Abstract
Pituitary volumes were measured in 55 first-episode schizophrenia patients at a baseline timepoint with 38 receiving a followup scan after antipsychotic treatment. Fifty-nine healthy volunteers had baseline scans with 34 receiving a followup scan. There were no baseline group differences in pituitary volumes or changes in volume following antipsychotic treatment.
Keywords: magnetic resonance imaging, antipsychotic treatment, psychosis
1. Introduction
The pituitary gland is part of the hypothalamic-pituitary-adrenal (HPA) axis, one of the primary biological systems that mediate the experience of stress (De Groot and Harris, 1950; Sam and Frohman, 2008). Abnormalities in HPA axis functioning have been implicated in schizophrenia and related psychotic disorders (Walker and Diforio, 1997), with multiple studies reporting HPA axis hyperactivity in the onset and exacerbation of psychotic symptoms (Muck-Seler et al., 1999; Ryan et al., 2004). Studies also demonstrate greater ACTH and cortisol among individuals experiencing their first psychotic episode (Ryan et al., 2003, 2004; Walsh et al., 2005 ).
Magnetic resonance imaging (MRI) studies investigating the pituitary in schizophrenia and related psychotic disorders have yielded mixed findings. Some groups reported larger (Pariante et al., 2004; Pariante et al., 2005; Takahashi et al., 2009) or smaller (Pariante et al., 2004; Upadhyaya et al., 2007) pituitary volume in patients compared to healthy controls, although two studies reported no group differences (Tournikioti et al., 2007; Nicolo et al., 2009). The use of antipsychotic medications could influence pituitary volume, possibly via the inhibition of corticotrophin releasing- hormone (CRH) gene activity (Basta-Kaim et al., 2006) or the stimulation of prolactin secreting cells (Meltzer and Fang, 1976; Gruen et al., 1978). Several MR imaging studies have supported the possibility of pituitary volume changes in association with antipsychotic treatment, although findings have been inconsistent regarding the direction of changes (Pariante et al., 2005; MacMaster et al., 2007; Nicolo et al., 2009). Such inconsistencies may be due to the heterogeneity of the samples including differences in prior antipsychotic drug exposure.
The goal of the current study was to compare pituitary volumes between patients experiencing a first-episode of schizophrenia with minimal or no prior antipsychotic drug exposure to healthy volunteers and to investigate possible changes in pituitary volume following antipsychotic treatment.
2. Methods
2.1 Subjects
The 55 patients in this study were participating in clinical trials investigating antipsychotic drug efficacy. Twenty-eight of the 55 patients were antipsychotic drug-naïve at the time of the baseline scan whereas 23 had a mean exposure of 8 days (SD=7) to typical or atypical antipsychotics prior to the baseline scan. Medication history prior to the baseline scan was unavailable for 4 patients. Thirty-eight of the 55 patients received followup scans after a median 22 weeks (range 16–169 weeks) of antipsychotic treatment with olanzapine, risperidone, clozapine, prolixin, perphenazine and/or haldol. We converted medications received between the two scan timepoints to chlorpromazine equivalents using the approaches described by Woods (2003) and Lehman and colleagues (2004). Medication data at the time of the second scan were unavailable for two patients as these patients terminated their clinical trials prior to the second scan. Diagnoses were based on the SCID for Axis I DSM-IV Disorders (First et al., 1998) supplemented by information from family members and clinicians. All patients met DSM-IV criteria for schizophrenia (n = 36), schizoaffective disorder (n = 6) or schizophreniform disorder (n = 13). Mean age at first psychotic symptoms was 22 (SD = 5.3).
Fifty-nine healthy volunteers were recruited from the community and were determined to have no Axis I disorders by the SCID-NP (First et al., 2001). Healthy volunteers received an initial baseline scan and a repeat scan following a median of 19 weeks (range 11–163 weeks). Exclusion criteria for all study participants included any serious medical condition, any treatment known to affect the brain or meeting DSM-IV criteria for mental retardation. All procedures were approved by the local IRB and written informed consent was obtained from all participants.
