Abstract
Aim
Although hematopoietic cell transplantation (HCT) arrests the cognitive decline in mucopolysaccharidosis type IH (Hurler syndrome, MPS IH), these children continue to have neuropsychological deficits as they age. Both compromised attention and effects on white matter have been observed in cancer patients who have had chemotherapy. Therefore, we explored the effects of disease and treatment on brain function in children with MPS I who have had HCT with those with attenuated MPS I treated with enzyme replacement therapy (ERT).
Methods
Subjects: 7 MPS IH participants at least 5 years post-HCT were compared with 7 attenuated participants who were treated with ERT. Measures: IQ, attention, spatial ability, and memory were assessed. Medical history and an unsedated MRI scan using diffusion tensor imaging (DTI) were acquired.
Results
Despite clinically equivalent IQ and memory, children with MPS IH had poorer attention span than those with attenuated MPS I as well as decreased fractional anisotropy (FA) of the corpus callosum. A relationship between attention scores and FA was found in the MPS IH group but not the attenuated group. FA was also related to frequency of medical events.
Interpretation
In children with MPS IH, both the treatment and the disease affect attention functions associated with poor white matter integrity.
Keywords: MPS I, hematopoietic cell transplant, attention span, cognition, diffusion tensor imaging, medical history
1. Introduction
We used neuropsychological measures and diffusion tensor imaging (DTI) to explore the late effects of hematopoietic cell transplant (HCT) in patients with severe MPS IH (Hurler syndrome), and found effects on attention and fractional anisotropy. HCT is the standard of care for children with MPS IH, halting the cognitive decline associated with the CNS disease [1]. While early HCT results in better cognitive development [2–4], specific long-term CNS residual problems have not been well defined. Parents describe attention and information processing difficulties, but these domains have not been explored. Because similar neurocognitive effects have been described that implicate white matter structure and function in patients receiving chemotherapy or HCT for leukemia [5–10], we investigated these effects in MPS IH.
Children with the same enzymatic deficiency, but with later onset, are Hurler-Scheie (intermediate) and Scheie (mild) syndromes with variable cognitive and somatic involvement. Because no definitive diagnostic criteria separate these two syndromes, for the purposes of this research, we designate them as attenuated or MPS IA. Enzyme replacement therapy (ERT) is the standard of care for MPS IA. The degree and cause of cognitive deficits in MPS IA are not well understood.
We explored the association of neurocognitive abilities with diffusion tensor imaging (DTI), a measure of white matter integrity. We hypothesized that deficits associated with the preparative regimen may explain some of the residual cognitive difficulties in the transplanted MPS IH group by interfering with the integrity and organization of the brain. We determined whether DTI values of fractional anisotropy and mean diffusivity are associated with neurocognitive functions across and within each group.
2. Methods
2.1 Participants
Ten participants with MPS IH treated with HCT were recruited from our blood and marrow transplant database. The criteria for inclusion were: 1) at least five years post- HCT, 2) physically and cognitively able to undergo 40 minutes in a scanner without sedation and 2 hours of neuropsychological testing, 3) hearing and vision adequate for neuropsychological testing, and 4) between 7 and 25 years of age. One participant’s data were excluded due to movement artifact and 2 were not included because the scanner DTI software was upgraded mid-study and DTI results would likely differ from those collected pre-upgrade.
Ten participants with MPS IA (Hurler-Scheie or Scheie syndrome) were recruited using the same criteria as above and had been on ERT for at least 3 months. Five were recruited from our clinic patients and five from collaborators at other institutions. DTI data from 7 of the 10 participants were collected prior to scanner upgrade and were included for analysis.
This protocol was approved by the IRB and consent and assent were obtained from parents and children. Transportation, imaging, and neuropsychological testing were supported by outside funds and each participant received a $100 stipend.
2.2. Measures
2.2.1 Diffusion Tensor Imaging
DTI is an imaging technique that measures the movement of water molecules within brain tissue and the extent to which this diffusion is isotropic (equal in all directions) or anisotropic (directionally restricted). The anisotropy level can be used to estimate axonal (white matter) organization in the brain. For instance, in well-myelinated axon bundles, water diffusion is restricted to the axis of the bundle and is less isotropic compared to cerebrospinal fluid in which water diffuses more freely in all directions. The DTI measure of anisotropy (fractional anisotropy – FA) has values ranging from 0 (isotropic) to 1 (anisotropic). Mean diffusivity (MD), a measure of overall diffusion, is also provided. More technical details about DTI can be found elsewhere [11,12].
Image Acquisition: Each participant had an unsedated scan using a research-dedicated 3T Siemens Trio scanner (Siemens, AG, Erlangen, Germany). During imaging, each patient watched a movie or listened to music which enhanced cooperation and minimized movements.
