Introduction
Penile Cancer is rare in developed countries but not uncommon in developing countries like India, Brazil and some of the African Countries [1]. In India, it is more commonly seen in costal Tamilnadu, probably related to poor Hygiene. 20–30 % of patients with high risk Penile Cancer harbour microscopic metastasis in the inguinal nodes at presentation [2–5]. Involvement of lymph nodes is one of the important prognostic factors. Radical inguinal lymphadenectomy provides local control of disease and has a survival advantage but is a morbid procedure. Attempts have been made to reduce the morbidity by modifying the template, technique and to preserve the saphenous vein [6–8]. Despite of careful dissection and maintaining vascularity to skin, and refinement of techniques, necrosis of the margins, lymphorrhea and other surgical morbidity is seen in upto 50 % of patients [9–11]. Rare consequence of margin necrosis and infection is catastrophic haemorrhage from femoral vessels. Video endoscopic inguinal lymphadenectomy (VEIL) is the procedure described to reduce the morbidity associated with open counterpart and is a minimally invasive approach, which reduces the pain ensures early recovery and gives cosmetically better outcome [1, 3, 6, 12–18]. Hence we will be describing the technique of VEIL and discussing the advantages and oncological outcome in our series.
Historical Aspects
VEIL procedure was developed based on the principles of endoscopic surgery for harvesting the saphenous vein for the purpose of coronary artery bypass graft, endoscopic surgery described for axillary lymphadenectomy and description of endoscopic subcutaneous modified lymph node dissection in Cadaveric models [19–22].
Indications for VEIL
Though VEIL is described in this article for carcinoma of Penis, Urethra and vulva, same surgical principles may be used for melanoma of the leg.
Surgical Steps
Anaesthesia
VEIL could be done with spinal, epidural or general anaesthesia. If bilateral VEIL is being planned, then epidural and or GA is a good option as it takes more operative time compared to unilateral VEIL.
Position of the Patient, Surgeon and Monitor
Patient is placed in supine position with thigh abducted and externally rotated for 30 degree at the hip joint. Sequential pneumatic compression device is applied to legs to prevent deep vein thrombosis. Surface marking of the femoral triangle is made for better orientation and to limit the dissection to femoral triangle.
Port Placement
Camera port site – 1 to 1.5 cm incision is made deep to Scarpa’s fascia, 2 cm caudal to the apex of the femoral triangle. Subscarpa’s plane is created either by sharp dissection with scissors or bluntly by inserting index finger, medially and laterally and towards the inguinal ligament to create adequate space for insertion of secondary ports at 5–6 cm medially and laterally at the level of the apex of femoral triangle. 5 and 10 mm ports are placed under the guidance of finger placed inside the dissected plane for the use of grasper and clip applicator or harmonic scalpel respectively right hand secondary port should be of 10 mm and left hand port is of 5 mm for right handed surgeon for the ease of applying clips. 10 mm camera port is inserted at the end and fixed to skin. Balloon port is preferred at camera port site as it will prevent leakage of carbon dioxide and can be pulled outside during early part of dissection for better visualization, as the space is less. All the ports are fixed to skin to prevent from slipping out.
Surgeon stands lateral to the patient’s ipsilateral leg and monitor placed contralaterally at the level of waist. Carbon dioxide pressure is kept initially to 15–16 mm of mercury to assist in dissection. Harmonic scalpel is useful in creating subscarpa’s plane by piercing the fat mechanically to create the right plane after identifying the glistening white Scarpa‘s fascia. Surface marking helps in identifying the boundaries of dissection and will give good orientation of site of dissection. Once subscarpa’s plane is dissected upto the level of inguinal ligament, external oblique aponeurosis is seen. Dissect laterally and medially to the boundaries of femoral triangle that completes the dissection of skin flap. Carbon dioxide pressure to be reduced to 5–6 mm of mercury to prevent development of subcutaneous emphysema of the abdomen and sometimes chest which is not of a concern, as it absorbs eventually in less than a day. The superficial nodes are seen towards the floor. Small venous tributaries encountered may be divided using harmonic scalpel using coagulation mode.
