Abstract
Genotypic analysis was performed on 48 Mycobacterium tuberculosis complex strains collected from a hospital in Dhaka city. Deletion analysis showed that the isolates were all M. tuberculosis; 13 of them were found to be of the “ancestral” type, while 35 were of the “modern” type, indicating that both endemic (ancestral type) and epidemic (modern type) strains cause tuberculosis in Bangladesh. Genotyping based on the spoligotype and variable-number tandem repeats (VNTR) of mycobacterial interspersed repetitive units (MIRU) was also done. A total of 34 strains (71%) were grouped by spoligotyping into nine different clusters; the largest comprised 15 isolates of the Beijing genotype, whereas the remaining eight clusters consisted of two to five isolates. MIRU-VNTR typing detected 32 different patterns among 44 tested strains, and the 15 Beijing strains were further discriminated by MIRU-VNTR typing (7 distinct patterns for the 15 isolates). These results indicate that MIRU-VNTR typing, along with spoligotyping and deletion analysis, can be used effectively for molecular epidemiological studies to determine ongoing transmission clusters; to our knowledge, this is the first report about the type of strains prevailing in Bangladesh.
Despite global efforts to combat tuberculosis (TB), the disease remains a major public health problem worldwide, especially in developing countries such as Bangladesh. Key factors in the control of TB are rapid detection and adequate therapy to arrest further transmission. Outbreaks of infectious disease often result from exposure to a common source of the etiologic agent (9). Generally, the etiologic agent causing an outbreak of infection is derived from a single cell whose progeny are genetically identical or closely related to the source organism. In epidemiological terms, the organisms involved in the outbreak are clonally related. DNA fingerprinting techniques now exist which identify specific strains of Mycobacterium tuberculosis (22). These techniques, along with conventional approaches, have become powerful tools in TB epidemiology. Unfortunately, epidemiological data for Bangladesh are scarce. Hence, monitoring the control of TB by epidemiological investigation is of utmost importance.
Large-scale genotyping of M. tuberculosis using IS6110 restriction fragment length polymorphism is labor-intensive and requires culturing of the slow-growing mycobacteria, and the results are sometimes difficult to compare among laboratories. After the completion of the genome sequence of M. tuberculosis H37Rv, comparative-genomics approaches greatly enhanced our understanding of the mechanisms of insertion and deletion of DNA and the resulting distribution of variable regions around the genomes of tubercle bacilli (4, 6, 7). Based on this knowledge, a deletion analysis system has been developed to differentiate the members of the M. tuberculosis complex (4, 23). There are 20 variable regions, of which 14 regions of difference (RD1 to RD14) were found to be absent from bacillus Calmette-Guérin (BCG) Pasteur relative to M. tuberculosis H37Rv (2, 7, 10). Six regions, H37Rv-related deletions (RvD1 to RvD5 and M. tuberculosis specific deletion 1 (TbD1), are absent from the M. tuberculosis H37Rv genome relative to other members of the M. tuberculosis complex. Based on the presence or absence of the TbD1 region, M. tuberculosis strains can be divided into “ancestral” and “modern” types. The Beijing, Haarlem, and African strains responsible for major epidemics are modern types (4, 16).
Spoligotyping is a PCR-based method which allows simultaneous detection and strain differentiation of M. tuberculosis present in clinical specimens without the need for culture (13). The method is based on strain-dependent hybridization patterns of in vitro-amplified DNA with multiple spacer oligonucleotides. This region contains multiple short 36-bp direct repeats (DRs) and nonrepetitive spacers, which are 35 to 41 bp in length, interspersed between the DRs. Spoligotyping is a rapid method that allows large numbers of isolates to be handled in a short time. The DRs are extremely well conserved among M. tuberculosis complex strains, making spoligotyping a specific method for the detection of M. tuberculosis complex members (13).
Recently, a typing method based on variable-number tandem repeats (VNTR) of genetic elements named mycobacterial interspersed repetitive units (MIRU) in 12 human minisatellite-like regions of the M. tuberculosis genome has been developed (20, 28). MIRUs are composed of 40- to 100-bp repetitive DNA sequences, dispersed in 41 intergenic regions of the M. tuberculosis complex genome (18, 29, 30). Twelve of these sites display polymorphisms in MIRU copy number among nonrelated M. tuberculosis isolates. Typing usingMIRUs is a PCR-based method where strains can be typed by a numerical code corresponding to the numbers of MIRUs in the different loci. Here, we apply a combination of all three different genotyping methods to a random collection of isolates of M. tuberculosis from Bangladesh.
MATERIALS AND METHODS
Mycobacterial strains and genomic DNA.
A total of 48 cases of TB were investigated (Table 1). Samples were collected randomly from patients attending the Institute of Diseases of Chest and Hospital (IDCH) between 1999 and 2000. IDCH is a national facility for the diagnosis and treatment of TB in Dhaka city. Treatment is free, and the hospital serves a large segment of the city's population. It also handles a substantial number of patients with complications referred from other hospitals and Thana Health Complexes in and outside Dhaka city. Twenty-six of the patients were from different parts of Dhaka city (Table 1). Others were from different regions of Bangladesh outside Dhaka (Table 1). Most of them (>70%) were from a low socioeconomic condition. Thirty-six were male, and 12 were female. Their ages ranged from 16 to 60 years (the mean age was 34 years). Twenty-two patients had a scar present, indicating that they were vaccinated with BCG during their childhood. Twenty patients had no scar, and the result was not recorded for the other six. All of the patients were sputum microscopy positive and culture positive. Thirty-three samples were from previously diagnosed patients who had been treated with antitubercular drugs for certain periods. Fifteen of the isolates were primary isolates. Drug sensitivity testing was performed on 39 samples.
TABLE 1.