Diffusion-weighted images were acquired with single-shot, spin echo echo-planar imaging (EPI) sequences using the following parameter settings: AC-PC aligned, oblique slices, TR/TE=11000/104 ms; slice thickness=2 mm; FoV=256; in-plane resolution= 2mm × 2 mm. Diffusion gradients were applied in 12 noncolinear directions (b=1000 mm2/s). One T2-weighted image volume without a diffusion gradient was also collected using the same imaging parameters (b = 0 mm2/s). Image volumes were averaged over three acquisitions to increase the signal-to-noise ratio and to reduce image artifacts. The total scanning time for DTI acquisition was 7 minutes 20 seconds. An additional scan was conducted to acquire a field map with the following parameters: TR/TE 700/4.62, flip angle=90, slice thickness =2mm, FoV 256, which lasted approximately 3 minutes.
Image Processing: The diffusion-weighted images were corrected for distortion, caused by magnetic field (B0) inhomogeneity/B0 susceptibility, eddy current and simple head motion, using the field map with an in-house program written in MATLAB [13]. The distortion-corrected images were processed using DTI Studio software [14]. Three eigenvalues and eigenvectors were obtained and fractional anisotropy (FA) and trace maps were calculated. Mean Diffusivity (MD) values were obtained from the trace maps (MD = Trace/3).
The corpus callosum (CC), a commissural fiber bundle that connects the two hemispheres, was chosen as the region of interest because anomalies and thinning have been described in MPS I [15–17]. The CC was traced manually on the mid-sagittal slice on a color-coded map (in which red, green and blue colors were assigned to right-left, anterior-posterior and superior-inferior fiber orientations, respectively). Measurements were made twice by the same rater and the average of the two measurements was used in the subsequent analyses. Intra-rater reliability (between first and second measure) calculated using Cronbach’s Alpha for FA was 0.965 and for MD was 0.970. Intraclass correlation for FA was 0.967 and for MD was 0.971.
2.2.2 Neuropsychological Testing
As we were particularly interested in functions mediated by white matter connections, we analyzed cognitive ability (Wechsler Abbreviated Scale of Intelligence -WASI) [18], attention and response speed (Test of Variables of Attention- TOVA) [19], and spatial perception (Judgment of Line Orientation- JLO) [20]. Memory (California Verbal Learning Test- CVLT) [21] was included as a contrast measure since memory is usually associated with gray matter function.
2.2.3 Medical Data
The type of medical history data collected can be found in Table 1 and transplant data in Supplementary Table 1. In addition, we collected data on presence of seizures, psychiatric problems and educational rehabilitation interventions. For the MPS IH group, number of transplants, presence of graft versus host disease (GVHD) and other medical events were also collected. For the MPS IA group, no pre-treatment clinical IQ or MRI data were available.
Table I.
Description of the samples
| MPS IH | MPS IA | |
|---|---|---|
| Mean age in years (standard deviation) | 12.6 (4.5) | 16.5 (4.6) |
| Gender: Females | 3/7 | 4/7 |
| Age at transplant | 14 months (range 5–20) | |
| Pre-transplant developmental quotient | 99 (range 83–114) | |
| Time from transplant in years: | 11.2 (4.7) | |
| Years of ERT | 5.19 (3.2) | |
| Medical Risk Factors | Frequency (n=7) | Frequency (n=7) |
| * More than four surgeries | 6/7 | 4/7 |
| Visual problems | ||
| Mild corneal clouding | 0/7 | 5/7 |
| Moderate corneal clouding | 4/7 | 1/7 |
| * Severe corneal clouding/impairment | 3/7 | 0/7 |
| * Hearing aids | 2/7 | 0/7 |
| Cervical cord compression | 0/7 | 3/7 |
| Pre HCT white matter abnormality | 4/6 | No data |
| * Ventriculoperitoneal shunt for hydrocephalus | 1/7 | 4/7 |
| Transplant related risks | ||
| * Two transplants | 1/7 | not applicable |
| * Radiation in prep regimen | 3/7 | not applicable |
| Number of Risk Factors | ||
| Number of Risks = 0 | 1 (14.3%) | 1(14.3%) |
| Number of Risks = 1 | 1 (14.3%) | 3(42.9%) |
| Number of Risks = 2 | 3 (42.9%) | 2 (28.6%) |
| Number of Risks = 3 | 0 | 1(14.3%) |
| Number of Risks = 4 | 1 (14.3%) | 0 |
| Number of Risks = 5 | 1 (14.3%) | 0 |
component of the summed score for Risk Factor Score.
To quantify medical risk from the medical data, we created a Risk Factor score that summed the number of factors that might cause compromise of white matter or affect neuropsychological test scores. These risks are indicated by asterisks in Table 1.
