Abstract
One of the greatest concerns in Chagas' disease is the absence of reliable methods for the evaluation of chemotherapy efficacy in treated patients. The tests available to evaluate cure after the specific treatment are the complement-mediated lysis (CoML) and flow cytometry tests, but they are not feasible for routine clinical use. In this study, we evaluated an enzyme-linked immunosorbent assay (ELISA) based on the recombinant Trypanosoma cruzi complement regulatory protein (rCRP) as a method to determine parasite clearance in comparison to the CoML and other methods such as conventional serology, hemoculture, and PCR in serum samples of 31 patients collected before and after the treatment, monitored for an average of 27.7 months after chemotherapy. The results showed that the percentage of patient samples that were positive by rCRP ELISA was reduced from 100 to 70.3, 62.5, 71.4, and 33.4% in the first, second, third, and fourth years after treatment, respectively, while the samples positive by CoML were reduced to 85.2, 81.2, 71.4, and 33.4% during the same period, demonstrating the same significant tendency in the reduction of positive samples. On the other hand, the conventional serology (CS) tests did not present this reduction. The percentage of samples positive by PCR was initially 77.4% and decreased to 55.5, 68.7, 47.7, and 50.0% at the fourth year after treatment, confirming the drastic clearance of circulating parasites after treatment. Our results strongly suggest that the rCRP ELISA was capable of detecting the early therapeutic efficacy in treated patients and confirmed its superiority over the CS tests and parasitologic methods.
Chagas' disease remains a major public health concern in regions of Latin America, where approximately 15 million people are infected (50). Following a brief acute phase, the infection can progress to a lifelong chronic phase in which the paucity of bloodstream parasites makes their direct detection difficult. Diagnosis of Chagas' disease generally occurs during the chronic phase by conventional serology (CS), e.g., indirect immunofluorescence, indirect hemagglutination, and enzyme-linked immunosorbent assay (ELISA). Detection of parasites in the blood during the chronic phase is difficult and generally accomplished by indirect methods such as xenodiagnosis and hemoculture (8, 12, 14, 15, 42). Although the sensitivity of these methods has been improved recently (10, 13, 20, 21, 25), they are laborious and involve the culture of live infectious parasites, besides being slow and time-consuming, thus limiting their usefulness.
Treatment of Chagas' disease patients with nitroimidazole-derivative drugs can prevent the pathological outcomes of chronic disease, and treatment has been associated with cure in some cases. Treatment of all acute-phase patients is therefore indicated; however, its efficacy in chronic-phase patients remains controversial (11). Nevertheless, treatment of patients in the early chronic phase, before the development of overt clinical symptoms, has been advocated. Evaluation of the efficacy of these and other drugs, particularly during the chronic phase, is hampered by the difficulty in determining parasite levels in the blood after treatment. In addition, long-term follow-up studies of some treated patients have demonstrated that CS tests may remain positive for several years despite repeated negative direct parasite detection tests, such as hemoculture or xenodiagnosis (18, 21). An alternative to CS testing as an assessment of parasite clearance has been proposed by Krettli and Brener (30), who demonstrated that antibody-dependent, complement-mediated lysis (CoML) is a more reliable indicator of parasitological cure. In extensive long-term patient studies, it was observed that sera from patients with positive hemocultures supported CoML of trypomastigotes, whereas sera from patients with repeated negative hemocultures had diminished or no lytic capacity (21). The presence of lytic antibodies has therefore been proposed as an indicator of an active ongoing infection and treatment failure (21, 28). This test also requires the use of live, infectious trypomastigotes and is thus not practical for routine use in the evaluation of chemotherapeutic agents or in the clinical management of chagasic patients (27).
One target of lytic antibodies is a 160-kDa glycoprotein present on the surface of trypomastigotes (34, 37). The 160-kDa protein functions to restrict alternative and classical complement activation and lysis of the parasites (37). The T. cruzi complement regulatory protein (CRP) binds to the complement components C3b and C4b, thus inhibiting assembly of the C3 convertase, the central enzyme in the complement cascade. Antibodies raised to the purified CRP were shown to lyse trypomastigotes in CoML assays, presumably by neutralizing its complement regulatory activity and allowing full complement activation (36). Immunoreactivity of chagasic patient sera with the CRP correlated with the results of the CoML test and was therefore proposed as a specific indicator of parasite clearance and drug efficacy (40). In these studies, the CRP was purified in low yield from infectious trypomastigotes, thus limiting the feasibility of routine use. The T. cruzi CRP has been cloned, and the recombinant protein has been purified from expression systems (4, 39). We have recently developed and characterized a highly specific and sensitive ELISA-based test for the diagnosis of Chagas' disease using the T. cruzi CRP recombinant form (rCRP) (35). Because of the critical need for rapid tests to be used in monitoring cure of chagasic patients after specific treatment, we compared the efficacy of the rCRP ELISA with CoML, CS, PCR, and hemoculture methods in the present study.
