Abstract
Fracture healing and fracture fixation in the context of osteoporosis is extremely difficult. To inhibit osteoclast-induced bone resorption and associated implant loosening in this pathology, we describe a local delivery strategy to delivery RNA interfering technology to bone sites to target and down-regulate osteoclast formation and function. Resorbable polymer, poly(lactic-co-glycolic acid) (PLGA) microparticles were exploited as a passive phagocyte-targeting carrier to deliver RANK siRNA to both osteoclast precursors and osteoclasts - the professional phagocytes in bone. These natural phagocytes internalize micron-sized particles while most other non-targeted cells in bone cannot. PLGA-siRNA microparticles were dispersed within biomedical grade calcium-based injectable bone cement clinically used in osteoporosis as a bone augmentation biomaterial for fragility fracture prevention and fixation. siRNA released from this formulation in vitro retains bioactivity against the cell target, RANK, in cultured osteoclast precursor cells, inhibiting their progression toward the osteoclastic phenotype. These data support the proof-of-concept to utilize a clinically relevant approach to locally deliver siRNA to phagocytes in bone and improve fragility fracture healing in the context of osteoporosis. This local delivery system delivers siRNA therapeutics directly to osteoporosis sites from clinically familiar injected bone augmentation materials but could be extended to other injectable biomaterials for local siRNA delivery.
Keywords: siRNA, osteoclasts, bone resorption, PLGA microparticles, calcium phosphate bone cement, osteoporosis, bone augmentation, local delivery
1. Introduction
Osteoporosis is a chronic disease characterized by low bone density and quality, responsible for fragility fractures in the hip, wrist and spine, and severely impairing patients’ quality of life.[1] Most osteoporotic fractures require operative reduction and stabilization, but fracture healing processes in the context of ongoing osteoporosis pathology are problematic. Low bone quality and excessive bone resorption decrease the mechanical strength necessary for acceptable, durable fracture healing. Clinical approaches to improve bone quality in osteoporosis fixation include reinforcement of porous, weakened bones prior to fracture or device implantation (i.e., prophylaxis) by direct augmentation of bone around the fracture and fixation hardware using injected prosthetic bulking materials.[2–6] The technique utilizes various biomaterials, including polymethylmethacrylate (PMMA), calcium phosphate substitutes (as bone cements, shaped blocks, coatings), various bone grafts, and modified implants.[7] Calcium phosphate cement (CPC), a ceramic chemically similar to natural bone’s mineral component, has attracted attention due to several unique properties, including intrinsic resorption and replacement with nature bone, deployment as viscous setting fluid matrices by local injection and acceptable in situ biocompatibility.[8–10]. In vivo degradation of CPC-based cements can vary widely depending on formulation and chemistry, induced by both passive physiologic dissolution and active cell-mediated phagocytosis.[11] To date, substantial experience supports the feasibility and value of using CPC as a drug carrier in bone augmentation. Pharmacologically active molecules of diverse chemistries are readily distributed within the liquid bone cement formulation prior to setting. Several drugs have been formulated for release from CPC, including antibiotics to decrease post-surgical infections,[12, 13] anticancer drugs to reduce tumorogenesis[14] and growth factors to promote bone healing.[15] However, release rates of encapsulated drugs are limited since most CPC formulations are dense monoliths and in vivo resorption is slow for degradation-controlled release. To enhance release and CPC degradation, soluble poragens are incorporated into the CPC to more rapidly dissolve and enhance CPC porosity and dissolution, and drug release.[16]
Degradable poly(lactic-co-glycolic acid) (PLGA) particles have been incorporated into cement to deliberately increase porosity and interconnectivity upon their degradation, facilitating further active and passive in vivo degradation of cement and enhancing bone in-growth at surgical sites.[8, 17] Resultant changes of CPC handling properties and cement setting are maintained within desired workable ranges.[8] This provides an opportunity to load both the CPC matrix and encapsulated PLGA particles with drug loads for controlled release strategies from the injectable hybrid biomaterial. PLGA is a well-studied degradable polymer for particle-based formulations: FDA-approved PLGA microsphere drug delivery products include Lupron Depot® (Abbott Laboratories), Trelstar® Depot (Watson Pharmaceuticals), Risperdal® ConstaTM (Ortho-McNeil-Janssen Pharmaceuticals) and Bydureon™ (Amylin Pharmaceuticals). Bioactive molecule microencapsulation for use in both animals and humans and versatile capacity for sustaining long-term controlled drug release in these therapeutic systems is well-established.[18, 19] As a dual-use drug delivery medium, PLGA particles have also been tested in combination with CPC to deliver protein bone growth factors (rhBMP-2) while also acting as a poragen.[20]
After a typical device implantation, large numbers of differentiating osteoclast precursors, monocyte macrophages, are recruited to the peri-implant area, especially at the bone-implant interface.[21, 22] In osteoporosis and osteopenias, enhanced bone resorption and osteoclastogenic activities often correlates with reduced bone-implant interface area and results in aseptic loosening and finally prosthetic failure. Specifically suppressing osteoclastic bone resorption activity in peri-implant area could improve initial implant stabilization. Significantly, smaller PLGA microparticles (i.e., <10 μm diameter) are selectively scavenged by host phagocytic cells, providing a particle size-selective cell-specific drug targeting and dosing mechanism.[23] Therefore, specific osteoclast and phagocyte cell uptake can be achieved by controlling PLGA particle sizes to those internalized by professional phagocytes while avoiding other intrinsically low-phagocytically active cell types (i.e., fibroblasts, osteoblasts, progenitor cells) in the tissue bed.
Gene silencing using siRNA has many potential therapeutic applications due to several advantages intrinsic to RNA interference (RNAi).[24] siRNAs are well-known for their very short circulating half-lives in vivo,[25] and current challenges in systemic targeting from parenteral siRNA formulations.[26] These unresolved issues have produced the current emphasis on local delivery of therapeutic siRNA as a more direct clinical goal,[27] also in many possible bone targets.[28] Two cytokines -- RANK ligand (RANKL) and macrophage colony-stimulating factor (M-CSF) -- are essential for osteoclastogenesis.[29, 30] RANKL interactions with RANK receptor on the surface of osteoclast precursors are critical for expression of osteoclast-specific genes,[31] bone resorption and survival of mature osteoclasts.[32] Osteoclastogenesis is negatively regulated by osteoprotegerin (OPG), which is also expressed by stromal cells and osteoblasts.[33] OPG is a decoy receptor that competes with RANK for binding RANKL.[34] In this RANKL/RANK/OPG axis, positive regulator RANKL and negative regulator OPG are coordinated through RANK interaction on osteoclast precursor cells in bone sites to regulate normal bone formation and degradation. We have shown recently that siRNA against RANK provides potent in vitro inhibition of osteoclast phenotypic functions and osteoclast-mediated bone resorption.[35] Here we describe a strategy to deliver RANK siRNA loaded in PLGA microspheres resident in an injectable CPC augmentation matrix for local delivery to low-density bone and specifically for suppressing bone-resident osteoclast formation and bone resorption activity in osteoporotic sites.
