Abstract
A diagnosis of cancer and subsequent treatments place demands on psychological adaptation. Behavioral research suggests the importance of cognitive, behavioral, and social factors in facilitating adaptation during active treatment and throughout cancer survivorship, which forms the rationale for the use of many psychosocial interventions in cancer patients. This cancer experience may also affect physiological adaptation systems (e.g., neuroendocrine) in parallel with psychological adaptation changes (negative affect). Changes in adaptation may alter tumor growth-promoting processes (increased angiogenesis, migration and invasion, and inflammation) and tumor defense processes (decreased cellular immunity) relevant for cancer progression and the quality of life of cancer patients. Some evidence suggests that psychosocial intervention can improve psychological and physiological adaptation indicators in cancer patients. However, less is known about whether these interventions can influence tumor activity and tumor growth-promoting processes and whether changes in these processes could explain the psychosocial intervention effects on recurrence and survival documented to date. Documenting that psychosocial interventions can modulate molecular activities (e.g., transcriptional indicators of cell signaling) that govern tumor promoting and tumor defense processes on the one hand, and clinical disease course on the other is a key challenge for biobehavioral oncology research. This mini-review will summarize current knowledge on psychological and physiological adaptation processes affected throughout the stress of the cancer experience, and the effects of psychosocial interventions on psychological adaptation, cancer disease progression, and changes in stress-related biobehavioral processes that may mediate intervention effects on clinical cancer outcomes. Very recent intervention work in breast cancer will be used to illuminate emerging trends in molecular probes of interest in the hope of highlighting future paths that could move the field of biobehavioral oncology intervention research forward.
I. Psychological Adaptation During The Cancer Experience
A. Psychological Challenges in Cancer
A diagnosis of cancer and its treatments are stressful. Chief concerns of patients once diagnosed with cancer involve fears of recurrence, being damaged by adjuvant therapy, not seeing children grow, premature death, and loss of social ties and activities (Stanton, 2006; Spencer et al., 1999). Emotional distress and negative affect states are common after a cancer diagnosis and treatment and contribute to poorer psychological well being especially if persisting after treatment (Cordova, Andrykowsky, Kenady et al., 1995). Frequently reported psychosocial phenomena during the cancer experience include increased anxiety, depressed mood, social disruption, and sleep and fatigue-associated disruption (Ganz, Desmond, Leedham et al., 2002; Stanton, 2006). Although quality of life (QoL) generally improves markedly after cancer treatment and into the survivorship period (Bloom, Petersen & Kang, 2007), this is not always the case (Stein, Syrjala & Andrykowski, 2008). Poorer psychological adaptation after diagnosis and during treatment predicts diminished QoL many years later (Carver et al., 2005; Steginga, Lynch, Hawkes, Dunn & Aitken, 2009; Wenzel, DeAlba, Habbal et al., 2005) and may be associated with physiological processes (e.g., neuroendocrine and immune system regulation) relevant for their health status and QoL (Antoni, Lutgendorf, Cole et al., 2006).
B. Cognitive, Behavioral and Social factors and Adaptation to Cancer
Individuals vary considerably in their psychological responses to and recovery from the stress of diagnosis and treatment of cancer. Work examining individual difference factors in psychological adaptation suggests the importance of cognitive, behavioral and social factors, which forms the rationale for many of the psychosocial interventions developed for cancer patients (see Antoni, 2003 for review). A small sample of these is now listed. Cognitive factors influencing how the experience of cancer is appraised (optimistic rational appraisals) may enhance adaptation and QoL during treatment, during the year after surgery, and several years later (Brothers & Andersen, 2009; Carver, Smith, Antoni et al., 2005). Behavioral factors (e.g., having relaxation skills during cancer treatment) are associated with less distress and better adaptation after cancer treatment (Andersen at al., 2007; Luebbert, Dahme, Hasenbring et al., 2001). Many other behavioral factors are relevant for adaptation and health outcomes including physical exercise, diet, and medication adherence as reviewed elsewhere (Andersen, Glaser & Kiecolt-Glaser, 1994; McGregor & Antoni, 2009). Social support may be associated with both adaptation to cancer (Talley, Molix, Schlegel & Bettencourt, 2010) and to longer-term health outcomes (Nausheen, Gidron, Peveler, & Moss-Morris, 2009; Pinquart & Duberstein, 2010), The support that cancer patients receive, often coming from spouse or family members, is both the most helpful to patients in managing distress and may also be the most harmful if mismanaged.(Figueiredo, Fries & Ingram, 2004; Friedman, Kalidas, Elledge et al., 2005; Wimberly, Carver, Laurenceau et al., 2005) Thus, interpersonal skills may be particularly important for these patients as they communicate their needs to their support network. Taken together, this work suggests that cancer patients may psychologically adapt better to the cancer experience if they possess the cognitive, behavioral and social skills necessary to meet the challenges of treatment. It is plausible that cancer patient’s physiological adaptation to stressors may also mirror their psychological adaptation.
II. Physiological Adaptation Processes Relevant During the Cancer Experience
Cancer diagnosis and treatment induce acute and chronic stress and reduced QoL, which may affect neuroimmune regulation promoting inflammatory processes that could contribute to both symptom exacerbation and metastasis (Antoni, Lutgendorf, Cole et al., 2006; Andersen, Kiecolt-Glaser & Glaser, 1994). As noted elsewhere in this Special Issue of Brain, Behavior and Immunity, chronic stress, negative affect and social adversity have also been associated with biobehavioral alterations (increased sympathetic nervous system [SNS] signaling, hypothalamic pituitary adrenal [HPA] axis dysregulation, inflammation and decreased cellular immunity), which could interact with the tumor microenvironment to promote factors favoring tumor growth (e.g., angiogenesis), invasion (e.g., tissue remodeling and epithelial-mesenchymal transition), and metastatic signaling (e.g., anoikis), during and after cancer treatment (Lutgendorf & Sood, 2011).
Animals with tumors and treated cancer patients show HPA axis dysregulation—including elevated total and nocturnal cortisol output and decreased diurnal variation—which may be aggravated by stress and negative affect and could promote inflammatory processes (Sephton & Spiegel, 2003). It is not clear to what extent HPA dysregulation derives from stress and depression-induced leukocyte glucocorticoid receptor resistance, or if it is secondary to tumor- or treatment-produced inflammatory products, or both. Chronic stress and negative affect may support inflammatory processes, both by stimulating pro-inflammatory cytokine secretion and disrupting HPA axis-related inflammatory control (Miller, Cohen & Ritchey, 2003). Importantly, altered diurnal cortisol dysregulation also relates to poorer survival in women with metastatic breast cancer (BCa) (Sephton et al., 2000).
Longitudinal studies show that while distress decreases after adjuvant therapy begins and quality of life improves after treatment for many cancer patients (Bloom, Petersen & Kang, 2007), lingering physical challenges such as fatigue can peak during treatment and persist thereafter in breast cancer patients (Schmidt, Chang-Claude, Vrieling, Flesch-Janys & Steindorf, 2012) and other cancer survivors (Stein, Syrjala & Andrykowski, 2008). In some breast cancer patients, distress levels may remain elevated vs matched healthy controls up to 15 months after diagnosis (Hinnen, Ranchor, Sandeman et al., 2008). In fact, there is a growing awareness of the need to screen for psychological distress in all cancer patients (Jacobsen, 2007). Some cancer patients’ ability to carry out daily activities decreases during and after treatment, distress may increase, which can trigger interpersonal strain, a cascade that may further deplete energy resulting in negative mood, disrupted sleep and fatigue. Because distress reactions appear to be a possible common denominator contributing to multiple abnormalities (decreased psychological adaptation, HPA axis, and cytokine dysregulation) characterizing cancer treatment, then one component of effective treatment might focus upon improving psychological adaptation via psychosocial intervention. Cancer patients with less social support also experience more anxiety, greater cortisol levels, and molecular evidence consistent with impaired transcription of glucocorticoid response genes, and increased activity of pro-inflammatory transcription control pathways (Lutgendorf, Sood & Antoni, 2010). This suggests that psychosocial interventions teaching stress management skills (relaxation and coping strategies) to decrease distress and interpersonal skills to build social support may be particularly relevant for cancer patients (Antoni, 2003).