Dissection of deep fascia started at the apex of femoral triangle. Carefully divide the fat and identify the deep fascia and cut using coagulation mode to effectively coagulate the maximum density of lymphatics which travel at this site. Identify the saphenous vein 2–3 cm medial to apex of femoral triangle and preserve it, if indicated. All the fibro fatty lympho areolar tissue with deep fascia is divided along the lateral and medial border of the the triangle. Right direction of dissection is confirmed by palpating the harmonic scalpel tip corresponding to the surface marking over the skin. Laterally sortoris muscle fibers and medially adductor longus muscle fibers will be seen. Divide the lympho areolar tissue and deep fascia from the underneath muscle fibers. 2–3 cm cephaloid to apex of femoral triangle pulsation of the femoral artery is seen. (Re arrange the sentence) Deep fascia covering the femoral vessels be divided to see the lymphatics running parallel to the artery and vein. Dividing these lymphatics could increase the post operative lymphorrhea and lymphedema. Femoral nerve seen lateral to the artery is identified and preserved. Once sapheno femoral junction is reached, saphenous vein is dissected off the fibro fatty lympho areolar tissue to preserve the vein to reduce the risk of lymphedema. Multiple venous tributaries enters the saphenous vein at this junction are divided between clips. Small arterial branches arise from femoral artery are divided between clips. Once we reach the inguinal ligament, medially spermatic cord is visualised, that is the limit of dissection and specimen is free now. Haemostasis is achieved and the specimen is removed through camera port. Incision may be increased if the specimen is bulky, which is expected in obese patients.
12–14 Fr suction drain is placed through the lateral port. Port sites closed with subcuticular absorbable sutures. Drain removed once the output in 24 h reduced to less than 10–20 ml.
Oral feeds resumed in 6–12 h depending on the type of anaesthesia (Figs. 1 and 2).
Fig. 1.
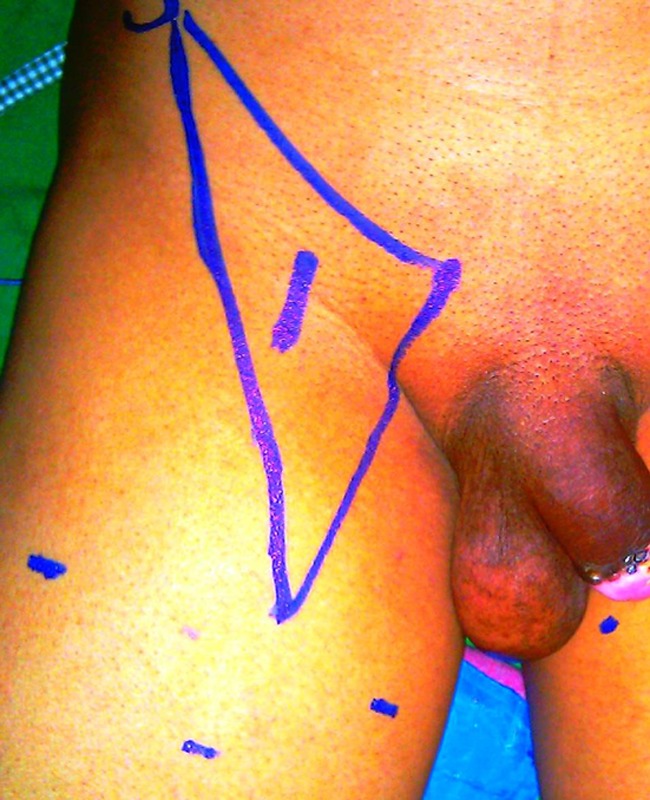
Surface marking of femoral triangle with sites for port placement and site of femoral artery
Fig. 2.
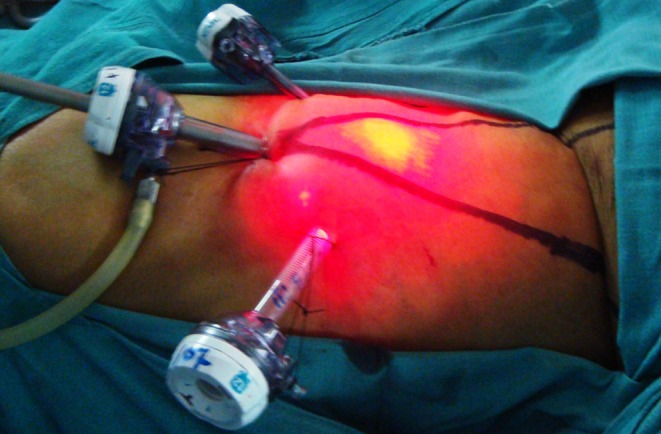
Ports placed for right VEIL with pneumo lifting the dissected skin flap and transillumination of light through the flap
Modifications
Superficial VEIL
This is the first part of the conventional VEIL described above, where the subscarpas plane is dissected all through the femoral triangle. Instead of cutting the deep fascia, and the fibrofatty lympho areolar tissue anterior to the deep fascia is removed, including the lymph nodes at the fossa ovalis. Saphenous vein could be preserved to reduce the morbidity. Superficial VEIL procedure is simple and the average operative time is 45 to 60 min. Specimen is subjected for frozen section. If the lymph nodes are positive for malignancy, then ileoinguinal lymphadenectomy is performed.