2.2 Magnetic Resonance (MR) Imaging Procedures
MR imaging exams were conducted on a GE 1.5T imaging system. One hundred twenty four contiguous coronal images (slice thickness = 1.5 mm) were acquired through the whole head using a 3D Fast SPGR sequence with IR Prep in a 256 × 256 matrix producing nominal in-plane resolution of 0.86mm × 0.86mm. Measurements were completed in MEDx (Sensor Systems, 1998) following alignment along the anterior and posterior commissures. Scans were mixed together randomly and no identifying information was available to the operator from the scan.
2.3 Measurement Delineation Criteria
Measurement of total intracranial contents included the total cerebrum, cerebrospinal fluid, cerebellum and brainstem. Inter-rater reliability between two raters as assessed by intra-class correlations [ICCs]) in 9 cases was 0.99. The pituitary was delineated on coronal images and included the posterior pituitary, but excluded the pituitary stalk. The lateral borders were the cavernous sinuses visualized by the presence of the internal carotid arteries. Superiorly the pituitary was bound by the diaphragma sella, which was well-demarcated and inferiorly bound by the sphenoid sinus. All cases were measured by two operators and the average measurement was used as the dependent variable in analyses. The intra-class correlation between two operators across all cases was 0.94 at baseline and 0.93 at followup.
2.4 Statistical Analyses
Group differences in age and education were examined using independent groups t tests. Chi-square tests were used to examine group differences in sex and handedness. Mixed models analyses were used to investigate group differences in pituitary volume with group (patient vs. healthy volunteer) and sex serving as between subjects factors. Timepoint (baseline vs. followup) served as a within subjects factor. We used Spearman rank order correlations to investigate the relationship between changes in pituitary volume and total cumulative exposure to antipsychotics (in chlorpromazine equivalents) between the two scan timepoints. Age and total intracranial volume served as statistical covariates in general linear models. Alpha was set to 0.05 (two-tailed).
3. Results
Patients and comparison subjects did not differ significantly in distributions of age, sex, handedness or total intracranial volume (p’s > 0.05), but as expected patients had significantly less education (t=−3.94; p=<0.001). Total intracranial volume was 1347 cm3 (SD = 160) in patients and 1353 cm3 (SD = 110) in healthy volunteers. Mean pituitary volumes for patients at the baseline and followup timepoints was 819 mm3 (SD = 137) and 832 mm3 (SD = 137), respectively. Mean pituitary volumes at the baseline and followup timepoints for healthy volunteers was 814 mm3 (SD = 186) and 835 mm3 (SD = 173), respectively.
Neither the main effect of group nor the group-by-time interaction were statistically significant. Similar findings were observed for both the subgroup of antipsychotic drug-naïve patients and a subgroup of patients who took only atypical antipsychotics between scans. There was no significant correlation between antipsychotic drug exposure (in chlorpromazine equivalents) and change in pituitary volume between the two scan timepoints across the entire cohort, in the subgroup of antipsychotic drug-naïve patients, or in the subgroup of patients who took only atypical antipsychotics. A main effect of sex (F=7.08, df = 1,109, p = 0.009) indicated that females had larger pituitary volumes overall compared to males with post-hoc analyses indicating this effect was evident at both the baseline (F=5.43, df=1,109, p = 0.02) and followup (F = 5.92, df = 1,67, p = 0.02) timepoints. Figure 1 shows the baseline pituitary volumes for males and females separately.
Figure 1.