2.3 Statistical Analysis
Patient characteristics were evaluated by MPS group. Differences between group means were evaluated with a t-test. Associations between DTI measures and neurological test scores were estimated using linear regression and evaluated against a t-distribution for confidence intervals and P-values. All statistical analyses were performed using R v2.13.1 [22].
3. Results
3.1 Medical Data
For the 7 participants with MPS IH, one required two transplants in order to obtain engraftment with the second transplant at 34 months of age. Age of HCT was not associated with any of the outcomes. For those who had HCT, all had Cyclosporine A and prednisone for prevention of graft-versus-host disease (GVHD). Only one patient had chronic GVHD (graft versus host disease) of a mild degree. One patient had a carrier donor. Four of 7 had radiation, but 3 of the 4 had a brain-sparing protocol. See Supplementary Table 1.
For MPS IA, all participants had been on ERT for at least 3 months (maximum = 6 years) with no adverse events. Notable in the results were that both groups had a large number of surgeries. The most common surgeries were various orthopedic procedures, ventilation tubes, tonsillectomy and adenoidectomy, hernia repair and carpal tunnel surgery. Moderate or severe corneal clouding was more frequent in the MPS IH group. Hydrocephalus with ventriculoperitoneal shunt was more common in the MPS IA group. See Table 1. None of the participants in either group had seizures. One MPS IA patient was on medication for significant psychiatric symptoms and one with MPS IH had intermittent anxiety symptoms but was untreated.
3.2 Outcome Measures
3.2.1. Neuropsychological Performance
The mean performance of the MPS IA participants was better than that of MPS IH (Table 2) but no significant differences were found on measures of intelligence or memory. Scores were severely impaired or the test was unable to be performed in participants with significant corneal clouding. However, all were able to perform other visual tests that had higher contrast or less visual detail. Between-group differences on the TOVA reached statistical significance for the TOVA d-prime variable which measures correct stimulus detection (p = 0.038).
Table 2.
Neuroimaging and neuropsychological test results by MPS I subgroup
| Covariate | Overall | MPS IA | MPS IH | Difference from MPS IA (95% CI) | P-value |
|---|---|---|---|---|---|
| Cognitive Ability: WASI Full Scale IQ | 81.4 (14.1) | 84.9 (14.6) | 77.9 (13.7) | −7.0 (−23.5, 9.5) | 0.374 |
| Cognitive Ability: WASI Verbal Scale IQ | 86.6 (10.2) | 87.9 (11.0) | 85.4 (9.95) | −2.4 (−14.7, 9.8) | 0.672 |
| Cognitive Ability: WASI Performance Scale IQ | 80.4 (15.8) | 84.1 (15.5) | 76.6 (16.3) | −7.6 (−26.1, 11.0) | 0.391 |
| Memory: California Verbal Learning Test | 84.2 (15.6) | 80.3 (19.1) | 88.1 (11.0) | 7.9 (−10.9, 26.6) | 0.370 |
| Spatial: Judgement of Line Orientation* | 70.8 (18.4) | 79.9 (19.1) | 61.7 (13.2) | −18.1 (−37.6, 1.3) | 0.065 |
| Attention: TOVA d prime | 77.7 (12.5) | 84.6 (13.0) | 70.9 (7.73) | −13.7 (−26.5, −0.9) | 0.038 |
| Attention: TOVA Omission Errors | 70.2 (28.3) | 82.0 (29.1) | 58.4 (23.9) | −23.6 (−54.7, 7.6) | 0.124 |
| Attention: TOVA Commission Errors | 88.6 (22.6) | 98.9 (11.4) | 78.3 (27.0) | −20.6 (−46.1, 5.0) | 0.100 |
| Reaction Time (to correct response): TOVA | 93.1 (18.6) | 97.7 (20.4) | 88.6 (16.9) | −9.1 (−31.0, 12.7) | 0.379 |
| Variability (of reaction time): TOVA | 79.5 (24.7) | 89.0 (29.4) | 70.0 (15.9) | −19.0 (−47.4, 9.4) | 0.165 |
|
| |||||
| Corpus Callosum Fractional Anisotropy | 0.57 (0.10) | 0.64 (0.05) | 0.51 (0.09) | −0.1 (−0.2, 0.0) | 0.008 |
| Corpus Callosum Mean Diffusivity | 1.04 (0.15) | 0.96 (0.11) | 1.13 (0.13) | 0.2 (0.0, 0.3) | 0.019 |
5 of 7 MPS IH and 1 of 7 MPS IA participants had the lowest possible score
3.2.2 DTI results
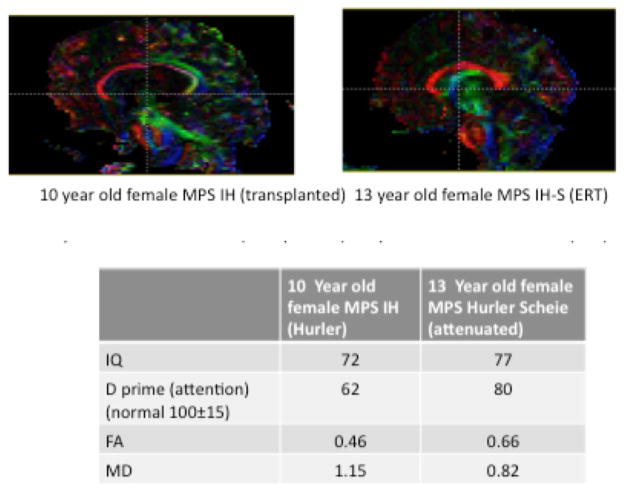
FA values in the midline corpus callosum were significantly higher on average among MPS IA participants compared to MPS IH. Conversely, MD values were significantly lower for MPS IA. See Table 2. Because age is associated with DTI measures of FA and MD23 and was found to differ between the two groups, differences were also evaluated adjusted for age with similar results. Examples of FA maps for an MPS IH and MPS I A of the same gender and approximate age can be seen in Figure 1.