Excision of Saphenous Vein
Excision of saphenous vein is done when enlarged lymph nodes are found on clinical examination or on imaging or when the risk of involvement of inguinal nodes by malignancy is high. This is done by dividing the vein between clips at the initial part of dissection medial to apex of femoral triangle and dividing the vein at its junction with the femoral vein by hemoloc clips, so as to remove the segment of vein traversing the femoral triangle. Occasionally encountered accessory saphenous vein is removed in the same way.
Bilateral VEIL
VEIL is done consecutively, one side after the other side. Technically it is possible to do bilateral VEIL simultaneously when 2 complete set of laparoendoscopic equipments and *operating team is available. Simultaneous one side VEIL and contralateral open radical inguinal lymphadenectomy is possible to reduce the operative time.
Robotic VEIL
Josephson described the Robot assisted VEIL using Da Vinci system by 3 ports. There are no differences in the technical steps. The lateral port is used either by robot for suction or retraction and by assistant for application of clips. Very few authors have done this. Long term outcome to claim the oncological superiority is not available, however all the advantages of robotic assisted procedures like ergonomic position for surgeon, 3 dimensional view with dept perception are the advantages. It adds to the cost of the procedure.
Laparo Endoscopic Single Site (LESS) VEIL
Described by Tobias Machado, has the advantage of single incision to reduce the pain and improve the cosmesis [23]. It is technically demanding and need special instruments used for LESS surgery.
Patient Methods and Results
Prospective data was collected from patients who underwent VEIL surgery between July 2007 and July 2011 at two Institutions. Patients with squamous cell carcinoma of penis, vulva and urethra with the adverse histological futures were selected for the study. All patients underwent VEIL after 2–4 weeks period, following the surgery for the primary tumour. Initially 3 patients with nonpalpable lymph nodes were selected for VEIL on one side and open radical inguinal lymphadenectomy was done contralaterally. This gave us confidence and enhanced the understanding of endoscopic anatomy and the numbers of nodes removed were comparable to open surgery without the morbidity of wound. Hence, bilateral VEIL was performed in subsequent patients. Preoperative, intraoperative, early post operative and follow up data was collected and shown in the Table 1. Patients with inguinal nodal metastasis underwent iliac lymphadenectomy. All the patients received deep vein prophylaxis (pneumatic sequential compression device perioperatively, early ambulation and low molecular heparin)
| At HCG-BIO | - 8 patients - 16 VEIL (all 8 were bilateral) |
| At RGCI and RC | - 14 patients- [23 VEIL-(9 bilateral and 5 unilateral)] |
| Total | -22 patients- 39 VEIL |
| 1 (2.56 %) patient underwent bilateral robotic VEIL and | |
| 1 (2.56 %) patient underwent unilateral robotic VEIL | |
| Total number of bilateral VEILs done from both centres- 17 | |
| All 5 (12.82 %) unilateral VEILs were done at RGCI and RC. | |
Table 1.
Demography details
| Total number of patients | 22 (19 males and 3 females) |
| Age | 37–76 years (Average age- 53 years) |
| Total number of VEIL | 39 |
| No. Of patients with Carcinoma Penis | 19 (48.71 %) |
| No. Of patients with Carcinoma Vulva | 2 (5.12 %) |
| Patients with Carcinoma urethra | 1 (2.56 %) |
| No. Of Robotic VEIL | 3 (7.69 %) (2 patients) |
| No. Of patients with palpable nodes | 8 sides (20.51 %) |
| No. Of patients with pathologically node positive | 9 sides (23.07 %) |
| Mean followup | 33.4 months (8–55 months) |
Results
Total 22 patients underwent 39 VEIL surgeries. VEIL was completed in all the patients without the need for conversion to open surgery.
Intraoperatively 1 (2.56 %) patient (first patient) developed abdominal and chest wall subcutaneous emphysema. 1 (2.56 %) patient developed bilateral skin flap necrosis on 3rd postoperative day. One side healed with secondary intention, other side required tensor fascialata rotation flap. One (2.56 %) patient had venous bleeding 6 h after surgery and was managed by compression dressing alone. Lymphocele had developed on 4 (10.25 %) sides requiring 1 to 4 aspirations at 1–3 weeks interval without any consequences. None (0 %) of our patients developed local recurrence till date.