MATERIALS AND METHODS
Human sera.
A total of 31 patients in the chronic phase of Chagas' disease were evaluated in this study. Patient blood samples were collected at the Ambulatório de Doença de Chagas, Hospital das Clínicas, Universidade Federal de Minas Gerais (Belo Horizonte, Minas Gerais, Brazil), and the patients were treated with benznidazole (N-benzyl-2-nitro-1-imidazolacetamide), which was given by mouth in daily doses of 5 mg/kg of body weight, twice a day for 60 days according to the rules of the technical management from the Brazilian Health Ministry (19), which suggests the treatment of the patients with the indeterminate form at the chronic phase of the disease. Samples were collected prior to treatment and at least once after chemotherapy with a minimum interval of 24 months between them. This study was carried out with the consent of the participants and was approved by the Ethics Committee of the Hospital das Clínicas/087/99, Universidade Federal de Minas Gerais.
ELISA.
The recombinant T. cruzi CRP was purified as previously reported (4, 34), and the rCRP ELISA was carried out as described elsewhere (35). Briefly, the 96-well plates were coated with 125 ng of the purified T. cruzi rCRP, and the assay was performed using the primary and secondary antibodies diluted at 1:200 and 1:7,500, respectively. The cutoff (CO) values were calculated for each plate as follows: CO = m + 2 SD, in which m is the absorbance average of the negative controls (n = 8) and SD is standard deviation.
CoML.
Lytic antibodies were detected by the CoML test (21, 29) as follows: 6 × 106 to 7 × 106 trypomastigotes per ml were incubated with human serum as complement source at 37°C for 30 min. After incubation, an aliquot was evaluated to assure total resistance to complement lysis in the absence of immune serum. Aliquots of the suspension of trypomastigotes (50 μl) and the test serum (50 μl), diluted two- and fourfold, were mixed and incubated at 37°C for 30 min and placed on ice. Fifty microliters of human serum as complement source was added to 50 μl of each sample, and the parasites were counted in a hemocytometer. The tubes were incubated at 37°C for 45 min and replaced on ice, and the parasites were recounted. The lysis percentage was calculated as 100 − (number of parasites in sample at 45 min) × 100/(initial number of parasites in sample).
Sera adsorption.
All patient serum samples were adsorbed with rabbit red cells for the removal of the nonspecific anti-Gal antibodies (46, 47). The adsorption was carried out as follows: 5 ml of rabbit blood was collected in vacuum tubes containing sodium heparin. Five milliliters of phosphate-buffered saline (PBS) 1× was added to the blood, and the mixture was cleared by centrifugation at 1,500 × g for 10 min. Cells were washed twice with PBS and recovered by centrifugation as described above. The cell pellet was resuspended in 4 ml of PBS 1×, and 400-μl aliquots of this solution were distributed in 1.5-ml Microfuge tubes, and the cells were collected by centrifugation for 5 min at 1,500 × g. For the adsorption, 200 μl of each patient serum was added to the red-cell pellet, mixed, and incubated at room temperature for 1 h. After incubation, the mixture was cleared by centrifugation as described above, and the supernatant was removed and stored at −20°C until use.
Hemoculture.
The technique was carried out on patient samples as described previously (13). Briefly, 30 ml of venous blood was collected in heparinized tubes and red cells were recovered from the plasma by centrifugation at 300 × g for 10 min at 4°C. The plasma supernatant was centrifuged at 900 × g at 4°C for 20 min, and 3 ml of liver infusion tryptose (LIT) medium (9) was added to the pellet. The packed red blood cells were washed once and resuspended in 6 ml of LIT, mixed, and distributed into six plastic tubes (Falcon), each containing 3 ml of LIT. All tubes were maintained at 27 to 28°C, mixed gently twice weekly, and examined monthly for up to 120 days. Ten microliters of each preparation was examined microscopically under 22-mm2 coverslips at a magnification of ×150.
Preparation of DNA for PCR.
DNA was prepared from 10 ml of blood taken at the time of hemoculture preparation as described before (2, 3). The samples were stored for 5 days at room temperature, boiled at 100°C for 15 min, and stored at 4°C, when a 200-μl aliquot of blood was collected from each sample and DNA extraction was performed (7, 22).
PCR conditions.
PCR amplification was performed with primers 121 [5′-AAATAATGTACGG(T/G)GAGATGCATGA-3′] and 122 (5′-GGTTCGATTGGGGTTGGTGTAATATA-3′), described by Degrave et al. (17) and synthesized by Operon Technologies, Inc.; these primers amplify a 330-bp fragment of the variable region of the T. cruzi kinetoplast DNA minicircle. The reaction was performed as described before (22), and the PCR products were visualized by 6% polyacrylamide gel electrophoresis after silver staining (41). All DNA extraction steps and reaction mixtures used for PCR were monitored and compared with positive and negative controls. To avoid contamination the reaction steps were performed in separate environments, using equipment and reagents destined exclusively to each stage. To test whether inhibition of the reaction was occurring, DNA from tissue culture-derived T. cruzi was obtained and used as a positive control. The size of the PCR products was monitored using a 100-bp-ladder molecular size marker (Promega Corp.).