It is plausible that poorer psychological adaptation to cancer could exacerbate stress-associated alterations in neuroendocrines, cellular immune function, and pro-inflammatory signaling which could promote cancer progression. To date there are very few studies that have experimentally demonstrated a stress-induced change in either immune system indicators or tumor growth factors that predicts survival and cancer recurrence in humans. Conducting such a test essentially involves using a psychosocial intervention to modulate psychological adaptation (decrease distress and adversity states and improve positive states) in cancer patients, monitoring changes in stress-associated biobehavioral processes (SNS activation, HPA axis regulation, inflammation and cellular immune functioning), processes that promote tumor growth (tissue modeling and invasion, angiogenesis, apoptosis, anoikis) and then following these cohorts of patients for evidence of effects on disease course (recurrence, mortality). This involves recruiting a cohort of diagnosed cancer patients into a trial of a psychosocial intervention at a critical juncture in the cancer continuum (e.g., at the time of treatment for primary disease or at the point of disease recurrence), monitoring for initial improvements in psychological adaptation (reduced distress and depression and increased positive states); and following them over months for intermediate changes in SNS and HPA activity, inflammation, cellular immunity in plasma, circulating immune cells, and tumor cells if possible; and then following them for 5–10 years for clinical outcomes. There is now exciting preliminary evidence that several of these biobehavioral changes may indeed follow from psychological interventions designed to help cancer patients adapt. Before detailing these studies we summarize the research demonstrating the efficacy of psychosocial interventions designed to help cancer patients adapt to treatment.
III. Psychosocial Intervention Effects on Psychological Adaptation, Stress-Related Biobehavioral Processes, and Cancer Progression
Because cognitive, behavioral and social factors can affect how cancer patients adapt to diagnosis and treatment for cancer, many investigators have evaluated the effects of psychosocial interventions on psychological adaptation during cancer treatment. These interventions were designed to cognitively modify outlook, stress appraisals and coping via cognitive behavioral therapy (CBT); behaviorally reduce tension, anxiety and distress through relaxation training, mindfulness, hypnosis, yoga, and other techniques; and interpersonally build skills like assertiveness and anger management, in a group format to improve perceived social support and communication. In sum, psychosocial interventions have been developed to allow cancer patients to learn relaxation and other anxiety reduction strategies, modify cognitive appraisals, enact positive reframing and acceptance coping strategies to decrease distress, and use interpersonal skills to build and maintain social support. As these interventions work to improve psychological adaptation to the stressors of cancer diagnosis and treatment they may directly (or indirectly through stress management) improve a myriad of health behaviors such as physical exercise, diet, sleep, and medication adherence, which can each in their own right, impact health outcomes in cancer patients (Andersen, Kiecolt-Glaser & Glaser, 1994). For a recent review of these pathways in the context of breast cancer see McGregor & Antoni (2009). The remainder of this review will focus on summarizing the empirical evidence accrued over the past 10 years for the effects of psychosocial interventions that modify cognitive, behavioral and social/interpersonal factors on psychological adaptation and how these changes in positive and negative psychological adaptation parallel alterations in stress-related biobehavioral processes, and cancer progression. Because the largest number of psychosocial intervention studies have involved women with breast cancer (BCa) (Newell et al., 2002) we will emphasize this work, though where relevant, we also highlight intervention studies conducted in patients with other cancers.
A. Interventions Targeting Stress Processes to Facilitate Psychological Adaptation
Over 300 trials of psychological interventions have been conducted in cancer patients over the past 50 years, and most have been conducted in women with BCa. Reviews covering the evidence published through the early 2000s (e.g., Newell et al, 2002) concluded that psychosocial interventions that teach relaxation and stress management, help patients ventilate feelings, improve coping strategies, and provide social support are able to improve QoL and help them manage pain and other physical symptoms. The sample sizes of these trials were generally small and efficacy varied as a function of intervention content and format (group vs individual delivery) (Newell et al., 2002). More recent studies with larger samples have generally supported positive effects for psychosocial interventions on QoL indicators in BCa patients (McGregor & Antoni, 2009). Among studies targeting non-metastatic BCa patients published in the past 10 years, group-based cognitive behavioral and coping skills training interventions (8–24 sessions) have been shown to decrease anxiety, depressed mood and improve QoL. Many effects were still apparent over a 12-month follow-up supporting the clinical utility of these approaches. Some of these interventions blended CBT and health education (Andersen et al., 2004), CBT and sleep improvement techniques (Savaard et al., 2005), or CBT and interpersonal skills training (e.g., cognitive behavioral stress management, CBSM, Antoni, Lechner, Kazi et al., 2006). For instance, BCa patients assigned to CBSM (vs a psychoeducational group) in the weeks after surgery but prior to the onset of adjuvant therapy showed medium to large effect size decreases in negative affect (d = 0.33) , thought intrusions (d = 1.22), rated anxiety (d = 0.74), and interpersonal disruption (d = 0.53), and increases in positive affect (d = 0.31), benefit finding (d = 0.82), and positive states of mind (d = 1.16) for up to one year (Antoni, Lechner, Kazi et al., 2006; Antoni, Wimberly, Lechner et al., 2006). These studies collectively provide strong evidence that group-based psychosocial interventions that target stress management during active treatment can reliably modulate indicators of stress, affect and adversity and support positive experiences for extended periods of time in cancer patients.
B. Psychosocial Intervention Effects on Disease Progression, Recurrence and Survival
One of the most controversial areas of psycho-oncology research has concerned the question of whether psychosocial interventions can affect the clinical course of cancer (Spiegel, 2011). A landmark study by Spiegel et al. (1989) indicated that women with metastatic BCa who received a 12–month group-based supportive expression therapy (SET) intervention (focused on emotional expression, social support provision, and encouraging acceptance of mortality and decreasing the anxiety surrounding death lived twice as long (approximately 1.5 years longer) as women assigned to standard cancer treatment. Over the subsequent two decades attempts to replicate these effects have been mixed. Three particular clinical trials have been published over the past 10 years, each evaluating the effects of 12-month group-based psychosocial interventions on disease recurrence and survival in women with metastatic disease (Goodwin et al., 2001; Kissane et al., 2007; Spiegel et al., 2007). In each of these trials women with metastatic BCa were assigned to a 12-month course of weekly group-based SET or standard care and two of them (Goodwin et al., 2001; Kissane et al., 2007) showed no survival advantage for women assigned to SET. However, in the Goodwin et al (2001) study intervention participants had higher levels of depression than controls at baseline, and depression has been shown elsewhere to be a negative prognostic indicator in this population (Giese-Davis, Collie, Rancourt et al., 2011). Although the Spiegel et al (2007) did not show an overall survival effect for SET, secondary analyses found that the subset of women with estrogen receptor (ER) negative tumors assigned to SET had greater survival (Spiegel et al., 2007). This suggested that while women with ER+ tumors may have had more effective medical treatment options available, women with ER-tumors (including those who are triple negative: ER-, PR-, Her2Neu-) could be the major beneficiaries of psychosocial interventions going forward. A subgroup analysis of the Kissane et al (2007) trial did not reveal a survival advantage for ER-women assigned to SET. In view of the post-hoc nature of the Spiegel et al analysis, and the lack of a similar finding in the Kissane et al trial, the role of ER status (and possibly other clinicopathologic characteristics) in moderating the effects of psychosocial interventions in women with metastatic breast cancer deserves further study. It is noteworthy that investigating the link between psychosocial adaptation and disease course in patients with advanced cancers such as metastatic BCa may be complicated by advanced disease, and having different treatment options available for different types of breast cancer. What do we know about the effects of psychosocial interventions on disease outcomes in patients who are recruited earlier in the disease process?
Two studies to date have used psychosocial interventions to modulate psychological adaptation in patients treated for primary disease, observed increases in biobehavioral (cellular immune) processes, and then followed patients for evidence of intervention effects on disease course (recurrence, mortality) for at least 10 years (Andersen et al., 2004; Andersen et al 2008; Fawzy, Kemeny, Fawzy et al., 1990; Fawzy, Cousins, Fawzy et al., 1990; Fawzy et al.,1993; Fawzy et al., 2003). In the first of these (Fawzy et al., 1990), patients with malignant melanoma were randomized to 6 weeks of structured group-based psychosocial intervention vs usual care. Intervention participants revealed increased active coping and decreased negative mood at 6 weeks (Fawzy, Cousins, Fawzy et al., 1990), increased interferon-stimulated natural killer cell cytotoxicity (NKCC) at 6 months (Fawzy, Kemeny, Fawzy et al., 1990), and decreased mortality and recurrence at 6 year (Fawzy et al., 1993) and 10 yr follow-up (Fawzy et al., 2003). While the changes in biobehavioral processes (NKCC) at 6-month follow-up did not predict the 6-yr clinical outcomes, intervention-associated increases in active coping did predict clinical outcomes. This suggested the possibility that other biobehavioral changes that may have occurred in tandem with increases in active coping (pro-angiogenic or pro-inflammatory processes) may have mediated the effects of this intervention on disease outcomes.