2. Materials and Methods
2.1 Reagents
PLGA (50:50 monomer ratio, molecular weight (MW 57 kDa) with lauryl ester end groups was purchased from Lakeshore Biomaterials (AL, USA). Branched polyethyleneimine (bPEI) (MW: 25kDa) and poly(vinyl alcohol) (PVA, average MW 30–70 kDa) were purchased from Sigma-Aldrich (USA). RNase-free water was prepared using diethyl pyrocarbonate (DEPC) (Sigma-Aldrich). RANK siRNA (sense 5′-GCGCAGACUUCACUCCAUAUU-3′, antisense 5′-UAU GGAGUGAAGUCUGCGCUU-3′) and RNase-free siRNA buffer were purchased from Dharmacon (CO, USA). DNA substitute for the siRNA molecule against RANK (siRNA analogue) (5′-GCGCAGACTTCACTCCATATT-3′, 5′-TATGGAGTGAAGTCTGCGCTT-3′) were purchased from Integrated DNA Technologies (IA, USA).
2.2 Primary murine cell harvesting and differentiation
C57BL/6 male mice (6–8-week-old, Jackson Labs, ME, USA) were maintained in a specific pathogen-free facility at the University of Utah. All procedures were performed as approved by the Institutional Animal Care and Use Committee, University of Utah. Bone marrow cells (BMC) were harvested from murine tibias and femurs of C57BL/6 male mice and differentiated into osteoclast precursors using previously described methods.[35] Briefly, BMCs were cultured in α-MEM (Gibco) containing 10% FBS and 1% penicillin-streptomycin (defined for all cell cultures as “complete media”) overnight at a density of 1×106 cells/ml. Non-adherent cells were harvested the next day and immediately seeded into 24-well tissue culture plates in complete media with 30 ng/ml M-CSF (R&D Systems) at a density of 1×106 cells per well. After 2 days of culture, attached cells were used as osteoclast precursors. To generate osteoclasts, precursor cells were incubated in 200ng/ml RANKL and 30ng/ml M-CSF (R&D Systems) in complete media (defined as “osteoclast-specific media”), refreshed every other day.45 RANKL was expressed using a previously described detailed procedure.[35]
2.3 Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was isolated using an RNeasy Mini Kit (Qiagen). Up to 4 μg of RNA was used to make cDNA with the SuperScript III 1st strand RT kit for PCR (Invitrogen). PCR primers were designed for RANK (5′-AGATGTGGTCTGCAGCTCTTCCAT-3′, 5′-ACACACTTCTTGCTGACTGGAGGT-3′) and cyclophilin B (housekeeping control, 5′-AGCGCTTCCCAGATGAGAACTTCA-3′, 5′-GCAATGGCAAAGGGTTTCTCCACT-3′) using Primerquest software and purchased from Integrated DNA Technologies (USA). PCR was performed with iTaq DNA polymerase in the iCycler Thermal cycler (Bio-Rad). PCR products were analyzed on ethidium bromide-stained TBE-based 2% agarose gels. The electrophoresis was run at 100 V for 30 min and the gel was visualized with Bio-Rad imaging system.
2.4 Preparation of siRNA/bPEI complexes and siRNA transfection in vitro
To prepare siRNA/bPEI complexes at various cation/anion (NP) charge ratios, 2 μl of 10 μM RANK siRNA aqueous solution was mixed with 2 μl of bPEI solutions of different concentrations that yield final mass ranging from 0.016 to 0.16 μg. The complex solutions were kept at room temperature for 20 min. Then, 4 μl of each complex solution was used for electrophoresis on ethidium bromide-stained TBE-based 2% agarose gels. The electrophoresis was run at 100 V for 20 min, followed by the visualization with UV light to assess the formation of siRNA-bPEI complexes. Cell transfections with siRNA/bPEI complexes at fixed NP ratios in complete media were performed subsequently. siRNA/bPEI complexes for each well were prepared by mixing 2.5 ul of 20 μM siRNA aqueous solution with 1.6, 1.2, 0.8 and 0 μl (NP 20, 15, 10 and 0) of 1 mg/ml bPEI, respectively, in a total volume of 20 μl with RNase-free water. After incubation at room temperature for 20 min, complete media was added to achieve the final volume of 1 ml, yielding a final amount of siRNA in each well of 0.67 μg. The dose was determined based on published results.[35, 36] siRNA transfections were always performed in complete media. After 24-h incubation at 37°C under 5% CO2, culture media was refreshed with 1 ml of complete media and transfected cells were further incubated. RNA was harvested 2 days later and RANK expression was analyzed by PCR.[35]
2.5 PLGA microparticle preparation
Microparticles of PLGA were prepared using a known double-emulsion solvent evaporation technique.[37] In brief, siRNA or siRNA analogue/bPEI complexes were prepared by mixing equal volumes of 33.5 μg of siRNA/siRNA analogue and 1 mg/ml of bPEI in RNase-free siRNA buffer. Complex-containing PLGA microspheres were prepared by dispersing the siRNA/bPEI aqueous solution into a solution of 50 mg PLGA dissolved in 2 ml methylene chloride with intense probe sonication (Biologics, Inc. Ultrasonic 3000, 20 s sonication with 10 s pause). Then, the water/oil dispersion was added into the 4% PVA solution while vortexing. The final w/o/w emulsion was mixed into 4 ml of 0.6% PVA solution and set for 3~4 hr at room temperature while stirring. The solidified microspheres were washed using RNase-free water three times followed by lyophilization.
2.6 PLGA particle characterization
2.6.1 Particle size distribution, zeta potential and surface morphology
Particle size distribution and zeta potential (surface charge) of PLGA microparticles with siRNA was determined using a Malvern Instruments Zetasizer Nano ZS (Westborough, USA) in 10 mM NaCl at pH 7.4. To further confirm measured particle sizes and surface morphology, scanning electron microscopy (SEM) was performed. PLGA microspheres were attached to a metal stub using conductive tape and coated with gold in a sputter coater. SEM images of coated samples were captured at 5 kV (Hitachi S2460N).
2.6.2 siRNA loading and encapsulation efficiency
Following a previously described procedure,[18] 3–5 mg PLGA particles were dissolved in DMSO. The siRNA was extracted by adding equal volumes of Tris-EDTA buffer (10 mM Tris-HCl containing 1 mM EDTA, pH 7.4). Aqueous layers were removed, and siRNA concentration was measured using the QuantlT™ PicoGreenTM assay (Invitrogen, USA). Encapsulation efficiency is calculated as the percent of the PLGA-extracted siRNA versus the initial feed siRNA amount. The siRNA was then precipitated from the aqueous solution by adding 0.3 M sodium acetate and 70% (v/v) ethanol. After 30 min incubation at −20°C, the solution was centrifuged at 12,000 rpm at 4°C for 30 min. The pellet was dried and analyzed on a 2% agarose gel.
2.6.3 Evaluation of in vitro siRNA controlled release
siRNA analogue loaded PLGA particles (1 mg) were suspended in 1 ml RNase-free siRNA buffer (pH 7.4), and kept at 37°C under constant gentle shaking for four weeks. To collect the supernatant, the suspension was centrifuged at 12,000 rpm for 15 min, and supernatant was removed for analysis followed by the replacement with the same volume of fresh buffer. The siRNA content in the supernatant was measured using the QuantlT™ PicoGreenTM assay.
2.6.4 Cytotoxicity assay
Murine BMCs were cultured in osteoclast-specific media with 1 mg/ml PLGA particles loaded with either RANK siRNA or the siRNA analogue negative control. After 3-day incubation in 37°C, cytotoxicity was measured using CellTiter-Blue Cell Viability Assay kit (Promega, USA).