Slot blot hybridization.
To confirm the specificity of the amplified product and increase the sensitivity of the protocol, PCR samples were subjected to the acid nucleic hybridization as described (45). When PCR alone is employed, 10 fg of parasite DNA was detected in polyacrylamide gel, whereas the limit of detection by hybridization was 0.1 fg (22).
Data analysis.
The positive percentage for each reaction and in each period of time was obtained from a contingency table 2×C, in which the data were classified as positive and negative and in five groups according to the period of time. The differences among the positive percentages, observed in the different periods of time for each reaction, were tested using the chi-square test as described by Snedecor and Cochran (43). To evaluate the positive percentage variation obtained for each reaction in the different periods of time after treatment, the data were converted using the arcsin-square root method and tested using the Pearson correlation coefficient and linear regression (43). The differences among the arcsin-square root tendencies of the positive reaction percentages obtained for each method were tested using covariance analysis (43). For every analysis we used the significance level of 0.05.
RESULTS
We compared the rCRP ELISA and other methods such as CoML, CS, PCR, and hemoculture to evaluate therapeutic efficacy for 31 treated chagasic patients. The average time after treatment was 27.7 months, and the patients were separated in five groups for test comparisons: before treatment (n = 31), until 12 months or first year (n = 27), between 13 and 24 months or second year (n = 16), from 25 to 36 months or third year (n = 21), and more than 37 months or fourth year (n = 6) after the treatment. The analysis was made by the comparison among the results obtained in the same methods by different periods of treatment.
The rCRP ELISA-positive percentage results reduced to 70.3, 62.5, 71.4, and 33.4% of the serum samples from the first, second, third, and fourth groups, respectively (Table 1). The CoML test before adsorption-positive percentages decreased to 96.2, 81.2, 85.7, and 86.4% of those patient serum groups after treatment. When these same samples were subjected to the adsorption protocol, the percentage of positive samples decreased to 85.2, 81.2, 71.4, and 33.4% for the different periods (Table 1). The comparison between the results produced by rCRP ELISA and CoML test using the patients' sera after adsorption showed that the recombinant ELISA presented a higher ability to detect the early therapeutic success or failure from 6 to 12 months after treatment. These results also showed the importance of the serum adsorption to reduce false-positive results due to the reaction against nonspecific anti-Gal antibodies. Furthermore, the results demonstrate that the rCRP ELISA and CoML were in agreement, particularly in the later time points after treatment.
TABLE 1.
Percentages of positive rCRP ELISA, CoML, hemoculture, PCR, and conventional serology tests on sera of chagasic patients
| Time (n)a | % Positiveb
|
|||||
|---|---|---|---|---|---|---|
| rCRP | CoML | CoML adsorbed | Hemoculture | PCR | CS | |
| Before treatment (31) | 100 | 100 | 100 | 51.6 | 64.5 | 100 |
| 1st yr (27) | 70.3 | 96.2 | 85.2 | 3.8 | 44.4 | 100 |
| 2nd yr (16) | 62.5 | 81.2 | 81.2 | 6.2 | 62.5 | 100 |
| 3rd yr (21) | 71.4 | 85.7 | 71.4 | 9.6 | 42.9 | 95.2 |
| 4th yr (6) | 33.4 | 86.4 | 33.4 | 0 | 33.4 | 83.3 |
n, number of serum samples.
χ2 values were as follows: for rCRP, 18.21; for CoML adsorbed, 16.12; and for hemoculture, 28.89. P values were as follows: for rCRP, 0.00112; for CoML, not significant; for CoML adsorbed, 0.0028; for PCR, not significant; and for conventional serology, not significant.
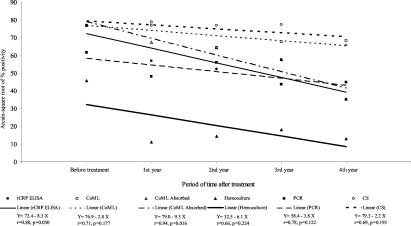
A significant reduction was observed in the positive hemoculture results from the first year after treatment. This test was positive in 51.6% of the patients before treatment, and this value decreased to 3.8, 6.2, 9.6, and 0.0% in the 4 years following treatment, respectively (Table 1). On the other hand the PCR followed by hybridization presented higher positive percentages, since this was positive in 77.4% of the patients before treatment and became negative in 45.5, 31.3, 52.3, and 50.0% of them in the four following years after treatment, but a significant difference among these percentages was not observed (Table 1). As expected, the results obtained by the CS tests did not show any reduction in the percentage of positive samples after the specific treatment. With the purpose to examine whether the linear regressions of arcsin-square root of positive percentage on years after treatment are the same in rCRP ELISA and other tests, we used the analysis of covariance, assuming homogeneity of residual variances (P = 0.170), and the results are shown in Fig. 1. No significant difference (P = 0.693) was observed compared to the regression coefficients obtained by hemoculture (β = −8.5), CS (β = −6.1), PCR (β = −3.8), and CoML (β = −5.4). The comparison among the regression coefficients obtained by CoML before (β = −5.4) and after (β = −11.9) the adsorption of sera demonstrated a significantly higher tendency toward positive percentage reduction when the test was performed with adsorbed samples (P = 0.041). No significant difference (P = 0.759) was shown among the regression coefficients between CoML using adsorbed sera (β = −11.9) and rCRP ELISA (β = −10.9).
