Abstract
Background
Candida infections are a leading cause of infectious disease-related death in infants supported with extracorporeal membrane oxygenation (ECMO). The ECMO circuit can alter drug pharmacokinetics (PK), thus standard fluconazole dosing in children on ECMO may result in suboptimal drug exposure. This study determined the PK of fluconazole in infants on ECMO.
Methods
Infants <120 days old received either intravenous fluconazole prophylaxis (25 mg/kg once a week) or treatment (12 mg/kg daily) while on ECMO. Paired plasma samples were collected pre- and post-oxygenator around doses 1 and 2 to calculate PK indices and describe oxygenator extraction. A 1-compartment model was fit to the data using non-linear regression. Surrogate pharmacodynamic targets for efficacy were evaluated.
Results
Ten infants were enrolled. After dose 1 (n=9), the median clearance was 17 mL/kg/h, the median volume of distribution was 1.5 L/kg, and the median exposure in the first 24 hours (AUC0–24) was 322 h*mg/L. After multiple doses (n=7), the median clearance was 22 mL/kg/h, the median volume of distribution was 1.9 L/kg, and the AUC0–24 was 352 h*mg/L. After dose 1, 78% of infants achieved the prophylaxis target, while only 11% achieved the therapeutic target. Oxygenator extraction of fluconazole was minimal (−2.0%, standard deviation 15.0), and extraction was not correlated with age of the ECMO circuit (rho= − 0.05). There were no adverse events related to fluconazole.
Conclusions
Infants on ECMO had higher volume of distribution but similar clearance when compared with historical controls not on ECMO. In infants on ECMO, a fluconazole dose of 25 mg/kg weekly provides adequate exposure for prophylaxis against Candida infections. However, higher doses may be needed for treatment.
Keywords: fluconazole, Candida, extracorporeal membrane oxygenation, pharmacokinetics, infants
Extracorporeal membrane oxygenation (ECMO) is a cardiopulmonary bypass device used in the pediatric critical care setting that is life-saving for infants with refractory cardiorespiratory failure. Blood is drained from the central venous system via a surgically placed cannula, pumped through an artificial lung (oxygenator) where oxygen is added and carbon dioxide is removed, and then oxygenated blood is returned to either the venous or arterial circulation. ECMO has been used successfully in multiple disease states including meconium aspiration syndrome, persistent pulmonary hypertension of the newborn, fulminant myocarditis, and sepsis.1 Despite these successes, infants supported with ECMO are at high risk for ECMO-related complications, including nosocomial infections.2
Invasive candidiasis is the second most common nosocomial infection in infants on ECMO and is often fatal.2,3 The incidence of fungal infections in patients on ECMO varies by center, with reports ranging from 0.7–10%.2,3.Standard treatment for invasive candidiasis in many patient populations consists of antifungal agents (e.g., fluconazole) and removal or replacement of intravascular catheters due to the organism’s ability to adhere to indwelling catheters.4 Catheter removal for infants on ECMO is impossible if the infant cannot be disconnected from the ECMO circuit; therefore, successful treatment of invasive candidiasis in infants on ECMO relies upon optimal antifungal drug therapy.
Optimal antifungal management for patients on ECMO depends on dosing that has not been previously described and can differ greatly from other populations due to the pharmacokinetic (PK) changes induced by the ECMO circuit. PK changes attributed to the ECMO circuit include increased volume of distribution and decreased clearance, but these vary by drug.5–8 This study describes the ECMO-related PK changes of fluconazole and their impact on pharmacodynamic (PD) targets for fungal prophylaxis and treatment of invasive candidiasis.
MATERIALS AND METHODS
This was a prospective, single-center, open-label PK and safety trial of fluconazole in infants supported with ECMO at Duke University Medical Center. Infants <120 postnatal days and supported with ECMO were enrolled. Infants were excluded if they had a history of hypersensitivity or severe vasomotor reaction to any triazole or had previously participated in the study. Infants received either prophylactic fluconazole per study protocol (25 mg/kg administered intravenously over 2 hours) or fluconazole per standard of care (12 mg/kg administered intravenously over 1 hour) if the child had a known or suspected fungal infection. Infants on prophylactic fluconazole continued to receive fluconazole 25 mg/kg once weekly for the duration of their ECMO course. Duration of treatment for infants with suspected or culture-proven fungal infection was at the discretion of the treating physician. This trial was approved by the institutional review board of Duke University Medical Center, registered with clinicaltrials.gov (NCT01169402), and conducted under a Food and Drug Administration investigational new drug application (#108314). Written informed consent was obtained from the legal guardian of each infant.
Sample Collection and Preparation
Up to 12 paired samples (200 μL whole blood per sample) were collected from the infant via an arterial catheter (Cout) and the ECMO circuit just before the oxygenator (Cin) for each infant at dose 1 and dose 2. Sampling times for all enrolled infants included a trough level 0–4 hours prior to the start of infusion and serial samples after the end of the infusion: 15 minutes (+/−15 minutes), 3 hours (+/−1 hour), 9 hours (+/−3 hours), 23 hours (+/−1 hour), and 47 hours (+/−1 hour). Samples were collected in ethylenediaminetetraacetic acid (EDTA) microcontainers and taken from a different site than the site used for fluconazole administration. Samples were processed immediately or placed on ice until processing. Plasma was separated via centrifugation (3000 g for 10 minutes at 4° C), manually aspirated, and transferred to polypropylene tubes. Plasma samples were frozen at −80° C until analysis.