Patient characteristics
| Strain | Age (yr) | Sexa | Treatment history | BCG scar | Sensitivityb to:
|
Origin | |||
|---|---|---|---|---|---|---|---|---|---|
| INH | RMP | EMB | SM | ||||||
| TB1 | 28 | M | Previously treated | Present | ND | ND | ND | ND | Dhaka |
| TB2 | 30 | M | Previously treated | Present | S | S | S | S | Dhaka |
| TB6 | 18 | M | New case | Absent | S | S | S | S | Dhaka |
| TB8 | 25 | M | New case | Present | ND | ND | ND | ND | Dhaka |
| TB9 | 60 | M | New case | Absent | S | S | S | S | Dhaka |
| TB10 | 50 | M | Previously treated | Present | ND | ND | ND | ND | Patuakhali |
| TB11 | 30 | M | New case | Present | ND | ND | ND | ND | Dhaka |
| TB12 | 56 | M | New case | Present | S | S | S | S | Natore |
| TB14 | 52 | M | Previously treated | Present | R | R | R | R | Rangpur |
| TB16 | 20 | F | New case | Present | ND | ND | ND | ND | Gazipur |
| TB19 | 50 | F | New case | Present | S | S | S | S | Dhaka |
| TB20 | 16 | M | New case | Absent | ND | ND | ND | ND | Narayanganj |
| TB21 | 22 | M | New case | Absent | S | S | S | S | Narayanganj |
| TB23 | 35 | M | New case | Present | S | S | S | S | Kishoreganj |
| TB24 | 20 | F | New case | Absent | ND | ND | ND | ND | Dhaka |
| TB27 | 26 | M | New case | Present | S | S | S | S | Dhaka |
| TB29 | 22 | F | New case | Present | S | S | S | S | Dhaka |
| TB30 | 20 | F | New case | Absent | S | S | S | S | Dhaka |
| TB32 | 35 | F | New case | Present | ND | ND | ND | ND | Dhaka |
| MA1 | 30 | M | Previously treated | Not known | S | S | S | S | Dhaka |
| MA2 | 32 | F | Previously treated | Absent | S | S | S | S | Dhaka |
| MA5 | 42 | M | Previously treated | Absent | S | S | S | S | Dhaka |
| MA6 | 32 | M | Previously treated | Absent | R | R | S | S | Shariatpur |
| MA7 | 50 | M | Previously treated | Absent | R | R | S | S | Noakhali |
| MA9 | 33 | M | Previously treated | Absent | R | R | S | S | Munshiganj |
| MA11 | 27 | M | Previously treated | Present | S | S | S | S | Comilla |
| MA12 | 38 | F | Previously treated | Absent | R | S | S | R | Dhaka |
| MA13 | 33 | F | Previously treated | Absent | S | S | S | S | Dhaka |
| MA16 | 35 | M | Previously treated | Absent | R | R | R | R | Dhaka |
| MA17 | 46 | M | Previously treated | Not known | R | S | R | S | Narayanganj |
| MA19 | 25 | M | Previously treated | Present | R | S | S | S | Narayanganj |
| MA20 | 40 | M | Previously treated | Absent | R | R | R | R | Kishoreganj |
| MA25 | 55 | M | Previously treated | Present | S | R | S | R | Dhaka |
| MA27 | 50 | M | Previously treated | Present | S | R | S | S | Shariatpur |
| MA28 | 40 | M | Previously treated | Absent | R | R | S | R | Narsingdi |
| MA29 | 30 | M | Previously treated | Present | R | S | S | S | Kishoreganj |
| MA31 | 31 | M | Previously treated | Absent | R | R | R | R | Narayanganj |
| MA32 | 28 | M | Previously treated | Absent | S | S | S | S | Dhaka |
| MA35 | 23 | M | Previously treated | Present | S | S | S | R | Dhaka |
| MA36 | 50 | M | Previously treated | Not known | S | S | S | S | Dhaka |
| MA37 | 45 | M | Previously treated | Present | S | S | S | S | Habiganj |
| MA38 | 39 | M | Previously treated | Absent | ND | ND | ND | ND | Comilla |
| MA39 | 22 | F | Previously treated | Present | R | R | S | R | Gazipur |
| MA41 | 45 | M | Previously treated | Absent | R | S | S | S | Dhaka |
| MA42 | 22 | M | Previously treated | Present | R | S | R | R | Shariatpur |
| MA45 | 17 | F | Previously treated | Not known | S | S | S | S | Dhaka |
| MA46 | 40 | M | Previously treated | Not known | S | S | S | S | Dhaka |
| MA48 | 20 | F | Previously treated | Not known | R | S | S | S | Dhaka |
M, male; F, female.
INH, isoniazid; RMP, rifampin; EMB, ethambutol; SM, streptomycin; R, resistant; S, sensitive; ND, not done.
Genomic DNA was obtained by resuspending mycobacterial colonies in 100 to 200 μl of distilled H2O and incubating them at 85°C for 30 min. After centrifugation of the suspension, the supernatant containing the DNA was removed and stored at −20°C until further use.
RD PCR analysis.
RD PCR analysis was done using the methods described by Brosch et al. (4). Sequences inside or flanking RD and RvD regions were obtained from the websites http://genolist.pasteur.fr/TubercuList/ and http://www.sanger.ac.uk/Projects/M bovis/. Primers that would amplify ∼500-bp fragments were designed by using the Primer3 website http://www-genome.wi.mit.edu/cgi-bin/primer3-www.cgi. A detailed list of all primer sequences used in this study is available at http://www.pnas.org/cgi/data/052548299/DC1/1. PCR amplifications with mixtures containing, per reaction, 1.25 μl of 10× PCR buffer [600 mM Tris HCL (pH 8.8), 20 mM MgCl2, 170 mM (NH4)2SO4, 100 mM β-mercaptoethanol], 1.25 μl of 20 mM nucleotide mix, 50 nM each primer, 1 to 10 ng of template DNA, 10% dimethyl sulfoxide, 0.2 U of Taq polymerase (Gibco-BRL), and sterile distilled water to 12.5 μl were performed on a PTC-100 amplifier (MJ Inc.) with an initial denaturation step of 90 s at 95°C followed by 35 cycles of 30 s at 95°C, 1 min at 58°C, and 4 min at 72°C. Sequence analysis of the katG codon 463 polymorphism was done as described previously (4).
Spoligotyping.