FIG. 1.
Comparison between the arcsin-square root of positive percentage values and linear regressions of results from each method obtained for the group of treated chronic chagasic patients before and during the 4 years after treatment. CoML absorbed, CoML with sera after adsorption.
DISCUSSION
In Chagas' disease, the evaluation of clinical and parasitologic cure has been the subject of much study and controversy, and the lack of a definitive method for evaluation of parasite control or clearance has impaired the development of improved treatments, particularly for the chronic phase (16). The CS tests, which are very important for the diagnosis of the chronic infection with T. cruzi, have limitations mainly when used as a criterion of therapeutic cure (26). This was verified in a follow-up study of 100 patients subjected to specific treatment; the cure level was only 8% if the negative CS was used as a criterion. However, 60% of these patients had repeatedly negative results by xenodiagnosis (18). In another 10-year follow-up study of 82 treated patients, only 9% of the patients could be considered cured on the basis of the negative CS results, but this value increased to 34% when the CoML results were considered. These patients, described as dissociated, were considered cured, and their hemoculture tests were repeatedly negative (21).
In the present study, we evaluated the use of an ELISA using the recombinant T. cruzi CRP protein to detect therapeutic efficacy in chronic chagasic patients after treatment. The CRP is a trypomastigote-specific protein that was found to be a principal effector molecule restricting complement-mediated lysis of the parasites, and it also elicits the production of lytic antibodies in humans (37, 38, 40). The lytic capacity of the anti-CRP antibodies is believed to be due to the neutralization of the CRP complement-inhibitory activity (36). The full-length cDNA encoding the T. cruzi CRP was previously isolated (39) and was stably transfected in mammalian cells, allowing its large-scale production and purification (4). The isolation of the T. cruzi CRP recombinant form was followed by its evaluation in an ELISA for the diagnosis of chronic chagasic patients. These results demonstrated the high sensitivity of the rCRP ELISA for diagnosis (100%) compared with the CS, CoML, and PCR tests, which are also highly sensitive (35).
As the T. cruzi CRP is a target of complement lytic antibodies and the CoML test has been shown to be the one of the best evaluation methods of an ongoing infection, we sought to determine whether the rCRP ELISA could effectively replace the CoML test and other diagnostic tests to monitor the therapeutic efficacy in treated chagasic patients. For this evaluation we used a group of 31 patients which were treated with benznidazole according to the guidelines of the Chagas' disease division of the Brazilian Health Ministry (19). We found that the percentage of positive blood samples in the rCRP ELISA declined in the years following treatment, showing a significant reduction from the first year after treatment (70.3%). The reduction of the percentage of positive samples was also observed in CoML test mainly by the third year posttreatment, when both methods showed comparable levels of positive percentages. Although there was some difference observed in the first 2 years, the comparison of linear regression of the positive percentage by year showed the absence of significant difference, confirming the high identity between results of these two methods. The comparison of the CoML results from sera before and after adsorption were used showed the necessity of the adsorption procedure to remove most of the nonspecific antibodies and consequently reduce the occurrence of false-positive results. This analysis confirmed a significant difference among their positive percentage reduction tendencies, as well if it were compared with the rCRP ELISA data. The reduction in the percentage of positive samples observed for the rCRP ELISA and the CoML tests in the fourth year posttreatment was not considered significant due to the low number of patients of this group (n = 6). However, an individual follow-up evaluation of the patients demonstrated that once the patients' rCRP ELISA tests became negative, this result remain unaltered for the subsequent samples. Negative serum conversion occurred in 37 of 58 treated children, compared to 3 negative children among the 54 in the placebo group using ELISA with a highly specific carbohydrate-rich trypomastigote antigen (1). In an examination of sera from children in the acute phase and in early chronic phase following treatment, other investigators reported similar declines in antibody recognition after several years of follow-up (1, 33, 44).
