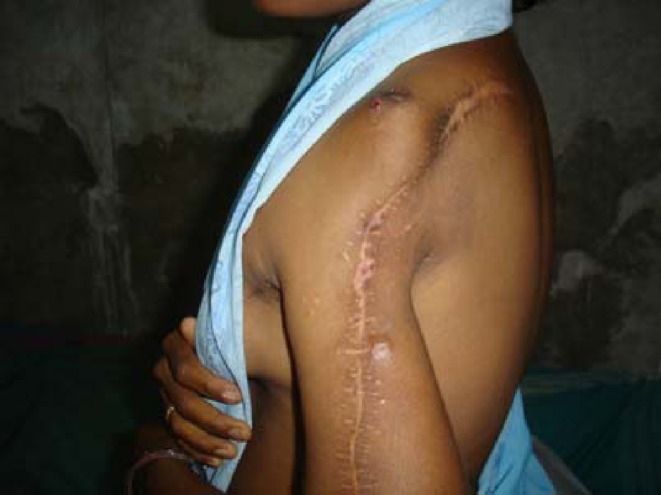
Post operative picture
The mean tumor size of the surgical group was 8.1 cm. Limb Salvage was possible in all but one patient with involvement of extremities. One patient with attempted limb salvage with vascular reconstruction using a PTFE graft underwent an above knee amputation in the early postoperative period following graft failure secondary to infection. Procedures undertaken were shown in the Table 2.
Table 2.
Procedures undertaken were shown in the table below
| Surgical procedure | No of patients |
|---|---|
| Wide monobloc excision | 21 |
| Scapulectomy | 4 |
| Wide local excision & centralization of ulna | 2 |
| Wide local excision with excision of metatarsals & bone grafting | 2 |
| Laparotomy and excision | 1 |
| Amputation | 1 |
| TOTAL | 31 |
Patterns of Recurrence
There were 14 recurrences (45.2 %) which were surgically salvaged. Various variables taken up for analyses of recurrences are given in Table 3.
Table 3.
Comparison between recurrent and disease free group
| Disease free group | Recurrence group | Time taken to recur (months) | ||
|---|---|---|---|---|
| No. of Patients | 25 | 6 | ||
| Site | Not significant | Not significant | ||
| Size (cm) | 9 | 9.5 | ||
| Median Age (years) | 29 | 35 | ||
| No. of primary tumor | Single | 22 | 4 | 16 |
| Multiple | 3 | 2 | 13 | |
| Margin Status | Positive | 2 | 4 | 15 |
| Negative | 23 | 2 | 11 | |
| Presentation | Primary | 16 | 2 | 13 |
| Recurrent | 9 | 4 | 16 | |
In the surgical group there were 5 positive margins and 1 close margin. Median follow-up time in our series is 5 year. Four patients were offered adjuvant radiation as per tumor board decision.
Adjuvant Therapy
Four patients received adjuvant Radiotherapy out of which 1 experienced recurrence which was surgically salvaged. Radiotherapy was given for cases with margin positivity, multiple tumors or multiple recurrences.
Adjuvant tamoxifen was given to 5 patients. Usually it is reserved for inoperable tumors and select patients with margin positivity. One patient with mesenteric fibromatosis has stable disease for the past 5 years with tamoxifen alone and another patient is on follow up 1 year after starting therapy. Three patients on adjuvant therapy were lost for follow up.
Discussion
On comparison of our findings with the World literature, we could find concordance in most of the variables barring a few. There were more boys in the pediatric age group. Only one patient—a young girl was found to have abdominal fibromatosis in contrast to World literature in which fertile females dominate. These, we think, might be due to small number of cases analyzed, but could also be due to different demographic trends in this part of the country.
Surgery was the mainstay of therapy for all operable cases of fibromatosis. This is confirmed in almost all the literature we reviewed. We did not offer adjuvant RT routinely to all patients because of the young age of the patients at presentation and considering the long term complications of radiation in this group of patients. Since the role of anti-estrogens and cytotoxic agents is not very clearly understood in the adjuvant setting they are not routinely used except in select circumstances.
Recurrence
In our analysis, we found sex, site, size, multiplicity at presentation had no influence on recurrence while recurrence at presentation (56 % recurrent vs. 24 % primary) and positive margin (66 % positive margin vs. 10 % free margin) had a strong association with recurrence.