Spoligotyping was performed as previously described by Kamerbeek et al. (13) with minor modifications. The DR region was amplified by PCR with oligonucleotide primers derived from the DR sequence. Mycobacterial genomic DNA was extracted from cultured cells as described previously (14, 15). Twenty-five microliters of the following reaction mixture were used for the PCR: 12.5 μl of HotStarTaq Master Mix (Qiagen; this solution provides a final concentration of 1.5 mM MgCl2 and 200 μM each deoxynucleoside triphosphate), 2 μl of each primer (20 pmol each), 5 μl of DNA solution (ca. 10 ng), and 3.5 μl of distilled water). The mixture was heated for 15 min at 96°C and subjected to 30 cycles of 1 min at 96°C, 1 min at 55°C, and 30 s at 72°C. The amplified product was hybridized to a set of 43 immobilized oligonucleotides, each corresponding to one of the unique spacer DNA sequences within the DR locus. After hybridization, the membrane was washed twice for 10 min in 2× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7])-0.5% sodium dodecyl sulfate at 60°C and then incubated in 1:4,000-diluted streptavidin-peroxidase conjugate (Boehringer) for 45 to 60 min at 42°C. The membrane was washed twice for 10 min in 2× SSPE-0.5% sodium dodecyl sulfate at 42°C and rinsed with 2× SSPE for 5 min at room temperature. Hybridizing DNA was detected by the enhanced chemiluminescence method (Amersham) (32, 33) and by exposure to X ray film (Hyperfilm ECL; Amersham) as specified by the manufacturer.
PCR and MIRU analysis.
PCRs were carried out by using the PCR reagent system (Gibco-BRL). Five microliters from fivefold-diluted DNA solutions was added to a final volume of 50 μl containing 0.2 μl of DNA polymerase (1 U), 0.2 mM each dATP, dCTP, dGTP, and dTTP, 5 μl of PCR buffer, 0.4 μM (2 μM for locus 7) primers and 1 to 3.5 mM MgCl2. The primers and MgCl2 concentrations used were as described by Mazars et al. (20). The PCR fragments were analyzed by agarose gel electrophoresis with 1.5% agarose. The sizes of the amplicons were estimated by comparison with 50- and 100-bp ladders. The MIRU copy number per locus was calculated by using the conventions described by Supply et al. (30).
RESULTS
Deletion analysis.
Forty-eight strains isolated from pulmonary TB patients were investigated by PCR-based deletion analysis using the 20 variable regions. Most of the RD regions were present in all the strains tested. RD1, RD2, RD4, RD7, RD8, RD9, RD10, RD12, RD13, RD14 and RvD1 were present in all strains (Table 2). The presence of these 10 RD regions, which are strictly conserved in M. tuberculosis, indicates that all the strains tested belonged to the species M. tuberculosis (4). The RD3 region was absent in 29 strains, and the product amplified using the second set of internal primers was smaller in 2 strains. RD11, was also absent from 8 of 48 strains tested. The RD3 and RD11 regions correspond to prophages phiRv1 and phiRv2 of M. tuberculosis H37Rv (7). RD5, which contains the genes plcA to plcC that encode proteins with phospholipase C activity, was absent from five strains. Such genes have been identified as hot spots for insertion of the mobile element IS6110 (35). Homologous recombination of two copies of IS6110 oriented in the same direction may cause deletion of the intervening sequences, and this mechanism contributes to the hypervariability of such genomic regions (5). RD6, containing IS1532 (10), was absent from 10 strains. These four regions were found to be absent from some of the M. tuberculosis strains investigated previously (4).
TABLE 2.
Deletion analysis and spoligotyping of Bangladeshi strain
| Strain | Presence of regiona:
|
||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RD 1 | RD 2 | RD 3 | RD 4 | RD 5B | RD 6 | RD 7 | RD 8 | RD 9 | RD 10 | RD 11 | RD 12 | RD 13 | RD 14 | 15-Be | RvD1 | RvD2 | RvD3 | RvD4 | RvD5 | TbD 1 | |
| Block 1 | |||||||||||||||||||||
| TB1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| TB32 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| MA25 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| MA31 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| TB16 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| TB29 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| TB23 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| TB21 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| TB27 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| TB30 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| TB19 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| TB2 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 |
| TB20 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 |
| Block 2 | |||||||||||||||||||||
| TB8 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | ND | 1 | 0 | 1 | 1 | 1 | 0 |
| MA9 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
| MA36 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
| TB24 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| MA5 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| MA29 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | ND | 1 | 0 | 0 | 1 | 1 | 0 |
| MA39 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 0 |
| MA41 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
| Block 3 | |||||||||||||||||||||
| MA6 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| MA11 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| MA19 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| MA35 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| MA42 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| MA45 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| MA46 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| MA17 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| MA27 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| MA32 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| MA37 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| TB6 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| MA1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| MA16 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| MA28 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 1 | 1 | 0 |
| Block 4 | |||||||||||||||||||||
| TB9 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
| TB10 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
| MA2 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
| MA7 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| MA12 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| MA13 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 |
| MA20 | 1 | 1 | 0 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 |
| TB14 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
| TB12 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
| MA38 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| MA48 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 1 | 0 |
| TB11 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 1 | 1 | 1 | 1 | 0 |
0, region not present; 1, region present; 2, both internal and flanking primers give product. IS-Be = IS 6110 Beijing strains; ND, not determined.
In a large number of the isolates tested (35 isolates), the TbD1 region was deleted, indicating that they are all modern strains of M. tuberculosis. However, 13 of 48 strains were found to harbor the TbD1 region (Table 2). As expected, the presence or absence of RvD regions was variable. RvD1 was present in all 48 strains, whereas RvD2 and RvD3 were highly variable in their presence. RvD2 and RvD3 were absent from 27 and 24 strains, respectively, with both RvD2 and RvD3 being deleted from 21 strains. Strains belonging to the Beijing cluster (see below) were shown to lack both RvD2 and RvD3, consistent with previous findings (4). Like RD5, RvD2 also contains a plc gene (plcD), and it has been shown that deletions in the RvD2 region can be as large as 20 kb in some M. tuberculosis strains (12). RvD2 and RvD3 are located very close to each other on the chromosome of M. tuberculosis H37Rv at positions 1989 and 1997 kb, respectively. As such, the constant absence of region RvD2 and RvD3 from M. tuberculosis strains belonging to the Bejing cluster suggests that these two regions may have been removed by a single deletion of a larger chromosomal region. RvD4 was present in 45 strains and absent from 3 strains. RvD5 was absent from one of the strains tested.