Andersen et al (2008) tested the effects of a group-based psychosocial intervention on survival and recurrence in 227 women with non-metastatic BCa who received the intervention just after surgery. Women were randomized to standard care vs 4 months of weekly group-based intervention and 8 months of monthly sessions. The intervention included relaxation and stress reduction exercises, coping skills training and health behavior change strategies related to diet and exercise. Intervention participants showed a significant reduction in overall and BCa specific mortality rates as well as 45% reduced risk of cancer recurrence at a median of 11 years follow-up (Andersen et al., 2008). Those who did recur were cancer free for an average of 6 months longer, after controlling for age, accrual site, disease stage and other clinicopathological factors, cancer treatment. There were no group differences in psychiatric medications and outside counseling received. Similarly those in the intervention revealed 56% less risk of death from BCa and 49% lower all-cause mortality risk at follow-up. Among those who died from BCa median survival time in the intervention group was 1.3 years longer (M = 6.1 yrs) than those assigned to standard care (M = 4.8 yrs). In addition to demonstrating effects of psychosocial intervention on clinical outcomes Andersen et al’s group have also provided some evidence for intervention effects on biobehavioral mechanisms that may explain these effects.
The Andersen et al psychosocial intervention produced alterations in some stress-related immune processes that could contribute to improved general health and possibly altered disease course. These included increases in cellular immunity measures (lymphocyte proliferative responses [LPR] to mitogens) (Andersen et al., 2004) over the 4-month pre-post intervention period. Women in the intervention did report decreased distress but also more healthy eating habits, reduced smoking rates, and differed in the range of chemotherapy doses received (Andersen et al., 2004). At the 12-month follow up health and toxicity items rated by oncology nurses revealed that intervention participants evidenced better health status based on staff ratings. Within the intervention condition, reductions in distress at the 4-month time point predicted better health at 12 months (Andersen et al., 2007a). This team also conducted analyses of blood samples collected at longer-term follow-up during which women were monitored for health status and disease recurrence. Specifically, in a subgroup of depressed women monitored over the survival follow-up period, those assigned to the intervention showed decreases in immunologic markers consistent with active infection or chronic inflammatory conditions (total white blood cells [WBC] and neutrophils) compared to controls (Thornton et al., 2009). Though speculative, these changes were posited by the authors to explain, in part, the effects of this intervention on better overall physical health status over the year after surgery (Andersen et al., 2007b), and disease outcomes (recurrence and survival) observed over the subsequent decade (Andersen et al., 2008).
Women whose cancer ultimately recurred revealed greater serum cortisol and greater levels of WBC and neutrophils 17 months prior to their recurrence compared to those who remained disease free suggesting that these immunological changes in the intervention group may have been relevant in explaining differences in clinical outcomes between groups (Thornton, Andersen & Carson, 2008). Interestingly those women who experienced a distal recurrence at this point had weaker cellular immune responses (NKCC, LPR to mitogens) and greater elevations in WBC compared to those who experienced a local recurrence. Thus one possible explanation for the positive effects of this intervention on recurrence may be the normalization of stress- and treatment-associated neuroendocrine and immunologic regulation during a critical period following treatment and preceding recurrence. Previously it has been suggested that optimizing neuroendocrine and immunologic status may mitigate the “seeding” of micro-metastatic cells after primary treatment (Ben-Eliyahu 2003) thereby optimizing the environment for residual disease to thrive in. Thus normalizing these processes may be critical following initial surgery and during recovery from adjuvant chemotherapy and radiation. Finally, this team followed women after the point of disease recurrence and observed a reduced risk of death over an 80-month follow-up among those who had been assigned to the intervention arm (Andersen, Thornton, Shapiro et al., 2010). During the 12-month period following recurrence the intervention group also showed improvements in psychological adaptation (decreased negative mood and increased social support) and greater lymphocyte proliferative responses to mitogens, and greater NKCC. This trial provides the best evidence to date that a psychosocial intervention that improves psychological adaptation (decreased distress) may increase cellular immune function (lymphocyte proliferation) early in treatment, and decrease the odds of mortality and recurrence at 7–11 years. Moreover this study is the first to demonstrate that in a subgroup of the most depressed women, intervention participants showed reductions in indicators of inflammation at 17 months prior to recurrence which were, in fact, predictive of recurrence. Finally this is the first study to show that having been in a psychosocial intervention may promote persisting positive changes in psychological adaptation, immune functioning and clinical outcomes after disease recurs. This trial provides preliminary support for the value of using psychosocial interventions to modulate psychological adaptation, monitoring changes in biobehavioral processes reflecting cellular immune functioning, inflammation and other tumor-promoting processes, and following cohorts systematically for effects on clinical outcomes.
B. Psychosocial Intervention Effects on Stress-Related Biobehavioral Processes during Breast Cancer treatment
Over the past 10 years, other trials have evaluated the effects of stress reduction techniques such as cognitive behavioral stress management [CBSM] and meditation-based stress reduction (MBSR). These interventions have shown salutary effects on psychological adaptation, neuroendocrine and immunologic indicators in patients recruited during their medical treatment (see McGregor & Antoni, 2009 for review of BCa studies). As noted previously CBSM is a group based approach that blends cognitive, behavioral and interpersonal skills training through in-session didactic and role playing activities and homework and daily practice of stress management techniques (Antoni, 2003). MBSR applies mindfulness meditation training as a stress management technique and is often delivered in a group format over weekly training sessions and a full-day or longer retreat. Briefly, the effects of CBSM and MBSR have included decreases in late afternoon serum cortisol levels and increases in lymphocyte proliferative response and Th1 cytokine production and Th1/Th2 production ratio (McGregor & Antoni, 2009; Antoni et al., 2009; Carlson et al., 2007; Witek-Janisek et al., 2008). Since the MBSR trials were not randomized clinical trials caution is in order when interpreting the validity of these findings. We now summarize the specific neuroendocrine and immune findings from studies in our lab using CBSM.
We studied women who had completed surgery for non-metastatic BCa and were preparing for the start of adjuvant therapy. They were recruited in the weeks after surgery, competed baseline assessments, and were assigned to either 10 weeks of group-based CBSM or a one-day psychoeducational control group. Women completed questionnaires and provided blood samples at 6 and 12 month follow-up. Women in CBSM reported improvements in negative and positive mood and a wide variety of quality of life indicators as well as decreases in late afternoon serum cortisol, and increases in IL-2 and IFN-γ production from anti-CD3 stimulated peripheral blood mononuclear cells (PBMCs) (Phillips et al., 2008; Antoni et al., 2009). Thus this form of intervention, which was previously associated with decreases in distress states, and increases in positive states, was also related to reductions in cortisol and increases in cellular immune function that are consistent with a hastened recovery from cancer treatment. Showing reductions in PM cortisol is important because flatter di-urnal cortisol slopes (due partly to higher PM levels) have been associated previously with decreased survival among women with metastatic BCa (Sephton et al., 2000). Intervention effects on Th1 cytokine production may be important for supporting cellular immune processes that are involved in tumor eradication, such as antigen presenting cells, cytotoxic-T-cells, and T regulatory cells (Disis & Lyerly, 2005).
In comparing the work in CBSM with the intervention of Andersen et al, which combined relaxation with CBT and health behavior change strategies, it is noteworthy that in each trial, distress and/or cortisol decreases were paralleled by either increased frequency of relaxation practice (Andersen et al., 2007b) or increased confidence in using relaxation to manage stress (Antoni et al., 2006; Phillips et al., 2011). This provides some insight into one of the possible “active ingredients” of these interventions (i.e., anxiety-tension reduction) and corresponding neuroendocrine changes that may explain their effects on immunologic indicators. Since these are correlational findings, however, it is necessary to conduct experiments to separate the effects of relaxation training from other aspects of multi-modal interventions such as CBSM. Across most of these psychosocial intervention studies using patients with non-metastatic BCa it is important to note that only trials showing psychological effects demonstrated physiological effects, and in some the magnitude of the changes in neuroendocrine and immune indicators paralleled the size of the psychological effects (McGregor & Antoni, 2009). It is yet to be determined whether the patients enrolled in these trials of psychosocial interventions that showed neuroendocrine and immune effects of stress management in women with primary breast cancer in the past 10 years (e.g., Antoni et al., 2009; Savaard et al., 2005; Witek-Janisek et al., 2008) will demonstrate less disease recurrence and greater survival as reported by Andersen et al. (2008). However, it seems worthwhile to invest in following these extant cohorts who have already completed the intervention phase.