2.6.5 Particle in vitro cell-specific uptake and intracellular degradation studies
Murine BMCs were cultured in complete media at 1×106 cells/ml for 24 hr and then incubated with 1 mg/ml PLGA particles for 3 days in this media. After three washes with PBS, live cell images were subjected to microscopic observation. Fluorescent dye-labeled PLGA particles were prepared by adding 0.5% (weight percentage vs. PLGA) 6-coumarin dye to the methylene chloride phase used in the PLGA microparticle fabrication, and microparticles were fabricated as described (vida supra).[38] Osteoclast precursor cells were seeded at 1×106 cells/well and incubated with 1 mg/ml fluorescently labeled PLGA particles in a 24-well plate in complete media containing M-CSF and RANKL at 37°C. Particle intracellular degradation studies were performed using fluorescence microscopy. Culture media was refreshed every other day. Transition of punctate into diffuse fluorescence indicates particle degradation within intracellular bodies.
2.6.6 Particle release of RANK siRNA and RANK knockdown efficiency in cell culture
Murine BMCs were incubated with PLGA particles in 24-well plates in osteoclast-specific media. The dose of siRNA per well (125 nM) was determined based on previous published results.[35] RANK siRNA-loaded PLGA particle-treated groups were compared with control groups treated with blank PLGA particles without siRNA, and also with siRNA analogue-loaded PLGA particle-treated groups. PLGA particles were diluted in 1 ml osteoclast-specific media and added to osteoclast cell cultures. Total RNA was isolated 4 days post-transfection using an RNeasy Mini Kit (Qiagen). PCR products were analyzed on ethidium bromide-stained TBE-based 2% agarose gels and visualized under UV light.
2.7 Loading of siRNA-containing PLGA particles into CPC
A commercially available clinical grade injectable CPC, a calcium sulfate hardening paste (Bone Plast, BP070) was gift from BioMet Osteobiologics (NJ, USA). The product is moldable to fit any structure and can be resorbed in 6–8 weeks by osteoclastic activity and chemical dissolution. Pure CPC cement was prepared according to the manufacturer’s instructions and used as the control. PLGA microparticles were loaded into dry CPC powder at 10% (w/w). This mixture was added into sterile 5-ml syringes (Becton Dickinson, NJ) filled with aqueous solution provided in the kit to achieve the liquid/powder ratio of 0.35 ml/g.[8] Subsequently, thorough mixing required manual stirring within the syringe barrel for 60 s. The resulting CPC suspension containing PLGA microparticles was then injected into a clean rubber mold (circular cylinder shape: 6 mm diameter, 6 mm height) to cure CPC samples in this shape.
2.8 Characterization of the PLGA-modified CPC cement
2.8.1 Cement Porosity
The total porosity of PLGA microparticle-loaded CPC was determined by heating CPC specimens at 650°C for 2 hr to pyrolyze the PLGA particles from the sample. CPC sample weight was measured before and after pyrolysis. Total porosity and macroporosity was calculated from the following equations[39]:
| [1] |
| [2] |
Equation [1] calculates macropores produced by PLGA particle voids. Equation [2] yields the sample total porosity, including micropores, representing the porosity of the pure CPC sample. mburnt is the weight of samples after PLGA removal by heating, v (cm3) is the volume of the sample, mpureCPC is the weight of pure CPC sample, and ρHAP (g/cm3) is the density of hydroxyapatite (3.156 g/cm3).[40]
2.8.2 CPC mechanical testing
CPC samples were vacuum-dried overnight. Compression strength was measured using an Instron (Instron TT-CM; Instron, Canton, USA). Samples were placed in the mechanical testing bench and compressive strength was measured at a crosshead speed of 0.5 mm/min. Mechanical stiffness was determined and compared between CPC alone and 10:90 PLGA/CPC samples.
2.8.3 CPC composite matrix morphology
Cross-sections of CPC samples (vacuum-dried overnight) were visualized for porosity and morphology by SEM.
2.9 RANK knockdown efficacy by siRNA released from modified cement in vitro
In order to fabricate CPC composite wafers to deploy into 24-well plate cell cultures for siRNA release and bioactivity assays in cell culture, 1 mg of PLGA microparticles carrying 0.67 μg of siRNA were mixed thoroughly with 9 mg CPC powder (10:90) on a sterilized plastic weighing plate, and dissolved in aqueous solution to reach the same liquid/powder ratio of 0.35 ml/g as used for mechanical samples. A flat medicine spoon was used to mold the CPC mixture into a flat disc. After curing the CPC into a hard wafer, a razor blade was used to cut and remove pieces of the CPC wafer to fit into wells of 24-well culture plate. Murine bone marrow cells were then seeded directly onto these CPC wafers at 1×106 per well in the wells. Cells were then incubated with 30 ng/ml M-CSF in complete media for the first two days and followed by incubation with osteoclast-specific media. Cell RNA was harvested 8 days post-treatment and subject to PCR. PCR products were analyzed on ethidium bromide-stained TBE-based 2% agarose gels run at 100 V for 30 min and visualized with UV light.
2.10 Cell imaging
Fluorescent images of adherent cells in culture were captured using a Coolsnap color CCD camera attached to a Nikon E600 microscope using Image Pro Plus 4.0 software. All images were captured using the same exposure time period and corrected for unequal field illumination.
2.11 Statistical analysis
Analysis of variance (ANOVA) followed by two-tailed student’s t-test was used for statistical analyses. All experiments were repeated three times with multiple replicates within each run. Results were considered statistically significant if p < 0.05.
3. Results
3.1 Optimization of RANK siRNA/bPEI complexes
To determine the NP threshold for stable siRNA polyplex formation, different amounts of bPEI were mixed with 0.14 nmol RANK siRNA at NP ratios of 0, 0.5, 1, 2, and 5. Figure 1A shows the migration of siRNA/bPEI complexes by gel electrophoresis. With NP ratios of 0, 0.5 and 1, siRNA bands migrate on the gel, indicating uncomplexed, excess siRNA. When the NP ratio was 2, the free siRNA band density was substantially weaker. When the NP ratio was 5, no siRNA migrates freely in the gel, indicating that all siRNA molecules are entrapped in bPEI complexes through electrostatic interactions. Knowing that an NP value of 5 was the threshold, RANK expression was assayed after cells were incubated with complexes with NP ratios of 10, 15 and 20 for 2 days. RANK gene expression started to decrease when the NP ratio was 15, and significant down-regulation was shown when the NP was 20 (Figure 1B). Therefore, an NP ratio of 20 was used for the following delivery experiments.
Figure 1. siRNA polyplex preparation.
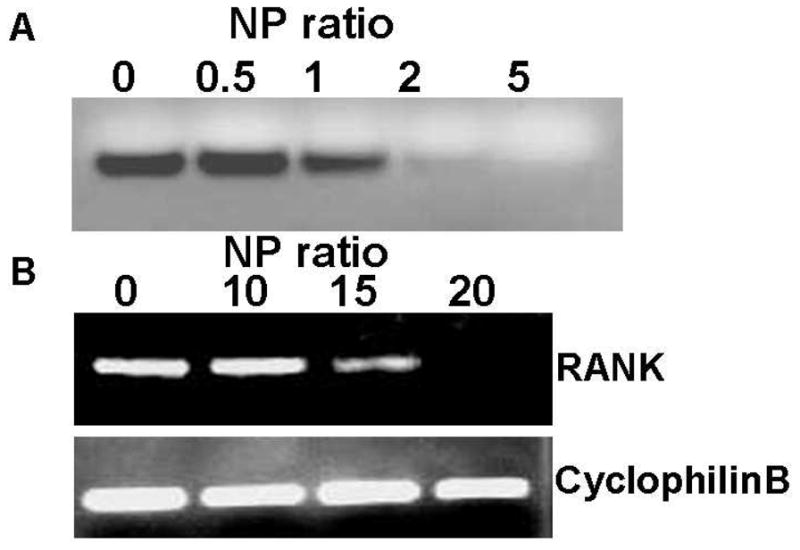
(A) Gel migration of RANK siRNA/bPEI complexes at different NP ratios (0–5). (B) RANK expression in BMC cultures 3 days post-BMC treatment with RANK siRNA/bPEI complexes at different NP ratios (0–20) in serum-based cell culture media.
