Abstract
PPT1-related neuronal ceroid lipofuscinosis (NCL) is a lysosomal storage disorder caused by deficiency in a soluble lysosomal enzyme, palmitoyl-protein thioesterase-1 (PPT1). Enzyme replacement therapy (ERT) has not been previously examined in a preclinical animal model. Homozygous PPT1 knockout mice reproduce the known features of the disease, developing signs of motor dysfunction at 5 months of age and death by around 8 months. In the current study, PPT1 knockout mice were treated with purified recombinant PPT1 (0.3 mg, corresponding to 12 mg/kg or 180 U/kg for a 25 g mouse) administered intravenously weekly either 1) from birth; or 2) beginning at 8 weeks of age. The treatment was surprisingly well tolerated and neither anaphylaxis nor antibody formation was observed. In mice treated from birth, survival increased from 236 to 271 days (p<0.001) and the onset of motor deterioration was similarly delayed. In mice treated beginning at 8 weeks, no increases in survival or motor performance were seen. An improvement in neuropathology in the thalamus was seen at 3 months in mice treated from birth, and although this improvement persisted it was attenuated by 7 months. Outside the central nervous system, substantial clearance of autofluorescent storage material in many tissues was observed. Macrophages in spleen, liver and intestine were especially markedly improved, as were acinar cells of the pancreas and tubular cells of the kidney. These findings suggest that ERT may be an option for addressing visceral storage as part of a comprehensive approach to PPT1-related NCL, but more effective delivery methods to target the brain are needed.
Keywords: enzyme replacement therapy, lysosomal storage disorder, Batten disease, infantile neuronal ceroid lipofuscinosis
1. INTRODUCTION
Deficiency in the enzyme palmitoyl protein thioesterase-1 (PPT1; EC 3.1.2.22) causes the human lysosomal storage disorder ceroid lipofuscinosis, neuronal-1 (CLN1), which is characterized by progressive blindness, cognitive and motor deterioration, and seizures. These eventually lead to a chronic vegetative state [1]. The enzyme is a small, globular hydrolase of the α/β type that removes fatty acids from cysteine residues in lipid-modified proteins during lysosomal protein degradation [2]. Over 60 mutations in the PPT1 gene in NCL patients have been reported [3]. While most patients come to medical attention around age 12–18 months, onset later in childhood and adulthood can occur, with the age of onset reflecting the level of residual enzyme activity [4–6]. In adults affected by the disorder, psychiatric symptoms precede the diagnosis and may be accompanied by cardiac signs and symptoms [5, 7–8]. While neurodegeneration dominates the clinical picture, storage material is found throughout most tissues, where it appears by light microscopy as a yellow-brown pigment (lipofuscin) that autofluoresces under ultraviolet illumination [9]. Striking accumulations in pancreas, thyroid, distal convoluted tubules and glomerular podocytes of the kidney, and macrophages in a variety of tissues, including the liver and spleen have been reported [10–11]. Because PPT1 is targeted to the lysosome via the canonical mannose 6-phosphate receptor pathway, enzyme replacement therapy (ERT) using exogenously administered enzyme can be envisioned as a treatment for the disorder, but has not previously been attempted in a preclinical model.
The PPT1 knockout mouse demonstrates the major features associated with the human disease, including autofluorescent storage and manifestations such as seizures, decline in motor performance and reduced lifespan [12–13]. The mice live about 235 days in the absence of treatment [12, 14]. The neuropathology [12–13, 15–20] and (to a lesser extent) the distribution of visceral storage [12–13] have been well described.
The aim of the current study was to determine the effect of high dose intravenous human recombinant PPT1 on motor performance, survival, and autofluorescent storage material in the brain and viscera of PPT1 knockout mice. Treatment was begun either from birth (post-natal day 0–1) or from 8 weeks of age, when mice are fully mature but exhibit no obvious signs of the disorder. We found that treatment from birth caused modest but statistically significant improvements in motor performance, survival, and brain pathology and marked improvements in visceral storage, whereas treatment beginning at 8 weeks reduces visceral storage only. The treatment was remarkably well tolerated and no anaphylaxis or antibody formation was detected.
2. MATERIALS AND METHODS
2.1 Human recombinant PPT1
Human recombinant PPT1 was prepared from an overproducing CHO clone as described [21]. The enzyme was stored in aliquots at a concentration of 5 mg/ml in phosphate-buffered saline containing 1 mM EDTA and 1 mM β-glycerol phosphate, and diluted to 1.5 mg/ml in the same buffer shortly before use. All injections were from the same lot. The specific activity of the lot was 15 U/mg (where 1 U = 1 μmole of 4-methylumbelliferyl-6-thiopalmitoyl-β-D-glucoside (MU-6S-Palm-βGlc) hydrolyzed per minute [22]). Mannose 6-phosphate receptor binding was 85% as determined by a column-binding assay [21]. The enzyme prevented [35S]cysteinyl thioester lipid accumulation in PPT1 deficient lymphoblasts in a mannose 6-phosphate dependent manner with an EC50 of 0.25 nM during an overnight incubation as determined using a previously developed assay [23]. A dose of 0.3 mg weekly (corresponding to 12.5 mg/kg for a 25 g mouse) was the highest feasible dose given the quantities available for the experiment, and was considered to be reasonable based on a previous pharmacokinetic and biodistribution study [21], which indicated that this dose would provide at least 20% of wild type activity in major organs (except the brain) for a minimum of 72 hours.
2.2 Mouse injections
PPT1 knockout mice were maintained as homozygous breeding stock on a C57BL/6 background, housed in a barrier facility and received food and water ad libitum. Treatment and vehicle groups were assigned randomly from litters born within a 2–3 day window after timed matings. For the groups receiving treatment from birth, mice received a single injection of 0.1 ml (0.15 mg) of human recombinant PPT1 (or vehicle alone) via superficial temporal vein on postnatal day 0. On days 7, 14, and 21, they received 0.3 mg intraperitoneally, and then 0.3 mg via tail vein injection beginning on day 28 (four weeks of age). They then received weekly injections of 0.3 mg via tail vein thereafter (corresponding to an average dose of ~14 mg/kg for female and ~11 mg/kg for male PPT1 knockout mice, respectively). For the two groups receiving injections beginning at 8 weeks, treatment with 0.3 mg was begun at 8 weeks of age via tail vein and repeated weekly thereafter. Concurrent groups of uninjected PPT1 knockout mice and uninjected C57BL/6 wild-type controls were maintained for comparison. Each experimental or control group consisted of 12–16 mice. All procedures were carried out under an IACUC-approved protocol at the University of Texas Southwestern Medical Center.