It is important to consider that psychosocial intervention effects on disease outcomes may be mediated by other stress-related behavioral and/or biologic processes. Some behavioral processes include improvements in health behaviors (more exercise, better nutrition, less alcohol consumption, better cancer treatment adherence) and whether psychosocial intervention participants actually receive more effective medical treatment (e.g., co-intervention effects). For instance it is worth noting that women assigned to the assessment arm of the Andersen et al trial revealed greater individual variability in chemotherapy dose intensity than those in the intervention arm (Andersen et al, 2008). Some biological processes that remain to be studied to explain the effects of psychosocial interventions on clinical outcomes in cancer patients include inflammation and those processes that may play a role in directly supporting tumor growth and metastasis, such as angiogenesis, tumor cell migration, tissue remodeling, and anoikis and resistance to apoptosis (Lutgendorf & Sood, 2011; Lutgendorf, Sood & Antoni, 2010). There is growing evidence that neuroendocrine hormones such as glucocorticoids and adrenergic hormones can influence communications between tumor, endothelial, and stromal cells which appears to be critical in modulating downstream signaling pathways important for disease progression (Lutgendorf, Sood & Antoni, 2010).
One paradigm for examining the effects of psychosocial interventions on cell signaling pathways that are relevant to human cancer progression is to examine whether psychosocial interventions with cancer patients are associated with transcriptional changes in circulating leukocytes reflecting molecular pathways that affect inflammation and cellular immune signaling as well as those that promote invasion and metastasis. For instance, given that chronic stress and negative affect are related to other biological processes such as inflammation, which may have relevance for cancer progression (Cole, 2009: Pierce, Ballard-Barbash, Bernstein et al., 2009), it is important to examine whether stress reduction interventions (e.g., CBSM) can affect inflammatory indicators and stress-sensitive neuroendocrine processes (e.g., glucocorticoid receptor sensitivity) that control inflammation within circulating leukocytes. It would also be intriguing to show that these interventions are associated with transcriptional changes in genes controlling cellular immunity (e.g., interferon activation pathways) within these cells. Molecular changes in signaling profiles related to tissue modeling and invasion would also provide evidence that these interventions are capable of working directly on stress-related tumor promoting activities in circulating leukocytes as a model for stress-induced effects in potential stromal cells interacting in the tumor microenvironment (Lutgendorf & Sood, 2011). Showing that these molecular changes happen in harmony with changes in psychological adaptation during and after psychosocial intervention would help identify some of the biobehavioral mechanisms underlying the link between stress modulation and the course of cancer. How close are we to demonstrating such phenomena?
D. Intervention Effects on Stress-Related Leukocyte Transcriptional Changes in Cancer Patients Undergoing Treatment
In an example of this emerging line of work we now summarize a recent study that examined (a) the association of psychological adaptation and leukocyte transcriptional dynamics in women who had recently completed surgery for primary BCa; and (b) the parallel effects of group-based CBSM on psychological adaptation and transcriptional changes as these women moved through their treatment and into the recovery period (Antoni, Lutgendorf, Blomberg et al., 2012). Using frozen cells from women who had previously participated in an RCT testing the effects of a 10-week CBSM intervention vs an active control (Antoni et al., 2009) we conducted genome-wide transcriptional profiling and bioinformatic analysis (Cole, 2009) at study entry, and 6- and 12-month follow-up. We found that greater negative affect and less positive affect was associated with greater than 50% differential expression of 201 genes, including upregulated expression of pro-inflammatory cytokines (IL1A, IL1B, IL6, TNF) and metastasis-promoting genes (e.g., those involved in tissue remodeling and epithelial-mesenchymal transition, LMNA, MMP9) (Antoni et al., 2012). Gene Ontology analyses confirmed that these transcripts were disproportionately involved in pro-inflammatory cytokine function and wound healing. This is the first evidence that individual differences in psychological adaptation status early in BCa treatment (2–10 weeks after surgery) are significantly associated with a leukocyte transcriptional profile reflecting an up-regulation of signaling pathways associated with inflammation, invasion and metastasis. These effects were independent of disease stage, time since surgery, sociodemographic factors and anxiolytics, pain and sleep medications, and anti-depressants.
We next found that women assigned to CBSM showed decreases in negative affect and increases in positive affect, and also showed altered expression of 91 genes by > 50% at 6–12 month follow-up (Antoni et al., 2012) (see Table 1 for description of genes, and magnitude of CBSM effects). These changes included down-regulation of 62 genes encoding pro-inflammatory cytokines, the prostaglandin-synthesis enzyme COX2, inflammatory chemokines and their receptors, and mediators of tissue remodeling and epithelial-mesenchymal transition. Women in CBSM also revealed 29 upregulated genes relevant for cellular immune responding including Type I interferon response, Type II interferon signaling, and interferon signal transduction. Real-time Polymerase Chain Reaction (RT-PCR) analysis confirmed microarray-indicated group differences in the between-group relative expression of a sample of transcripts audited.
Table 1.
Valence and magnitude of cognitive behavioral stress management (CBSM) intervention effects on genomic indicators representing different biological pathways relevant to carcinogenesis over a 6–12 month period in women with breast cancer
| Biological Pathway1 | Genomic Indicator | Cell Type Origin2 | Valence | Magnitude (CBSM: control fold diff) |
|---|---|---|---|---|
| Inflammation | ||||
| Pro-inflammatory cytokine | IL1A, IL1B, IL6 | Mo, pDC | down-regulation | 0.35 – 0.59 |
| Pro-inflammatory chemokines and their receptors | CCL2, CCL3, CCL3L1 CCL3L3, CCL4L1, CCL4L2, CCL7, CXCL1, CXCL2, CXCR7 | Mo, pDC | down-regulation | 0.41 – 0.61 |
| Prostaglandin-synthesis enzyme | PTGS2 (a COX2 marker) | Mo, pDC | down-regulation | 0.46 |
| Metastasis Promotion | ||||
| Tissue remodeling/epithelial-mesenchymal transition | G0S2, LMNA, MMP9, OSM | Mo, pDC | down-regulation | 0.55 – 0.63 |
| Cellular Immunity | ||||
| Type I interferon response | IFIT1, IFIT2, IFIT3, IFIT44, IFIT44L, ISG15, MX2, OAS2, OAS3 | Mo | up-regulation | 1.68 – 2.08 |
| Type II interferon signaling | IFNG | Mo | up-regulation | 1.54 |
| Interferon signal transduction | STAT1, STAT2 | Mo | up-regulation | 1.51 – 1.58 |
Inferred from TeLis bioinformatics program (Cole, 2009)
Inferred from Transcript Origin Analysis (Cole et al., 2011)
Mo: Monocyte pDC: plasmacytoid dendritic cell
We then used promoter-based bio-informatic analyses to infer the families of genes contributing to these alterations using TELiS bioinformatic analysis of transcription factor-binding motif (TFBM) distributions in promoters of differentially expressed genes (Cole, 2009). Our results appeared mediated by decreased activity of the transcription factors (TFs) for nuclear factor-kappa B (NFkB/Rel) and the Globin Transcription Factor (GATA) family and increased activity of interferon response factors. We also found that the women in CBSM showed increased expression of genes controlling the glucocorticoid receptor (GR) relative to controls. Parallel analyses of gene transcription controlling for concurrent serum cortisol levels showed an over-representation of GR response elements in the promoters of CBSM-up-regulated genes. Differential transcription of genes bearing GR response elements was not attributable to differential expression of genes encoding the GR. These findings are provocative and suggest that the effect of CBSM on gene profiles reflecting a down-regulation of inflammatory signaling co-occur in tandem with an upregulation of GR. Since chronic stress has been proposed to down-regulate GR and up-regulate inflammatory (NFκB) signaling in other populations (Miller, Chen, Sze et al., 2008) it is plausible that the transcriptional changes in neuroendocrine and inflammatory factors observed after CBSM are mediated by the decreases in chronic stress and negative affect as noted above. Transcript Origin Analyses (Cole et al., 2011) implicated monocytes and plasmacytoid dendritic cells (pDCs) as the most likely cells involved in CBSM-induced transcriptional changes with up-regulated genes deriving predominately from monocytes and down-regulated transcripts associated with both monocytes and pDCs. The effects of CBSM on gene expression profiles persisted when controlling for clinicopathological, cancer treatment-related, and psychiatric medications, as well as potential sociodemographic and behavioral confounders (Antoni et al., 2012).