Considering the high sensitivity and specificity of the CoML test in detecting lytic antibodies and the absence of significant difference between the positive percentage reduction tendencies of this test and the rCRP ELISA in the posttherapeutic patient evaluation, we conclude that the rCRP ELISA is an efficient and specific indicator of parasite clearance and suggest its use as a cure criterion. This elevated correlation between these two tests corroborates the previous data obtained by Norris et al. (40), which demonstrated the total concordance among the CoML test and the immunoprecipitation using the native T. cruzi CRP in nontreated, dissociated, and cured chronic chagasic patients.
The results obtained by the CS tests did not become negative in the first 2 years posttreatment, and in the following years, although some patient samples became negative, these results were neither statistically significant nor consistent. These results confirmed the persistence of the CS antibodies in the patients' sera for several years after treatment as previously shown (21, 24, 27, 28). The hemoculture test presented a very high level of negative results from the first year after treatment, reaching 100% in the last year of follow-up. However, this reduction cannot be considered a reliable indicator of cure, since it has a relatively low sensitivity, especially in treated patients where the benznidazole action leads to a rapid destruction of T. cruzi bloodstream trypomastigotes forms and is not efficient in all patients (21, 24). It may therefore produce false-negative results in cases of low-level parasitemia, yielding inconclusive results posttreatment.
PCR, considered to be a highly sensitive method to detect T. cruzi in blood of chronic chagasic patients (10, 22, 31, 35), also resulted in a positive percentage reduction during the years posttreatment. Our results corroborate previous data that have suggested the PCR is an excellent technique for posttherapeutic evaluation of treated chagasic patients and also for monitoring T. cruzi persistence during the specific treatment (6, 23). The PCR technique may be a rapid and safe indicator of the susceptibility of the parasite to the action of the drugs, allowing early modification of therapy in cases of resistance or reactivation of chagasic infection (32). The data presented here show the superiority of the immunological tests over parasitological ones, particularly the high sensitivity of the rCRP ELISA. The differences of PCR sensitivity could be explained by the intermittent presence and quantity of circulating parasites at the time of blood collection as shown previously (10, 11). Moreover, different populations of T. cruzi with high genetic variability are found in different regions of endemicity and may be related to biological variability as virulence or susceptibility to the immune response, influencing parasitemia levels in humans (5). In studies carried out in different areas of endemicity of countries such as Bolivia and Brazil, where T. cruzi II stocks are frequent, PCR showed higher sensitivity than it did in areas of the Amazonian Basin, where only T. cruzi I stocks circulate (48, 49, 51).
In conclusion, our results demonstrate that the rCRP ELISA test was superior to other established methods as a specific marker of therapeutic efficacy in treated chronic chagasic patients. In addition, the easy handling and development of the assay opened new perspectives for its use in routine clinical laboratories and blood banks, since its large-scale production would likely make this assay available at low cost.
Acknowledgments
This work was supported by CNPq (Conselho Nacional de Pesquisa e Desenvolvimento Científico e Tecnológico), CAPES (Coordenação de Aperfeiçoamento de Pessoal do Ensino Superior), FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais), and the National Institutes of Health (Public Health Service grant AI-32719).
We thank Orlando Carlos Magno and Afonso Da Costa Viana for technical assistance, Renato Saphler Avelar for the harvest of blood from the patients, and Renato Assuncao for suggestions on the statistical analysis.
REFERENCES
- 1.Andrade, A. L. S. S., F. Zicker, R. M. Oliveira, S. Almeida-Silva, A. O. Luquetti, L. R. Travassos, I. C. Almeida, S. S. Andrade, J. G. Andrade, and C. M. Martelli. 1996. Randomized trial of efficacy of benznidazole in treatment of early Trypanosoma cruzi infection. Lancet 348:1407-1413. [DOI] [PubMed] [Google Scholar]