3.2 Characterization of PLGA microspheres
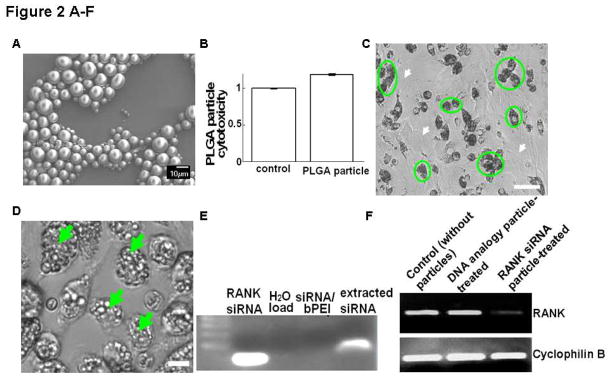
PLGA SEM images (Figure 2A) revealed that microspheres displayed the expected spherical morphology with an average size of 5.45 ± 0.88 μm as determined from light scattering particle size analysis. The zeta potential of PLGA microparticles was −21.07 ± 1.42 mV under these conditions. The siRNA encapsulation efficiency in these PLGA particles using the double-emulsion method is 80.75 ± 4.1% (n=3).
Figure 2. Physicochemical and functional properties of siRNA encapsulated in PLGA microparticles.
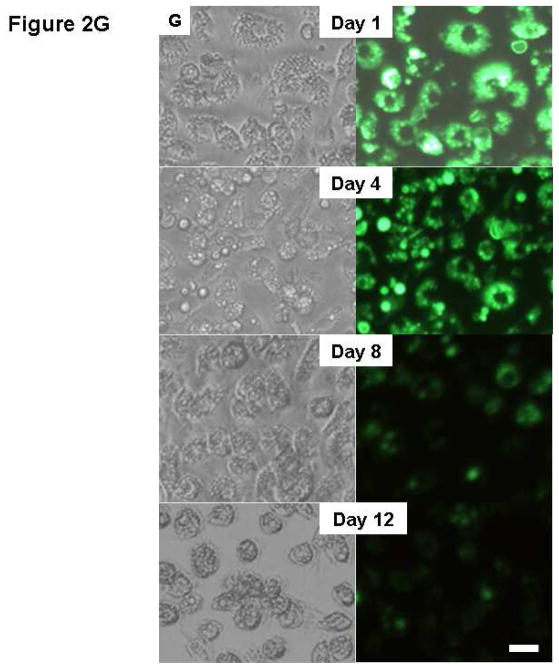
(A) SEM image of PLGA particles. (B) Murine BMC cell cytotoxicity of RANK siRNA/bPEI complexes incorporated in PLGA particles in serum-based cultures after 3 days. (C) a, In vitro cell-specific uptake 3 day-incubation of BMCs with PLGA microparticles. (circles: monocytes; white arrows: non-phagocytes, i.e. fibroblasts). Bar=50 μm; (D) in vitro cell uptake of PLGA microparticles 3 days post-incubation with murine primary monocytes (arrows: internalized particles). Bar=10 μm; (E) Gel electrophoresis comparison of RANK siRNA extracted from PLGA particles in cells versus control stock siRNA. (F) Significant down-regulation of RANK in murine bone marrow cell (BMC) cultures by siRNA-loaded PLGA particles. RANK siRNA analogue-loaded PLGA particles served as negative control. (G) Degradation of fluorescently labeled PLGA particles in BMC cultures over time (left, bright field; right, fluorescent images). Bar=25 μm.
3.3 RANK knockdown effects, cytotoxicity and intracellular degradation of PLGA microspheres
Cytotoxicity from PLGA microparticle exposure to cells in culture was determined: no significant cell toxicity differences were observed between controls without treatment and cells treated with particles (Figure 2B). After three days of incubation with the BMC pool, PLGA particles were observed in the cytoplasm of phagocytic cells, primarily monocyte macrophages (Figure 2C), very comparable to phagocytosis results for purified primary monocyte macrophages incubated with PLGA particles (Figure 2D). Gel electrophoresis showed that the charge and molecular weight of RANK siRNA extracted from PLGA particles was similar to the control stock siRNA (Figure 2E). RNA was harvested from murine-derived primary bone marrow cells incubated with microparticles for 4 days and RANK expression was analyzed. Cells incubated with RANK siRNA-loaded PLGA particles showed significant down-regulation of RANK expression, compared with controls without microparticle incubation and cells treated with microparticles loaded with siRNA analogues as controls (Figure 2F). After 12 days in culture, the majority of microspheres disappeared and the enclosed fluorescence marker was distributed evenly throughout the cell volume, indicating release by particle degradation (Figure 2G).
3.4 In vitro release of siRNA analogues of RANK siRNA from PLGA microparticles
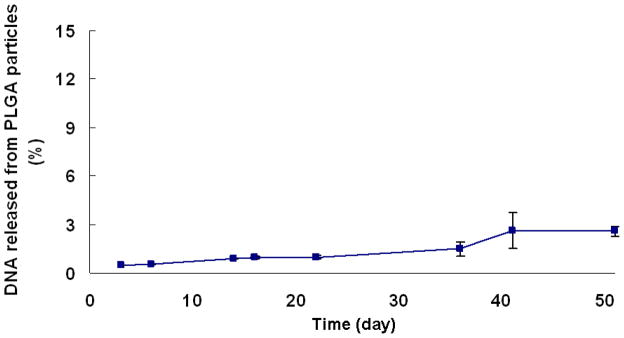
Murine RANK siRNA analogue-loaded (i.e., oligoDNA) PLGA microparticles were used to evaluate the in vitro release profile of siRNA from microparticles due to similar physical chemistry and structure, higher stability and lower cost of oligoDNA compared to siRNA. Little or no DNA release was observed from PLGA microparticles over 50 days in culture media alone (avg. ± SD, n=3, Figure 3).
Figure 3. Release kinetics of RANK siRNA analogue (oligoDNA) from PLGA microparticles.
One mg PLGA particles were incubated in 1ml RNase-free siRNA buffer (Dharmacon) (n=3, error bars represent SD).
3.5 Characterization of PLGA microparticle/CPC composite matrix
Table 1 shows calculated results for microparticle porosity and macroporosity (avg. ± SE, n=3). Pure CPC samples had a porosity of 55%, while the PLGA-loaded CPC samples (10% w/w particle load) displayed a higher porosity of 63%. The macroporous fraction introduced by PLGA particles was 35%.
Table 1.