Figure 1.
FA maps for a MPS IH and a MPS IA participants matched for age and gender. The figures below show the assigned colors for FA in the white matter tracts for the following directions: red= medio-lateral (left-right), green = anterior-posterior (front-back) and blue=superior-inferior (up-down). Data below the figure are from these two participants.
3.2.3. Association between DTI and medical risks
Medical risk score was calculated by adding up the number of risks for each participant as indicated in Table 1. Scores ranged from 0 to 5. Pearson correlations were calculated between number of risk factors and each of FA and MD. The correlation of FA and risk factors for all participants was r = −0.63 (p <.01). The correlation with MD and risk factors was 0.64 (p<.01).
3.2.4 Association between neuropsychological scores and DTI
Associations between neuropsychological scores and DTI measures (FA and MD) were evaluated for the entire sample as well as for each subgroup with an interaction. Analyses were conducted adjusted for age thereby adjusting for any residual effects, or other potential confounders also associated with age even though all tests were scored using age-based norms.
We found no significant association of IQ or memory scores with any variable. For the whole sample, FA in the corpus callosum was significantly associated with better attention as measured by TOVA d-prime, TOVA Omission errors, and TOVA Variability (Supplementary Table 2). In addition, a trend toward significance was observed for TOVA Reaction time. MD was significantly associated with lower scores for TOVA Omission Errors but not the other TOVA variables.
Next, we examined an interaction by the MPS IA and MPS IH groups (Table 3). For the MPS IA group, only TOVA Omission Errors was associated with FA. However, it is worth noting that this result is primarily driven by one of the seven participants with MPS IA due to the lack of variation in FA values in this group. MD was not associated with any of the TOVA measures. Thus, for the MPS IA sample, neither FA nor MD was associated with attention as measured by the TOVA.
Table 3.
Association between DTI measures (FA and MD) and neuropsychological measures evaluated separately, adjusted for age within MPS IA and MPS IH.
| Outcome | Covariate | Difference (95% CI) | P-value |
|---|---|---|---|
| TOVA d′ | Age (per year) | 0.11 (−1.40, 1.63) | 0.884 |
| FA (per tenth): MPS IA | 5.26 (−13.07, 23.58) | 0.574 | |
| FA (per tenth): MPS IH | 6.98 (−3.59, 17.55) | 0.196 | |
|
| |||
| Omission Errors | Age (per year) | 0.35 (−2.47, 3.18) | 0.806 |
| FA (per tenth): MPS IA | 39.21 (5.01, 73.41) | 0.025 | |
| FA (per tenth): MPS IH | 21.96 (2.23, 41.70) | 0.029 | |
|
| |||
| Commission Errors | Age (per year) | 1.06 (−2.17, 4.30) | 0.521 |
| FA (per tenth): MPS IA | −8.72 (−47.89, 30.45) | 0.663 | |
| FA (per tenth): MPS IH | −1.46 (−24.06, 21.14) | 0.899 | |
|
| |||
| TOVA Reaction Time | Age (per year) | −1.50 (−3.90, 0.90) | 0.220 |
| FA (per tenth): MPS IA | −11.11 (−40.12, 17.90) | 0.453 | |
| FA (per tenth): MPS IH | 18.56 (1.81, 35.30) | 0.030 | |
|
| |||
| Variability | Age (per year) | −3.01 (−5.87, −0.16) | 0.038 |
| FA (per tenth): MPS IA | −3.04 (−37.59, 31.51) | 0.863 | |
| FA (per tenth): MPS IH | 24.81 (4.87, 44.74) | 0.015 | |
|
| |||
| TOVA d′ | Age (per year) | 0.38 (−1.01, 1.76) | 0.597 |