Fluconazole extraction by the ECMO oxygenator was calculated using the paired samples collected from the ECMO circuit just prior to the oxygenator (Cin) and from the patient (Cout) using the equation: [(Cin − Cout)/Cin] * 100. Any extraction levels greater than 3 standard deviations above or below the mean extraction were considered outliers and were excluded from the analysis. Venous (Cin) samples were used for all PK analyses.
ECMO Circuit Configuration
The ECMO circuit configuration was consistent across all infants and included tubing (Sorin Smart®, Sorin Group, Denver, CO), a centrifugal pump (Sorin Revolution®, Sorin Group, Denver, CO), and a hollow fiber membrane oxygenator (Quadrox-iD Adult® or Quadrox-iD Pediatric®, MAQUET Cardiovascular, Wayne, NJ). A hemofilter (Sorin DHFO.2®, Sorin Group, Denver, CO) was used if the infant required hemofiltration or hemodialysis. The circuit prime volume was 450 mL for the Quadrox-iD Adult® oxygenator (infants 2, 3, 5, 7, 9, 10) and 250 mL for the Quadrox-iD Pediatric® oxygenator (infants 1, 4, 6, 8).
Analytic Procedures and Analyses
Plasma fluconazole concentrations were determined using a validated liquid chromatography-tandem mass spectrometry assay.9 The lower limit of quantification was 0.01 mg/L; intraday and interday precision ranged from 2.84% to 10.8% and 5.27% to 11.5%, respectively, within the concentration range of the standard curve (0.01 to 10 mg/L).
The primary outcomes were the fluconazole PK indices in infants on ECMO (clearance, volume of distribution) and the number of infants achieving the proposed PD target for fungal prophylaxis (fluconazole time above the minimum inhibitory concentration [T>MIC; 4 mg/L] for >50% of the dosing interval).10 In addition, the proportion of infants achieving the surrogate PD target for treatment (area under the curve from 0–24 hours [AUC0–24] > 400 mg*h/L)11 of invasive candidiasis was calculated. This was performed because the dose evaluated in this study has previously been evaluated for treatment of candidiasis.12 The secondary outcome was fluconazole extraction by the ECMO circuit. The PK indices of fluconazole were examined with descriptive statistics.
PK data were analyzed by nonlinear regression with the most appropriate model using WinNonlin v. 6.2 (Pharsight Co., St. Louis, MO). The model fit was evaluated using successful minimization, diagnostic plots, goodness of fit as assessed by the Akaike Information Criterion, and precision of the parameter estimates. AUC0–24 was computed by the linear trapezoidal method using simulated data predicted from the 1-compartment model. The T>MIC was estimated and expressed as a percentage of the dose interval using the following equation:
where Clast is last simulated fluconazole concentration from the terminal elimination phase using the predicted data from the 1-compartment model; MIC is the minimum inhibitory concentration (4 mg/L); k is the elimination rate constant derived from the 1-compartment model; tlast is time of last fluconazole concentration using the predicted data from the 1-compartment model; and tMIC is estimated time above the MIC. For an infant on standard-of-care fluconazole (12 mg/kg daily), AUC0–24 and T>MIC were calculated using the simulated data after administration of a 25 mg/kg dose using the clearance and volume of distribution estimates from the 1-compartment model.
The relationships between fluconazole clearance and serum creatinine on the day of dose, as well as the fluconazole volume of distribution and the ECMO prime volume were explored with scatter plots. Differences in PK indices between doses were evaluated using Wilcoxon signed-rank test. Correlation between ECMO circuit extraction and time was measured with Spearman’s rank correlation coefficient. The sample size was based on the ability to provide reasonable estimates for safety. It was determined a priori that, with a sample size of 8, the 95% confidence interval (CI) for an adverse event (AE) in 1 infant within the patient population was 0–53%. STATA 11 (College Station, TX) was used to perform the statistical analyses. In all cases, a P value of <0.05 was considered statistically significant.
Safety
The safety of fluconazole was assessed by monitoring the frequency, intensity, and relationship to study drug of AEs while on study drug and for 7 days after the last dose. The results of clinical laboratory tests performed within 72 hours of study drug administration and weekly throughout the monitoring period were recorded. The primary AE of interest was liver toxicity assessed by the evaluation of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) values measured at least weekly during study drug administration. AEs were graded according to the National Cancer Institute’s Common Terminology Criteria and reported as mild (grade 1), moderate (grade 2), severe (grade 3), life-threatening (grade 4), or fatal (grade 5). Causality (unrelated and possibly, probably and definitely related) of AEs and study drug was determined by the principal investigator and the treating physician. AEs determined to be probably or definitely related to study drug were followed until resolution. The safety data were summarized descriptively.