2.3 Rotarod assessments of motor performance
For motor coordination testing, mice were tested on a Rotarod (model 755, IITC Life Science Inc., Woodland Hills, CA) at 5 weeks and 3, 5, 6, 7 and 8 months of age and the latency to fall in seconds recorded. Trials lasted a maximum of 60 seconds. At each age, mice underwent a pretest trial on a stationary rod, followed by two test trials on the constant speed Rotarod (3 rpm) for each of three consecutive days. The maximum latency to fall in the two test trials on the final day of testing was reported as the final outcome measure. For logistical reasons, mice treated beginning at eight weeks (vehicle and ERT), wild type and untreated knockout mice were tested as one cohort and mice treated starting from birth (vehicle and ERT) were tested using the same conditions and apparatus as a separate cohort approximately two months later. A three-factor mixed ANOVA model was used to examine the differences in time on the Rotarod comparing treatment groups, age at start of treatment, and time of measurement (at 6, 7, and 8 months).
2.4 Survival
Mice were assessed weekly for body weight and more frequently for general health. Mice were sacrificed when a loss of greater than 10% of highest weight recorded was noted for two consecutive weeks, when animals could not right themselves, or were moribund (poorly responsive to tactile stimulation). The observer was blinded as to assignment of treatment groups. A Kaplan-Meier log-rank test was used to determine whether there was an overall significant difference among these groups and pair-wise comparisons were performed using a Bonferroni correction to alpha (.05 divided by the number of comparisons). A significance level of .05 was used for the overall test and .05/4=0.0125 for the pair-wise group comparisons.
2.5 Tissue processing and fluorescence microscopy
Mice were anesthetized with Avertin and perfused transcardially with cold heparinized physiological saline followed by 4% formaldehyde, freshly prepared from paraformaldehyde, in PBS, pH 7.4. All tissues excluding brain were harvested, fixed overnight, transferred to 50 mM Tris, pH 6.5, and then dehydrated, paraffin embedded, and sectioned according to standard protocols. Serial sections (5 μM) were deparaffinized and stained with routine hematoxylin/eosin for pathological evaluation, or left unstained and coverslipped with Vectashield (Vector Laboratories) for evaluation of autofluorescent pigment (excitation 470 ± 20 nm, emission 525 ± 25 nm). Fixation, permeabilization, and staining runs were carried out in exact parallel to ensure comparative significance between groups. Brains were postfixed overnight and cryoprotected in 30% sucrose in 50 mM TBS, pH 7.6 and 40μm coronal sections were cut on a Microm HM430 Freezing Microtome (Microm International GmbH, Wallendorf, Germany).
2.6 Neuropathology
Cresyl violet staining and immunohistochemistry was performed as previously described [17, 20]. For cresyl violet staining, a one in six series of coronal brain sections were mounted onto chrome-gelatin coated slides and allowed to dry overnight. Sections were then stained in 0.05% cresyl violet solution for 45 minutes at 60°C, differentiated in alcohol, cleared in xylene and coverslipped in DPX. A one in six series of free floating sections from each brain were immunostained for markers of astrocytosis (rabbit anti-GFAP, 1:4000 dilution, Dako Ltd, cat #Z0334) and microglial activation (rat anti-mouse CD68, 1:2000 dilution, AbD Serotec, cat #MCA1957). Sections were quenched for endogenous peroxidase activity with 1% H2O2 in TBS, washed and blocked in 15% normal serum (Vector Laboratories) diluted in TBS with 0.3% Triton-X100 (TBS-T). The normal serum was directed against the host species of the secondary antibody. After blocking, sections were incubated overnight at 4°C in primary antibody diluted in 10% normal serum in TBS-T. Subsequently, sections were washed and incubated at room temperature in biotinylated secondary antibody (biotinylated swine anti-rabbit IgG, DAKO, cat #E0353; biotinylated rabbit anti-rat IgG, Vector Laboratories, cat #BA-4001), followed by washing and incubation in Vectastain Elite ABC solution (Vector Laboratories) before visualization with DAB (Sigma).
Histological measurements of cortical thickness, volume and neuron counts were performed as previously described [17, 20] on cresyl violet stained sections using StereoInvestigator software (MBF Bioscience, Williston, VT). All of the cortical measurement data presented are for the primary somatosensory barrel field cortex (S1BF), which we have shown to be affected relatively early and to a greater extent than other cortical regions. This is a well-defined neuropathological phenotype, which we have selected to use in other studies as a standardized measure for judging therapeutic efficacy of a variety of different approaches [24–25]. Thresholding image analysis was carried out on GFAP and CD68 stained sections. Thirty non-overlapping images from three consecutive sections were captured from a defined region, with all parameters of light intensity, video camera setup and calibration settings kept constant. Images were analyzed using Image Pro Plus software (Media Cybernetics, Chicago, IL) by choosing an appropriate threshold that selected the foreground immunoreactivity above background. All analysis was done blinded to genotype and treatment group. A one-way ANOVA with a Bonferroni post-hoc test used to determine if there were significant differences between the groups.
2.7 Determination of serum mouse anti-human PPT1 antibodies by ELISA
Purified recombinant human PPT1 protein (50 ng per well) in 100 mM sodium bicarbonate/carbonate buffer, pH 9.5, was absorbed onto wells in a 96-well microtiter plate (BD Bioscience Cat#353279) then incubated with a blocking solution consisting of 5% casein acid hydrolysate (Sigma) in PBS-T (phosphate-buffered saline containing 0.1% Tween). Serum was diluted with PBS, added to the coated plate and incubated at room temperature to allow antibody binding. After one hour of incubation, the plate was washed to remove non-specific binding serum components. Secondary horseradish-peroxidase (HRP) conjugated antibody (donkey anti-mouse IgG, Jackson ImmunoResearch, catalog #715-035-150) at 1:10,000 dilution was added to each well and incubated at room temperature for 1 h. After washing with PBS-T, an HRP substrate, TMB (BioFX, cat#TMBW-1000-01) was added at 50 μl per well, incubated 5–10 minutes at room temperature for color development. The reaction was stopped by adding 10% HCl. The absorbance was measured at 450 nm.
To prepare positive controls for the ELISA assay, two PPT1 knockout mice were injected intraperitoneally with 40 μg each of recombinant human PPT1 in Freund’s complete adjuvant and boosted twice at 3 week intervals with 20 μg of antigen in Freund’s incomplete adjuvant. Mouse serum was collected from these animals and from a random sample of PPT1 knockout mice in the experimental groups at time points indicated in the Figure Legends.