The propensity of aggressive fibromatosis to locally recur is related to its infiltrative nature, which makes it difficult for the surgeon to grossly identify the true extent of disease. These tumors lack a pseudo capsule and display nonpalpable diffusion along muscle bundles and fascial planes. This may justify the high recurrence rate of the disease after adequate surgery. Posner et al., Goy et al. and Spear et al. identified positive resection margins as the most important Independent predictive factor of local recurrence [6–9]. But in a study by Merchant et al. [4] and in few other studies positive margin does not affect the outcome adversely. Gronchi et al. [18] speculates that the recurrence rate highly depends on inherent characteristics of the disease, which might be more or less aggressive on its own, so that it might recur or not rather independent of the quality of surgery.
In Gronchi et al’s series [18], patients with large tumors located at the extremities or girdles had a higher risk of recurrence, regardless of whether they were excised with positive or negative resection margins. We did not find any such association between site and recurrence.
Unfortunately, there are relatively few large series that outline the actuarial local recurrence free survival rates stratified by microscopic margin status for patients treated by surgery alone. Comparison of local recurrence-free survival rates following surgery in Desmoid tumors is shown in Table 4: [10]
Table 4.
Comparison of local RFS after surgery in various institutions
| Institution | No of patients | Follow-up (months) | Local RFS | |
|---|---|---|---|---|
| R0 | R1 | |||
| Massachusetts General Hospital | 51 | 12–59 | 77 | 56 |
| MDACC | 122 | 113 | 73 | 46 |
| MSKCC | 128 | 88 | 86 | 49 |
| Govt Royapettah Hospital | 36 | 60 | 90 | 44 |
The experience of these institutions suggest that local control rates for patients who are treated by surgery alone is in the order of 50 % when R1 resection is made compared to approximately 75 % with R0 resection.
The most intriguing aspect of our recurrence pattern is the time to recur. Although positive margin in our series was associated with high recurrence rate, the duration taken for the tumor to recur in both the positive margin group and negative margin group did not show any significant difference. The number of tumor at presentation, single vs. multiple and the presentation, primary vs. recurrent, also did not show any significant difference in the time taken to recur. Although we could not find any literature evidence to support this fact, we believe this fact reiterates that recurrences in fibromatosis may be more influenced by the biology of the disease (Table 5).
Table 5.
A table comparing our data with the western literature is shown below
| Variables | Our study | World literature |
|---|---|---|
| Mean Age | 27 | 25–35 |
| Sex (F:M) | 1.25:1 | 1.6:1 |
| Children | 13.8(%) | |
| Sex ratio(F:M) | 1:4 | 1:1 |
| Tumor | ||
| Number | 6 | |
| Size | 8.8 cm(3–20) | |
| Site distribution | ||
| Abdominal | 2.8 %(1) | 25 % |
| Extra-abdominal | 86.1 % (31) | 50 % |
| Intra-abdominal | 11.1 %(4) | 15 % |
| Recurrence | ||
| No of recurrence | 45.2 %(14) | |
| Margin positive cases | 66 % (4) | 60 % |
| Recurrence after | ||
| Primary surgery | 30 % | |
| Recurrent surgery | 56 % |
Pattern of Recurrence
Pattern of recurrence in fibromatosis can also be quiet different from other tumors. Recurrences can be within the surgical field and also away from it. The recurrence of the tumor away from the field of surgery, more proximally within the same myotome was not classically observed in our series. In our series one patient who had a tumor in the origin of coracho brachialis after surgical excision experienced recurrence at lower end of the muscle about 10 cm away from the primary tumor and subsequent recurrence in pectoralis major muscle. Here the recurrence pattern is first distal to the index lesion and then proximal to it within the same myotome. Whether this is due to intra muscular microscopic infiltration of the tumor along the muscle spindles or a genetic field defect in the affected muscles is unknown. In few patients the recurrences can be just outside the radiation field. One of our patients experienced a recurrence just outside the radiation field.
Multicentric Fibromatosis –A New Entity
Fong et al. has described an entity where multiple lesions are found typically confined to one anatomical region of the body [11]. In most cases the second growth is found proximal to the first growth. This is found in 5 % of the patients diagnosed to have extra abdominal fibromatosis.