RESULTS
Between August 2010 and July 2011, 10 infants with a median age of 19 days (range 1–113 days) supported with ECMO were enrolled in the study, received at least 1 dose of fluconazole, and were included in the safety analysis (see Table, Supplemental Digital Content 1, which shows infant demographics). Nine infants received prophylactic intravenous fluconazole (25 mg/kg weekly infused over 2 hours), and 1 infant (#7) was treated for presumed fungal infection with standard-of-care fluconazole (12 mg/kg intravenously daily infused over 1 hour). PK samples were collected from the 9 infants on prophylaxis around the first dose, and the 6 infants who remained on ECMO for more than 1 week were also sampled around the second dose. Infant #7, who received fluconazole 12 mg/kg/day, had PK samples collected around the final seventh dose. Thirty percent of infants were female, 60% were white, and 10% were Hispanic. Three infants were born pre-term. At time of cannulation, median corrected age was 41 weeks (range 36–53 weeks). Hemofiltration and/or continuous veno-venous hemodialysis (CVVHD) was used in 3 infants. Overall serum creatinine was similar between first dose and multiple dose evaluations, although 2 infants receiving hemofiltration or CVVHD had a 3-fold increase in their serum creatinine between dose 1 and 2. The renal dysfunction in infant #3 was multifactorial, while that in infant #6 was due to intrinsic renal dysfunction following cardiac arrest. In both cases, renal dysfunction occurred prior to initiation of hemofiltration.
Pharmacokinetics
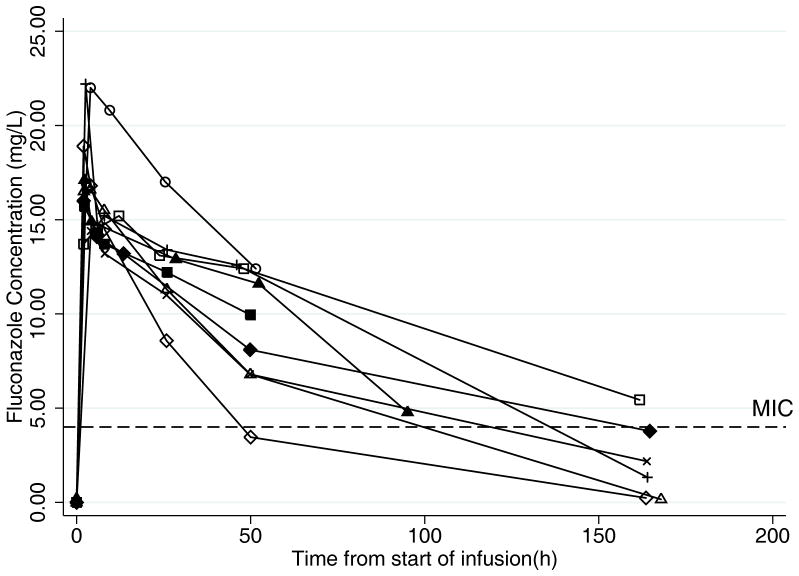
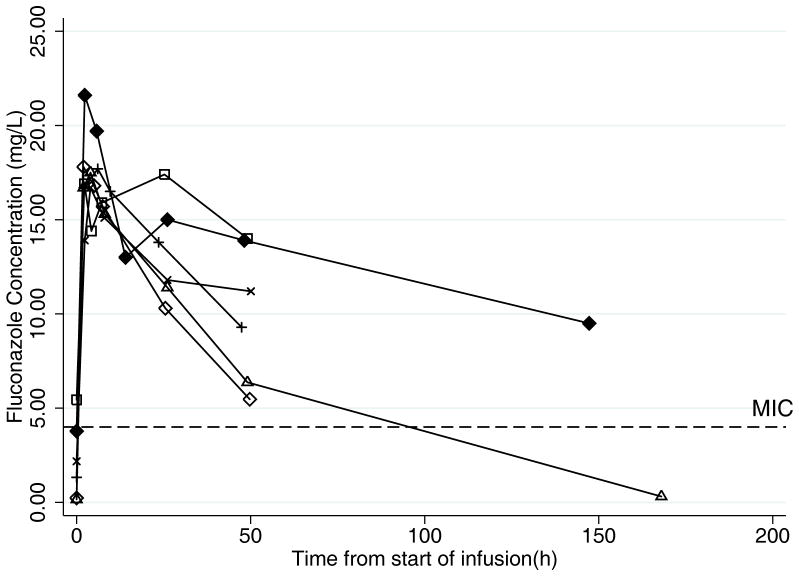
PK data were evaluated from 62 plasma samples around the first dose and 47 samples after multiple doses. The plasma concentrations after the first dose and after multiple doses are shown in Figure 1. The PK indices after first and multiple doses are presented in Table 1. A 1-compartment model with zero-order infusion appropriately described the data, and PK indices were estimated with high precision as evidenced by median clearance coefficient of variation of 10.2% (range 2.0–25.8%) and median volume of distribution coefficient of variation of 5.7% (range 1.4–16.1%). After the first dose, the median (interquartile range [IQR]) clearance was 17 mL/kg/h (14, 22), the median volume of distribution was 1.5 L/kg (1.3, 1.7), and the median AUC0–24 was 322 mg*h/L (307, 343). After multiple doses, the median (IQR) clearance was 22 mL/kg/h (11, 33), the median volume of distribution was 1.9 L/kg (1.4, 2.2), and the AUC0–24 was 352 mg*h/L (344, 399). Fluconazole clearance was inversely related to serum creatinine (see Figure, Supplemental Digital Content 2, which shows weight-normalized fluconazole clearance after dose 1 and multiple doses versus serum creatinine). Neither fluconazole clearance (P=0.92) nor serum creatinine (P=0.67) was significantly different between the first and multiple doses. There was no relationship between the fluconazole volume of distribution and amount of the volume of exogenous blood needed to prime the ECMO circuit (data not shown). A relationship between postnatal age and clearance and gestational age and clearance was not observed during visual inspection of scatter plots (data not shown). The volume of distribution of fluconazole did not differ significantly between dose 1 and multiple doses (P=0.14). The median fluconazole trough concentration before dose 2 was 1.8 mg/L (range 0.2–5.4).