Importantly, the genes down-regulated by CBSM included many of the transcripts that were also up-regulated in women with greater negative and less positive affect at baseline. This suggests a specificity of CBSM impact on psychological adaptation-associated genes, specifically those involved in inflammation and tissue remodeling. The immune cell types most likely mediating CBSM transcriptional alterations--antigen presenting myeloid cells—have previously been linked to distress states (Cole et al., 2011). Bioinformatic inferences of TF activity (GATA- and NF-κB/Rel-family TFs) associated with CBSM-induced transcriptional alterations have been linked to stress and SNS signaling in prior work as well (Miller, Chen, Fok et al., 2009, Lutgendorf, Degeest, Sung et al., 2009). Findings also suggest that CBSM induced GR activation, possibly representing a reversal of the distress-related GR transcriptional down-regulation shown in other work on chronic stress (Miller et al., 2008). Because these effects persisted after controlling for individual differences in circulating cortisol levels (which CBSM has shown to decrease, Phillips et al., 2008), they suggest that CBSM affects GR target gene expression primarily by enhancing GR functional sensitivity (i.e., reversing stress-induced GR desensitization) (Stark, Avitsur, Padgett et al, 2001). If this is true it opens the possibility that CBSM and other stress reduction interventions may modulate inflammatory signaling by mitigating stress-induced GR down-regulation in the context of cancer treatment, and possibly in other chronic medical and psychiatric conditions.
Although these findings are provocative, it must be kept in mind that they are not definitive since only a partial sample of cases from a prior RCT were available for analyses. If in fact CBSM was capable of causing the changes in gene expression observed in this study it is unclear whether the accompanying changes in CNS-mediated mood changes precede or follow from changes in leukocyte signaling. It is plausible that pro-inflammatory cytokines derived from activated monocytes may signal to the brain to causally affect neural function and mood (Harrison, Brydon, Walker et al., 2009; Eisenberger, Berkman, Inagaki et al., 2010), thus inducing a bi-directional regulatory circuit that could explain associations between inflammation and CNS-mediated distress processes. These results justify future randomized trials of similar psychosocial interventions in cancer patients utilizing transcriptional analyses of specific leukocyte subsets, possibly along with functional assays designed to probe the communication between neuroendocrines (e.g., glucocorticoids) and specific leukocyte subpopulations (e.g., monocytes) and their association with longer-term clinical outcomes in order to explore whether the effects of psychosocial interventions on these signaling pathways are relevant to cancer disease progression.
Beginning this line of work with BCa patients makes sense because (a) this is a high prevalence disease and provides the availability of larges samples for clinical trials and mechanism analysis, (b) because most of the evidence showing psychosocial intervention effects on clinical outcomes in cancer populations were in BCa (e.g., Andersen et al., 2008; Spiegel et al., 1989), (c) the first work showing intervention effects on transcriptional changes has been done in BCa (Antoni et al., 2012), and (d) the growing understanding of the role of processes such as inflammation in promoting BCa disease progression (Cole, 2009). Reductions in pro-inflammatory cytokines (e.g., IL1A) and NF-κB activity are notable in the context of BCa because chronic inflammation is believed to contribute to BCa progression and recurrence after treatment. (Pierce, Ballard-Barbash, Bernstein et al., 2009). CBSM also downregulated the expression of specific genes known to play a role in cancer progression and metastasis (e.g., MMP9), a pattern of change which is associated with reduced progression of cancer (Hanahan & Weinberg, 2011). It also appears that the women receiving CBSM show contemporaneous increases in gene expression associated with a recovery of interferon-mediated cellular immunity, which may be relevant for immunosurveillance of cancer micro-metastases (Benish & Ben Eliyahu et al., 2010) or opportunistic infections during and after adjuvant treatment. Together these data suggest that in addition to its beneficial effects on psychological adaptation, CBSM can also alter immune cell gene expression in a manner that may both reverse the biological impact of experienced stress during treatment and could potentially influence disease progression in BCa patients (Lutgendorf, Sood & Antoni, 2010).
E. Psychosocial Intervention Effects in Other Cancer Populations
There is no reason to believe that these psychosocial intervention effects are restricted to BCa. Since other cancer models have shown strong evidence for the influence of stress physiology and neuroendocrines on angiogenesis, invasion, and inflammation in tumor and stromal cells (e.g., Ovarian cancer, Lutgendorf & Sood, 2011) it is reasonable to examine the effects of psychosocial interventions on other types of cancer. The effects of psychosocial interventions on biobehavioral processes in populations other than BCa patients have only rarely been studied. One hallmark study noted previously, showed that a 6-week group-based psychosocial intervention focused on coping skills and interpersonal support was associated with improved mood, increased NKCC, and greater survival and disease-free interval in patients with malignant melanoma followed up to 10 years (Fawzy et al., 2003). More recently, immunological effects of some novel psychosocial interventions have been reported in conjunction with other populations including men with prostate cancer and women with gynecological cancers. One study showed a 2-session stress management intervention (deep breathing, guided imagery and adaptive coping skills) offered to men prior to surgery for prostate cancer related to decreases in mood disturbance and increases in NKCC one week pre- to 48 hrs post-surgery (Cohen, Parker, Vence et al., 2011). This is an interesting finding in view of prior work showing that a psychosocial intervention initiated prior to surgery was associated with improved 10-year survival in patients treated for gastrointestinal cancer (Kuchler, Bestmann, Rappat et al., 2007). Another study showed that telephone-delivered psychosocial counseling intervention is associated with improved QoL and a shift toward a more Th1/Th2 cytokine bias in women with cervical cancer (Nelson, Wenzel, Osann et al., 2008). Finally, an integrative medicine approach—Healing Touch—was associated with a greater preservation of NKCC in women with cervical cancer as they went through chemoradiation treatments as compared to controls (Lutgendorf, Mullen-Houser, Russell et al., 2011). There is also evidence that palliative care intervention may decrease pain and depression on the one hand and increase survival in metastatic lung cancer patients (Temel et al., 2010). All of this work deserves replication in larger samples and longer follow-ups to determine whether these interesting biobehavioral effects during treatment predict longer-term clinical outcomes.
F. Exploring Other Clinical Health Outcomes in Cancer Patients
When designing studies of psychosocial interventions in cancer patients it is reasonable to consider other stress-related clinical health outcomes beyond survival and disease recurrence such as the incidence of moderate to severe opportunistic infections (OI) during and after the completion of surgical and adjuvant therapy as well as the effects of cancer treatment on “late effects” including insulin resistance-related cardiovascular disease and diabetes, conditions, which in turn, may also influence cancer treatment efficacy and disease progression (Pal & Hurria, 2010). Surgery and other cancer-related treatment may compromise the immune system sufficiently to place treated cancer patients at risk for opportunistic disease during or after the treatment period (Antoni, Schneiderman & Penedo, 2006). Stress-related changes in upper respiratory infections, reactivation of latent herpesvirus infections, and the progression of virally-associated neoplastic processes are well established. Stress reduction interventions have been shown to modify neuroendocrine (decreased urinary cortisol and NE) and immune (increased naïve T cell reconstitution, decreased antibody titers to herpesviruses, and decreased viral load) parameters as well as clinical disease (cervical neoplasia, Antoni, Pereira et al., 2008) in persons with infectious disease such as Human Immunodeficiency Virus (HIV) (for review see Carrico & Antoni, 2008).