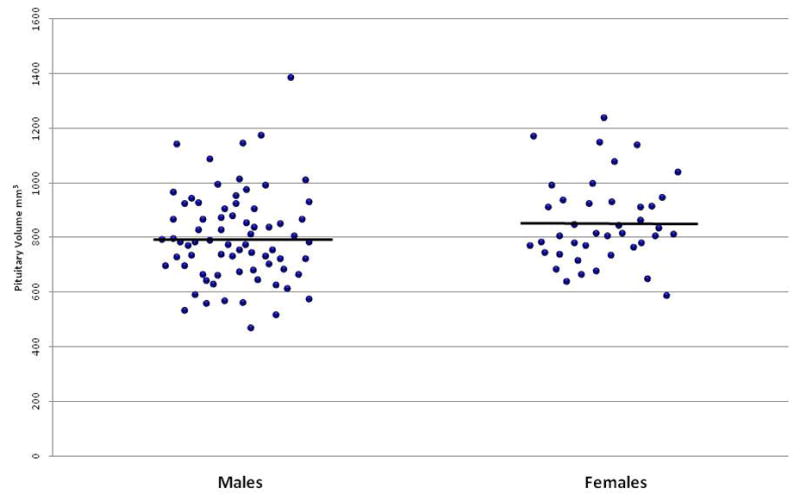
Baseline pituitary volumes in males and females
4. Discussion
We did not observe group differences in pituitary volume between patients and healthy volunteers at a baseline timepoint with similar negative effects observed among the antipsychotic drug-naïve patients. The lack of significant group differences at the baseline timepoint is consistent with several other studies reporting no significant group differences in pituitary volume in first episode (Nicolo et al., 2009) and chronic (Tournikioti et al., 2007) patients with schizophrenia. Additionally, we did not observe short-term pituitary volume changes following general antipsychotic treatment in patients experiencing a first-episode of schizophrenia. Differences in antipsychotics and dosages might account for differences in findings (Pariante et al., 2005; MacMaster et al., 2007; Nicole et al., 2009).
We identified a significant effect of sex on pituitary volume across patients and healthy volunteers, which was evident at both the baseline and followup timepoints. Our data are consistent with findings from several studies that reported a significant effect of sex on pituitary volume (Takano et al., 1999; Pariante et al., 2004; 2005; MacMaster et al., 2007; Tournikioti et al., 2007; Upadhyaya et al., 2007; Nicolo et al., 2009; Takahashi et al., 2009;). The functional significance of larger pituitary volume in females is unknown, but may have implications for sex differences in psychiatric disorders that implicate dysregulation of the HPA axis in their neurobiology including depression (e.g. Amsterdam et al., 1989) and anxiety (Gustafsson et al., 2008).
In sum, our data neither support a role for abnormal pituitary volume in the pathogenesis of first-episode schizophrenia nor changes in volume in association with antipsychotic treatment. Study limitations include the possibility of an ascertainment bias in that very ill patients may not have been available for a followup scan and the lack of direct neuroendocrine measures.
Acknowledgments
Role of the Funding Source
Funding for this study was provided by NIMH Grants MH60374 (Dr. Bilder), MH074543 (Dr. Kane), MH60004 (Dr. Robinson), K23 DA015541 (Dr. Sevy) and M01 RR018535 (NSLLIJ Research Institute General Clinical Research Center). The NIMH had no further role in study design, in the collection, analysis and interpretation of data, in the writing of the report, and in the decision to submit the paper for publication.
Footnotes
Contributors
Drs. Bilder and Robinson designed the study and wrote the protocols. Dr. Christian performed the measurements. Drs. Sevy and Gunduz-Bruce treated the patients in the clinical trials. Ms. Napolitano and Dr. Szeszko performed the statistical analyses. Drs. Gruner and Szeszko wrote the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
Drs. Gruner, Christian and Gunduz-Bruce declare no conflicts of interest. Dr. Szeszko has received compensation from Boehringer Ingelheim. Dr. Sevy has received consulting fees from Abbott. Dr. Bilder has received consulting fees and/or honoraria from Janssen Pharmaceutica, Sumitomo, Pfizer, Cogtest, Cypress Bioscience, Vanda, Dainipon Sumitomo, Johnson & Johnson, Merck and Roche. Dr. Robinson receives grant support from Bristol-Myers Squibb and Janssen and compensation from Astra Zeneca, Lundbeck and MedAvante.