3. RESULTS
3.1 Effect of ERT on motor performance and survival
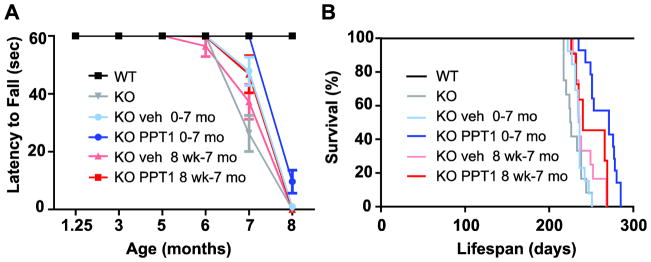
Groups of 12–16 PPT1 knockout mice were treated intravenously on a weekly schedule with human recombinant PPT1 (or vehicle alone) beginning either shortly after birth or starting at 8 weeks of age. Concurrent uninjected PPT1 knockout and wild-type mice were included in the trial for comparison. Rotarod performance using a constant speed paradigm was assessed at 5 weeks and 3, 5, 6, 7, and 8 months. The maximum latency to fall was used in the analysis (maximum of 2 trials, 60 sec trials) (Fig. 1A). Wild-type mice performed maximally (60 sec) at all time points and showed 100% survival. Mice treated with PPT1 performed significantly better than those treated with vehicle alone (p=0.0014), and mice treated beginning at birth performed significantly better than mice treated from 8 weeks of age (p=0.0004). However, the gains were modest, reflecting a delay in motor decline of a few weeks. Similar effects were seen on survival (Fig. 1B). Overall, differences among groups were statistically significant (log rank test, p < 0.0001), with mice treated with recombinant PPT1 from birth living significantly longer (median number of days to death (±SE), 271±16.6 vs. 236±1.3, respectively, p<0.0001), but not if treated from 8 weeks of age (240±12.8 vs. 235±1.7, p=0.15).
Fig. 1.
Effect of ERT on (A) latency to fall from a rotating rod (Rotarod); and (B) survival. Recombinant human PPT1 was administered weekly via tail vein injection, beginning either on post-natal day 1 or at 8 weeks of age as indicated. The dose was 0.15 mg on post-natal day 1 and 0.3 mg thereafter. (A) Rotarod performance. Statistical analysis employed a 3-factor mixed ANOVA model to examine the differences in time on the Rotarod comparing treatment groups, age at start of treatment, and time of measurement (6, 7, and 8 mo). Mice treated with PPT1 performed significantly better than those treated with vehicle alone (least square means, 39.4±1.2 sec vs. 33.7±1.2 sec, p=0.0014), and mice treated beginning at birth performed significantly better than mice treated from 8 weeks (least square means, 39.7±1.1 sec vs. 33.3±1.3, p=0.0004). (B). Survival. Treatment from birth prolonged survival from 236 to 271 days (a 15% increase, p< 0.0001). Treatment beginning at 8 weeks did not significantly prolong survival.
3.2 Effect of ERT on neuropathology
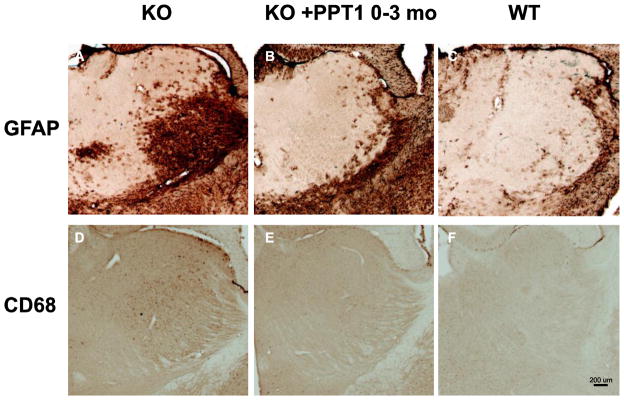
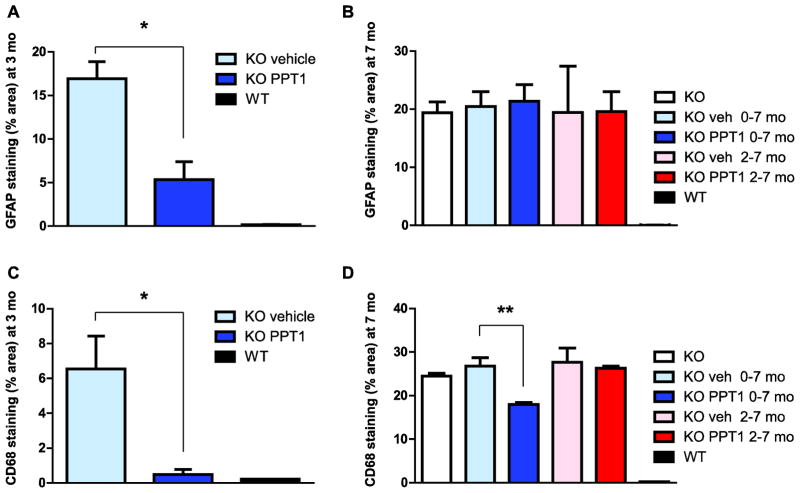
Three mice chosen at random from each group were sacrificed for detailed pathological examination of brain, eye, heart, lung, kidney, liver, spleen, pancreas, spinal cord, small intestine, and skeletal muscle at 3 or 7 months of age, and tissues were collected from the remaining mice at terminal sacrifice. Particular attention was paid to brain neuropathology as it is the most important target organ in humans. Measurements of cortical thickness, volume, and analysis of immunostaining using markers of astrocytosis (GFAP) and microglial activation (CD68) were performed. In previous studies of the PPT1 knockout mouse, the earliest detected pathological change is astrocytosis, which is first detected in the thalamus, and which is followed by a wave of microglial activation ([17, 20]). In mice treated from shortly after birth to the age of 3 months, astrocytosis (Fig. 2A) and microglial activation (Fig. 2B) in the thalamus were markedly attenuated. These data, as well as representative sections from mice examined at 7 months and mice treated from 8 weeks to 7 months, are quantified in Fig. 3. As shown in Fig. 3(A and C), mice treated beginning at birth and examined at 3 months showed marked reductions in GFAP and CD68 immunoreactivity. However, despite continued treatment, GFAP immunoreactivity (Fig. 3B) continued to increase so that no treatment effect was apparent by 7 months of age. A significant attenuation persisted in CD68 microgliosis in the mice treated beginning at birth (Fig. 3D). When analyzed at age 7 months, there were no significant differences in treated vs. untreated mice in cortical thickness, volume (cortex, hippocampus, striatum or thalamus), or neuron counts (results not shown). Furthermore, in mice treated from the age of 8 weeks to 7 months, there were significant differences in thickness, volume, and GFAP and CD68 staining between wild-type and untreated knockout mice, but no treatment effects were observed (results not shown).
Fig. 2.