FIGURE 1.
Fluconazole concentration time profiles. A. Dose 1: Plasma concentration-time profiles in young infants (n=9) after receiving first dose of intravenous fluconazole 25 mg/kg. Concentrations at time=0 hours are concentrations prior to first dose. Pharmacodynamic (PD) target for prevention of fungal infection is serum concentration > minimum inhibitory concentration (MIC) (4 mg/L) for 84 hours (50% of the dosing interval). B. Dose 2: Plasma concentration-time profiles in young infants after receiving second dose of intravenous fluconazole 25 mg/kg (n=6). Infant #7 who received treatment fluconazole (12 mg/kg daily) is not represented in this figure. Concentrations at time=0 hours are trough concentrations prior to the second dose. PD target for prevention of fungal infection is serum concentration >MIC (4 mg/L) for 50% of the dosing interval, which was 84 hours for the once-weekly 25 mg/kg dose.
TABLE 1.
Pharmacokinetic Indices
| ID | First Dose | Multiple Doses | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Dose (mg/kg) | CL (mL/h/kg) | V (L/kg) | t1/2 (h) | T>MIC (%) | AUC0–24 (h* mg/L) | Dose (mg/kg) | CL (mL/h/kg) | V (L/kg) | t1/2 (h) | T>MIC (%) | AUC0–24 (h* mg/L) | |
| 1 | 25 | 19 | 1.3 | 47 | 63 | 367 | 25 | 22 | 1.5 | 46 | 61 | 385 |
| 2 | 25 | 22 | 1.7 | 55 | 61 | 288 | 25 | 19 | 1.9 | 68 | 83 | 352 |
| 3 | 25 | 10 | 1.7 | 116 | 132 | 324 | 25 | 9 | 2.3 | 189 | 233 | 399 |
| 4 | 25 | 17 | 1.5 | 60 | 75 | 343 | - | - | - | - | - | - |
| 5 | 25 | 33 | 1.4 | 29 | 39 | 322 | 25 | 33 | 1.4 | 29 | 38 | 347 |
| 6 | 25 | 16 | 1.7 | 75 | 85 | 307 | 25 | 11 | 1.9 | 115 | 152 | 424 |
| 7* | - | - | - | - | - | - | 12 | 22 | 2.2 | 56 | 438 | 299† |
| 8 | 25 | 14 | 1.7 | 79 | 91 | 316 | - | - | - | - | - | - |
| 9 | 25 | 44 | 1.3 | 21 | 28 | 303 | 25 | 35 | 1.4 | 27 | 39 | 344 |
| 10 | 25 | 11 | 1.2 | 76 | 106 | 423 | - | - | - | - | - | - |
|
| ||||||||||||
| Median | 17 | 1.5 | 60 | 75 | 322 | 22 | 1.9 | 56 | 83 | 352 | ||
| IQR | 14, 22 | 1.3, 1.7 | 47, 76 | 61, 91 | 307, 343 | 11, 33 | 1.4, 2.2 | 37, 92 | 39, 233 | 344, 399 | ||
Infant 7 was on fluconazole per standard of care (12 mg/kg IV daily), and PK sampling occurred after the final (seventh) dose. All other multiple-dose data were obtained during the infants’ second dose.
T>MIC and AUC0–24 for infant 7 were obtained by simulating exposure after a dose of 25 mg/kg using clearance and volume of distribution derived from the 1-compartment model.
CL indicates clearance; V, volume of distribution; t1/2, half-life; T>MIC, percentage of the dosing interval that fluconazole concentration was above the minimum inhibitory concentration of 4 mg/L (PD target was 50% of dosing interval); AUC0–24,area under the curve from 0–24 hours (PD target 400 h*mg/L); IQR, interquartile range.