References
- Abelson JL, Khan S, Liberzon I, Young EA. HPA axis activity in patients with panic disorder: review and synthesis of four studies. Depression and Anxiety. 2007;24:66–76. doi: 10.1002/da.20220. [DOI] [PubMed] [Google Scholar]
- Amsterdam JD, Maislin G, Gold P, Winokur A. The assessment of abnormalities in hormonal responsiveness at multiple levels of the hypothalamic-pituitary-adrenocortical axis in depressive illness. Psychoneuroendocrinology. 1989;14:43–62. doi: 10.1016/0306-4530(89)90055-3. [DOI] [PubMed] [Google Scholar]
- Basta-Kaim A, Budziszewska B, Jaworska-Feil L, Tetich M, Kubera M, Leskiewicz M, Otczyk M, Lason W. Antipsychotic drugs inhibit the human corticotrophin-releasing-hormone gene promoter activity in neuro-2A cells- an involvement of protein kinases. Neuropsychopharmacology. 2006;31:853–865. doi: 10.1038/sj.npp.1300911. [DOI] [PubMed] [Google Scholar]
- De Groot J, Harris GW. Hypothalamic control of the anterior pituitary gland and blood lymphocytes. Journal of Physiology III. 1950:335–346. doi: 10.1113/jphysiol.1950.sp004483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruen PH, Sachar EJ, Langer G, Altman N, Leifer M, Frantz A, Halpern FS. Prolactin responses to neuroleptics in normal schizophrenic subjects. Archives of General Psychiatry. 1978;35:108–116. doi: 10.1001/archpsyc.1978.01770250110011. [DOI] [PubMed] [Google Scholar]
- Gustafsson PE, Gustafsson PA, Ivarsson T, Nelson N. Diurnal cortisol levels and cortisol response in youths with obsessive-compulsive disorder. Neuropsychobiology. 2008;57:14–21. doi: 10.1159/000123117. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Biometrics Research Department, New York State Psychiatric Institute; (SCID-I/NP, version 2.0, 2/01 revision) Non-patient Edition. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders. Biometrics Research Department, New York State Psychiatric Institute; (SCID-I/P, version 2.0, 8/98 revision) Patient Edition. [Google Scholar]
- Lehman AF, Kreyenbuhl J, Buchanan RW, Dickerson FB, Dixon LB, Goldberg R, Green-Paden LD, Tenhula WN, Boerescu D, Tek C, Sandson N, Steinwachs DM. The schizophrenia patient outcomes research team (PORT): updated treatment recommendations 2003. Schizophrenia Bulletin. 2004;30:193–217. doi: 10.1093/oxfordjournals.schbul.a007071. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, El-Sheikh R, Upadhyaya AR, Nutche J, Rosenberg DR, Keshavan M. Effect of antipsychotics on pituitary gland volume in treatment-naïve first episode schizophrenia: a pilot study. Schizophrenia Research. 2007;92:207–210. doi: 10.1016/j.schres.2007.01.022. [DOI] [PubMed] [Google Scholar]
- MacMaster FP, Keshavan M, Mirza Y, Carrey N, Upadhyaya AR, El-Sheikh R, Buhagiar CJ, Taormina SP, Boyd C, Lynch M, Rose M, Ivey J, Moore GJ, Rosenberg DR. Development and sexual dimorphism of the pituitary gland. Life Sciences. 2007;80:940–944. doi: 10.1016/j.lfs.2006.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY, Fang VS. The effect of neuroleptics on serum prolactin in schizophrenic patients. Archives of General Psychiatry. 1976;33:279–286. doi: 10.1001/archpsyc.1976.01770030003001. [DOI] [PubMed] [Google Scholar]
- Muck-Seler D, Pivac N, Jakovljevic M, Brzovic Z. Platelet secretion, plasma cortisol, and dexamethasone suppression test in schizophrenic patients. Biological Psychiatry. 1999;45:1433–1439. doi: 10.1016/s0006-3223(98)00174-7. [DOI] [PubMed] [Google Scholar]