| MD (per tenth): MPS IA | 2.75 (−5.10, 10.60) | 0.492 | |
| MD (per tenth): MPS IH | −4.07 (−11.24, 3.10) | 0.266 | |
|
| |||
| Omission Errors | Age (per year) | 1.45 (−1.69, 4.60) | 0.366 |
| MD (per tenth): MPS IA | −10.16 (−27.92, 7.60) | 0.262 | |
| MD (per tenth): MPS IH | −12.21 (−28.43, 4.01) | 0.140 | |
|
| |||
| Commission Errors | Age (per year) | 0.83 (−2.03, 3.69) | 0.571 |
| MD (per tenth): MPS IA | 6.51 (−9.64, 22.66) | 0.429 | |
| MD (per tenth): MPS IH | −1.33 (−16.08, 13.41) | 0.860 | |
|
| |||
| TOVA Reaction Time | Age (per year) | −0.47 (−3.08, 2.15) | 0.727 |
| MD (per tenth): MPS IA | −0.66 (−15.42, 14.09) | 0.930 | |
| MD (per tenth): MPS IH | −6.16 (−19.63, 7.31) | 0.370 | |
|
| |||
| Variability | Age (per year) | −1.70 (−4.84, 1.45) | 0.290 |
| MD (per tenth): MPS IA | 1.80 (−15.96, 19.56) | 0.843 | |
| MD (per tenth): MPS IH | −8.52 (−24.73, 7.70) | 0.303 | |
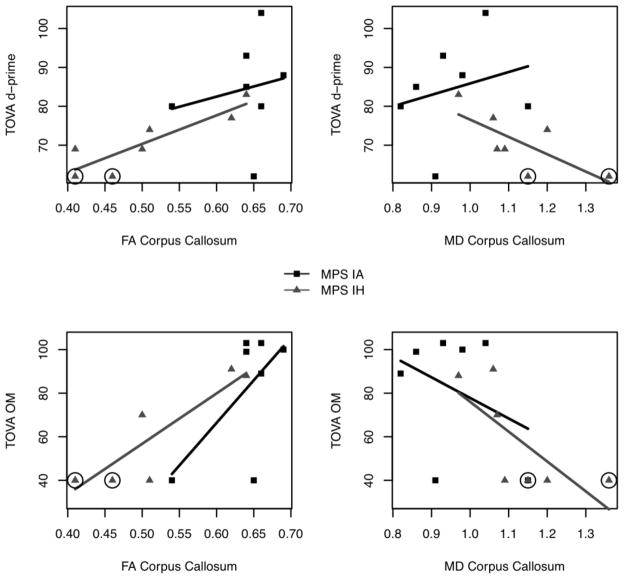
Notably, for the MPS IH group, FA of the corpus callosum but not MD was significantly related to TOVA Reaction Time, Omission Errors, and Variability See Figures 2 and Supplementary Figure 1. Thus, for the MPS IH sample, lower FA values were associated with poorer performance on several TOVA measures of attention. Note that those MPS IH participants with the largest number of medical risk factors (4 and 5) had the lowest scores on TOVA variables and FA (See circled values on Figure 2 and Supplementary Figure 1).
Figure 2.
The association of TOVA d-prime and omission errors with fractional anisotropy (FA) and MD (mean diffusivity) for the MPS IH and MPS IA groups. Circled values refer to 2 MPS IH participants with 4 or 5 medical risk factors.
4. Discussion
MPS I has an incidence of about one in 100,000 live births characterized by accumulation of excess heparan and dermatan sulfate due to deficient alpha-L-iduronidase enzyme activity [24]. Damage to multiple organs occurs for the entire range of severity although children with MPS IH have earlier onset of symptoms and CNS effects. Although hydrocephalus, hearing and vision difficulties as well as severe motor involvement contribute to poor cognition and impaired language, the slowing and decline of cognitive and language development in MPS IH is thought to occur independently of these problems [25,26]. Affected children develop normally in the first months of life; a slowing in cognitive development occurs at one year that accelerates and is evident by age 2 years [24,25]. By age 3 years, without treatment most children with MPS IH are cognitively impaired. A correlation of −0.58 has been found between mental ability and age in untreated children [25]. Median life expectancy without treatment is 5.3 with most children dying by 10 years of age [25]. Unlike other forms of MPS, children with MPS IH do not have significant behavioral difficulties until late in their disease course [25,26].
Hematopoietic cell transplant (HCT) arrests CNS deterioration and ameliorates disease in soft tissue [2–4,27–29]. Survival and continued new learning in children with MPS IH has been demonstrated but with residual deficits [30]. While previous studies have indicated steeper developmental growth curves in children transplanted under two years of age, many of these children continue to have difficulty in their school performance. Could it result from a combination of disease and HCT treatment effects on the brain?