Pharmacodynamic Targets
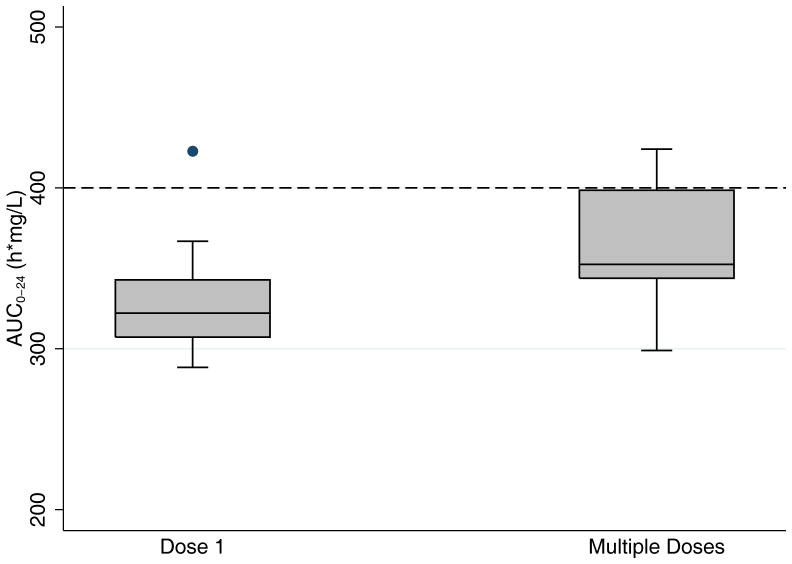
After the first dose, 7 out of 9 infants (78%) achieved the prophylaxis target of T>MIC (4 mg/L) for >84 hours, which was 50% of the dosing interval.10 Only 1 infant (11%) achieved the therapeutic target for treatment of invasive candidiasis of AUC0–24 > 400 h*mg/L (Figure 2).11 After multiple doses of fluconazole, 5 out of 7 infants (71%) achieved the prophylaxis target, and none achieved the therapeutic target. None of the infants developed a fungal infection while enrolled in the study.
FIGURE 2.
Median (interquartile range) fluconazole exposure in the first 24 hours (AUC0–24) after dose 1 and multiple doses. Pharmacodynamic target for therapy is AUC0–24 >400 h*mg/L. AUC0–24 indicates area under the curve from 0–24 hours.
ECMO Circuit Extraction
A total of 210 samples were included in the extraction analysis after 2 paired samples (1.9%) were identified as outliers and excluded from the analysis.. Mean fluconazole extraction across the oxygenator was −2.0% (standard deviation 15.0), but there was wide dispersion around the mean. Extraction by the oxygenator did not correlate with the age of the ECMO circuit (rho= − 0.05).
Safety
There were 11 adverse events in 6 infants, including 3 deaths. None of the AEs were probably or definitely related to study drug (see Table, Supplemental Digital Content 3, which shows adverse events). Three infants had elevated AST or ALT levels. In all 3 cases, the transaminase levels were elevated prior to initiation of study fluconazole and declined to normal levels while on study drug. Of the 3 infants who died, 2 of the deaths were attributed to severe congenital heart disease, and 1 death was attributed to intracranial hemorrhage.
DISCUSSION
Invasive candidiasis can be a devastating complication of ECMO. Invasive candidiasis is difficult to treat in this setting and is associated with higher mortality compared with infants on ECMO without invasive candidiasis.2,3 Infants on ECMO with invasive disease require optimal dosing of antifungal drugs and may benefit from fungal prophylaxis. To prevent or treat invasive infection, the first step is to determine exposure of a candidate drug. This requires at least 1 PK and safety study in infants supported by ECMO because the ECMO circuit can substantially alter the PK of drugs through the addition of large volumes of exogenous blood to prime the ECMO circuit, adhesion of drug to components of the ECMO circuit, and renal insufficiency common in infants on ECMO.13
The complexity of the ECMO circuit and its impact on dosing have been observed for drugs that require therapeutic drug monitoring such as gentamicin7,14–16 and vancomycin.8,17,18 PK data of antifungals in ECMO, however, are extremely scarce. Two case reports suggested that voriconazole and caspofungin may be extracted by the ECMO circuit, resulting initially in subtherapeutic exposure with standard dosing, although voriconazole concentrations became supra-therapeutic over time, consistent with decreased clearance.19,20 The voriconazole data were supported by an ex vivo ECMO model indicating that 71% of a voriconazole dose was adsorbed by the ECMO circuit within 3 hours of administration.21
In this PK trial of fluconazole in infants on ECMO, volume of distribution was 50–90% higher and clearance was similar in infants on ECMO compared with a similar cohort of critically ill infants not on ECMO,12 resulting in lower fluconazole exposure (AUC0–24) (Table 2). The increased volume of distribution in infants on ECMO is consistent with physiology: infants have a total blood volume of ~80 mL/kg (estimated range for our cohort 160–500 mL), and 250–450 mL of blood are required to prime the ECMO circuit. Thus, the circuit effectively doubles the native blood volume of these infants. Clearance was inversely related to serum creatinine. This relationship was also expected as the kidneys constitute the primary fluconazole elimination pathway. Therefore, renal function should be considered when dosing fluconazole in this population. However, fluconazole clearance can be affected by the concurrent use of hemofiltration or CVVHD. In adults supported with CVVHD, higher doses of fluconazole were required to maintain adequate exposure due to effective drug removal by the CVVHD system.22 Three of the infants enrolled in the present study received hemofiltration, and 1 received CVVHD. While the small sample size limits generalization of results (these 3 infants had the lowest, median, and highest fluconazole clearances, respectively), infant #3 who was supported with both hemofiltration and CVVHD had a threefold rise in serum creatinine between the start of dose 1 and start of dose 2, the lowest clearance, and the highest volume of distribution. This pattern of renal insufficiency is common in children on ECMO and supports the hypothesis that, for renally cleared drugs, a larger dose administered less frequently is appropriate.