ERT reduces histological markers of glial activation in the thalamus (VPL/VPM) in mice treated from birth and assessed at 3 months of age. A–C, GFAP immunohistochemistry (marker of astrocytosis). In untreated mutant mice intense localized astrocytosis is evident within the ventral posterior thalamic nucleus (VPM/VPL), but in mice treated from birth this astrocytosis is markedly reduced and resembles that seen in age-matched control mice. D–F. Consistent with published data, a low level of microglial activation is evident as pale CD68 immunoreactivity in the same region of the thalamus 3 month old untreated mutant mice, but is virtually absent in mice treated from birth, appearing similar to untreated controls.
Fig. 3.
Quantitation of GFAP and CD68 immunohistochemical staining of thalamus. Photomicrographs similar to those shown in Fig. 2 were analyzed by thresholding analysis (Image Pro Plus, Media Cybernetics). Three mice in each group were analyzed at three months of age (panels A, C) or at 7 months of age (panels B and D). Mice received weekly PPT1 either starting at birth or at 8 weeks of age as indicated. GFAP staining was reduced and CD68 staining was nearly abolished in mice treated from birth and analyzed at three months (panels, left). Although treatment continued, the improvement in GFAP staining was lost by 7 months, whereas a statistically significant reduction in CD68 staining remained (panels, right). Data is presented as mean ± standard error of the mean.
3.3 Effect of ERT on visceral pathology
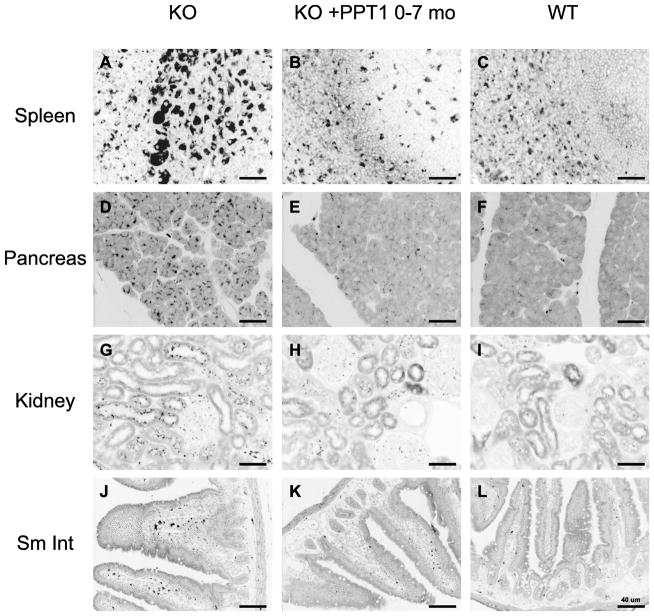
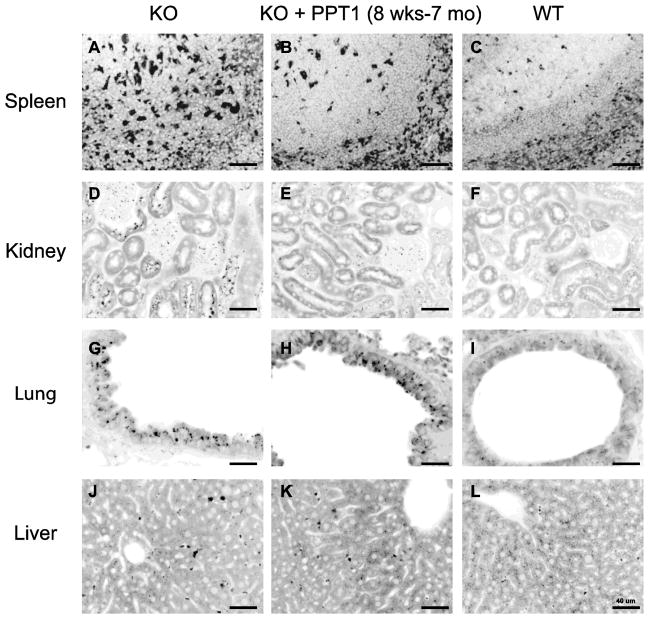
The effect of ERT on visceral accumulation of autofluorescence storage material is shown in Figs. 4–6. (In these inverted images, the autofluorescence signal appears in black whereas underlying tissue can be seen in gray). Fig. 4 shows representative images of spleen, pancreas, kidney and small intestine of mice treated from birth to age 7 months. As shown in Fig. 4, panels A–C, copious autofluorescence is demonstrated in macrophages in PPT1 knockout mice in and around follicular areas of the spleen, which are effaced in some areas. ERT markedly improved this pathology (Fig. 4, panel B). Abundant storage material was also present throughout the pancreas, which was essentially eliminated by enzyme treatment (Fig. 4, panels D–F). In the kidney (Fig. 4, panels G–I) there was substantial clearance of storage from kidney tubular cells, whereas glomerular deposits, interestingly, were not much affected. In the small intestine (Fig. 4, panels J–L), autofluorescence was readily demonstrated in interstitial areas, and these were also cleared by treatment.
Fig. 4.
ERT reduces autofluorescent storage material in spleen, pancreas, kidney and small intestine in PPT1 knockout mice treated from birth to 7 months. Representative images from tissues from 3 mice in each group are shown. Similar results were seen for all mice at terminal sacrifice, which occurred around 9 months in ERT-treated mice and 8 months in vehicle treated mice (not shown).
Fig. 6.
ERT reduces autofluorescent storage material in spleen and kidney but not lung and liver in mice treated from 8 weeks to 7 months. Representative images from tissues from 3 mice in each group are shown. Similar results were seen for all mice at terminal sacrifice (not shown).
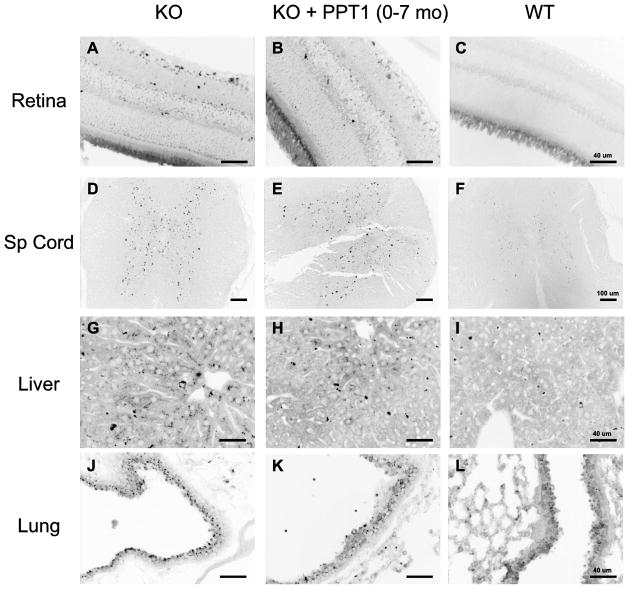
Some peripheral tissues were unaffected by PPT1 treatment and others had so little storage that treatment effects could not be assessed (Fig. 5). Deposits in the retina and spinal cord were similar in treatment vs. control groups (Fig. 5, A–F). In the liver, deposits were scant. Storage in Kupfer cells was improved in ERT-treated mice, but parenchymal storage was rare, and found occasionally even in wild-type mice, particularly in peri-portal areas. Accumulation of storage material in epithelial cells of the upper airways was striking in the knockout mice (a finding not previously reported). However, a treatment effect, if any, was difficult to assess because a moderate degree of autofluorescence was also seen in wild-type mice in some airways (Fig. 5J–L). Similar findings in autofluorescent storage material in the visceral organs were seen in the groups of mice treated starting at the age of 8 weeks (Fig. 6). Near complete resolution of storage in the spleen and kidney tubules was observed (Fig. 6, panels A–F), whereas little change was seen in lung and liver (Fig. 6, panels G–L).