MPS IA is characterized by later onset and less CNS involvement [28,31]. Scheie syndrome has a late onset with no easily discernible effects on the CNS. Hurler-Scheie syndrome is an intermediate form with onset in middle childhood with learning difficulties of undetermined nature and severity. Children with MPS IA are treated with ERT; enzyme does not cross the blood brain barrier to any significant degree but no need for CNS treatment was assumed presumably because no decline in cognition was thought to be present. However, evidence for a high incidence of speech language problems [31] and clinical observations indicating decline in cognitive skills suggests more cognitive involvement in MPS IA participants than previously considered [32,33].
In this exploratory project, differences were hypothesized between MPS IH and MPS IA that might clarify abnormalities of brain structure and function due to disease severity or treatment.
Group Differences: The children with MPS IH did not differ from those with MPS IA in cognitive ability or memory; for both groups the mean IQ was about one standard deviation below the mean. The low mean IQ in the MPS IA group dispels any notion that their CNS is entirely intact. While long term cognitive and adaptive deficits in MPS IH are well known [30,34], educational and vocational expectations may need to be altered for MPS IA with attention to their cognitive limitations. Four of 7 MPS IA participants had shunts for hydrocephalus. Three had cord compression in contrast to none of the MPS IH participants who presumably have decreased risk due to HCT. In such a small sample it is hard to determine the impact of these conditions, but future studies with a larger sample should consider their contribution to lowered cognitive ability.
TOVA d prime, a measure of signal detection and attention span, was significantly poorer in the MPS IH group (in the impaired range) compared to the MPS IA group (in the low average range). While omission and commission errors (both components of d prime), were two and one standard deviations lower for the MPS IH group compared to MPS IA group, respectively, group variability and small sample size may have contributed to the lack of statistical significance for these individual variables.
FA when controlled for age was significantly different between the MPS IH and the MPS IA groups with the former showing lower FA, suggesting poorer white matter integrity in the region of interest. MD was also significantly different with higher values indicating more diffusion in the MPS IH group. These results are consistent with other research in pediatric cancer patients8–10 indicating that patients who undergo HCT have long-term white matter compromise with decreased FA and white matter volumes. As we do not have normal controls, we cannot determine whether the MPS IA values are normal or fall between the normal and MPS IH values.
Relationship between DTI and neuropsychological results: For the combined sample, FA was significantly associated with TOVA d prime (attention), errors of omission, and variability. MD values were associated only with omission errors. Analyzing the groups separately indicated that the associations between DTI measures and measures of attention were driven primarily by the MPS IH group. The association of TOVA d prime and reaction time with FA in the MPS IH group was significant; poorer stimulus detection and slower reaction time were associated with lower FA in the corpus callosum. Poorer connectivity in the pathways that mediate visual attention and processing is likely. Inattention and inefficient processing have been described consistently by the parents of children with MPS IH, but not MPS IA.
While HCT can be life saving and prevent cognitive decline in MPS IH, risks to the CNS may exist from the treatment itself. In children with ALL treated with chemotherapy or HCT, presumably without a ‘brain disease,’ late treatment effects have been found in executive function, speed of processing, and decreased FA [5–10].
HCT treatment requires ablation of the bone marrow with the use of chemotherapy, and sometime radiation. While the negative effects of radiation especially at greater than 1800 cGy are well known [35], all but one of our patients had a brain-sparing protocol and was below 1400 cGY to minimize effects on cognition. To reduce the negative effects of the preparative regimen yet preserve the benefit of HCT to MPS IH patients, new protocols that are reduced in intensity offer hope of preserving cognitive function in these patients.
Medical risk factors were associated with FA across the whole group, although the association was stronger in the MPS IH group. The two participants with the most medical risk factors (4 and 5) had lowest TOVA scores and FA. Thus, in MPS IH, both disease severity and HCT treatment have a negative effect on attention functions which are associated with poor white matter integrity.
This study is limited by a small sample with resultant lack of power to detect differences, lack of control group, and a wide age range. In addition, we recognize that patients with attenuated MPS I are not a perfect comparison group. While they did not have HCT treatment, they had less severe disease. A comparison group with neither MPS nor HCT treatment would not have allowed us to examine either of those factors.
While obtaining larger samples in rare diseases is a challenge, we are currently conducting a confirmatory prospective longitudinal study with a larger sample as well as a control group to further explore the role of specific disease, treatment and risk factors in the long-term outcomes of children with MPS I.
Supplementary Material
Highlights.
Attention problems were found in MPS IH compared to attenuated MPSI.
Fractional anisotropy was lower in MPS IH than in attenuated MPS
Fractional anisotropy was associated with attention problems in MPS IH
Fractional anisotropy was associated with frequency of medical events
Transplant and disease severity affects white matter connectivity and attention
Acknowledgments
The study was supported by grants from Genzyme Corporation, Biomarin Pharmaceuticals, Minnesota Medical Foundation, the Ryan Foundation and the Lysosomal Disease Network NIH U54NS065768 and the resources of the Center for Magnetic Resonance Research and the Center for Neurobehavioral Development at the University of Minnesota.