Summary results of the CPC matrix porosity measurements
| Average mass (g) | Density (g/cm5) | porosity (%) | Macroporosity (%) | |
|---|---|---|---|---|
| Microporous cement | 0.2522 ± 0.0045 | 1.770 ± 0.016 | 55.02 ± 0.42 | ---- |
| 10:90 PLGA/CPC | 0.2241 ±0.0066 | 1.572±0.023 | 63.68±0.56 | 35.23±0.99 |
3.6 CPC morphology and mechanical characteristics
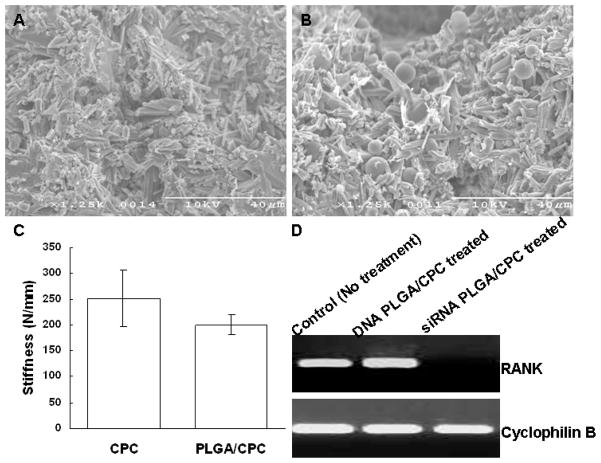
Figures 4A and 4B show SEM images of the cross-sections of pure CPC and CPC with PLGA particles, respectively, within the cured 10:90 PLGA/CPC matrix. PLGA microparticles were distributed homogeneously within the CPC matrix (Figure 4B). CPC matrix stiffness is expressed as maximum compressive load (N) versus maximum compressive extension (mm). Control samples (CPC alone) showed a stiffness of 251.8±27.14 N/mm (avg. ±SD). The 10% PLGA-incorporated CPC samples had an average value of 200.5±9.57 N/mm (avg. ±SD). No significant difference in stiffness was observed between the CPC alone and the 10:90 CPC samples (Figure 4C).
Figure 4. Characteristics of CPC matrices: morphology, mechanical and functional properties.
(A) SEM of cross-section of pure cured CPC, (B) SEM of cross-section of PLGA/CPC composite, (C) Comparison of mechanical stiffness between CPC alone and 10:90 PLGA/CPC samples. (D) Significantly reduced RANK expression in murine BMC cultures by treatment with RANK siRNA-PLGA particle-loaded CPC matrices for 8 days. RANK siRNA analogue-loaded PLGA/CPC matrices served as negative control with no observable effects in cultures.
3.7 Down-regulation of RANK gene expression by RANK siRNA-loaded PLGA/CPC composite microparticles
Murine BMC RNA was harvested 8 days after these cells were seeded on CPC wafers. PCR results shows significant suppression of RANK expression in cells incubated with RANK siRNA-loaded PLGA/CPC compared with control without particle treatments and siRNA analogue-loaded PLGA/CPC matrices (Figure 4D).
4. Discussion
Increasing incidence of osteoporotic fragility fractures and their prolonged healing requirements post-fixation constitute major clinical challenges that require improved clinical solutions for better osteoporosis patient outcomes. Though bisphosphonate drugs show some efficacy against generalized osteoporosis, their severe suppression of bone turnover largely inhibits fracture healing.[41, 42] Currently, no therapy has yet produced significant improvements in osteoporotic bone augmentation for improved fragility fracture healing. RNAi is a new promising drug class with unique mechanisms to selectively target pathological pathways. Yet, effective siRNA delivery to target tissues is difficult, mandating primarily local or topical routing to produce therapeutic efficacy to date. Importantly, while siRNA has been targeted to bone systemically,[43] siRNA has not yet been studied to ameliorate osteoclast-mediated bone loss in the context of fragility fractures and fixation tooling. This opportunity was explored in this study with in vitro knockdown of RANK in murine BMCs by siRNA using PLGA particle-loaded injectable bone augmentation biomaterials (CPC) clinically applied in osteoporosis as delivery matrices.
As RANK is recognized to play an essential role in regulating osteoclastogenesis, it is a natural target for siRNA in the context of osteoporosis therapy.[44] Activation of RANK by its ligand, RANKL, is required for the formation and activation of osteoclasts.[45, 46] Our previous RANK siRNA delivery methods [35] show specific siRNA silencing of RANK in both macrophage-like secondary cell line cultures (RAW264.7) and primary murine BMC cultures. Significantly, RANK siRNA also inhibited both osteoclast formation and bone resorption in vitro. These data collectively support proof-of-concept to deliver therapeutic RANK siRNA to osteoclasts locally to inhibit their metabolic activity in bone without necrosis.[35] Therefore, this current study further developed this concept for locally delivered siRNA targeting RANK from injectable bone cement, assessing in vitro siRNA delivery and RANK knockdown efficacy from PLGA particles within CPC bone augmentation materials to target osteoclasts and osteoclast precursors and suppress osteoclast-mediated excessive bone resorption.
To increase the stability and loading efficiency of siRNA in PLGA microparticles, RANK siRNA was complexed with bPEI at different NP ratios (Figure 1) because of bPEI’s known utility as a non-viral nucleic acid delivery vector.[47–49] The siRNA/bPEI migration assay demonstrates that siRNA forms stable complexes with bPEI when the NP ratio ≥ 5. Transfection of RANK siRNA in mouse BMCs in serum-based cultures with these polyplexes was monitored by PCR two days post-transfection. Compared to controls without treatment, RANK gene expression in BMC cultures showed no significant gene expression reduction when the NP ratio was 10, but began to decrease when the NP ratio equaled 15. Significant RANK gene silencing was achieved when the NP ratio reached 20, indicating that siRNA specifically down-regulates RANK message RNA expression in BMCs. Therefore, a NP ratio of 20 was used for subsequent experiments. RANK knockdown effect was comparable to use of commercial cationic DharmaFECT 4 transfection reagent in our previous published results.[35]
For maximum particle loading and protection of siRNA payloads from nucleases, 4% (w/v) PVA was used for PLGA microparticle fabrication.[37, 50] Slight modifications to the emulsion fabrication process, including the time and power of sonication, facilitated microparticle size control to within the desired range of 1~10 μm needed to selectively target particle uptake exclusively by professional bone phagocytes.[23] PEI has been proven previously to increase oligoDNA encapsulation in PLGA particles at least 2-fold relative to that without PEI, especially for pre-formed PEI-oligoDNA polyplexes, and to increase cellular accumulation of nanoparticles.[51] For this study, the use of bPEI improves the siRNA PLGA particle encapsulation efficiency to 81%, comparable with previous published encapsulation efficiency of pre-formed siRNA-PEI in PLGA particles.[51] Cytotoxicity from siRNA-loaded PLGA particles was analyzed by culturing BMCs with PLGA particles in serum-containing complete media at minimum effective doses determined previously.[35] No significant cytotoxicity was observed (Figure 2). Electrophoresis results showed that the extracted siRNA from these particles maintained a similar charge, migration and molecular weight as the uncomplexed siRNA. Consistent with literature precedent,[23] microparticles were readily phagocytosed by monocyte macrophages compared to non-phagocytic cell cultures (Figure 2C), establishing this particle size-dependent local targeting mode at peri-implant sites known to attract bone phagocytes. Gradual intracellular particle degradation was observed using fluorescence imaging. Complete microparticle degradation was achieved in 12 days of incubation in cell cultures (Figure 2).