Fig. 5.
ERT did not reduce autofluorescent storage material in retina, spinal cord, liver parenchyma, and lung in PPT1 knockout mice treated from birth to 7 months. Representative images from tissues from 3 mice in each group are shown. Similar results were seen for all mice at terminal sacrifice (not shown).
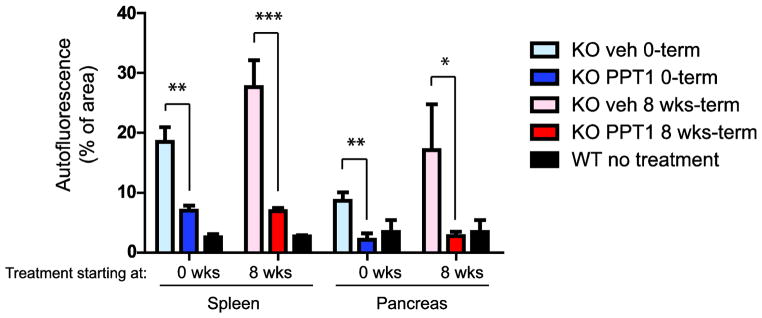
Quantitation of autofluorescence (by threshold analysis of images) was straightforward for spleen and pancreas and was performed for all available mice at terminal sacrifice (shown in Fig. 7). This analysis revealed essentially complete normalization of autofluorescence in pancreas and a 70–80% reduction of autofluorescence in spleen for animals treated either from birth or from 8 weeks. The effect of treatment was highly significant (p<0.0001).
Fig. 7.
Quantitation of autofluorescent storage material in spleen and pancreas, with PPT1 treatment beginning either on post-natal day 1 or at 8 weeks. Quantitative thresholding analysis was performed on 10 randomly selected fields from tissues of each of 3–6 mice at terminal sacrifice. Age-matched wild-type mice are shown as controls.
3.4 Adverse reactions and antibody formation
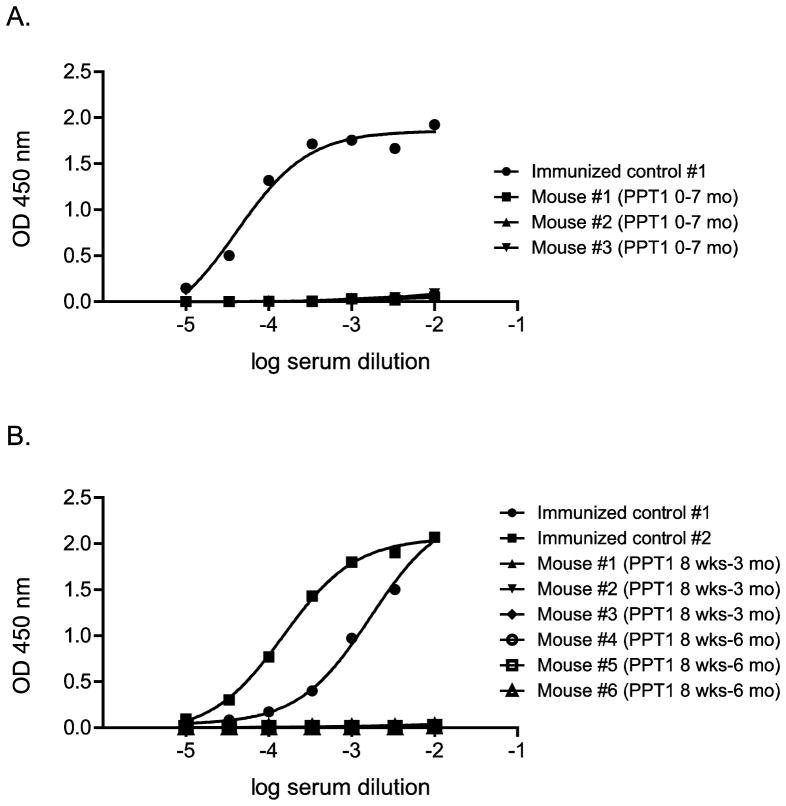
Somewhat surprisingly, no adverse reactions to injection of human recombinant PPT1 in the mice were observed, and no antibodies to the human recombinant protein could be demonstrated by ELISA. However, antibodies were easily raised in PPT1 knockout mice using a standard immunization protocol involving the use of adjuvant (Fig. 8).
Fig. 8.
Absence of anti-human PPT1 antibody formation detected by ELISA in ERT-treated PPT1 KO mice. A, mice treated continuously beginning at post-natal day 1 and analyzed at 7 months. B, mice treated beginning at 8 weeks and analyzed at either 3 or 6 months. Two PPT1 KO mice that were immunized with human recombinant PPT1 by conventional methods (repeated intraperitoneal injection, initially with adjuvant) were used as positive controls.
4. DISCUSSION
Several important points relevant to the treatment of PPT1-related NCL can be drawn from the current study. First, intravenous administration of high-dose recombinant PPT1 was well tolerated in the mouse model. Anaphylaxis and antibody formation, frequently limiting in other mouse models of lysosomal storage disorders, including late infantile NCL [26], were not seen. We speculate that this was due either to the high-dose protocol, leading to tolerance, or possibly due to an inherent lack of immunogenicity of human PPT1 in the mouse (although antibodies could be formed in the presence of adjuvant using standard immunization protocols). This could be tested directly by challenging chronically treated animals with PPT1 and adjuvant in a future study. The method used to create the knockout construct (introduction of a stop codon at the extreme 3′ terminus, just upstream of a key catalytic residue) may also have contributed to the lack of antibody response, as a low (but undetectable) level of translation arising from the disrupted gene may have provided some degree of tolerance.