Conclusion
VEIL is minimally invasive approach to radical inguinal lymph node dissection to reduce skin related morbidity compared with open radical inguinal lymphadenectomy. It is possible and feasible in low volume palpable inguinal nodes. It duplicates the principles of open surgery and reduces the skin related complications. Cosmetically VEIL is better accepted and reduces the hospital stay. Long term oncological results are not available. Randomized multi institutional studies are required to prove its efficacy over open counterpart.
Discussion
VEIL duplicates every oncological principles of open surgery without having the incision close to the groin to reduce the morbidity related to the wound. It is possible to remove lymphatic tissue from the complete template. Intra operative frozen section may be asked for when planning limited template or superficial lymphadenectomy. Saphenous vein could be preserved to reduce the lymph edema, as in open surgery.
Skin related complications like necrosis and lymphorrhea through the wound are avoided as necrosis is very rare and lymph can be collected through the drain. Pain is probably less due to smaller wounds as in any minimally invasive procedures, however randomized studies are required to prove this.
Interim oncological results appear to be similar to open procedure but long term outcome is not available, which is essential in any malignancy. VEIL definitely reduces hospital stay and is better accepted by the patient.
The number of lymph nodes removed by VEIL, when compared to that removed by the open radical inguinal Lymphadenectomy done at our centre before the initiation of study were comparable. Overall outcome of our series, when compared with the literature published till date is also comparable, that shows the effectiveness of VEIL and hence we may expect similar long term oncological outcome and better cosmetic results in future.
References
- 1.Tobias-Machado M, Starling ES, Oliveira ABP, Pompeo AC, Wroclawski ER. 5-years experience with Video Endoscopic Inguinal Lymphadenectomy (VEIL): learning curve and technical variations of a new procedure. Journal of Andrological Sciences. 2009;16:25–32. [Google Scholar]
- 2.Ficarra V, Zattoni F, Cunico SC, Galetti TP, Luciani L, Fandella A, et al. Gruppo Uro-Oncologico del Nord Est (Northeast Uro-Oncological Group) Penile Cancer Project. Lymphatic and vascular embolizations are independent predictive variables of inguinal lymph node involvement in patients with squamous cell carcinoma of the penis: Gruppo Uro-Oncologico del Nord Est (Northeast Uro-Oncological Group) Penile Cancer data base data. Cancer. 2005;103:2507–16. doi: 10.1002/cncr.21076. [DOI] [PubMed] [Google Scholar]
- 3.Ornellas AA, Correia Seixas AL, Marota A, Wisnescky A, Campos F, Moraes JR. Surgical treatment of invasive squamous cell carcinoma of the penis: retrospective analysis of 350 cases. J Urol. 1994;151:1244–1249. doi: 10.1016/s0022-5347(17)35222-9. [DOI] [PubMed] [Google Scholar]
- 4.Srinivas V, Morse MJ, Herr HW. y cols.: Penile cancer: relation of extent of nodal metastasis to survival. J Urol. 1987;137:880. doi: 10.1016/s0022-5347(17)44281-9. [DOI] [PubMed] [Google Scholar]
- 5.Kattan MW, Ficarra V, Artibani W, et al. Nomogram predictive of cancer specific survival in patients undergoing partial or total amputation for squamous cell carcinoma of the penis. J Urol. 2006;175:2103–2108. doi: 10.1016/S0022-5347(06)00313-2. [DOI] [PubMed] [Google Scholar]
- 6.Catalona WJ. Modified inguinal lymphadenectomy for carcinoma of the penis with preservation of saphenous veins: technique and preliminary results. J Urol. 1988;140:306–310. doi: 10.1016/s0022-5347(17)41589-8. [DOI] [PubMed] [Google Scholar]
- 7.d’Ancona CA, Lucena RG, Querne FA, Martins MH, Denardi F, Netto NR., Jr Long-term follow-up of penile carcinoma treated with penectomy and bilateral modified inguinal lymphadenectomy. J Urol. 2004;72:498–501. doi: 10.1097/01.ju.0000132239.28989.e1. [DOI] [PubMed] [Google Scholar]