Spoligotyping.
All 48 isolates were analyzed by spoligotyping (Table 2). A cluster was defined as two or more isolates from different patients with identical spoligotype patterns, whereas nonclustered patterns were referred to as unique. The largest cluster (ST6) consists of 15 strains, one cluster contains five strains (ST5), and there are seven other clusters (ST1, ST2, ST3, ST4, ST7, ST8, and ST9) comprising two strains each. Fourteen isolates (29%) exhibited unique (nonclustered) patterns. All strains of the largest cluster comprising 15 isolates contained only 9 of the 43 spacer sequences tested. They showed a spoligotype pattern with hybridization only to the 3′-terminal spacer 35 to 43, which is characteristic of the Beijing genotype. In all strains identified as the Beijing type, RD3, RvD2, and RvD3 regions were found to be absent by deletion analysis. We examined 46 strains for the presence of the Beijing genotype-specific IS6110 in the dnaA-dnaN region (16). This marker was present only in the largest cluster of strains identified as Beijing members by spoligotyping and deletion analysis (Table 2).
The 48 isolates analyzed by deletion analysis and spoligotyping were organized in four blocks according to their degree of relatedness (Table 2). Block 1comprised the 13 ancestral strains having TbD1 present. M. bovis strains usually lack spacer sequences 39 to 43 (16). The spoligotypes of three strains in block 1, MA25 and MA31, lacking spacers 40 to 43, and TB29, lacking spacers 34 to 43, highly resembled those of M. bovis, but the presence of region RD9 and some other RD regions that are not contained in the genome of M. bovis confirmed that these strains were indeed M. tuberculosis strains.
All 13 strains in block 1 belonged to phylogenetic group 1 defined by Sreevatsan et al. (27) based on the katG codon 463 (CTG) sequence polymorphism. It is noteworthy that the great majority of these strains (85%) carried spacer 33, which was absent from all other tested M. tuberculosis strains, whereas spacers 29 to 32 were missing (Table 2). It seems that this particular combination is a characteristic part of the spoligotypes common in M. tuberculosis strains that have region TbD1 still present; therefore they have been named ancestral M. tuberculosis strains (4).
In contrast, block 2 contains eight strains from phylogenetic group 1 with TbD1 deleted. These isolates showed high similarity in their spoligotype pattern for spacers 23 to 43 to those from Beijing strains, which are regrouped in block 3. The major difference between the spoligotypes of strains from block 2 and the Bejing strains in block 3 is that many of the spacers from 1 to 22 are still present in strains from block 2 whereas they are absent from Bejing strains, which characteristically harbor only spacers 35 to 43 (Table 2).
Block 4 contains strains of phylogenetic group 2 or 3 of Sreevatsan et al. (27), showing the katG463 (where 463 is the codon number) sequence CGG. Interestingly, all these strains lacked spacers 33 to 36 but had the flanking spacers present. This spoligotype signature is typical of strains from phylogenetic groups 2 and 3 (4, 25). According to data from a large spoligotype collection, strains with this characteristic combination (spacer 32 present, spacers 33 to 36 absent, and spacer 37 present) represent the great majority of M. tuberculosis strains isolated throughout the world (26).
Genotyping by MIRU-VNTR.
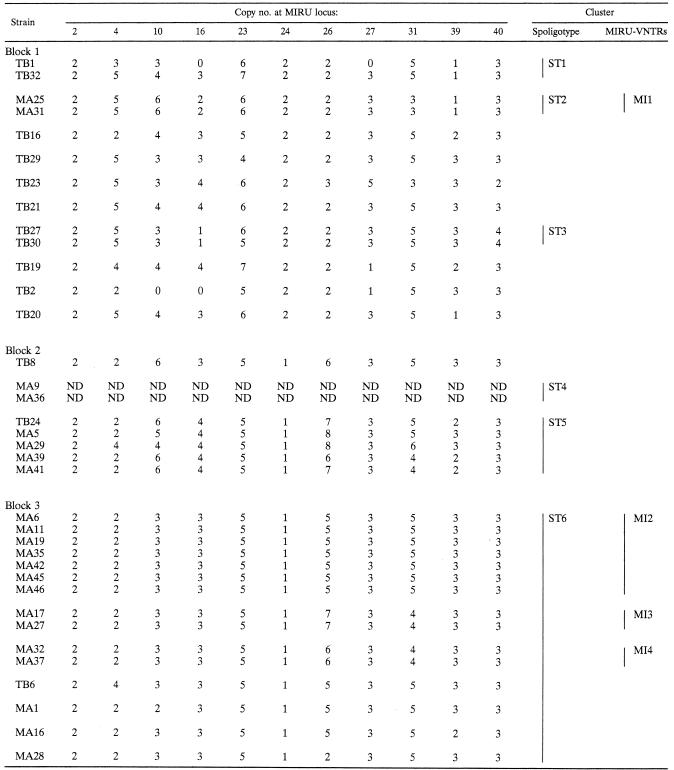
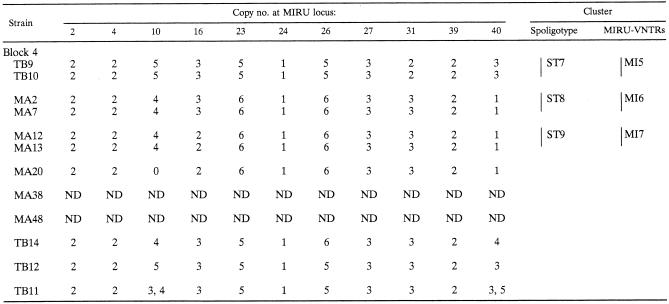
Forty-four of the 48 isolates were also typed using the MIRU-VNTR typing method. This detected 32 different patterns (Table 3), 19 of which were grouped into seven clusters. The largest cluster comprised seven strains (MI2), and six clusters comprised two strains each. Twenty-five strains had unique patterns. The 15 Beijing strains clustered in block 3 by spoligotyping were divided into seven different MIRU genotypes. Eleven of them were grouped into three MIRU-VNTR patterns, and two groups comprised two identical strains. The remaining four had unique MIRU patterns (Table 3). Unexpectedly, most of the strains (38 strains) failed to give a PCR product with primers for the MIRU 20 locus and only 6 strains gave similar-sized bands (data not shown). Since the reason for the failure of amplification at this locus remains unclear, the results of MIRU locus 20 were not included in the present MIRU-VNTR typing analysis. Another unusual finding was that TB11 isolates gave double bands of different sizes for MIRU loci 10 and 40 (Table 3). Although it is possible that the patient was coinfected with two different strains, this result is still surprising, given that the DNA was prepared from a single colony. Both MIRU 10 and 40 are highly polymorphic.