Although multicentre fibromatosis confined to one anatomical region is a known entity, we describe a new hitherto undescribed non random pattern of distribution of multicentric extremity fibromatosis.
Two male patients presented with multiple fibromatosis of lower limb distributed in gluteus muscles, quadriceps, muscles of popliteal fossa and a lesion in between metatarsals in the foot. One patient underwent wide excision of all lesions in a staged manner and is now undergoing treatment for recurrence at two places (left Thigh and popliteal fossa). The other patient, who has had the disease for the past 10 years, refused any form of treatment. In both patients the tumors were around the hip and knee joint and both of them had the disease for more than 10 years. Fong et al’s [11] observation of second growth arising proximal to the first one was not seen in both patients. The new lesions arose both proximal and distal to the index lesion.
One female patient had multiple lesions in the upper limb distributed along Pectoralis major, Biceps, Corachobrachialis and between the metacarpals. These tumors were found arising around the shoulder and elbow joint and again the new lesions arose both proximal and distal to the index lesion.
In all the three patients the lesions were separated widely and found to cross the joints as well. This fact rules out the possibility of skip metastasis or a contiguous spread from the index lesion. The nonrandom pattern of distribution especially the tumors arising between the metacarpals and metatarsal is striking and is not possible with any known local infiltrative patterns.
We believe the muscle groups involved in this form of fibromatosis acquire a genetic defect and some other environmental or hormonal factor makes the tumors manifest itself at a later age. Further studies on these patients may open new avenues in epidemiology.
Adjuvant Therapy
Four of our patients received adjuvant RT out of which 1 experienced recurrence which was salvageable by revision surgery. Recommendations for adjuvant radiation have not been standardized. Interpretation of the limited literature in the use of EBRT in the management of Desmoid tumors is hampered by the rarity of the lesion and the absence of margin-specific actuarial local recurrence rates for large series of consecutively treated patients.
Even though some groups give adjuvant radiation [12–14] to all postoperative patients irrespective of margin status, we followed the policy of giving radiation to only those patients who are margin positive, multiple tumors and recurrent tumors. The reason is that RT does not guarantee high local control and the young patients we treated might still be salvaged by RT should recurrences occur later.
We offer Tamoxifen to all inoperable tumors[15–17]. One of our patients with mesenteric fibromatosis is having stable disease for the past 5 years. We could not come to any conclusion on the efficacy of tamoxifen since three out of patients are lost for follow-up. We also offer tamoxifen in adjuvant setting for patients who had experienced multiple recurrences. The efficacy of tamoxifen in adjuvant setting could not be analyzed in our series as the number is very small. We are yet to find a clinical situation in which cytotoxic chemotherapy can be considered. The data substantiating the role of endocrine, cytotoxic and NSAIDS are mostly retrospective or non-randomized single institution studies.
Conclusion
Aggressive fibromatosis is an enigma occupying the twilight zone between benign and malignant behavior. From our Institute’s experience we conclude that recurrence in fibromatosis is atleast partly independent of management options and depends on the inherent biology of the tumor. We suggest surgery as the primary modality with radiation reserved for select patients with margin positivity, inoperable tumors, and multiple tumors. Since the disease has a long natural history, a wait and watch policy can be offered in select patients for giving adjuvant RT since new lesions could arise away from the field of surgery. Myotomic and non-random patterns of recurrence could also be reasons.
The paucity in number of international studies with adequate follow-up might be due to the rarity and the slow growing nature of the tumor. There had never been a randomized controlled study comparing different treatments and recurrence patterns undertaken anywhere in the world. Multi-institutional prospective RCTs and subsequent pooled data analysis may optimize the treatment protocol for this enigmatic and rare disease.