In vitro release profiles of DNA from PLGA microparticles have been shown to be dependent on the molecular mass of the PLGA; increasing PLGA molecular weight reduced DNA release rate.[52] Little or no release of DNA from PLGA particles was shown from PLGA microspheres where the PLGA molecular weight was greater than 30kDa.[53] OligoDNA release results from PEI polyplexes in PLGA microparticles are consistent with previous results (Figure 3). Only 3% of loaded oligoDNA, as a stable siRNA analogue for this study, was released from the PLGA particles of molecular weight 57 kDa over 7 weeks in buffer. High molecular weight PLGA particles protect encapsulated DNA and maintain the particle integrity until particles are released from CPC to target cell candidates by CPC degradation. Clear differences are observed between particle degradation-release of nucleic acid payloads within cells (i.e., 12 days, Figure 2G) versus that to water (negligible release after 50 days to water, Figure 3) possibly facilitated by lysosomal degradation. siRNA analogue/bPEI polyplex-loaded PLGA particles possess similar polyplex charge as RANK siRNA and the same particle surface charge when loaded into PLGA particles (Table 1). However, as expected, the actual siRNA/bPEI PLGA particles effectively reduced RANK expression in BMC cultures, compared with control siRNA analogue/bPEI-loaded PLGA particles, indicating that decreased RANK expression was due to specific siRNA taken-up by phagocytes and subsequent gene sequence-specific RANK message knockdown. The retention of physicochemical and functional integrity of RANK siRNA during the encapsulation also suggests that the polyplex polymer effectively protects siRNA during particle fabrication. Taken together, these results support the utility of siRNA/bPEI-loaded PLGA microparticles for specific suppression of RANK expression in osteoclast precursors in serum-based cultures of NP ratios of 20.
Studies have shown that cement scaffold microporosity largely improved bone in-growth by enhancing protein absorption to this enlarged surface area, promoting increased osteoblast attachment and improving microenvironmental cement solubility.[54] This concept is extended to PLGA microparticles encapsulating RANK siRNA within CPC as an injectable composite structural and drug delivery material. The design mandates particle-selective delivery to professional host-resident phagocytes responsible for bone loss in osteoporosis, and application of the CPC matrix as a local bone augmentation material adjunct to osteoporotic fixation. Addition of 20% (w/w) PLGA particles is the maximum loading to maintain composite cement injectability and retain all CPC physical parameters within surgically workable ranges,[17, 20, 55, 56], but properties of CPC containing 10% PLGA microspheres are closer to CPC alone.[8] Therefore, 10% (w/w) PLGA particle/CPC composite formulations were selected for siRNA delivery in this study. After cement curing and setting, the PLGA/CPC formulation formed a rigid scaffold with homogeneously distributed PLGA microspheres. Addition of these PLGA microparticles significantly increased the porosity of the cement (see Table 1), implying the improvement of interconnectivity within the cement. Significantly, as long as pore interconnectivity is obtained, PLGA particle release will not be dependent on CPC degradation or dissolution kinetics, yielding a sustained release system with some limited versatility based on particle loading. In addition, such interconnectivity also can accelerate overall aqueous phase access, penetration and degradation of CPC. Matrix mechanical properties could also be compromised with higher PLGA particle incorporation. Based on mechanical testing results from the 10% formulations of PLGA-loaded CPC, no significant differences were observed in stiffness between CPC alone and PLGA/CPC composites at their as-cured initial stage (See Figure 4C).
For cell cultures, thin, flat CPC composite wafers were used to accelerate PLGA particle release to produce an experimentally accessible cell culture period. Successful knockdown of the RANK gene was evident in osteoclast precursor phagocytic cells with RANK siRNA/PEI PLGA particle-incorporated CPC. This proves the capability of this bone targeting delivery system to enter phagocytes and alter specific target message. Unchanged RANK expression observed from siRNA analogue/bPEI PLGA-incorporated CPC and blank CPC control cell cultures supports the desired specificity of knockdown by released siRNA.
We have previously shown specific siRNA-induced suppression of RANK expression resulted in significant inhibition of both osteoclast formation and also model bone resorption in both secondary RAW264.7 and primary cultures in vitro.[35] With this correlation, RANK expression was used here as the evaluation indicator for siRNA uptake and resulting phagocyte inhibition. PLGA particle release must start with the particles exposed on the CPC matrix surface and the newly generated CPC pores then increase interconnectivity over time to facilitate further particle release and make the cement more porous and susceptible for further degradation. In vitro studies have shown that the overall medium pH for 10:90 PLGA/CPC samples decreased to pH 4.4 after 12 weeks’ incubation, ostensibly from PLGA acid by-product production. Significant pH reductions began after week 4.[16] However, this in vitro release profile will be very different from in vivo kinetics, due to body fluid transport, bone mechanical stress influences, and individual pathological conditions. Local bone tissue systems could be buffered sufficiently, depending on the deployed CPC volume and the CPC-PLGA intrinsic weak acid-base interactions.
Future studies will exploit local siRNA in vivo delivery to osteoporotic bone models from bone augmentation devices and injected liquid depots. This strategy seeks to provide a clinically relevant approach to improve osteoporotic fragility fracture healing using current clinical bone augmentation strategies, while also remaining amenable to local siRNA therapeutic drug delivery directly from bone defects and common fracture fixation hardware. Many RNA targets are possible with this approach.[28]
5. Conclusions
This study supports the dual concept of applying the unique combination of injectable bone-augmenting CPC and local siRNA cell-targeted PLGA particle delivery with siRNA cell uptake targeted to bone phagocytes from CPC matrices injected into bone augmentation sites. We show that siRNA encapsulated within PLGA microspheres does not significantly impair its functional integrity in target message knockdown in bone phagocyte cultures. As a result, targeted phagocytic cell-specific delivery can be achieved by controlling PLGA particle sizes within the micron range. RANK expression has been specifically knocked down in osteoclasts and their precursor phagocytic cells when delivered in several ways to cell cultures, resulting in inhibited bone resorption.[35] Phagocytosis-specific cell delivery of siRNA against RANK using particles selectively targets osteoclastic phenotypes. This is important in the context of treating bone augmentation zones where osteoporosis has produced low bone density and facilitates CPC augmentation injection. In addition, resulting PLGA particle release and implant degradation produces CPC macropores known to facilitate further particle release, and promote bone in-growth, simultaneously with RANK siRNA release. This strategy is intended to both suppress bone resorption in peri-implant areas needed to facilitate healing and enhance mechanical stabilization processes for fixation of fragility fractures using clinically common bone augmentation strategies for local siRNA delivery to bone.
Acknowledgments
The authors appreciate the generous gift of the RANKL plasmid expression construct from Professor M. F. Manolson, University of Toronto, Canada and donated CPC samples from BioMet. We thank I. Zharov, University of Utah, for access to a ceramic oven, and J. Wolchok for his assistance with mechanical testing. This work was partially supported by NIH grant EB00894 and a SEED grant from the University of Utah.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Yuwei Wang, Email: Yuwei.wang@utah.edu.
Kenny K. Tran, Email: kynam@u.washington.edu.
Hong Shen, Email: hs24@u.washington.edu.
David Grainger, Email: david.grainger@utah.edu.