Cancer survivors might also be at elevated risk for the development of co-morbidities that are associated with alterations in insulin metabolism (abdominal obesity, dyslipidemia, cardiovascular disease and diabetes mellitus) as delayed or “late effects” of successful cancer treatment (Basaria, Muller, Carducci, Egan & Dobs, 2006; Thomson, Thompson, Wright-Bea et al., 2009). For instance, adult survivors of childhood cancer show increased risk for metabolic syndrome indicators (hypertension, hypertriglyceridemia, abdominal obesity) over a 17-yr follow-up period (Hoffman, Derdak, Bernstein et al., 2008). It has also been established that comorbidities before cancer treatment can affect clinical outcomes (decreased survival, increased disease recurrence) (Patnaik, Byers, DiGuiseppi & Denberg, 2011). For instance, conditions such as diabetes mellitus, may increase the risk for disease recurrence in colon cancer (Pal & Hurrier, 2010), while obesity, another sign of altered insulin metabolism, has been associated with reduced efficacy of aromatase inhibitors in ER+ breast cancer patients (Sestak, Distler, Forbes et al., 2010). Interestingly metformin, an insulin metabolism modulator, has been shown to have potential as a cancer therapeutic, especially in breast and colon cancers, which are associated with hyperinsulinemia (Dowling, Goodwin & Stambolic, 2011). To the extent that psychosocial factors and stress physiology can affect the pathogenesis of comorbidities related to insulin metabolism (Chrousos, 2000; Vitaliano, Scanlon, Zhang, Savage, Hirsch & Siegler, 2002; Schneiderman, Antoni, Penedo & Ironson, 2010) then psychological interventions, including stress management, may mitigate the risk of and effects of these conditions in cancer survivorship. It seems reasonable that psychosocial intervention may be able to affect the risk of opportunistic disease and some of the co-morbidities listed here in treated cancer patients and may yield definitive results in much shorter follow-up periods than are required for documenting effects on disease recurrence and survival.
G. Caveats and Methodological Considerations
The cancer diagnosis and treatment may induce acute and chronic stress and reduced quality of life. These changes may be accompanied or followed by poor compliance to medical regimens, increases in negative health behaviors and decreases in positive health behaviors, which may combine with physiologic responses to stress to encourage local and metastatic disease progression. A major challenge in psychosocial intervention research in oncology is finding ways to minimize and account for the confounding effects of different disease characteristics and cancer treatments on biobehavioral indicators and clinical outcomes (Bovjberg, 1991; van der Pompe, Antoni & Heijnen, 1998). Using the example of breast cancer, differences in clinicopathological characteristics can have prognostic significance (stage, HER2-neu+/−, ER/PR+/−) (Biganzoli & Boracchi, 2004; Woodward, Storm, Tucker, McNeese, 2003), and in at least one trial, were found to moderate the effects of a psychological intervention on clinical outcomes (Spiegel et al., 2007).
Effects of adjuvant therapy can vary over short periods and regimens are tailored from patient to patient. There is likely inter-individual variation in neuroimmunological responses attributable to surgery, chemotherapy, radiation, immunomodulators, anti-emetics, and hormonal treatments (e.g, Tamoxifen), to name a few of the major classes of treatment. This can affect the decisions investigators make abut the timing of their measurements. Decisions about the timing of an intervention also depend on what level of prevention the investigator is targeting (Miller et al., 2009). Determining the timing of psychosocial intervention onset and measurement intervals involves minimizing confounders of biobehavioral readouts by working around known treatment regimens and monitoring the medical complications that occur during and after treatment is completed. This involves balancing the potential clinical yield of peri-treatment timing against the rendering of questionable findings. In cancer patients receiving adjuvant treatments, can we deliver a psychosocial intervention during a patient’s peak point of stress and anxiety and still get interpretable neuroendocrine and immune results? Is it better to collect pre-intervention baseline data before the surgery or adjuvant therapy begins, intervene during active treatment, and wait for patients to complete the regimen before collecting follow-up data to observe the shape of their recovery curve, and continue to follow them over clinically meaningful periods? This latter approach may minimize some confounders of medical treatments but many powerful medical treatments have unclear post-treatment side effect kinetics. Because some have hypothesized that the critical period for stress-mediated immunosuppression increasing the risk of breast cancer metastatic spread is in the weeks after surgery (Ben-Eliyahu, 2003), it seems as though some intervention research in breast cancer patients may benefit by contending with these timing issues head on as in the case of the Andersen et al (2008) trial. Going forward, potential confounds might be minimized in psychosocial intervention trials by controlling for elapsed days since diagnosis and surgery for the pre-intervention measures, elapsed days since most recent adjuvant treatment for intercurrent follow-ups, as well as careful monitoring of frequency/dosage of regimen, treatment actually received, corticosteroids, anti-emetics, non-steroidal anti-inflammatory agents, and other medications patients are receiving at each time point.
Although research on the efficacy of psychosocial interventions on disease progression in diagnosed cancer patients is promising, identifying vulnerable populations of patients who will most benefit from these interventions is an important direction for future research. Such high-risk populations might be identified through psychosocial criteria (e.g., depressed, highly distressed, socially isolated, a socially disadvantaged minority group member) or because of a biological factor (e.g., hereditary risk, stage of disease, tumor receptor status, or having a specific co-morbid condition like HIV/AIDS). Understanding how physical and psychosocial challenges and responses manifest differently across the cancer continuum from initial diagnosis, to curative and adjuvant treatment, to early survivorship, to disease recurrence, and to the emergence of late effects and the need for palliative care (Miller, Bowen, Croyle & Rowland, 2009) will be key in tailoring the content of psychosocial interventions developed for different cancer populations.
Examining new ways to deliver these interventions so that they have greater reach may utilize technological advances in telecommunications (web-based and mobile-phone-based delivery, Heckman, Barcikowski, Ogles et al., 2006) in combination with community-based participatory research methods to reach those populations that are unable or hesitant to receive psychosocial intervention in a medical facility. Using remote collection of biological samples and psychosocial data in home-delivered intervention trials may have real limits but could play a role in expanding the reach of intervention research in cancer populations in real-world settings. However, before these applications are developed it is important to identify the optimal format (group, couples, family, or individual-based) and dosage (number, frequency and duration of training sessions and maintenance sessions) of face-to-face psychosocial interventions needed to influence the psychological adaptation and biobehavioral processes necessary to produce a clinical outcome. It is important to note that some of the most impressive effects of psychosocial interventions on clinical cancer outcomes in breast cancer have been achieved with very high intensity interventions spanning 12 months of group-based therapeutic contact (Andersen et al., 2008; Spiegel et al., 1989), though work in malignant melanoma has documented effects on clinical outcomes with a much shorter intervention of 6 weeks (Fawzy et al., 1993). It would be critical to see if other brief psychosocial interventions (as few as 2 sessions) that have demonstrated effects on psychological adaptation and biobehavioral indicators (e.g., Cohen et al., 2011) are also associated with positive clinical outcomes over time.
Finally, intervention research designed to elucidate biologic pathways linking psychological adaptation processes to cancer health outcomes has focused mostly on a limited number of neuroendocrine and immune indicators (for review see McGregor & Antoni, 2009). It is important to expand our observations to explore the cellular, biochemical, and molecular activities underlying tumor development and progression that have been related to stress factors and other psychosocial phenomena in the past 10 years, and which may operate in concert with stress-related immunologic changes in influencing disease outcomes (Lutgendorf & Sood, 2011). As attention in cancer research has shifted to a greater emphasis on the tumor microenvironment, it is now reasonable to examine how psychosocial processes and interventions affect the stromal cells (e.g., circulating and tumor-associated myeloid cells) that could interact in the tumor microenvironment and how such changes relate to the clinical course of disease.
Conclusion
The evidence available to date demonstrates the ability of many different psychosocial interventions to improve responses to the stress and adversity of the cancer experience in order to improve psychological adaptation. Improvements in psychological adaptation (decreased negative affect and social disruption and increased positive affect and quality of life) have been linked to an improved physiological profile during and after treatment, which may increase the odds for disease-free survival in some cancers. It is crucial to both establish the reliable effects of these interventions on clinical outcomes (recurrence and survival) in more cancer populations and a wider representation of the biobehavioral processes that might explain these effects. Emerging technologies now allow us to expand biobehavioral research in oncology by applying microarray and bioinformatics analyses of immune and tumor cell transcriptional activity. This could illuminate the juncture of neuroimmune communications underlying inflammatory and tumor promoting cell signaling. Several leukocyte gene expression profiles that have been consistently tied to stress and adversity on the one hand, and inflammation and tumor metastasis on the other, are accessible for researchers conducting clinical trials of psychosocial interventions in cancer patients. By co-examining intervention-associated changes in psychological adaptation and leukocyte transcriptional changes, and longer term follow-ups of clinical disease, one could amass systematic biobehavioral evidence for how these interventions work in patients undergoing cancer treatment.