TABLE 3.
MIRU-VNTR analysis of M. tuberculosis isolateslegend
Vertical bars correspond to spoligotype (ST) and MIRU-VNTR (MI) clusters. ND, not determined.
DISCUSSION
The aim of this study was to characterize the strains causing TB in Bangladesh by using three different molecular typing methods: RD-based deletion analysis, spoligotyping, and MIRU-VNTR typing. A total of 48 strains were studied, which were clinical isolates from adult pulmonary TB patients treated in a hospital in Dhaka during different periods. Deletion analysis using RD1 to RD14 revealed that all the samples investigated were M. tuberculosis. The use of primers internal to the RD regions, in parallel with primers flanking these regions, allowed us to identify possible false-positive or false-negative PCR amplification and contributed to the reproducibility and accuracy of the results. One very interesting finding of the study was that 13 of the 48 M. tuberculosis strains tested had the TbD1 region intact, indicating that a considerable number of such strains transmit TB in Bangladesh. TbD1, the “M. tuberculosis specific deletion 1” was originally identified as a 2,153-bp region that was specifically lacking from the mmpL6 genes of almost all M. tuberculosis strains (4). In contrast, all other members of the M. tuberculosis complex have this 2.1-kb region present. However, a few M. tuberculosis strains included in the initial analysis contained the TbD1 region, and these strains were named ancestral type M. tuberculosis strains because they belong to a lineage of strains that divided from all other M. tuberculosis strains before the deletion of TbD1 occurred (4). From an evolutionary standpoint, it seems that this group of strains is quite distant from all other M. tuberculosis strains, which is also reflected by the particular spoligotypes of these isolates. As shown in Table 2, most of these strains have the following spoligotype signature, i.e., absence of spacers 29 to 32, presence of spacer 33, and absence of spacer 34. The finding that this section of the spoligotype is conserved in most ancestral M. tuberculosis strains allows one to predict the presence or absence of TbD1 by simple analysis of the spoligotype.
By consulting the spoligotype database at the Pasteur Institute of Guadeloupe, which contains more than 3,300 spoligotypes from M. tuberculosis strains isolated in different parts of the world (26), it is possible to identify strains that resemble ancestral M. tuberculosis strains. However, the number of isolates with such spoligotypes is small. The few ancestral M. tuberculosis strains that have previously been analyzed had a very low copy number of IS6110 and were isolated from East African or Indian patients (16); it has been suggested that such strains may originate from foci of endemic infection (4, 24). Bangladesh has long been a region where TB is endemic. The large number of ancestral-type M. tuberculosis strains (13 of 48, represented in block 1 of Table 2) identified in the present study suggests that, indeed, these strains belong to the endemic strain pool, and that they have probably persisted in this region for a considerable time. Unfortunately, it is difficult to calculate with precision the time that has elapsed since their divergence from the putative common ancestor. However, some indication is available now from studies of mummified human remains. Zink et al. recently analyzed mycobacterial DNA sequences from Egyptian mummies that were at least 2,500 years old and were characterized by spinal and rib lesions pathognomonic for TB (36). Interestingly, the spoligotypes of these amplified mycobacterial DNAs showed many characteristics of “modern” M. tuberculosis strains of genetic group 2 or 3, similar to the ones depicted in block 4 of Table 2, i.e., absence of spacers 33 to 36 and presence of the flanking spacers 32 and 37. Considering the clonal structure of the M. tuberculosis strain population (31), with little or no exchange of genetic material between different lineages of M. tuberculosis, this observation suggests that the branches of ancestral and modern M. tuberculosis strains, both prevalent in Bangladesh, separated more than 2,500 years ago and represent phylogenetically quite distinct populations of M. tuberculosis strains present in the same geographical region.
The modern M. tuberculosis strains comprise representatives of major epidemics like the Beijing, Haarlem, and African clusters. The majority of M. tuberculosis strains of the Beijing family originated from the province of Beijing in China, and strains of this family were found to dominate in neighboring countries such as Mongolia, South Korea, Thailand, and Vietnam (1, 34). In contrast to the dominance of the Beijing genotype in many Asian countries, a low frequency (3%) of this genotype was reported among the strains from India (21). Strains of the Beijing family have also been found in Europe, Africa, and the United States. The “W” strain, which caused a large outbreak of multidrug-resistant TB in New York and other U.S. cities, belongs to the Beijing family (16).
In the neighboring countries in Asia, rates of infection with the Beijing family strains are higher than those in the more distant countries, suggesting that the Beijing family may have radiated from the Beijing area to other regions. The factor which was responsible for the selection and dissemination of the Beijing strains is not known, but there is evidence that Beijing strains, like strain HN878, are hypervirulent, as demonstrated by the unusually early death of infected immunocompetent mice (19). Beijing strains are more common in areas where BCG vaccination coverage is extensive. Most countries in Southeast Asia have used BCG vaccination for the past two to six decades. It has been suggested that BCG vaccination may have favored the selection of M. tuberculosis strains that resist BCG-induced immunity (11). The high prevalence of Beijing-type M. tuberculosis strains in Bangladesh may be linked to a similar phenomenon.