Footnotes
Synopsis: We suggest surgery as the primary modality with radiation reserved for select patients
References
- 1.Alman BA, Pajerski ME, Diaz-Cano S, Corboy K, Wolfe HJ. Aggressive fibromatosis (desmoid tumor) is a monoclonal disorder. Diagn Mol Pathol. 1997;6:98–101. doi: 10.1097/00019606-199704000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Wara WM, Phillips TL, Hill DR, et al. Desmoid tumors—treatment and prognosis. Radiology. 1977;124:225–226. doi: 10.1148/124.1.225. [DOI] [PubMed] [Google Scholar]
- 3.Li M, Cordon-Cardo C, Gerald WL, Rosai J. Desmoid fibromatosis is a clonal process. Hum Pathol. 1996;27:939–943. doi: 10.1016/S0046-8177(96)90221-X. [DOI] [PubMed] [Google Scholar]
- 4.Merchant NP, Lewis JJ, Leung DH, Woodruff JM, Brennan MF (2012) Extremity and trunk desmoid tumors: a multifactorial analysis of outcome. Cancer [PubMed]
- 5.Wold LE, Weiland LH. Tumefactive fibro-inflammatory lesions of the head and neck. Am J Surg Pathol. 1983;7:477–482. doi: 10.1097/00000478-198307000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Posner MC, Shiu MH, Newsome JL, Hajdu SI, Gaynor JJ, Brennan MF. The desmoid tumor: not a benign disease. Arch Surg. 1989;124:191–196. doi: 10.1001/archsurg.1989.01410020061010. [DOI] [PubMed] [Google Scholar]
- 7.Goy BW, Lee SP, Fu YS, Selch MT, Eilber F. Treatment results of unresected or partially resected desmoid tumors. Am J Clin Oncol. 1998;21(6):584–590. doi: 10.1097/00000421-199812000-00011. [DOI] [PubMed] [Google Scholar]
- 8.Spear MA, Jennings LC, Mankin HJ, Spiro IJ, Springfield DS, Gebhardt MC, Rosenberg AE, Efird JT. Suit HD management of aggressive fibromatoses. Int J Radiat Oncol Biol Phys. 1998;40(3):637–645. doi: 10.1016/S0360-3016(97)00845-6. [DOI] [PubMed] [Google Scholar]
- 9.Suit HD, Spiro IJ, Spear M. Benign and low-grade tumors of the soft tissues: role for radiation therapy. Cancer Treat Res. 1997;91:95–105. doi: 10.1007/978-1-4615-6121-7_7. [DOI] [PubMed] [Google Scholar]
- 10.Abeloff M, ArmiTAGE. J, Niederhuber. J Abeloff’s Clinical Oncology, 4th edition, May-2008
- 11.Fong Y, Rosen PP, Brennan MF. Multifocal desmoids. Surgery. 1993;114:902–906. [PubMed] [Google Scholar]
- 12.Easter DW, Halasz NA. Recent trends in the management of desmoids tumors. Summary of 19 cases and review of the literature. Ann Surg. 1989;210:765–769. doi: 10.1097/00000658-198912000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leibel SA, Wara WM, Hill DR, et al. Desmoid tumors: local control and patterns of relapse following radiation therapy. Int J Radiat Oncol Biol Phys. 1983;9:1167–1171. doi: 10.1016/0360-3016(83)90175-X. [DOI] [PubMed] [Google Scholar]
- 14.Sherman NE, Romsdahl M, Evans H, Zagars G, Oswald MJ. Desmoid tumors: a 20-year radiotherapy experience. Int J Radiat Oncol Biol Phys. 1990;19:37–40. doi: 10.1016/0360-3016(90)90131-3. [DOI] [PubMed] [Google Scholar]
- 15.Kinzbrunner B, Ritter S, Domingo J, Rosenthal CJ. Remission of rapidly growing desmoid tumors after tamoxifen therapy. Cancer. 1983;52:2201–2204. doi: 10.1002/1097-0142(19831215)52:12<2201::AID-CNCR2820521204>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 16.Waddell WR, Gerner RE, Reich MP. Nonsteroid anti-inflammatory drugs and tamoxifen for desmoid tumors and carcinoma of the stomach. J Surg Oncol. 1983;22:197–211. doi: 10.1002/jso.2930220314. [DOI] [PubMed] [Google Scholar]
- 17.Wilcken N, Tattersall MH. Endocrine therapy for desmoid tumors. Cancer. 1991;68:1384–1388. doi: 10.1002/1097-0142(19910915)68:6<1384::AID-CNCR2820680634>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 18.Gronchi A, Casali PG, Mariani L. Quality of surgery and outcome in extra-abdominal aggressive Fibromatosis: a series of patients surgically treated at a single institution. J Clin Oncol. 2003;21(7):1390–1397. doi: 10.1200/JCO.2003.05.150. [DOI] [PubMed] [Google Scholar]