- Nicolo J, Berger G, Garner BA, Velakoulis D, Markulev C, Kerr M, McGorry PD, Proffitt T, McConchie M, Pantelis C, Wood SJ. The effect of atypical antipsychotics on pituitary gland volume in patients with first-episode psychosis: A longitudinal MRI study. Schizophrenia Research. 2009 Nov 4; doi: 10.1016/j.schres.2009.10.005. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Pariante CM, Dazzan P, Danese A, Morgan KD, Brudaglio F, Morgan C, Fearon P, Orr K, Hutchinson G, Pantelis C, Velakoulis D, Jones PB, Leff J, Murray RM. Increased pituitary volume in antipsychotic-free and antipsychotic-treated patients of the AEsop first-onset psychosis study. Neuropsychopharmacology. 2005;30:1923–1931. doi: 10.1038/sj.npp.1300766. [DOI] [PubMed] [Google Scholar]
- Pariante CM, Vassilopoulou K, Velakoulis D, Phillips L, Soulsby B, Wood SJ, Brewer W, Smith DJ, Dazzan P, Yung AR, Zervas IM, Christodoulou GN, Murray R, McGorry PD, Pantelis C. Pituitary volume in psychosis. British Journal of Psychiatry: the Journal of Mental Science. 2004;185:5–10. doi: 10.1192/bjp.185.1.5. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Collins P, Thakore JH. Impaired fasting glucose tolerance in first-episode, drug-naïve patients with schizophrenia. American Journal of Psychiatry. 2003;160:284–289. doi: 10.1176/appi.ajp.160.2.284. [DOI] [PubMed] [Google Scholar]
- Ryan MC, Sharifi N, Condren R, Thakore JH. Evidence of basal pituitary-adrenal overactivity in first episode, drug naïve patients with schizophrenia. Psychoneuroendocrinology. 2004;29:1065–1070. doi: 10.1016/j.psyneuen.2003.08.011. [DOI] [PubMed] [Google Scholar]
- Sachar EJ, Kanter SS, Buie D, Engle R, Mehlman R. Psychoendocrinology of ego disintegration. American Journal of Psychiatry. 1970;126:1067–1078. doi: 10.1176/ajp.126.8.1067. [DOI] [PubMed] [Google Scholar]
- Sam S, Frohman LA. Normal physiology of hypothalamic pituitary regulation. Endocrinology and Metabolism Clinics of North America. 2008;37:1–22. doi: 10.1016/j.ecl.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Suzuki M, Velakoulis D, Lorenzetti V, Soulsby B, Zhou SY, Nakamura K, Seto H, Kurachi M, Pantelis C. Increased pituitary volume in schizophrenia spectrum disorders. Schizophrenia Research. 2009;108:114–121. doi: 10.1016/j.schres.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Takano K, Utsunomiya H, Ono H, Ohfu M, Okazaki M. Normal development of the pituitary gland: Assessment with three-dimensional MR volumetry. AJNR American Journal of Neuroradiology. 1999;20:312–315. [PMC free article] [PubMed] [Google Scholar]
- Tournikioti K, Tansella M, Perlini C, Rambaldelli G, Cerini R, Versace A, Andreone N, Dusi N, Balestrieri M, Malago R, Gasparini A, Brambilla P. Normal pituitary volumes in chronic schizophrenia. Psychiatry Research: Neuroimaging. 2007;154:41–48. doi: 10.1016/j.pscychresns.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Upadhyaya AR, El-Sheikh R, MacMaster FP, Diwardkar VA, Keshavan MS. Pituitary volume in neuroleptic-naïve schizophrenia: a structural MRI study. Schizophrenia Research. 2007 Feb;90(1–3):266–273. doi: 10.1016/j.schres.2006.09.033. [DOI] [PubMed] [Google Scholar]
- Walker EF, Diforio D. Schizophrenia: A neural diathesis-stress model. Psychological Review. 1997;104:667–685. doi: 10.1037/0033-295x.104.4.667. [DOI] [PubMed] [Google Scholar]
- Walsh P, Spelman L, Sharifi N, Thakore JH. Male patients with paranoid schizophrenia have greater ACTH and cortisol secretion in response to metoclopramide-induced AVP release. Psychoneuroendocrinology. 2005;30:431–437. doi: 10.1016/j.psyneuen.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. Journal of Clinical Psychiatry. 2003;64:663–667. doi: 10.4088/jcp.v64n0607. [DOI] [PubMed] [Google Scholar]