References
- 1.Dong L, Huang Z, Cai X, Xiang J, Zhu YA, Wang R, et al. Localized delivery of antisense oligonucleotides by cationic hydrogel suppresses TNF-alpha expression and endotoxin-induced osteolysis. Pharm Res. 2011;28:1349–56. doi: 10.1007/s11095-010-0334-0. [DOI] [PubMed] [Google Scholar]
- 2.Tanzer M, Karabasz D, Krygier JJ, Cohen R, Bobyn JD. The Otto Aufranc Award: bone augmentation around and within porous implants by local bisphosphonate elution. Clin Orthop Relat Res. 2005;441:30–9. doi: 10.1097/01.blo.0000194728.62996.2d. [DOI] [PubMed] [Google Scholar]
- 3.von der Linden P, Gisep A, Boner V, Windolf M, Appelt A, Suhm N. Biomechanical evaluation of a new augmentation method for enhanced screw fixation in osteoporotic proximal femoral fractures. J Orthop Res. 2006;24:2230–7. doi: 10.1002/jor.20299. [DOI] [PubMed] [Google Scholar]
- 4.Cook SD, Salkeld SL, Patron LP, Barrack RL. The effect of demineralized bone matrix gel on bone ingrowth and fixation of porous implants. J Arthroplasty. 2002;17:402–8. doi: 10.1054/arth.2002.32169. [DOI] [PubMed] [Google Scholar]
- 5.Sumner DR, Turner TM, Purchio AF, Gombotz WR, Urban RM, Galante JO. Enhancement of bone ingrowth by transforming growth factor-beta. J Bone Joint Surg Am. 1995;77:1135–47. doi: 10.2106/00004623-199508000-00001. [DOI] [PubMed] [Google Scholar]
- 6.Tanzer M, Harvey E, Kay A, Morton P, Bobyn JD. Effect of noninvasive low intensity ultrasound on bone growth into porous-coated implants. J Orthop Res. 1996;14:901–6. doi: 10.1002/jor.1100140609. [DOI] [PubMed] [Google Scholar]
- 7.Larsson S. Treatment of osteoporotic fractures. Scand J Surg. 2002;91:140–6. doi: 10.1177/145749690209100202. [DOI] [PubMed] [Google Scholar]
- 8.Habraken WJEM, Wolke JGC, Mikos AG, Jansen JA. Injectable PLGA microsphere/calcium phosphate cements: physical properties and degradation characteristics. J Biomater Sci Polymer Edn. 2006;17:1057–74. doi: 10.1163/156856206778366004. [DOI] [PubMed] [Google Scholar]
- 9.Ginebra MP, Traykova T, Planell JA. Calcium phosphate cements: competitive drug carriers for the musculoskeletal system? Biomaterials. 2006;27:2171–7. doi: 10.1016/j.biomaterials.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 10.Wu F, Su J, Wei J, Guo H, Liu C. Injectable bioactive calcium-magnesium phosphate cement for bone regeneration. Biomed Mater. 2008;3:44105. doi: 10.1088/1748-6041/3/4/044105. [DOI] [PubMed] [Google Scholar]
- 11.Jarcho M. Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop Relat Res. 1981:259–78. [PubMed] [Google Scholar]
- 12.Takechi M, Miyamoto Y, Ishikawa K, Nagayama M, Kon M, Asaoka K, et al. Effects of added antibiotics on the basic properties of anti-washout-type fast-setting calcium phosphate cement. J Biomed Mater Res. 1998;39:308–16. doi: 10.1002/(sici)1097-4636(199802)39:2<308::aid-jbm19>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 13.Ratier A, Gibson IR, Best SM, Freche M, Lacout JL, Rodriguez F. Setting characteristics and mechanical behaviour of a calcium phosphate bone cement containing tetracycline. Biomaterials. 2001;22:897–901. doi: 10.1016/s0142-9612(00)00252-0. [DOI] [PubMed] [Google Scholar]
- 14.Otsuka M, Matsuda Y, Fox JL, Higuchi WI. A novel skeletal drug delivery system using self-setting calcium phosphate cement. 9: Effects of the mixing solution volume on anticancer drug release from homogeneous drug-loaded cement. J Pharm Sci. 1995;84:733–6. doi: 10.1002/jps.2600840614. [DOI] [PubMed] [Google Scholar]
- 15.Blom EJ, Klein-Nulend J, Klein CP, Kurashina K, van Waas MA, Burger EH. Transforming growth factor-beta1 incorporated during setting in calcium phosphate cement stimulates bone cell differentiation in vitro. J Biomed Mater Res. 2000;50:67–74. doi: 10.1002/(sici)1097-4636(200004)50:1<67::aid-jbm10>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 16.Habraken WJEM, WGGC, Mikos AG, Jansen JA. Injectable PLGA microsphere/calcium phosphate cements: physical properties and degradation characteristics. J Biomater Sci Polymer Edn. 2006;17:1057–74. doi: 10.1163/156856206778366004. [DOI] [PubMed] [Google Scholar]
- 17.Link DP, van den Dolder J, van den Beucken JJ, Cuijpers VM, Wolke JG, Mikos AG, et al. Evaluation of the biocompatibility of calcium phosphate cement/PLGA microparticle composites. J Biomed Mater Res A. 2008;87:760–9. doi: 10.1002/jbm.a.31831. [DOI] [PubMed] [Google Scholar]
- 18.Woodrow KA, Cu Y, Booth CJ, Saucier-Sawyer JK, Wood MJ, Saltzman WM. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat Mater. 2009;8:526–33. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Panyam J, Labhasetwar V. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliv Rev. 2003;55:329–47. doi: 10.1016/s0169-409x(02)00228-4. [DOI] [PubMed] [Google Scholar]
- 20.Ruhe PQ, Hedberg EL, Padron NT, Spauwen PH, Jansen JA, Mikos AG. rhBMP-2 release from injectable poly(DL-lactic-co-glycolic acid)/calcium-phosphate cement composites. J Bone Joint Surg Am. 2003;85-A(Suppl 3):75–81. doi: 10.2106/00004623-200300003-00013. [DOI] [PubMed] [Google Scholar]
- 21.Kadoya Y, al-Saffar N, Kobayashi A, Revell PA. The expression of osteoclast markers on foreign body giant cells. Bone Miner. 1994;27:85–96. doi: 10.1016/s0169-6009(08)80211-5. [DOI] [PubMed] [Google Scholar]
- 22.Sabokbar A, Fujikawa Y, Neale S, Murray DW, Athanasou NA. Human arthroplasty derived macrophages differentiate into osteoclastic bone resorbing cells. Ann Rheum Dis. 1997;56:414–20. doi: 10.1136/ard.56.7.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirota K, Hasegawa T, Hinata H, Ito F, Inagawa H, Kochi C, et al. Optimum conditions for efficient phagocytosis of rifampicin-loaded PLGA microspheres by alveolar macrophages. J Control Release. 2007;119:69–76. doi: 10.1016/j.jconrel.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Achenbach TV, Brunner B, Heermeier K. Oligonucleotide-based knockdown technologies: antisense versus RNA interference. Chembiochem. 2003;4:928–35. doi: 10.1002/cbic.200300708. [DOI] [PubMed] [Google Scholar]
- 25.Soutschek J, Akinc A, Bramlage B, Charisse K, Constien R, Donoghue M, et al. Therapeutic silencing of an endogenous gene by systemic administration of modified siRNAs. Nature. 2004;432:173–8. doi: 10.1038/nature03121. [DOI] [PubMed] [Google Scholar]