TABLE 2.
Pharmacokinetic Indices in Infants on ECMO after the First Dose of Intravenous Fluconazole 25 mg/kg Compared with Historical Controls not on ECMO who Received 1 Dose of Intravenous Fluconazole 25 mg/kg12
| ECMO | Non-ECMO | P | |
|---|---|---|---|
| V (L/kg) | 1.5 (1.3, 1.7) | 1.0 (0.8, 1.4) | 0.02 |
| CL (ml/h/kg) | 17 (14, 22) | 18 (14, 22) | 0.57 |
| t1/2 (h) | 60 (47, 76) | 39 (24, 79) | 0.49 |
| AUC0–24 (h*mg/L) | 322 (307, 343) | 476 (366, 492) | 0.01 |
Data for historical controls were extracted from Piper et al. minus their infant on ECMO and compared using Wilcoxon signed-rank test.
ECMO indicates extracorporeal membrane oxygenation; V, volume of distribution; CL, clearance; t1/2, half-life; AUC0–24,area under the curve from 0–24 hours. All values are median (interquartile range).
Fluconazole undergoes minimal extraction by the ECMO circuit, and that extraction is not influenced by the age of the ECMO circuit. This finding is consistent with the low lipophilicity of fluconazole. Prior studies of drugs administered during ECMO have shown that increased lipophilicity is associated with increased adsorption by the circuit.23 Therefore, the findings from this study should not be extrapolated to other antifungal agents within and outside of class. Echinocandins have excellent activity against Candida species and penetrate biofilms, an appealing quality in a population for whom catheter removal is impossible. However, as a class, they are nearly 100% protein-bound and highly lipophilic, which may predispose them to high adhesion to the ECMO circuit.
PK/PD indices for fluconazole prophylaxis are not well defined. Dosing for fluconazole prophylaxis in immuno-compromised adults ranges from 50–200 mg/day, targeting an AUC0–24 of 50–200 mg*h/L, though with these strategies some resistance to fluconazole has developed. In premature infants, doses of 3–6 mg/kg given daily twice per week have been shown to decrease the incidence of invasive candidiasis without concurrent development of resistance, though the power to detect differences in resistance in these studies was low.24–26 A murine model of invasive candidiasis showed that resistance could be prevented if fluconazole serum concentrations were kept above the MIC for >50% of the dosing interval.10 The typical MIC of Candida species in children ranges from 0.25 to 4 mg/L.24–27
In this trial of fluconazole prophylaxis of infants on ECMO, fluconazole 25 mg/kg weekly resulted in exposures comparable to those seen in prophylaxis trials in adults and children with malignancy28 and premature infants.24–26 Further, at this dose, fluconazole serum concentrations were kept above the MIC of 4 mg/L for 50% of the dosing interval in over 70% of infants on ECMO. Fluconazole 25 mg/kg weekly should be adequate to prevent invasive candidiasis in infants supported with ECMO and prevent development of resistance.
However, a fluconazole dose of 25 mg/kg did not achieve the necessary exposure to treat invasive candidiasis (AUC0–24 >400 mg*h/L) in this population on ECMO. This is in contrast to a cohort of critically ill children not on ECMO, in whom this therapeutic target was achieved in 70% of infants who received a fluconazole loading dose of 25 mg/kg.12 Fluconazole PK is linear up to 40 mg/kg;29,30 thus, a fluconazole loading dose of 30–40 mg/kg would be needed to achieve an AUC0–24 effective to treat Candida infection in infants on ECMO. Only 1 infant in this study received daily fluconazole treatment doses (12 mg/kg/day), which limits the assessment of exposures achieved after repeat daily dosing. A fluconazole therapeutic regimen should be prospectively evaluated.
The decision to use fluconazole prophylaxis in children on ECMO should be based on individual risk factors and center-specific incidence of fungal infection. The overall rate of fungal infection for all ECMO centers is relatively low at 2 per 1000 ECMO days.2 Prophylaxis for a low-prevalence infection must be weighed against the devastating consequences of and difficulty treating a fungal infection in this population.3 A multi-center trial evaluating the epidemiology of fungal infection and efficacy of antifungal prophylaxis in children on ECMO is urgently needed.