- 8.Ferreira U, Ribeiro MA, Reis LO, Prudente A, Matheus WE. Senthinel lymph node biopsy in penile cancer: a comparative study using modified inguinal dissection. Int Braz J Urol. 2008;34:725–30. doi: 10.1590/S1677-55382008000600007. [DOI] [PubMed] [Google Scholar]
- 9.Abi-Aad AS, Kernion JB. Controversies in ileoinguinal lymphadenectomy for cancer of penis. Urol Clin N Am. 1992;19:319–324. [PubMed] [Google Scholar]
- 10.Bevan-Thomas R, Slaton JW, Pettaway CA. Contemporary morbidity from lymphadenectomy for penile squamous cell carcinoma. The M.D. Anderson cancer center experience. J Urol. 2002;167:1638–1642. doi: 10.1016/S0022-5347(05)65169-5. [DOI] [PubMed] [Google Scholar]
- 11.Nelson BA, Cookson MS, Smith JA, Jr, Chang SS. Complications of inguinal and pelvic lymphadenectomy for squamous cell carcinoma of penis: a contemporary series. J Urol. 2004;172:494–497. doi: 10.1097/01.ju.0000131453.52463.8f. [DOI] [PubMed] [Google Scholar]
- 12.Tobias-Machado M, Tavares A, Molina WR, Jr, Zambon JP, Medina JA, Forseto PH, Jr, Juliano RV, Wroclawski ER. Video endoscopic inguinal lymphadenectomy (VEIL): initial case report and comparison with open radical procedure. Arch Esp Urol. 2006;59(8):849–852. doi: 10.4321/S0004-06142006000800020. [DOI] [PubMed] [Google Scholar]
- 13.Tobias-Machado M, Tavares A, Molina WR, Jr, Forseto PH, Jr, Juliano RV, Wroclawski ER. Video endoscopic inguinal lymphadenectomy (VEIL): minimally invasive resection of inguinal lymph nodes. International Braz J Urol. 2006;32(3):316–321. doi: 10.1590/S1677-55382006000300012. [DOI] [PubMed] [Google Scholar]
- 14.Tobias-Machado M, Tavares A, Ribeiro Silva MN, Molina WR, Jr, Forseto PH, Juliano RV, Wroclawski ER. Can video endoscopic inguinal lymphadenectomy achieve a lower morbidity than open lymph node dissection in penile cancer patients? J Endourol. 2008;22(8):1687–1692. doi: 10.1089/end.2007.0386. [DOI] [PubMed] [Google Scholar]
- 15.Tobias-Machado M, Tavares A, Ornellas AA, Molina WR, Jr, Juliano RV, Wroclawski ER. Video endoscopic inguinal lymphadenectomy: a new minimally invasive procedure for radical management of inguinal nodes in patients with penile squamous cell carcinoma. J Urol. 2007;177:953–958. doi: 10.1016/j.juro.2006.10.075. [DOI] [PubMed] [Google Scholar]
- 16.Favorito LA, Nardi AC, Ronalsa M, Zequi SC, Sampaio FJ, Glina S. Epidemiologic study on penile cancer in Brazil. Int Braz J Urol. 2008;34:587–91. doi: 10.1590/S1677-55382008000500007. [DOI] [PubMed] [Google Scholar]
- 17.Thyavihally Y, Tongaonkar H. Video Endoscopic Inguinal Lymphadenectomy (VEIL): our initial experience. Urology. 2008;72:S106–7. doi: 10.1016/j.urology.2008.08.310. [DOI] [Google Scholar]
- 18.Raghunath SK, Rawal Sudhir, Samir Khanna (2008) Video abstract, VEIL technique. USICON 2008
- 19.Bishoff JA, Lackland AFB, Basler JW, Teichman JM, Thompson IM. Endoscopy subcutaneous modified inguinal limph node dissection (ESMIL) for squamous cell carcinoma of the penis. J Urol. 2003;169(Suppl 4):78. [Google Scholar]
- 20.Folliguet TA, Bret E, Moneta A, Musumeci S, Laborde F. Endoscopic saphenous vein harvesting versus ‘open’ technique. A prospective study. Eur J Cardiothoracic Surg. 1998;13:662–666. doi: 10.1016/S1010-7940(98)00092-X. [DOI] [PubMed] [Google Scholar]
- 21.Dardour JC, Ktorza T. Endoscopic deep periorbital lifting: study and results based on 50 consecutive cases. Aesthetic Plast Surg. 2000;24:292–8. doi: 10.1007/s002660010049. [DOI] [PubMed] [Google Scholar]
- 22.Avrahami R, Nudelman I, Watenberg S, Lando O, Hiss Y, Lelchuk S. Minimally invasive surgery for axillary dissection. Cadaveric feasibility study. Surg Endoscopy. 1998;12:466–8. doi: 10.1007/s004649900707. [DOI] [PubMed] [Google Scholar]
- 23.Tobias-Machado M. Laparo endoscopic single site (LESS) VEIL J. Endourology. 2011;25(4):607–10. doi: 10.1089/end.2010.0269. [DOI] [PubMed] [Google Scholar]