Although it is unclear when the optimal time to initiate these interventions would be within cancer treatment it seems plausible to begin by randomizing patients in the early post-diagnostic, post-surgical or post-recurrence period, and establish a cohort of patients who can be followed systematically for changes in psychological adaptation, biobehavioral indicators and heath status over time. One can then correlate intervention-associated “early and later changes” in biobehavioral processes with clinical course of disease over the subsequent period of years. Using a few of the intervention studies conducted in breast cancer we have suggested some of the ways one might precede along these lines.
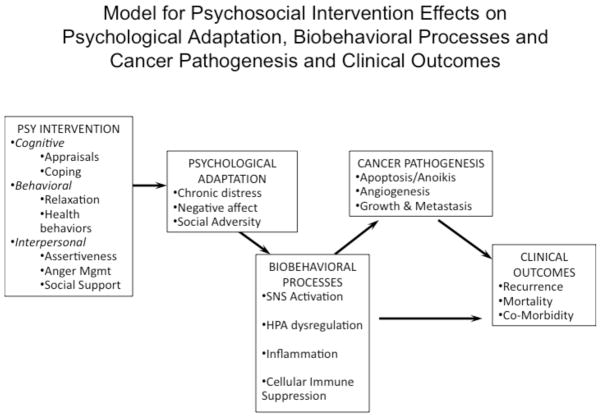
Figure 1 presents of model for guiding this line of thinking by listing some of the cognitive, behavioral and interpersonal intervention components, and indicators of psychological adaptation, biobehavioral processes, cancer pathogenesis, and health outcomes that might be considered by those designing psychosocial intervention studies in the future. Implicit in this model is a consideration of the many potential confounders that may be operating on each of these sets of variables, a discussion of which is beyond the scope of this review. It is reasonable to propose that psychosocial interventions that address cancer patient’s focal concerns in the period before and after surgery for primary disease may reduce stress-associated exacerbations of biobehavioral processes that could promote disease progression. Emerging work suggests the possibility that providing psychosocial interventions shortly after the diagnosis of recurrent disease may also facilitate psychological adaptation and affect biobehavioral processes and/or clinical outcomes (Andersen et al. 2010).
Figure 1.
This model suggests an approach for tracking changes in psychological adaptation, biobehavioral processes and cancer pathogenesis and clinical outcomes following a psychosocial intervention designed to address cognitive, behavioral and interpersonal processes as intervention targets to modulate cancer patients’ stress responses during cancer treatment. Psychosocial interventions are hypothesized to decrease chronic stress, negative affect and social adversity. Improvements in psychological adaptation are hypothesized to facilitate decreases in SNS activation, HPA axis dysregulation, inflammation and cellular immune deficits. These alterations in stress-related biobehavioral processes may decrease the likelihood of cancer pathogenic processes associated with tumor cell survival, growth, invasion and metastasis, which could precede clinical outcomes such as disease recurrence, co-morbidities and mortality. Alterations in stress-related biobehavioral processes may also influence clinical outcomes (e.g., co-morbidities) independent of the cancer pathogenic processes listed.
Basic research and preliminary intervention studies suggest that stress factors and psychological interventions may modulate biobehavioral processes in patients diagnosed with ovarian, cervical and prostate cancers. The next step is to test whether these changes predict the clinical course of these diseases. It is important to understand that though these conditions are collectively referred to as cancers, they are different diseases with different causes, promoters, treatments, and prognostic course after initial treatment and recurrence. Creating a rationale for examining psychosocial intervention effects on health outcomes in each of these conditions will require linking the most well-validated intervention approaches to improved psychological adaptation in parallel with changes in biobehavioral processes that are known to be related to the pathophysiology of each of these separate diseases. The trajectory toward optimal health outcomes in the long term may begin with differential recovery from medical treatment for primary disease or newly diagnosed recurrent disease as measured in psychological and physiological adaptation indicators that can be targeted through pre-emptive psychosocial intervention.
HIGHLIGHTS.
We review effects of psychosocial interventions on psychological adaptation, cancer outcomes, and biobehavioral processes that may mediate effects.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen BL, Thornton L, Shapiro C, Farrar W, Mundy B, Yang H, Carson W. Biobehavioral, immune and health benefits following recurrence for psychological intervention participants. Clinical Cancer Research. 2010;16:3270–3278. doi: 10.1158/1078-0432.CCR-10-0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Yang HC, Farrar WB, et al. Psychologic intervention improves survival for breast cancer patients: A randomized clinical trial. Cancer. 2008;113:3450–3458. doi: 10.1002/cncr.23969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a).Andersen BL, Farrar WB, Golden-Kreutz D, et al. Distress reduction from a psychological intervention contributes to improved health for cancer patients. Brain Behav Immun. 2007;21:953–961. doi: 10.1016/j.bbi.2007.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (b).Andersen B, Shelby R, Golden-Kruetz D. RCT of a psychological intervention for persons with cancer. I. Mechanisms of change. J Consult Clin Psychol. 2007;75:927–938. doi: 10.1037/0022-006X.75.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen BL, Kiecolt-Glaser JK, Glaser R. A biobehavioral model of cancer stress and disease course. American Psychologist. 1994;49:389–404. doi: 10.1037//0003-066x.49.5.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen B, Farrar WB, Golden-Kreutz DM, Glaser R, Emery CF, Crespin TR, et al. Psychological, behavioral, and immune changes after a psychological intervention: A clinical trial. J Clin Oncol. 2004;22:3570–80. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf S, Cole S, Dhabhar F, Sephton S, McDonald P, Stefanek M, Sood A. The influence of biobehavioral factors on tumor biology: pathways and mechanisms. Nature Reviews Cancer. 2006;6:240–248. doi: 10.1038/nrc1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH. Stress Management Intervention for Women With Breast Cancer. Washington D.C: American Psychological Association Press; 2003. [Google Scholar]
- Antoni MH, Lechner SC, Kazi A, Wimberly S, Gluck S, Carver CS. How stress management improves quality of life after breast cancer treatment. J Consult Clin Pyschol. 2006;74:1143–1152. doi: 10.1037/0022-006X.74.6.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Wimberly SR, Lechner SC, Kazi A, Sifre T, Urcuyo KR, Phillips K, Smith RG, Petronis VM, Guellati S, Wells KA, Blomberg B, Carver CS. Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. Am J Psychiatry. 2006;163(10):1791–1797. doi: 10.1176/ajp.2006.163.10.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Lechner S, Diaz A, Vargas S, Holley H, Phillips K, McGregor BA, Carver CS, Blomberg B. Cognitive behavioral stress management effects on psychosocial and physiological adaptation in women undergoing treatment for breast cancer. Brain, Behavior and Immunity. 2009;23:159–166. doi: 10.1016/j.bbi.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Lutgendorf S, Blomberg B, Carver CS, Lechner S, Diaz A, Stagl J, Arevalo J, Cole S. Cognitive-behavioral stress management reverses anxiety-related leukocyte transcriptional dynamics. Biological Psychiatry. 2012;71:366–372. doi: 10.1016/j.biopsych.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni MH, Schneiderman N, Penedo F. Behavioral interventions: Immunologic mediators and disease outcomes. In: Ader R, Glaser R, Cohen N, Irwin M, editors. Psychoneuroimmunology. 4. NY: Academic; 2006. pp. 675–703. [Google Scholar]
- Antoni MH, Pereira DB, Marion I, Ennis N, Andrasik MP, Rose R, et al. Stress management effects on perceived stress and cervical neoplasia in low-income HIV-infected women. J Psychosom Res. 2008;65:389–401. doi: 10.1016/j.jpsychores.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaria S, Muller DC, Carducci MA, Egan J, Dobs AS. Hyperglycemia and insulin resistance in men with prostate carcinoma who receive androgen-deprivation therapy. Cancer. 2006;106:581–588. doi: 10.1002/cncr.21642. [DOI] [PubMed] [Google Scholar]