- 26.Bumcrot D, Manoharan M, Koteliansky V, Sah DW. RNAi therapeutics: a potential new class of pharmaceutical drugs. Nat Chem Biol. 2006;2:711–9. doi: 10.1038/nchembio839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cejka D, Losert D, Wacheck V. Short interfering RNA (siRNA): tool or therapeutic? Clin Sci (Lond) 2006;110:47–58. doi: 10.1042/CS20050162. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Grainger DW. RNA therapeutics targeting osteoclast-mediated excessive bone resorption. Adv Drug Deliv Rev. 2012 doi: 10.1016/j.addr.2011.09.002. http://www.ncbi.nlm.nih.gov/pubmed/21945356. [DOI] [PMC free article] [PubMed]
- 29.Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93:165–76. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- 31.Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13:2412–24. doi: 10.1101/gad.13.18.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lacey DL, Tan HL, Lu J, Kaufman S, Van G, Qiu W, et al. Osteoprotegerin ligand modulates murine osteoclast survival in vitro and in vivo. Am J Pathol. 2000;157:435–48. doi: 10.1016/S0002-9440(10)64556-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Luthy R, et al. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–19. doi: 10.1016/s0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 34.Yasuda H, Shima N, Nakagawa N, Mochizuki SI, Yano K, Fujise N, et al. Identity of osteoclastogenesis inhibitory factor (OCIF) and osteoprotegerin (OPG): a mechanism by which OPG/OCIF inhibits osteoclastogenesis in vitro. Endocrinology. 1998;139:1329–37. doi: 10.1210/endo.139.3.5837. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y, Grainger DW. siRNA knock-down of RANK signaling to control osteoclast-mediated bone resorption. Pharm Res. 2010;27:1273–84. doi: 10.1007/s11095-010-0099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y, Panasiuk A, Grainger DW. Small interfering RNA knocks down the molecular target of alendronate, farnesyl pyrophosphate synthase, in osteoclast and osteoblast cultures. Molecular Pharmaceutics. 2011;8:1016–24. doi: 10.1021/mp100374n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbas AO, Donovan MD, Salem AK. Formulating poly(lactide-co-glycolide) particles for plasmid DNA delivery. J Pharm Sci. 2008;97:2448–61. doi: 10.1002/jps.21215. [DOI] [PubMed] [Google Scholar]
- 38.Walter E, Dreher D, Kok M, Thiele L, Kiama SG, Gehr P, et al. Hydrophilic poly(DL-lactide-co-glycolide) microspheres for the delivery of DNA to human-derived macrophages and dendritic cells. J Control Release. 2001;76:149–68. doi: 10.1016/s0168-3659(01)00413-8. [DOI] [PubMed] [Google Scholar]
- 39.Habraken WJ, Zhang Z, Wolke JG, Grijpma DW, Mikos AG, Feijen J, et al. Introduction of enzymatically degradable poly(trimethylene carbonate) microspheres into an injectable calcium phosphate cement. Biomaterials. 2008;29:2464–76. doi: 10.1016/j.biomaterials.2008.02.012. [DOI] [PubMed] [Google Scholar]
- 40.Majling Ján, PZa, Palová Angela, Svetík Stefan. Sintering of the ultrahigh pressure densified hydroxyapatite monolithic xerogels. Journal of Materials Research. 1997;12:198–202. [Google Scholar]
- 41.Yang KH, Won JH, Yoon HK, Ryu JH, Choo KS, Kim JS. High concentrations of pamidronate in bone weaken the mechanical properties of intact femora in a rat model. Yonsei Med J. 2007;48:653–8. doi: 10.3349/ymj.2007.48.4.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sebba A. Osteoporosis: how long should we treat? Curr Opin Endocrinol Diabetes Obes. 2008;15:502–7. doi: 10.1097/MED.0b013e328317ca83. [DOI] [PubMed] [Google Scholar]
- 43.Zhang G, Guo B, Wu H, Tang T, Zhang BT, Zheng L, et al. A delivery system targeting bone formation surfaces to facilitate RNAi-based anabolic therapy. Nat Med. 2012;18:307–14. doi: 10.1038/nm.2617. [DOI] [PubMed] [Google Scholar]
- 44.Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Yano K, et al. RANK is the essential signaling receptor for osteoclast differentiation factor in osteoclastogenesis. Biochem Biophys Res Commun. 1998;253:395–400. doi: 10.1006/bbrc.1998.9788. [DOI] [PubMed] [Google Scholar]
- 45.Li J, Sarosi I, Yan XQ, Morony S, Capparelli C, Tan HL, et al. RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci U S A. 2000;97:1566–71. doi: 10.1073/pnas.97.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, et al. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–23. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 47.Choung S, Kim YJ, Kim S, Park HO, Choi YC. Chemical modification of siRNAs to improve serum stability without loss of efficacy. Biochem Biophys Res Commun. 2006;342:919–27. doi: 10.1016/j.bbrc.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 48.Wang DA, Narang AS, Kotb M, Gaber AO, Miller DD, Kim SW, et al. Novel branched poly(ethylenimine)-cholesterol water-soluble lipopolymers for gene delivery. Biomacromolecules. 2002;3:1197–207. doi: 10.1021/bm025563c. [DOI] [PubMed] [Google Scholar]
- 49.Jiang G, Park K, Kim J, Kim KS, Oh EJ, Kang H, et al. Hyaluronic acid-polyethyleneimine conjugate for target specific intracellular delivery of siRNA. Biopolymers. 2008;89:635–42. doi: 10.1002/bip.20978. [DOI] [PubMed] [Google Scholar]
- 50.Capan Y, Woo BH, Gebrekidan S, Ahmed S, DeLuca PP. Preparation and characterization of poly (D, L-lactide-co-glycolide) microspheres for controlled release of poly(L-lysine) complexed plasmid DNA. Pharm Res. 1999;16:509–13. doi: 10.1023/a:1018862827426. [DOI] [PubMed] [Google Scholar]
- 51.Patil Y, Panyam J. Polymeric nanoparticles for siRNA delivery and gene silencing. Int J Pharm. 2009;367:195–203. doi: 10.1016/j.ijpharm.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alonso MJ, Cohen S, Park TG, Gupta RK, Siber GR, Langer R. Determinants of release rate of tetanus vaccine from polyester microspheres. Pharm Res. 1993;10:945–53. doi: 10.1023/a:1018942118148. [DOI] [PubMed] [Google Scholar]
- 53.Wang D, Robinson DR, Kwon GS, Samuel J. Encapsulation of plasmid DNA in biodegradable poly(D, L-lactic-co-glycolic acid) microspheres as a novel approach for immunogene delivery. J Control Release. 1999;57:9–18. doi: 10.1016/s0168-3659(98)00099-6. [DOI] [PubMed] [Google Scholar]
- 54.Woodard JR, Hilldore AJ, Lan SK, Park CJ, Morgan AW, Eurell JA, et al. The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity. Biomaterials. 2007;28:45–54. doi: 10.1016/j.biomaterials.2006.08.021. [DOI] [PubMed] [Google Scholar]
- 55.Link DP, van den Dolder J, Jurgens WJ, Wolke JG, Jansen JA. Mechanical evaluation of implanted calcium phosphate cement incorporated with PLGA microparticles. Biomaterials. 2006;27:4941–7. doi: 10.1016/j.biomaterials.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 56.Bohner M, Baroud G. Injectability of calcium phosphate pastes. Biomaterials. 2005;26:1553–63. doi: 10.1016/j.biomaterials.2004.05.010. [DOI] [PubMed] [Google Scholar]