Second, intravenously administered PPT1 prevented accumulation of autofluorescent storage material in a number of tissues examined. The response in the spleen was striking and consistent with previous experience in enzyme replacement using mannose 6-phosphate modified enzymes. Pancreatic involvement with lysosomal storage, while it occurs [27], is not often commented upon in relation to enzyme replacement therapy and our model provided an opportunity to demonstrate a positive response to a mannose 6-phosphate- modified enzyme in this tissue. The utilization of the mannose 6-phosphate receptor pathway by the pancreas for lysosomal enzyme targeting can be inferred from exocrine pancreatic involvement in I-cell disease [28] and the high abundance of mannose 6-phosphate receptors in the exocrine pancreas [29–30]. Our data is consistent with these findings and show a particular efficacy of a mannose 6-phosphorylated enzyme in this tissue.
One limitation of the current study is that cardiac autofluorescence could not be assessed due to high background autofluorescence when tissues are processed by standard methods. Cardiac involvement is of particular concern in the NCLs [7–8, 31]. Subtle valvular abnormalities of the aortic root have been described in PPT1 deficient mice [13]. In order to specifically address cardiac involvement, a separate study dedicated to the heart would be needed, ideally in conjunction with echocardiographic imaging during disease progression.
Finally, we have shown that brain neuropathology in the mouse model can be ameliorated by exogenously administered enzyme, albeit only during the perinatal period. Urayama, et al. [32–33] have shown that mannose 6-phosphate receptor-mediated transport of lysosomal enzymes across the blood-brain barrier is developmentally regulated in the mouse, and that lysosomal enzymes can penetrate brain tissue when administered between birth and 3 weeks of age. Our results are consistent with this finding, showing that administration of PPT1 beginning at birth leads to about a one-month delay in the development of brain pathological findings, decline in motor performance, and survival. While it is possible that the mice could be doing better because of visceral improvements, the data showing similar visceral improvements in mice treated beginning at birth as opposed to eight weeks, and the lack of known musculoskeletal pathology in the disease, argue that the motor improvements have a neurological basis. Our results demonstrate clearly that pathology in the PPT1 deficient mouse brain is responsive to exogenously administered PPT1 during the first few weeks of life. When PPT1 was administered after 8 weeks, we saw no effects on the brain, despite reports of increased blood brain permeability in the PPT1 knockout mouse at 6 months when measured by a highly sensitive gadolinium tracer method [34]. Apparently the increased permeability associated with inflammation was not sufficient to allow enough enzyme to enter the brain and positively impact the disease course.
We cannot rule out that a higher dose may have been more efficacious in the mouse. From a technical standpoint, higher doses of PPT1 are certainly possible. PPT1 can be prepared in concentrations up to 10 mg/ml (data not shown), corresponding to a possible dose which is 6.6 times more concentrated than the dose used here. (We have not attempted making higher concentrations, so the limits of solubility are not known). Therefore, it may be of interest to attempt higher doses in the mouse as more material becomes available. However, high-dose intravenous administration of lysosomal enzymes has thus far not been successful in the human in the context of other neuronopathic lysosomal storage diseases [35].
Quite recently, enzyme replacement therapy delivered directly to the cerebrospinal fluid has been attempted in several large animal models of other LSDs affecting the brain, with favorable results [36–40]. As a result, human clinical trials are in progress for mucopolysaccharidosis I (Hurler-Scheie syndrome, ClinicalTrials.gov #NCT00852358), Hunter syndrome (NCT00920647) and San Filippo syndrome (NCT01155778). Therefore, future studies could explore the effect of human recombinant PPT1 administered intrathecally to the PPT1-deficient mouse model and to the canine PPT1-deficient model that was recently described [41]. In addition, enzyme replacement may be useful for addressing visceral disease in conjunction with CNS-directed therapies in preclinical and clinical development, such as gene therapy [14, 24–25, 42] and human neural stem cell transplantation [43–44].
Highlights.
PPT1 knockout mice received intravenous weekly human PPT1 (0.3 mg) starting at birth or 8 weeks.
The treatment was well tolerated and neither anaphylaxis nor antibody formation was observed.
Survival increased and the onset of motor deterioration was delayed in mice treated from birth.
Substantial clearance of autofluorescent storage from visceral organs was observed.
Acknowledgments
The authors wish to thank Dr. Mark Sands for advice in the experimental design and instruction in the superficial temporal vein injection technique, Dr. James Richardson and John Shelton of the UT Southwestern Molecular Pathology Core for advice and preparation of visceral tissue specimens and microscopy, and Ami Pettersen for performing the behavioral tests. This work was funded by Taylor’s Tale, the Batten Disease Support and Research Association, the National Institute for Neurological Disorders and Stroke (NS036867) (S.L.H.), the Batten Disease Family Association, the Natalie Fund and the Bletsoe Family (J.C.).
ABBREVIATIONS USED
- NCL
neuronal ceroid lipofuscinosis
- PPT1
palmitoyl-protein thioesterase-1
- CLN1
ceroid lipofuscinosis, neuronal-1
- GROD
granular osmiophilic deposits
- FBS
fetal bovine serum
- MU-6S-Palm-βGlc
4-methylumbelliferyl-6-thiopalmitoyl-β-D-glucoside
- PBS
phosphate-buffered saline
- Man 6-P
mannose 6-phosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vesa J, Hellsten E, Verkruyse LA, Camp LA, Rapola J, Santavuori P, Hofmann SL, Peltonen L. Mutations in the palmitoyl protein thioesterase gene causing infantile neuronal ceroid lipofuscinosis. Nature. 1995;376:584–587. doi: 10.1038/376584a0. [DOI] [PubMed] [Google Scholar]