The samples for this study were collected from patients coming to a hospital from different parts of Dhaka city and also from areas outside Dhaka. There was no apparent epidemiological link among the strains shown to be identical by both spoligotyping and MIRU typing. Strains in the same cluster came from patients from different regions of the country. Although it is difficult to draw conclusions about the transmission pattern of TB in a particular area of Bangladesh, these results suggest that a number of cases may be due to recent transmission. Beijing strains were isolated from patients from different regions of Bangladesh, suggesting that these virulent strains are aggressively spreading throughout the country. Almost all of the strains of Beijing type were from patients who were previously treated with antitubercular drugs for certain periods. Many of them were from patients whose treatment had failed. Due to lack of information about the previous episode and the treatment history, it was not possible to confirm whether these were cases of reactivation or reinfection. Of 15 Beijing strains, 8 were found to be resistant to one or more antitubercular drugs, although we cannot differentiate between primary and secondary drug resistance since all patients were previously treated with antitubercular drugs. In earlier work, it has been shown that Beijing strains are often associated with drug-resistant TB (3, 8, 17, 21). Further studies using a larger sample size in a particular area are needed to investigate the incidence of Beijing-type strains in Bangladesh and to determine the transmission dynamics of TB caused by these virulent strains in the community. It is also of interest that drug resistance appears less common among ancestral strains, where only two cases were found among the 13 strains examined (Tables 1 and 2).
Most of the strains (38 strains) gave no PCR product with primers for MIRU locus 20; only 6 strains have two copies of the MIRU 20 locus present (data not shown). These results are very different from those of other studies done elsewhere (20, 28, 30) and should be further investigated. Since the MIRU 20 locus is less polymorphic and more than 90% discrimination can be obtained without its use, we have excluded MIRU 20 from our present analysis. Fifteen strains in the Beijing cluster identified by spoligotyping were further discriminated by MIRU-VNTR analysis. Eleven of them clustered into four groups, each consisting of two to seven strains, and four were found to have unique patterns. All MIRU-VNTR patterns of Beijing family strains were highly similar, differing only in copy numbers for one or two loci, indicating that M. tuberculosis isolates grouped into the Beijing family by spoligotyping also have a similar grouping pattern when other genetic markers like MIRU-VNTR typing are used. As shown in Table 2, this study also revealed that most spoligotype patterns of M. tuberculosis strains contain characteristic signatures which are specific for certain subpopulations. These characteristic signatures correlate strongly with the results of deletion analysis and the presence of katG463 alleles. The correct identification of clinical isolates and the appropriate estimation of the phylogenetic relatedness among various M. tuberculosis strains prevalent in a given geographic area is of utmost importance for epidemiological investigations and represents a prerequisite for the identification of emerging clones. As such, it is evident that MIRU-VNTR analysis has the potential to discriminate among the strains prevailing in Bangladesh and, in combination with spoligotyping and RD analysis, fulfills the above-mentioned requirements for performing effective molecular epidemiological studies.
Table 2a.
| Presence of spacerb:
| ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 | 40 | 41 | 42 | 43 |
| X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | X | . | X | X | X | X | X | . | X | X | X |
| X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | X | . | X | X | X | X | X | . | X | X | X |
| X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | X | . | X | X | X | X | X | . | . | . | . |
| X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | X | . | X | X | X | X | X | . | . | . | . |
| X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | X | . | X | X | X | X | X | X | X | X | X |
| X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | X | X | X | X | X | . | . | . | . | X | . | . | . | . | . | . | . | . | . | . |
| X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | X | X | X | X | X | . | . | . | . | X | . | X | X | X | X | X | X | X | X | X |
| X | . | . | X | . | X | X | X | X | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | X | . | X | X | X | X | X | X | X | X | X |
| X | . | . | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | X | . | X | X | . | . | . | X | X | X | X |
| X | . | . | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | X | . | X | X | . | . | . | X | X | X | X |
| X | X | X | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | X | . | . | . | . | X | . | X | X | . | X | X | X | X | X | X |
| X | X | X | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X |
| X | X | X | X | X | X | X | X | X | X | . | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | . | . | . | X | X | X | X | X | . | X | X | X |
| X | X | X | . | . | . | . | X | X | X | X | X | X | X | X | X | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| X | X | X | . | . | . | . | X | X | X | X | X | X | X | X | X | X | X | X | X | . | X | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| X | X | X | . | . | . | . | X | X | X | X | X | X | X | X | X | X | X | X | X | . | X | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| X | X | X | . | . | . | . | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| X | X | X | . | . | . | . | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| X | X | X | . | . | . | . | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| X | X | X | . | . | . | . | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| X | X | X | . | . | . | . | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | X | X | X | X | X | X | X | X | X |
| X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | . | . | X | . | . | . | . | X | X | X | X | X | X | X |
| X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | . | . | X | . | . | . | . | X | X | X | X | X | X | X |
| X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | X | X | X | X | X | X | X | X | . | . | . | . | X | X | X | X | X | X | X |
| X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | X | X | X | X | X | X | X | X | . | . | . | . | X | X | X | X | X | X | X |
| X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | X | X | X | X | X | X | X | X | . | . | . | . | X | X | X | X | X | X | X |
| X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | X | X | X | X | X | X | X | X | . | . | . | . | X | X | X | X | X | X | X |
| X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | X | X | X | X | X | X | X | X | . | . | . | . | X | X | X | X | X | X | X |
| X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | X | X | X | X | X | X | . | X | . | . | . | . | X | X | X | X | X | X | X |
| X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | X | X | X | X | X | X | . | X | . | . | . | . | X | X | X | X | X | X | X |
| X | X | X | . | . | X | X | X | X | . | X | X | X | . | X | X | X | X | X | X | X | X | X | X | X | X | . | X | X | X | X | X | . | . | . | . | X | X | X | X | X | X | X |
| X | X | X | . | . | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | X | X | X | X | X | X | X |
| X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | X | . | . | . | . | X | X | X | X | X | X | X |
., spacer not present; X, spacer present.
Acknowledgments
We thank André Raynouard of the French Embassy in Dhaka, S. I. Khan of Dhaka University, and Firdausi Qadri of ICDDR,B for their continued help and support. We thank Philip Supply, Institut Pasteur de Lille, for his advice in performing MIRU experiments.