Supplementary Material
Acknowledgments
Conflicts of interest and sources of funding: Dr. Watt receives support from NICHD (5T32HD043029-09), NIGMS (1T32GM086330-01A1), and the Thrasher Research Fund for his work in pediatric clinical pharmacology. Dr. Smith receives support from the U.S. government for his work in pediatric and neonatal clinical pharmacology (Government Contract HHSN267200700051C, PI: Benjamin), from NICHD (1K23HD060040-01), from AHRQ (1R18AE000028-01), and from industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp). Dr. Cohen-Wolkowiez receives support from NICHD 1K23HD064814-01 and the Thrasher Research Fund for his work in pediatric clinical pharmacology and from Pfizer Inc. for neonatal and pediatric drug development. Dr. Benjamin receives support from the U. S. government for his work in pediatric and neonatal clinical pharmacology (1R01HD057956-02, 1R01FD003519-01, 1U10-HD45962-06, 1K24HD058735-01; he is the principal investigator of the Pediatric Trials Network—Government Contract HHSN275201000002I); the nonprofit organization Thrasher Research Foundation for his work in neonatal candidiasis (http://www.thrasherresearch.org); and from industry for neonatal and pediatric drug development (http://www.dcri.duke.edu/research/coi.jsp). Dr. Brouwer receives support from NIGMS for her work in clinical pharmacology and mechanisms of altered hepatic disposition of anionic drugs (3R01GM41935-19).
The authors would like to thank the Thrasher Research Fund for support and would like to acknowledge the staff and research team in the pediatric critical care units at Duke University for their outstanding disposition, support, and dedication to this study.
References
- 1.Extracorporeal Life Support Organization (ELSO) Extracorporeal Life Support Registry Report: International Summary. Ann Arbor, MI: ELSO; 2010. [Google Scholar]
- 2.Bizzarro MJ, Conrad SA, Kaufman DA, Rycus P Extracorporeal Life Support Organization Task Force on Infections, Extracorporeal Membrane Oxygenation. Infections acquired during extracorporeal membrane oxygenation in neonates, children, and adults. Pediatr Crit Care Med. 2011;12:277–281. doi: 10.1097/PCC.0b013e3181e28894. [DOI] [PubMed] [Google Scholar]
- 3.Gardner AH, Prodhan P, Stovall SH, et al. Fungal infections and antifungal prophylaxis in pediatric cardiac extracorporeal life support. J Thorac Cardiovasc Surg. 2011 Dec 15; doi: 10.1016/j.jtcvs.2011.12.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Eppes SC, Troutman JL, Gutman LT. Outcome of treatment of candidemia in children whose central catheters were removed or retained. Pediatr Infect Dis J. 1989;8:99–104. [PubMed] [Google Scholar]
- 5.Veinstein A, Debouverie O, Grégoire N, et al. Lack of effect of extracorporeal membrane oxygenation on tigecycline pharmacokinetics. J Antimicrob Chemother. 2011 Dec 29; doi: 10.1093/jac/dkr550. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 6.Wildschut ED, de Hoog M, Ahsman MJ, Tibboel D, Osterhaus AD, Fraaij PL. Plasma concentrations of oseltamivir and oseltamivir carboxylate in critically ill children on extracorporeal membrane oxygenation support. PLoS One. 2010;5:e10938. doi: 10.1371/journal.pone.0010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhatt-Mehta V, Johnson CE, Schumacher RE. Gentamicin pharmacokinetics in term neonates receiving extracorporeal membrane oxygenation. Pharmacotherapy. 1992;12:28–32. [PubMed] [Google Scholar]
- 8.Mulla H, Pooboni S. Population pharmacokinetics of vancomycin in patients receiving extracorporeal membrane oxygenation. Br J Clin Pharmacol. 2005;60:265–275. doi: 10.1111/j.1365-2125.2005.02432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu D, Wade KC, Paul DJ, Barrett JS. A rapid and sensitive LC-MS/MS method for determination of fluconazole in human plasma and its application in infants with Candida infections. Ther Drug Monit. 2009;31:703–709. doi: 10.1097/FTD.0b013e3181b20b40. [DOI] [PubMed] [Google Scholar]