- 2.Lu JY, Hofmann SL. Thematic review series: lipid posttranslational modifications. Lysosomal metabolism of lipid-modified proteins. J Lipid Res. 2006;47:1352–1357. doi: 10.1194/jlr.R600010-JLR200. [DOI] [PubMed] [Google Scholar]
- 3.Kousi M, Lehesjoki AE, Mole SE. Update of the mutation spectrum and clinical correlations of over 360 mutations in eight genes that underlie the neuronal ceroid lipofuscinoses. Hum Mutat. 2012;33:42–63. doi: 10.1002/humu.21624. [DOI] [PubMed] [Google Scholar]
- 4.Das AK, Becerra CH, Yi W, Lu JY, Siakotos AN, Wisniewski KE, Hofmann SL. Molecular genetics of palmitoyl-protein thioesterase deficiency in the U.S. J Clin Invest. 1998;102:361–370. doi: 10.1172/JCI3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Diggelen OP, Thobois S, Tilikete C, Zabot MT, Keulemans JL, van Bunderen PA, Taschner PE, Losekoot M, Voznyi YV. Adult neuronal ceroid lipofuscinosis with palmitoyl-protein thioesterase deficiency: first adult-onset patients of a childhood disease. Ann Neurol. 2001;50:269–272. doi: 10.1002/ana.1103. [DOI] [PubMed] [Google Scholar]
- 6.Ramadan H, Al-Din AS, Ismail A, Balen F, Varma A, Twomey A, Watts R, Jackson M, Anderson G, Green E, Mole SE. Adult neuronal ceroid lipofuscinosis caused by deficiency in palmitoyl protein thioesterase 1. Neurology. 2007;68:387–388. doi: 10.1212/01.wnl.0000252825.85947.2f. [DOI] [PubMed] [Google Scholar]
- 7.Hofman IL, van der Wal AC, Dingemans KP, Becker AE. Cardiac pathology in neuronal ceroid lipofuscinoses--a clinicopathologic correlation in three patients. Eur J Paediatr Neurol. 2001;5(Suppl A):213–217. doi: 10.1053/ejpn.2000.0465. [DOI] [PubMed] [Google Scholar]
- 8.Fealey ME, Edwards WD, Grogan M, Orszulak TA. Neuronal ceroid lipofuscinosis in a 31-year-old woman presenting as biventricular heart failure with restrictive features. Cardiovasc Pathol. 2009;18:44–48. doi: 10.1016/j.carpath.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 9.Mole SE, Williams RE, Goebel HH. Correlations between genotype, ultrastructural morphology and clinical phenotype in the neuronal ceroid lipofuscinoses. Neurogenetics. 2005;6:107–126. doi: 10.1007/s10048-005-0218-3. [DOI] [PubMed] [Google Scholar]
- 10.Haltia M, Rapola J, Santavuori P. Infantile type of so-called neuronal ceroid lipofuscinosis. Part II. Histological and electron microscopic studies. Acta Neuropath. 1973;26:157–170. doi: 10.1007/BF00697751. [DOI] [PubMed] [Google Scholar]
- 11.Haltia M, Rapola J, Santavuori P, Keranen A. Infantile type of so-called neuronal ceroid-lipofuscinosis. 2. Morphological and biochemical studies. J Neurol Sci. 1973;18:269–285. doi: 10.1016/0022-510x(73)90076-2. [DOI] [PubMed] [Google Scholar]
- 12.Gupta P, Soyombo AA, Atashband A, Wisniewski KE, Shelton JM, Richardson JA, Hammer RE, Hofmann SL. Disruption of PPT1 or PPT2 causes neuronal ceroid lipofuscinosis in knockout mice. Proc Natl Acad Sci U S A. 2001;98:13566–13571. doi: 10.1073/pnas.251485198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galvin N, Vogler C, Levy B, Kovacs A, Griffey M, Sands MS. A murine model of infantile neuronal ceroid lipofuscinosis-ultrastructural evaluation of storage in the central nervous system and viscera. Pediatr Dev Pathol. 2008;11:185–192. doi: 10.2350/07-03-0242.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffey MA, Wozniak D, Wong M, Bible E, Johnson K, Rothman SM, Wentz AE, Cooper JD, Sands MS. CNS-directed AAV2-mediated gene therapy ameliorates functional deficits in a murine model of infantile neuronal ceroid lipofuscinosis. Mol Ther. 2006;13:538–547. doi: 10.1016/j.ymthe.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Kielar C, Wishart TM, Palmer A, Dihanich S, Wong AM, Macauley SL, Chan CH, Sands MS, Pearce DA, Cooper JD, Gillingwater TH. Molecular correlates of axonal and synaptic pathology in mouse models of Batten disease. Hum Mol Genet. 2009;18:4066–4080. doi: 10.1093/hmg/ddp355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macauley SL, Wozniak DF, Kielar C, Tan Y, Cooper JD, Sands MS. Cerebellar pathology and motor deficits in the palmitoyl protein thioesterase 1-deficient mouse. Exp Neurol. 2009;217:124–135. doi: 10.1016/j.expneurol.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kielar C, Maddox L, Bible E, Pontikis CC, Macauley SL, Griffey MA, Wong M, Sands MS, Cooper JD. Successive neuron loss in the thalamus and cortex in a mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol Dis. 2007;25:150–162. doi: 10.1016/j.nbd.2006.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lei B, Tullis GE, Kirk MD, Zhang K, Katz ML. Ocular phenotype in a mouse gene knockout model for infantile neuronal ceroid lipofuscinosis. J Neurosci Res. 2006;84:1139–1149. doi: 10.1002/jnr.21008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jalanko A, Vesa J, Manninen T, von Schantz C, Minye H, Fabritius AL, Salonen T, Rapola J, Gentile M, Kopra O, Peltonen L. Mice with Ppt1(Deltaex4) mutation replicate the INCL phenotype and show an inflammation-associated loss of interneurons. Neurobiol Dis. 2005;18:226–241. doi: 10.1016/j.nbd.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 20.Bible E, Gupta P, Hofmann SL, Cooper JD. Regional and cellular neuropathology in the palmitoyl protein thioesterase-1 null mutant mouse model of infantile neuronal ceroid lipofuscinosis. Neurobiol Dis. 2004;16:346–359. doi: 10.1016/j.nbd.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 21.Lu JY, Hu J, Hofmann SL. Human recombinant palmitoyl-protein thioesterase-1 (PPT1) for preclinical evaluation of enzyme replacement therapy for infantile neuronal ceroid lipofuscinosis. Mol Genet Metab. 2010;99:374–378. doi: 10.1016/j.ymgme.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Diggelen OP, Keulemans JL, Winchester B, Hofman IL, Vanhanen SL, Santavuori P, Voznyi YV. A rapid fluorogenic palmitoyl-protein thioesterase assay: pre- and postnatal diagnosis of INCL. Mol Genet Metab. 1999;66:240–244. doi: 10.1006/mgme.1999.2809. [DOI] [PubMed] [Google Scholar]