This work was funded in part by grants from the European Union (QLK2-CT-1999-01093 and QLRT-CT-2000-00630); the Department of the Environment, Food, and Rural Affairs (Great Britain); the Institut Pasteur (PTR 35); the Association Française Raoul Follereau; the Gates-GoB award 2001; USAID (grant no. HRN-A-00-96-9005-00) and the UNDP/World Bank/WHO special program for Research and Training in Tropical Diseases (TDR).
REFERENCES
- 1.Anh, D. D., M. W. Borgdorff, L. N. Van, N. T. Lan, T. van Gorkom, K. Kremer, and D. van Soolingen. 2000. Mycobacterium tuberculosis Beijing genotype emerging in Vietnam. Emerg. Infect. Dis. 6:302-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Behr, M. A., M. A. Wilson, W. P. Gill, H. Salamon, G. K. Schoolnik, S. Rane, and P. M. Small. 1999. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science 284:1520-1523. [DOI] [PubMed] [Google Scholar]
- 3.Bifani, P. J., B. Mathema, Z. Liu, S. L. Moghazch, B. Shopsin, B. Tempalski, J. Driscol, R. Frothingham, J. M. Musser, P. Alcabes, and B. N. Kreiswirth. 1999. Identification of a W variant outbreak of Mycobacterium tuberculosis via population-based molecular epidemiology. JAMA 282:2321-2327. [DOI] [PubMed] [Google Scholar]
- 4.Brosch, R., S. V. Gordon, M. Marmiesse, P. Brodin, C. Buchrieser, K. Eiglmeier, T. Garnier, C. Gutierrez, G. Hewinson, K. Kremer, L. M. Parsons, A. S. Pym, S. Samper, D. van Soolingen, and S. T. Cole. 2002. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc. Natl. Acad. Sci. USA 99:3684-3689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brosch, R., W. J. Philipp, E. Stavropoulos, M. J. Colston, S. T. Cole, and S. V. Gordon. 1999. Genomic analysis reveals variation between Mycobacterium tuberculosis H37Rv and the attenuated M. tuberculosis H37Ra strain. Infect. Immun. 67:5768-5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brosch, R., A. S. Pym, S. V. Gordon, and S. T. Cole. 2001. The evolution of mycobacterial pathogenicity: clues from comparative genomics. Trends Microbiol 9:452-458. [DOI] [PubMed] [Google Scholar]
- 7.Cole, S. T., R. Brosch, J. Parkhill, T. Garnier, C. Churcher, D. Harris, S. V. Gordon, K. Eiglmeier, S. Gas, C. E. Barry III, F. Tekaia, K. Badcock, D. Basham, D. Brown, T. Chillingworth, R. Connor, R. Davies, K. Devlin, T. Feltwell, S. Gentles, N. Hamlin, S. Holroyd, T. Hornsby, K. Jagels, B. G. Barrell, et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 8.Diaz, R., K. Kremer, P. E. de Haas, R. I. Gomez, A. Marrero, J. A. Valdivia, J. D. van Embden, and D. van Soolingen. 1998. Molecular epidemiology of tuberculosis in Cuba outside of Havana, July 1994-June 1995: utility of spoligotyping versus IS6110 restriction fragment length polymorphism. Int. J. Tuberc. Lung Dis. 2:743-750. [PubMed] [Google Scholar]
- 9.French, A. L., S. F. Welbel, S. E. Dietrich, L. B. Mosher, P. S. Breall, W. S. Paul, F. E. Kocka, and R. A. Weinstein. 1998. Use of DNA fingerprinting to assess tuberculosis infection control. Ann. Intern. Med. 129:856-861. [DOI] [PubMed] [Google Scholar]
- 10.Gordon, S. V., R. Brosch, A. Billault, T. Garnier, K. Eiglmeier, and S. T. Cole. 1999. Identification of variable regions in the genomes of tubercle bacilli using bacterial artificial chromosome arrays. Mol. Microbiol. 32:643-655. [DOI] [PubMed] [Google Scholar]
- 11.Hermans, P. W., F. Messadi, H. Guebrexabher, D. van Soolingen, P. E. de Haas, H. Heersma, H. de Neeling, A. Ayoub, F. Portaels, D. Frommel, et al. 1995. Analysis of the population structure of Mycobacterium tuberculosis in Ethiopia, Tunisia, and The Netherlands: usefulness of DNA typing for global tuberculosis epidemiology. J. Infect. Dis. 171:1504-1513. [DOI] [PubMed] [Google Scholar]
- 12.Ho, T. B., B. D. Robertson, G. M. Taylor, R. J. Shaw, and D. B. Young. 2000. Comparison of Mycobacterium tuberculosis genomes reveals frequent deletions in a 20 kb variable region in clinical isolates. Yeast 17:272-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamerbeek, J., L. Schouls, A. Kolk, M. van Agterveld, D. van Soolingen, S. Kuijper, A. Bunschoten, H. Molhuizen, R. Shaw, M. Goyal, and J. van Embden. 1997. Simultaneous detection and strain differentiation of Mycobacterium tuberculosis for diagnosis and epidemiology. J. Clin. Microbiol. 35:907-914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kolk, A. H., A. R. Schuitema, S. Kuijper, J. van Leeuwen, P. W. Hermans, J. D. van Embden, and R. A. Hartskeerl. 1992. Detection of Mycobacterium tuberculosis in clinical samples by using polymerase chain reaction and a nonradioactive detection system. J. Clin. Microbiol. 30:2567-2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kox, L. F., D. Rhienthong, A. M. Miranda, N. Udomsantisuk, K. Ellis, J. van Leeuwen, S. van Heusden, S. Kuijper, and A. H. Kolk. 1994. A more reliable PCR for detection of Mycobacterium tuberculosis in clinical samples. J. Clin. Microbiol. 32:672-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kremer, K., D. van Soolingen, R. Frothingham, W. H. Haas, P. W. Hermans, C. Martin, P. Palittapongarnpim, B. B. Plikaytis, L. W. Riley, M. A. Yakrus, J. M. Musser, and J. D. van Embden. 1999. Comparison of methods based on different molecular epidemiological markers for typing of Mycobacterium tuberculosis complex strains: interlaboratory study of discriminatory power and reproducibility. J. Clin. Microbiol. 37:2607-2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kurepina, N. E., S. Sreevatsan, B. B. Plikaytis, P. J. Bifani, N. D. Connell, R. J. Donnelly, D. van Sooligen, J. M. Musser, and B. N. Kreiswirth. 1998. Characterization of the phylogenetic distribution and chromosomal insertion sites of five IS6110 elements in Mycobacterium tuberculosis: non-random integration in the dnaA-dnaN region. Tubercle Lung Dis 79:31-42. [DOI] [PubMed] [Google Scholar]