- 2.Ávila, H. A., A. M. Gonçalves, N. S. Nehme, C. M. Morel, and L. Simpson. 1990. Schizodeme analysis of Trypanosoma cruzi stocks from South and Central America by analysis of PCR amplified minicircle variable region sequence. Mol. Biochem. Parasitol. 42:175-188. [DOI] [PubMed] [Google Scholar]
- 3.Ávila, H. A., D. S. Sigman, L. M. Cohen, R. C. Millikan, and L. Simpson. 1991. Polymerase chain reaction amplification of Trypanosoma cruzi kinetoplast minicircle DNA isolated from whole blood lysates: diagnosis of chronic Chagas' disease. Mol. Biochem. Parasitol. 48:211-222. [DOI] [PubMed] [Google Scholar]
- 4.Beucher, M., W. S. F. Meira, V. Zegarra, L. M. C. Galvão, E. Chiari, and K. A. Norris. 2003. Expression and purification of functional, recombinant Trypanosoma cruzi complement regulatory protein. Protein Exp. Purif. 27:19-26. [DOI] [PubMed] [Google Scholar]
- 5.Brenière, S. F., M. F. Bosseno, F. Noireau, N. Yacsik, P. Liegeard, C. Aznar, and M. Hontebeyrie. 2002. Integrate study of a Bolivian population infected by Trypanosoma cruzi, the agent of Chagas disease. Mem. Inst. Oswaldo Cruz 97:289-295. [DOI] [PubMed] [Google Scholar]
- 6.Britto, C., C. Silveira, M. A. Cardoso, P. Marques, A. Luquetti, V. Macedo, and O. Fernandes. 2001. Parasite persistence in treated chagasic patients revealed by xenodiagnosis and polymerase chain reaction. Mem. Inst. Oswaldo Cruz 96:823-826. [DOI] [PubMed] [Google Scholar]
- 7.Britto, C., M. A. Cardoso, P. Wincker, and C. M. Morel. 1993. A simple protocol for the physical cleavage of Trypanosoma cruzi kinetoplast DNA present in blood samples and its use in polymerase chain reaction (PCR)-based diagnosis of chronic Chagas disease. Mem. Inst. Oswaldo Cruz 88:171-172. [DOI] [PubMed] [Google Scholar]
- 8.Bronfen, B., F. S. A. Rocha, G. B. N. Machado, M. M. Perillo, A. J. Romanha, and E. Chiari. 1989. Isolamento de amostras do Trypanosoma cruzi por xenodiagnóstico e hemocultura de pacientes na fase crônica da doença de Chagas. Mem. Inst. Oswaldo Cruz 84:237-240. [DOI] [PubMed] [Google Scholar]
- 9.Camargo, E. P. 1964. Growth and differentiation in Trypanosoma cruzi. Origin of metacyclic trypanosomes in liquid media. Rev. Inst. Med. Trop. São Paulo 6:93-100. [PubMed] [Google Scholar]
- 10.Castro, A. M., A. O. Luquetti, A. Rassi, G. G. Rassi, E. Chiari, and L. M. C. Galvão. 2002. Blood culture and polymerase chain reaction for the diagnosis of the chronic phase of human infection with Trypanosoma cruzi. Parasitol. Res. 88:894-900. [DOI] [PubMed] [Google Scholar]
- 11.Castro, C., and A. Prata. 2000. Absence of both circadian rhythm and Trypanosoma cruzi periodicity with xenodiagnosis in chronic chagasic individuals. Rev. Soc. Bras. Med. Trop. 33:427-430. [DOI] [PubMed] [Google Scholar]
- 12.Chiari, E., and J. C. P. Dias. 1975. Nota sobre uma nova técnica de hemocultura para diagnóstico parasitológico na doença de Chagas na sua fase crônica. Rev. Soc. Bras. Med. Trop. IX:133-136. [Google Scholar]
- 13.Chiari, E., J. C. P. Dias, M. Lana, and C. A. Chiari. 1989. Hemocultures for the parasitological diagnosis of human chronic Chagas' disease. Rev. Soc. Bras. Med. Trop. 22:19-23. [DOI] [PubMed] [Google Scholar]
- 14.Chiari, E., and Z. Brener. 1966. Contribuição ao diagnóstico parasitológico da doença de Chagas na sua fase crônica. Rev. Inst. Med. Trop. São Paulo 8:134-138. [PubMed] [Google Scholar]
- 15.Coura, J. R., L. L. Abreu, H. P. F. Wilcox, N. Anunziato, and W. Petana. 1991. Evaluation of the xenodiagnosis of chronic Chagas patients infected ten years or over in an area where transmission has been interrupted—Iguatama and Pains, west Minas Gerais State, Brazil. Mem. Inst. Oswaldo Cruz 86:395-398. [DOI] [PubMed] [Google Scholar]
- 16.Coura, J. R., and S. L. Castro. 2002. A critical review on Chagas disease chemotherapy. Mem. Inst. Oswaldo Cruz 97:3-24. [DOI] [PubMed] [Google Scholar]
- 17.Degrave, W., S. P. Fragoso, C. Britto, H. van-Heuverswyn, G. Z. Kidane, M. A. B. Cardoso, R. U. Mueller, L. Simpson, and C. M. Morel. 1988. Peculiar sequence organization of kinetoplast DNA minicircles from Trypanosoma cruzi. Mol. Biochem. Parasitol. 27:63-70. [DOI] [PubMed] [Google Scholar]
- 18.Ferreira, H. O. 1990. Treatment of undetermined form of Chagas disease with nifurtimox and benznidazole. Rev. Soc. Bras. Med. Trop. 23:209-211. [DOI] [PubMed] [Google Scholar]
- 19.Fundação Nacional de Saúde. 1997. Tratamento etiológico da doença de Chagas-Brasília. Coordenação de controle de doenças transmitidas por vetores—gerência técnica de doença de Chagas, 2nd ed. 32. Fundação Nacional de Saúde, Rio de Janeiro, Brazil.