- 23.Lu JY, Verkruyse LA, Hofmann SL. Lipid thioesters derived from acylated proteins accumulate in infantile neuronal ceroid lipofuscinosis: correction of the defect in lymphoblasts by recombinant palmitoyl-protein thioesterase. Proc Natl Acad Sci U S A. 1996;93:10046–10050. doi: 10.1073/pnas.93.19.10046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Macauley SL, Roberts MS, Wong AM, McSloy F, Reddy AS, Cooper JD, Sands MS. Synergistic effects of central nervous system-directed gene therapy and bone marrow transplantation in the murine model of infantile neuronal ceroid lipofuscinosis. Ann Neurol. 2012 doi: 10.1002/ana.23545. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Roberts MS, Macauley SL, Wong AM, Yilmas D, Hohm S, Cooper JD, Sands MS. Combination small molecule PPT1 mimetic and CNS-directed gene therapy as a treatment for infantile neuronal ceroid lipofuscinosis. J Inherit Metab Dis. 2012 doi: 10.1007/s10545-011-9446-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chang M, Cooper JD, Sleat DE, Cheng SH, Dodge JC, Passini MA, Lobel P, Davidson BL. Intraventricular enzyme replacement improves disease phenotypes in a mouse model of late infantile neuronal ceroid lipofuscinosis. Mol Ther. 2008;16:649–656. doi: 10.1038/mt.2008.9. [DOI] [PubMed] [Google Scholar]
- 27.Hammel I, Alroy J. The effect of lysosomal storage diseases on secretory cells: an ultrastructural study of pancreas as an example. J Submicrosc Cytol Pathol. 1995;27:143–160. [PubMed] [Google Scholar]
- 28.Elleder M, Martin JJ. Mucolipidosis type II with evidence of a novel storage site. Virchows Arch. 1998;433:575–578. doi: 10.1007/s004280050292. [DOI] [PubMed] [Google Scholar]
- 29.Brown WJ, Farquhar MG. The mannose-6-phosphate receptor for lysosomal enzymes is concentrated in cis Golgi cisternae. Cell. 1984;36:295–307. doi: 10.1016/0092-8674(84)90223-x. [DOI] [PubMed] [Google Scholar]
- 30.Tooze J, Hollinshead M, Ludwig T, Howell K, Hoflack B, Kern H. In exocrine pancreas, the basolateral endocytic pathway converges with the autophagic pathway immediately after the early endosome. J Cell Biol. 1990;111:329–345. doi: 10.1083/jcb.111.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reske-Nielsen E, Baandrup U, Bjerregaard P, Bruun I. Cardiac involvement in juvenile amaurotic idiocy--a specific heart muscle disorder. Histological findings in 13 autopsied patients. Acta Pathol Microbiol Scand A. 1981;89:357–365. doi: 10.1111/j.1699-0463.1981.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 32.Urayama A, Grubb JH, Sly WS, Banks WA. Developmentally regulated mannose 6-phosphate receptor-mediated transport of a lysosomal enzyme across the blood-brain barrier. Proc Natl Acad Sci U S A. 2004;101:12658–12663. doi: 10.1073/pnas.0405042101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Urayama A, Grubb JH, Sly WS, Banks WA. Mannose 6-phosphate receptor-mediated transport of sulfamidase across the blood-brain barrier in the newborn mouse. Mol Ther. 2008;16:1261–1266. doi: 10.1038/mt.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saha A, Sarkar C, Singh SP, Zhang Z, Munasinghe J, Peng S, Chandra G, Kong E, Mukherjee AB. The blood-brain barrier is disrupted in a mouse model of infantile neuronal ceroid lipofuscinosis: amelioration by resveratrol. Hum Mol Genet. 2012 doi: 10.1093/hmg/dds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lachmann RH. Enzyme replacement therapy for lysosomal storage diseases. Curr Opin Pediatr. 2011;23:588–593. doi: 10.1097/MOP.0b013e32834c20d9. [DOI] [PubMed] [Google Scholar]
- 36.Vuillemenot BR, Katz ML, Coates JR, Kennedy D, Tiger P, Kanazono S, Lobel P, Sohar I, Xu S, Cahayag R, Keve S, Koren E, Bunting S, Tsuruda LS, O’Neill CA. Intrathecal tripeptidyl-peptidase 1 reduces lysosomal storage in a canine model of late infantile neuronal ceroid lipofuscinosis. Mol Genet Metab. 2011;104:325–337. doi: 10.1016/j.ymgme.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 37.Vite CH, Wang P, Patel RT, Walton RM, Walkley SU, Sellers RS, Ellinwood NM, Cheng AS, White JT, O’Neill CA, Haskins M. Biodistribution and pharmacodynamics of recombinant human alpha-L-iduronidase (rhIDU) in mucopolysaccharidosis type I-affected cats following multiple intrathecal administrations. Mol Genet Metab. 2011;103:268–274. doi: 10.1016/j.ymgme.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen A, Vogler C, McEntee M, Hanson S, Ellinwood NM, Jens J, Snella E, Passage M, Le S, Guerra C, Dickson P. Glycosaminoglycan storage in neuroanatomical regions of mucopolysaccharidosis I dogs following intrathecal recombinant human iduronidase. APMIS. 2011;119:513–521. doi: 10.1111/j.1600-0463.2011.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dierenfeld AD, McEntee MF, Vogler CA, Vite CH, Chen AH, Passage M, Le S, Shah S, Jens JK, Snella EM, Kline KL, Parkes JD, Ware WA, Moran LE, Fales-Williams AJ, Wengert JA, Whitley RD, Betts DM, Boal AM, Riedesel EA, Gross W, Ellinwood NM, Dickson PI. Replacing the enzyme alpha-L-iduronidase at birth ameliorates symptoms in the brain and periphery of dogs with mucopolysaccharidosis type I. Sci Transl Med. 2010;2:60ra89. doi: 10.1126/scitranslmed.3001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kondagari GS, King BM, Thomson PC, Williamson P, Clements PR, Fuller M, Hemsley KM, Hopwood JJ, Taylor RM. Treatment of canine fucosidosis by intracisternal enzyme infusion. Exp Neurol. 2011;230:218–226. doi: 10.1016/j.expneurol.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 41.Sanders DN, Farias FH, Johnson GS, Chiang V, Cook JR, O’Brien DP, Hofmann SL, Lu JY, Katz ML. A mutation in canine PPT1 causes early onset neuronal ceroid lipofuscinosis in a Dachshund. Mol Genet Metab. 2010;100:349–356. doi: 10.1016/j.ymgme.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Griffey M, Bible E, Vogler C, Levy B, Gupta P, Cooper J, Sands MS. Adeno-associated virus 2-mediated gene therapy decreases autofluorescent storage material and increases brain mass in a murine model of infantile neuronal ceroid lipofuscinosis. Neurobiol Dis. 2004;16:360–369. doi: 10.1016/j.nbd.2004.03.005. [DOI] [PubMed] [Google Scholar]
- 43.Guillaume DJ, Huhn SL, Selden NR, Steiner RD. Cellular therapy for childhood neurodegenerative disease. Part I: rationale and preclinical studies. Neurosurg Focus. 2008;24:E22. doi: 10.3171/FOC/2008/24/3-4/E21. [DOI] [PubMed] [Google Scholar]
- 44.Selden NR, Guillaume DJ, Steiner RD, Huhn SL. Cellular therapy for childhood neurodegenerative disease. Part II: clinical trial design and implementation. Neurosurg Focus. 2008;24:E23. doi: 10.3171/FOC/2008/24/3-4/E22. [DOI] [PubMed] [Google Scholar]