- 18.Magdalena, J., A. Vachee, P. Supply, and C. Locht. 1998. Identification of a new DNA region specific for members of Mycobacterium tuberculosis complex. J. Clin. Microbiol. 36:937-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Manca, C., L. Tsenova, A. Bergtold, S. Freeman, M. Tovey, J. M. Musser, C. E. Barry III, V. H. Freedman, and G. Kaplan. 2001. Virulence of a Mycobacterium tuberculosis clinical isolate in mice is determined by failure to induce Th1 type immunity and is associated with induction of IFN-alpha/beta. Proc. Natl. Acad. Sci. USA 98:5752-5757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazars, E., S. Lesjean, A. L. Banuls, M. Gilbert, V. Vincent, B. Gicquel, M. Tibayrenc, C. Locht, and P. Supply. 2001. High-resolution minisatellite-based typing as a portable approach to global analysis of Mycobacterium tuberculosis molecular epidemiology. Proc. Natl. Acad. Sci. USA 98:1901-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mistry, N. F., A. M. Iyer, T. D'Souza D, G. M. Taylor, D. B. Young, and N. H. Antia. 2002. Spoligotyping of Mycobacterium tuberculosis isolates from multiple-drug-resistant tuberculosis patients from Bombay, India. J. Clin. Microbiol. 40:2677-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Olive, D. M., and P. Bean. 1999. Principles and applications of methods for DNA-based typing of microbial organisms. J. Clin. Microbiol. 37:1661-1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parsons, L. M., R. Brosch, S. T. Cole, A. Somoskovi, A. Loder, G. Bretzel, D. Van Soolingen, Y. M. Hale, and M. Salfinger. 2002. Rapid and simple approach for identification of Mycobacterium tuberculosis complex isolates by PCR-based genomic deletion analysis. J. Clin. Microbiol. 40:2339-2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radhakrishnan, I., M. Y. K, R. A. Kumar, and S. Mundayoor. 2001. Implications of low frequency of IS6110 in fingerprinting field isolates of Mycobacterium tuberculosis from Kerala, India. J. Clin. Microbiol. 39:1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Soini, H., X. Pan, A. Amin, E. A. Graviss, A. Siddiqui, and J. M. Musser. 2000. Characterization of Mycobacterium tuberculosis isolates from patients in Houston, Texas, by spoligotyping. J. Clin. Microbiol. 38:669-676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sola, C., I. Filliol, M. C. Gutierrez, I. Mokrousov, V. Vincent, and N. Rastogi. 2001. Spoligotype database of Mycobacterium tuberculosis: biogeographic distribution of shared types and epidemiologic and phylogenetic perspectives. Emerg. Infect. Dis. 7:390-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sreevatsan, S., X. Pan, K. E. Stockbauer, N. D. Connell, B. N. Kreiswirth, T. S. Whittam, and J. M. Musser. 1997. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc. Natl. Acad. Sci. USA 94:9869-9874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Supply, P., S. Lesjean, E. Savine, K. Kremer, D. van Soolingen, and C. Locht. 2001. Automated high-throughput genotyping for study of global epidemiology of Mycobacterium tuberculosis based on mycobacterial interspersed repetitive units. J. Clin. Microbiol. 39:3563-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Supply, P., J. Magdalena, S. Himpens, and C. Locht. 1997. Identification of novel intergenic repetitive units in a mycobacterial two-component system operon. Mol. Microbiol. 26:991-1003. [DOI] [PubMed] [Google Scholar]
- 30.Supply, P., E. Mazars, S. Lesjean, V. Vincent, B. Gicquel, and C. Locht. 2000. Variable human minisatellite-like regions in the Mycobacterium tuberculosis genome. Mol. Microbiol. 36:762-771. [DOI] [PubMed] [Google Scholar]
- 31.Supply, P., R. M. Warren, A. L. Banuls, S. Lesjean, G. D. Van Der Spuy, L. A. Lewis, M. Tibayrenc, P. D. Van Helden, and C. Locht. 2003. Linkage disequilibrium between minisatellite loci supports clonal evolution of Mycobacterium tuberculosis in a high tuberculosis incidence area. Mol. Microbiol. 47:529-538. [DOI] [PubMed] [Google Scholar]
- 32.van Soolingen, D., P. E. de Haas, P. W. Hermans, P. M. Groenen, and J. D. van Embden. 1993. Comparison of various repetitive DNA elements as genetic markers for strain differentiation and epidemiology of Mycobacterium tuberculosis. J. Clin. Microbiol. 31:1987-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Soolingen, D., P. W. Hermans, P. E. de Haas, D. R. Soll, and J. D. van Embden. 1991. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J. Clin. Microbiol. 29:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Soolingen, D., L. Qian, P. E. de Haas, J. T. Douglas, H. Traore, F. Portaels, H. Z. Qing, D. Enkhsaikan, P. Nymadawa, and J. D. van Embden. 1995. Predominance of a single genotype of Mycobacterium tuberculosis in countries of east Asia. J. Clin. Microbiol. 33:3234-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vera-Cabrera, L., M. A. Hernandez-Vera, O, Welsh, W. M. Johnson, and J. Castro-Garza. 2001. Phospholipase region of Mycobacterium tuberculosis is a preferential locus for IS6110 transposition. J. Clin. Microbiol. 39:3499-3504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zink, A. R., C. Sola, U. Reischl, W. Grabner, N. Rastogi, H. Wolf, and A. G. Nerlich. 2003. Characterization of Mycobacterium tuberculosis complex DNAs from Egyptian mummies by spoligotyping. J. Clin. Microbiol. 41:359-367. [DOI] [PMC free article] [PubMed] [Google Scholar]