- 20.Galvão, L. M. C., J. R. Cançado, D. F. Rezende, and A. U. Krettli. 1989. Hemocultures from chronic chagasic patients using EDTA or heparin as anticoagulants. Braz. J. Med. Biol. Res. 22:841-843. [PubMed] [Google Scholar]
- 21.Galvão, L. M. C., R. M. B. Nunes, J. R. Cançado, Z. Brener, and A. U. Krettli. 1993. Lytic antibody titre as a means of assessing cure after treatment of Chagas disease: a 10 years follow-up study. Trans. R. Soc. Trop. Med. Hyg. 87:220-223. [DOI] [PubMed] [Google Scholar]
- 22.Gomes, M. L., A. M. Macedo, A. R. Vago, S. D. J. Pena, L. M. C. Galvão, and E. Chiari. 1998. Trypanosoma cruzi: optimization of polymerase chain reaction for detection in human blood. Exp. Parasitol. 88:28-33. [DOI] [PubMed] [Google Scholar]
- 23.Gomes, M. L., L. M. C. Galvão, A. M. Macedo, S. D. J. Pena, and E. Chiari. 1999. Chagas' disease diagnosis: comparative analysis of parasitologic, molecular and serologic methods. Am. J. Trop. Med. Hyg. 60:205-210. [DOI] [PubMed] [Google Scholar]
- 24.Gontijo, E. D., L. M. C. Galvão, and S. Eloi-Santos. 1999. Chagas disease: criteria of cure and prognosis. Mem. Inst. Oswaldo Cruz 94(Suppl. I):357-362. [DOI] [PubMed] [Google Scholar]
- 25.Junqueira, A. C. V., E. Chiari, and P. Wincker. 1996. Comparison of polymerase chain reaction with two classical parasitological methods for diagnosis of Chagas disease patients in an endemic region of north-eastern Brazil. Trans. R. Soc. Trop. Med. Hyg. 90:129-132. [DOI] [PubMed] [Google Scholar]
- 26.Krautz, G. M., L. M. C. Galvão, J. R. Cançado, A. Guevara-Espinoza, A. Ouaissi, and A. U. Krettli. 1995. Use of a 24-kilodalton Trypanosoma cruzi recombinant protein to monitor cure of human Chagas' disease. J. Clin. Microbiol. 33:2086-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krettli, A. U., J. R. Cançado, and Z. Brener. 1984. Criterion of cure of human Chagas' disease after specific chemotherapy: recent advances. Mem. Inst. Oswaldo Cruz 79:157-164. [Google Scholar]
- 28.Krettli, A. U., J. R. Cançado, and Z. Brener. 1982. Effect of specific chemotherapy on levels of lytic antibodies in Chagas' disease. Trans. R. Soc. Trop. Med. Hyg. 76:334-340. [DOI] [PubMed] [Google Scholar]
- 29.Krettli, A. U., P. Weisz-Carrington, and R. S. Nussenzweig. 1979. Membrane-bound antibodies to bloodstream Trypanosoma cruzi in mice: strain differences in susceptibility to complement-mediated lysis. Clin. Exp. Immunol. 37:416-423. [PMC free article] [PubMed] [Google Scholar]
- 30.Krettli, A. U., and Z. Brener. 1982. Resistance against Trypanosoma cruzi associated to anti-living trypomastigote antibodies. J. Immunol. 128:2009-2012. [PubMed] [Google Scholar]
- 31.Lages-Silva, E., E. Crema, L. E. Ramirez, A. M. Macedo, S. D. Pena, and E. Chiari. 2001. Relationship between Trypanosoma cruzi and human chagasic megaesophagus: blood and tissue parasitism. Am. J. Trop. Med. Hyg. 65:435-441. [DOI] [PubMed] [Google Scholar]
- 32.Lages-Silva, E., L. E. Ramirez, M. L. Silva-Vergara, and E. Chiari. 2002. Chagasic meningoencephalitis in a patient with acquired immunodeficiency syndrome: diagnosis, follow-up, and genetic characterization of Trypanosoma cruzi. Clin. Infect. Dis. 34:118-123. [DOI] [PubMed] [Google Scholar]
- 33.Luquetti, A. O. 1999. Evolution of knowledge on the etiological diagnosis of chagasic infection. Mem. Inst. Oswaldo Cruz 94(Suppl. I):283-284. [DOI] [PubMed] [Google Scholar]
- 34.Martins, M. S., L. Hudson, A. U. Krettli, J. R. Cançado, and Z. Brener. 1985. Human and mouse sera recognize the same polypeptide associated with immunological resistance to Trypanosoma cruzi infection. Clin. Exp. Immunol. 61:343-350. [PMC free article] [PubMed] [Google Scholar]
- 35.Meira, W. S. F., L. M. C. Galvão, E. D. Gontijo, G. L. L. Machado-Coelho, K. A. Norris, and E. Chiari. 2002. Trypanosoma cruzi complement regulatory protein: a novel antigen for use in an enzyme-linked immunosorbent assay for diagnosis of Chagas’ disease. J. Clin. Microbiol. 40:3735-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norris, K. A., B. Bradt, N. Cooper, and M. So. 1991. Characterization of a Trypanosoma cruzi C3 binding protein with functional and genetic similarities to the complement regulatory protein, decay accelerating factor. J. Immunol. 147:2240-2247. [PubMed] [Google Scholar]