We thank the participants for their enthusiastic cooperation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
O. Evren Guler, Email: guler@augsburg.edu.
Kyle Rudser, Email: rudser@umn.edu.
Kendra Bjoraker, Email: Kendra.Bjoraker@childrenscolorado.org.
Chester Whitley, Email: whitley@umn.edu.
Jakub Tolar, Email: tolar003@umn.edu.
Paul Orchard, Email: orcha001@umn.edu.
Kathleen Thomas, Email: thoma114@umn.edu.
References
- 1.Boelens J, Prasad V, Tolar J, Wynn R, Peters C. Current International Perspectives on Hematopoietic Stem Cell Transplantation for Inherited Metabolic Disorders. Pediatr Clin North Am. 2010;57:123–145. doi: 10.1016/j.pcl.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 2.Peters C, Balthazor M, Shapiro EG, et al. Outcome of unrelated donor bone marrow transplantation in 40 children with Hurler syndrome. Blood. 1996;87:4894–4902. [PubMed] [Google Scholar]
- 3.Peters C, Shapiro EG, Anderson J, et al. Hurler syndrome: II Outcome of HLA-genotypically identical sibling and HLA- haploidentical related donor bone marrow transplantation in fifty-four children. The Storage Disease Collaborative Study Group. Blood. 1998;91:2601–2608. [PubMed] [Google Scholar]
- 4.Aldenhoven M, Boelens JJ, de Koning TJ. The clinical outcome of Hurler syndrome after stem cell transplantation. Biol Blood Marrow Transplant. 2008;14:485–498. doi: 10.1016/j.bbmt.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 5.Anderson FS, Kunin-Batson AS, Perkins JL, et al. White versus gray matter function as seen on neuropsychological testing following bone marrow transplant for acute leukemia in childhood. Neuropsychiat Dis Treatment. 2008:283–288. doi: 10.2147/ndt.s2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campbell LK, Scaduto M, Sharp W, et al. A Meta-Analysis of the Neurocognitive Sequelae of Treatment for Childhood Acute Lymphocytic Leukemia. Pediatr Blood Cancer. 2007;49:65–73. doi: 10.1002/pbc.20860. [DOI] [PubMed] [Google Scholar]
- 7.Anderson FS, Kunin-Batson AS. Neurocognitive Late Effects of Chemotherapy in Children: The Past 10 Years of Research on Brain Structure and Function. Pediatr Blood Cancer. 2009;52:159–164. doi: 10.1002/pbc.21700. [DOI] [PubMed] [Google Scholar]
- 8.Reddick WE, Shan ZY, Glass JO, et al. Smaller white-matter volumes are associated with larger deficits in attention and learning among long-term survivors of acute lymphoblastic leukemia. Cancer. 2006;106:941–949. doi: 10.1002/cncr.21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khong PL, Leung LH, Fung AS, et al. White matter anisotropy in post-treatment childhood cancer survivors: Preliminary evidence of association with neurocognitive function. J Clin Oncol. 2006;24:884–890. doi: 10.1200/JCO.2005.02.4505. [DOI] [PubMed] [Google Scholar]
- 10.Aukema EJ, Caan MW, Nienke O, et al. White matter fractional anisotropy correlates with speed of processing and motor speed in young childhood cancer survivors. Int J Radiation Oncology Biol Phys. 2009;74:837–843. doi: 10.1016/j.ijrobp.2008.08.060. [DOI] [PubMed] [Google Scholar]
- 11.Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: Concepts and applications. J Magn Reson Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 12.Mori S, Zhang J. Principles of Diffusion Tensor Imaging and Its Applications to Basic Neuroscience Research. Neuron. 2006;51:527–539. doi: 10.1016/j.neuron.2006.08.012. [DOI] [PubMed] [Google Scholar]
- 13.MATLAB. The Mathworks, Inc; Natick, MA: http://www.mathworks.com. [Google Scholar]
- 14.Jiang H, Mori S. DTI Studio. John Hopkins University; Baltimore, MD: http://www.mristudio.org. [Google Scholar]
- 15.Gabrielli O, Polonara G, Regnicolo L, et al. Correlation between cerebral MRI abnormalities and mental retardation in patients with mucopolysaccharidoses. Amer J Med Genet. 2004;12:224–31. doi: 10.1002/ajmg.a.20515. [DOI] [PubMed] [Google Scholar]
- 16.Lee C, Dineen TE, Brack M, et al. The mucopolysaccharidoses: characterization by cranial MR imaging. Am J Neuroradiol. 1993;14:1285–1292. [PMC free article] [PubMed] [Google Scholar]