- 10.Andes D, Forrest A, Lepak A, Nett J, Marchillo K, Lincoln L. Impact of antimicrobial dosing regimen on evolution of drug resistance in vivo: fluconazole and Candida albicans. Antimicrob Agents Chemother. 2006;50:2374–2383. doi: 10.1128/AAC.01053-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wade KC, Benjamin DK, Jr, Kaufman DA, et al. Fluconazole dosing for the prevention or treatment of invasive candidiasis in young infants. Pediatr Infect Dis J. 2009;28:717–723. doi: 10.1097/INF.0b013e31819f1f50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Piper L, Smith PB, Hornik CP, et al. Fluconazole loading dose pharmacokinetics and safety in infants. Pediatr Infect Dis J. 2011;30:375–378. doi: 10.1097/INF.0b013e318202cbb3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dagan O, Klein J, Gruenwald C, Bohn D, Barker G, Koren G. Preliminary studies of the effects of extracorporeal membrane oxygenator on the disposition of common pediatric drugs. Ther Drug Monit. 1993;15:263–266. doi: 10.1097/00007691-199308000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Cohen P, Collart L, Prober CG, Fischer AF, Blaschke TF. Gentamicin pharmacokinetics in neonates undergoing extracorporal membrane oxygenation. Pediatr Infect Dis J. 1990;9:562–566. doi: 10.1097/00006454-199008000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Munzenberger PJ, Massoud N. Pharmacokinetics of gentamicin in neonatal patients supported with extracorporeal membrane oxygenation. ASAIO Trans. 1991;37:16–18. doi: 10.1097/00002480-199101000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Southgate WM, DiPiro JT, Robertson AF. Pharmacokinetics of gentamicin in neonates on extracorporeal membrane oxygenation. Antimicrob Agents Chemother. 1989;33:817–819. doi: 10.1128/aac.33.6.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buck ML. Vancomycin pharmacokinetics in neonates receiving extracorporeal membrane oxygenation. Pharmacotherapy. 1998;18:1082–1086. [PubMed] [Google Scholar]
- 18.Hoie EB, Swigart SA, Leuschen MP, et al. Vancomycin pharmacokinetics in infants undergoing extracorporeal membrane oxygenation. Clin Pharm. 1990;9:711–715. [PubMed] [Google Scholar]
- 19.Ruiz S, Papy E, Da Silva D, et al. Potential voriconazole and caspofungin sequestration during extracorporeal membrane oxygenation. Intensive Care Med. 2009;35:183–184. doi: 10.1007/s00134-008-1269-3. [DOI] [PubMed] [Google Scholar]
- 20.Spriet I, Annaert P, Meersseman P, et al. Pharmacokinetics of caspofungin and voriconazole in critically ill patients during extracorporeal membrane oxygenation. J Antimicrob Chemother. 2009;63:767–770. doi: 10.1093/jac/dkp026. [DOI] [PubMed] [Google Scholar]
- 21.Mehta NM, Halwick DR, Dodson BL, Thompson JE, Arnold JH. Potential drug sequestration during extracorporeal membrane oxygenation: results from an ex vivo experiment. Intensive Care Med. 2007;33:1018–1024. doi: 10.1007/s00134-007-0606-2. [DOI] [PubMed] [Google Scholar]
- 22.Yagasaki K, Gando S, Matsuda N, et al. Pharmacokinetics and the most suitable dosing regimen of fluconazole in critically ill patients receiving continuous hemodiafiltration. Intensive Care Med. 2003;29:1844–1848. doi: 10.1007/s00134-003-1980-z. [DOI] [PubMed] [Google Scholar]
- 23.Wildschut ED, Ahsman MJ, Allegaert K, Mathot RA, Tibboel D. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. 2010;36:2109–2116. doi: 10.1007/s00134-010-2041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Donowitz LG. Fluconazole prophylaxis against fungal colonization and infection in preterm infants. N Engl J Med. 2001;345:1660–1666. doi: 10.1056/NEJMoa010494. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman D, Boyle R, Hazen KC, Patrie JT, Robinson M, Grossman LB. Twice weekly fluconazole prophylaxis for prevention of invasive Candida infection in high-risk infants of <1000 grams birth weight. J Pediatr. 2005;147:172–179. doi: 10.1016/j.jpeds.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 26.Manzoni P, Stolfi I, Pugni L, et al. A multicenter, randomized trial of prophylactic fluconazole in preterm neonates. N Engl J Med. 2007;356:2483–2495. doi: 10.1056/NEJMoa065733. [DOI] [PubMed] [Google Scholar]
- 27.Kicklighter SD, Springer SC, Cox T, Hulsey TC, Turner RB. Fluconazole for prophylaxis against candidal rectal colonization in the very low birth weight infant. Pediatrics. 2001;107:293–298. doi: 10.1542/peds.107.2.293. [DOI] [PubMed] [Google Scholar]
- 28.van Burik JA, Ratanatharathorn V, Stepan DE, et al. Micafungin versus fluconazole for prophylaxis against invasive fungal infections during neutropenia in patients undergoing hematopoietic stem cell transplantation. Clin Infect Dis. 2004;39:1407–1416. doi: 10.1086/422312. [DOI] [PubMed] [Google Scholar]
- 29.Anaissie EJ, Kontoyiannis DP, Huls C, et al. Safety, plasma concentrations, and efficacy of high-dose fluconazole in invasive mold infections. J Infect Dis. 1995;172:599–602. doi: 10.1093/infdis/172.2.599. [DOI] [PubMed] [Google Scholar]
- 30.Humphrey MJ, Jevons S, Tarbit MH. Pharmacokinetic evaluation of uk-49,858, a metabolically stable triazole antifungal drug, in animals and humans. Antimicrob Agents Chemother. 1985;28:648–653. doi: 10.1128/aac.28.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.