- 37.Norris, K. A., G. Harth, and M. So. 1989. Purification of a Trypanosoma cruzi membrane glycoprotein which elicits lytic antibodies. Infect. Immun. 57:2372-2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Norris, K. A., and J. E. Schrimpf. 1994. Biochemical analysis of the membrane and soluble forms of the complement regulatory protein of Trypanosoma cruzi. Infect. Immun. 62:236-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Norris, K. A., J. E. Schrimpf, and M. J. Szabo. 1997. Identification of the gene family encoding the 160-kilodalton Trypanosoma cruzi complement regulatory protein. Infect. Immun. 65:349-357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Norris, K. A., L. M. C. Galvão, J. E. Schrimpf, J. R. Cançado, and A. U. Krettli. 1994. Humoral immune response to the Trypanosoma cruzi complement regulatory protein as an indicator of parasitologic clearance in human Chagas' disease. Infect. Immun. 62:4072-4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santos, F. R., S. D. J. Pena, and J. T. Epplen. 1993. Genetic and population study of a Y-linked tetranucleotide repeat DNA polymorphism with a simple non-isotopic technique. Hum. Genet. 90:655-656. [DOI] [PubMed] [Google Scholar]
- 42.Schenone, H., E. Alfaro, and A. Rojas. 1974. Bases y rendimiento del xenodiagnóstico en la infección chagásica humana. Bol. Chil. Parasitol. 29:24-26. [PubMed] [Google Scholar]
- 43.Snedecor, G. W., and W. G. Cochran. 1989. Statistical methods, 8th ed. Iowa State University Press, Ames.
- 44.Sosa Estani, S., E. L. Segura, A. M. Ruiz, E. Velazquez, B. M. Porcel, and C. Yampotis. 1998. Efficacy of chemotherapy with benznidazole in children in the indeterminate phase of Chagas' disease. Am. J. Trop. Med. Hyg. 59:526-529. [DOI] [PubMed] [Google Scholar]
- 45.Sturm, N. R., W. Degrave, C. M. Morel, and L. Simpson. 1989. Sensitive detection and schizodeme classification of Trypanosoma cruzi cells by amplification of kinetoplast minicircle DNA sequences: use in diagnosis of Chagas' disease. Mol. Biochem. Parasitol. 33:205-214. [DOI] [PubMed] [Google Scholar]
- 46.Towbin, H., G. Rosenfelder, J. Wieslander, J. L. Ávila, M. Rojas, A. Szarfman, K. Esser, H. Nowack, and R. Timpl. 1987. Circulating antibodies to mouse laminin in Chagas' disease, American cutaneous leishmaniasis and normal individuals recognize terminal galactosyl α(1→3) galactose epitopes. J. Exp. Med. 166:419-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Umezawa, E. S., M. S. Nascimento, and A. M. S. Stolf. 2001. Enzyme-linked immunosorbent assay with Trypanosoma cruzi excreted-secreted antigens (TESA-ELISA) for serodiagnosis of acute and chronic Chagas' disease. Diagn. Microbiol. Infect. Dis. 39:169-176. [DOI] [PubMed] [Google Scholar]
- 48.Wincker, P., J. Telleria, M. F. Bosseno, M. A. Cardoso, P. Marques, N. Yaksic, C. Aznar, P. Liegeard, M. Hontebeyrie, F. Noireau, C. M. Morel, and S. F. Brenière. 1997. PCR-based diagnosis for Chagas' disease in Bolivian children living in an active transmission area: comparison with conventional serology and parasitological diagnosis. Parasitology 114:367-373. [DOI] [PubMed] [Google Scholar]
- 49.Wincker, P., M. F. Bosseno, C. Britto, N. Yaksic, M. A. Cardoso, C. M. Morel, and S. F. Brenière. 1994. High correlation between Chagas' disease serology and PCR-based detection of Trypanosoma cruzi kinetoplast DNA in Bolivian children living in an endemic area. FEMS Microbiol. Lett. 124:419-424. [DOI] [PubMed] [Google Scholar]
- 50.World Health Organization. 2002. Control of Chagas disease. Report of a W.H.O. expert committee. W. H. O. Tech. Rep. Ser. 905:1-109. [PubMed] [Google Scholar]
- 51.Zingales, B., R. P. Souto, R. H. Mangia, C. V. Lisboa, D. A. Campbell, J. R. Coura, A. Jansen, and O. Fernandes. 1998. Molecular epidemiology of American trypanosomiasis in Brazil based on dimorphisms of rRNA and mini-exon gene sequences. Int. J. Parasitol. 28:105-112. [DOI] [PubMed] [Google Scholar]