- 17.Matheus G, Castillo M, Smith JK, et al. Brain MRI findings in patients with mucopolysaccharidosis types I and II and mild clinical presentation. Neuroradiology. 2004;46:666–672. doi: 10.1007/s00234-004-1215-1. [DOI] [PubMed] [Google Scholar]
- 18.Wechsler D. Wechsler Abbreviated Scale of Intelligence. Harcourt Assessments; San Antonio, TX: 1999. [Google Scholar]
- 19.Greenberg LM, Kindschi C. TOVA Test of Variables of Attention: Clinical Guide. Universal Attention Disorders; Los Alamitos, CA: 1999. [Google Scholar]
- 20.Benton AL, de Hamsher KS, Varney NR, et al. Contributions to neuropsychological assessment. Oxford University Press; New York: 1983. [Google Scholar]
- 21.Delis D, Kramer JH, Kaplan E, et al. The California Verbal Learning Test- Childrens Edition Manual. Psychological Corporation; San Antonio TX: 1994. [Google Scholar]
- 22.R Development Core Team R. A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna Austria: 2011. URL http://www.R-project.org/ [Google Scholar]
- 23.Barnea-Goraly N, Menon V, Eckert M, et al. White Matter Development During Childhood and Adolescence: A Cross- sectional Diffusion Tensor Imaging Study. Cerebral Cortex. 2005;15:1848–1854. doi: 10.1093/cercor/bhi062. [DOI] [PubMed] [Google Scholar]
- 24.Muenzer J, Wraith JE, Clarke LA the International Consensus Panel on the Management and Treatment of Mucopolysaccharidosis I. Mucopolysaccharidosis I: Management and Treatment. Pediatrics. 2009;123:19–29. doi: 10.1542/peds.2008-0416. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro E, Balthazor M. Metabolic and neurodegenerative disorders of childhood. In: Taylor G, Ris D, Yeates K, editors. Pediatric Neuropsychology: Research, Theory and Practice. Vol. 2000. Guilford Press; New York: 2000. pp. 171–205. [Google Scholar]
- 26.Shapiro E. Risk Factors Contributing To Neurolinguistic Development Following Hematopoietic Cell Transplant In Hurler Syndrome. Australian Society for the Study of Brain Impairment & International Neuropsychological Society (INS) Annual Meeting; Brisbane, Australia. 2004. [Google Scholar]
- 27.Wraith JE. The mucopolysaccharidoses: a clinical review and guide to management. Arch Dis Child. 1995;72:263–267. doi: 10.1136/adc.72.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Muenzer J, Fisher A. Advances in the Treatment of Mucopolysaccharidosis Type I. N Engl J Med. 2004;350:1932–1934. doi: 10.1056/NEJMp048084. [DOI] [PubMed] [Google Scholar]
- 29.Staba SI, Escolar ML, Poe M, et al. Cord-blood transplants from unrelated donors in patients with Hurler’s syndrome. N Engl J Med. 2004;350:1960–1969. doi: 10.1056/NEJMoa032613. [DOI] [PubMed] [Google Scholar]
- 30.Souillet G, Guffon N, Maire I, et al. Outcome of 27 patients with Hurler’s syndrome transplanted from either related or unrelated haematopoietic stem cell sources. Bone Marrow Transplant. 2003;31:1105–1117. doi: 10.1038/sj.bmt.1704105. [DOI] [PubMed] [Google Scholar]
- 31.Vijay S, Wraith JE. Clinical presentation and follow-up of patients with the attenuated phenotype of mucopolysaccharidosis type I. Acta Paediat. 2005;94:872–877. doi: 10.1111/j.1651-2227.2005.tb02004.x. [DOI] [PubMed] [Google Scholar]
- 32.Elkin TD, Megason G, Robinson A, et al. Longitudinal neurocognitive outcome in an adolescent with Hurler-Scheie syndrome. Neuropsychiat Dis Treatment. 2006;2:381–384. doi: 10.2147/nedt.2006.2.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bjoraker K, Charnas L, Whitley CB, et al. The natural history of cognitive abilities in Hurler-Scheie syndrome: Eight years longitudinal evaluation of three siblings treated with recombinant human alpha –L-iduronidase (laronidase, Aldurazyme®). American Society of Human Genetics; Salt Lake City UT. 2005. [Google Scholar]
- 34.Bjoraker K, Delaney K, Peters C, et al. Long Term Outcomes of Adaptive Functions for Children with MPS I. J Beh Dev Peds. 2006;27:290–296. doi: 10.1097/00004703-200608000-00002. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence YR, Li XA, el Naqa I, et al. Radiation dose-volume effects in the brain. Int J Radiat Oncol Biol Phys. 2010;76:S20–27. doi: 10.1016/j.ijrobp.2009.02.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.