- Ben-Eliyahu S. The promotion of tumor metastasis by surgery and stress: Immunological basis and implications for Psychoneuroimmunology. Brain Behavior Immun. 2003;17:S27–38. doi: 10.1016/s0889-1591(02)00063-6. [DOI] [PubMed] [Google Scholar]
- Benish M, Ben-Eliyahu S. Surgery as a double-edged sword: A clinically feasible approach to overcome the metastasis-promoting effects of surgery by blunting stress and prostaglandin responses. Cancers. 2010;2:1929–1951. doi: 10.3390/cancers2041929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biganzoli E, Boracchi P. Old and new markers for breast cancer prognosis: the need for integrated research on quantitative issues. European Journal of Cancer. 2004;40:1803–1806. doi: 10.1016/j.ejca.2004.04.026. [DOI] [PubMed] [Google Scholar]
- Bovjberg D. Psychoneuroimmunology: Implications for oncology? Cancer. 1991;67:828–832. doi: 10.1002/1097-0142(19910201)67:3+<828::aid-cncr2820671413>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Brothers BM, Andersen BL. Hopelessness as a predictor of depressive symptoms for breast cancer patients coping with recurrence. Psycho-Oncology. 2009;18:267–275. doi: 10.1002/pon.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson LE, Speca M, Faris P, Patel KD. One year pre-post intervention follow-up of psychological, immune, endocrine and blood pressure outcomes of mindfulness-based stress reduction (MBSR) in breast and prostate cancer outpatients. Brain Behav Immun. 2007;21(8):1038–1049. doi: 10.1016/j.bbi.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Carrico A, Antoni MH. The effects of psychological interventions on neuroendocrine hormone regulation and immune status in HIV-positive persons: A review of randomized controlled trials. Psychosom Med. 2008;70:575–84. doi: 10.1097/PSY.0b013e31817a5d30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, Smith RG, Antoni MH, Petronis VM, Weiss S, Derhagopian RP. Optimistic personality and psychosocial well-being during treatment predict psychosocial well-being among long-term survivors of breast cancer. Health Psychology. 2005;24:508–516. doi: 10.1037/0278-6133.24.5.508. [DOI] [PubMed] [Google Scholar]
- Chrousos G. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int J Obesity and Related Metabolic Disorder. 2000;24:S550–555. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- Cohen L, Parker O, Vence L, Savary C, Kentor D, Pettaway C, Babaian R, Pisters L, Miles B, Wei Q, Wiltz L, Patel T, Radvanyi L. Presurgical stress management improves postoperative immune function in men with prostate cancer undergoing radical prostatectomy. Psychosomatic Medicine. 2011;73 :218–225. doi: 10.1097/PSY.0b013e31820a1c26. [DOI] [PubMed] [Google Scholar]
- Cole SW, Hawkley LC, Arevalo JM, Cacioppo JT. Transcript origin analysis identifies antigen-presenting cells as primary targets of socially regulated gene expression in leukocytes. Proc Natl Acad Sci U S A. 2011;108:3080–3085. doi: 10.1073/pnas.1014218108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW. Social regulation of human gene expression. Current Directions in Psychological Science. 2009;18 :132–137. doi: 10.1111/j.1467-8721.2009.01623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole SW. Chronic inflammation and breast cancer recurrence. J Clin Oncol. 2009;27:3418–3419. doi: 10.1200/JCO.2009.21.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova MJ, Andrykowski MA, Kenady DE, McGrath PC, Sloan DA, Redd WH. Frequency and correlates of posttraumatic-stress-disorder-like symptoms after treatment for breast cancer. J Consult Clin Psychology. 1995;63:981–6. doi: 10.1037//0022-006x.63.6.981. [DOI] [PubMed] [Google Scholar]
- Dowling R, Goodwin P, Stambolic V. Understanding the benefit of metformin use in cancer treatment. PMC Medicine. 2011;9:33. doi: 10.1186/1741-7015-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disis ML, Lyerly HK. Global role of the immune system in identifying cancer initiation and limiting disease progression. J Clin Oncol. 2005;23(35):8923–8925. doi: 10.1200/JCO.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Berkman ET, Inagaki TK, Rameson LT, Mashal NM, Irwin MR. Inflammation-induced anhedonia: endotoxin reduces ventral striatum responses to reward. Biological Psychiatry. 2010;68:748–754. doi: 10.1016/j.biopsych.2010.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawzy FI, Cousins N, Fawzy NW, Kemeny ME, Elashoff R, Morton D. A structured psychiatric intervention for cancer patients, I: changes over time in methods of coping and affective disturbance. Arch Gen Psychiatry. 1990;47:720–725. doi: 10.1001/archpsyc.1990.01810200028004. [DOI] [PubMed] [Google Scholar]
- Fawzy FI, Kemeny ME, Fawzy NW, Elashoff R, Morton D, Cousins N, Fahey JL. A structured psychiatric intervention for cancer patients, II: changes over time in immunological measures. Arch Gen Psychiatry. 1990;47:729–735. doi: 10.1001/archpsyc.1990.01810200037005. [DOI] [PubMed] [Google Scholar]
- Fawzy F, Canada A, Fawzy S. Malignant melanoma: Effects of a brief, structured psychiatric intervention on survival and recurrence at 10-year follow-up. Arch Gen Psychiatry. 2003;60:100–103. doi: 10.1001/archpsyc.60.1.100. [DOI] [PubMed] [Google Scholar]
- Fawzy FI, Fawzy NW, Hyun CS, Elashoff R, Guthrie D, Fahey JL, Morton DL. Malignant melanoma. Effects of an early structured psychiatric intervention, coping, and affective state on recurrence and survival 6 years later. Arch Gen Psychiatry. 1993;50:681–689. doi: 10.1001/archpsyc.1993.01820210015002. [DOI] [PubMed] [Google Scholar]
- Figueiredo MI, Fries E, Ingram KM. The role of disclosure patterns and unsupportive social interactions in the well-being of breast cancer patients. Psycho-oncology. 2004;13:96–105. doi: 10.1002/pon.717. [DOI] [PubMed] [Google Scholar]
- Friedman LC, Kalidas M, Elledge R, Chang J, Romero C, Husain I, et al. Optimism, social support, and psychosocial functioning among women with breast cancer. Psycho-Oncology. 2005;15:595–603. doi: 10.1002/pon.992. [DOI] [PubMed] [Google Scholar]
- Ganz P, Desmond K, Leedham B, Rowland J, Meyerowitz B, Belin T. Quality of life in long-term. Disease-free survivors of breast cancer: a Follow-up study. J National Cancer Institute. 2002;94:39–49. doi: 10.1093/jnci/94.1.39. [DOI] [PubMed] [Google Scholar]
- Giese-Davis J, Collie K, Rancourt KM, et al. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clinical Oncology. 2011;29:413–20. doi: 10.1200/JCO.2010.28.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin PJ, Leszcz M, Ennis M. The effect of group psychosocial support on survival in metastatic breast cancer. N Engl J Med. 2001;345:1719–26. doi: 10.1056/NEJMoa011871. [DOI] [PubMed] [Google Scholar]
- Harrison NA, Brydon L, Walker C, Gray MA, Steptoe A, Critchley HD. Inflammation causes mood changes through alterations in subgenual cingulate activity and mesolimbic connectivity. Biological Psychiatry. 2009;66:407–414. doi: 10.1016/j.biopsych.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- Heckman TG, Barcikowski R, Ogles B, Suhr J, Carlson B, Holroyd K, Garske J. A telephone-delivered coping improvement group intervention for middle-aged and older adults living with HIV/AIDS. Ann Behav Med. 2006;32:27–38. doi: 10.1207/s15324796abm3201_4. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Derdak J, Bernstein D, Reynolds J, Avila N, Gerber L, Steinberg S, Chrousos G, Mackall C, Mansky P. Metabolic syndrome traits in long-term survivors of pediatric sarcoma. Pediatric Blood and Cancer. 2008;50:341–346. doi: 10.1002/pbc.21363. [DOI] [PubMed] [Google Scholar]
- Hinnen C, Ranchor A, Sandeman R, Snijders T, Hagedoom M, Coyne J. Course of distress in breast cancer patients, their partners, and matched control couples. Annals of Behavioral Medicine. 2008;36:141–148. doi: 10.1007/s12160-008-9061-8. [DOI] [PubMed] [Google Scholar]
- Jacobsen P. Screening of psychological distress in cancer patients: challenges and opportunities. J Clin Oncol. 2007;25:4526–4527. doi: 10.1200/JCO.2007.13.1367. [DOI] [PubMed] [Google Scholar]
- Kissane D, Grabsch B, Clarke D. Supportive-expressive group therapy for women with metastatic breast cancer: Survival and psychosocial outcomes from a randomized controlled trial. Psycho-Oncol. 2007;16:277–86. doi: 10.1002/pon.1185. [DOI] [PubMed] [Google Scholar]
- Kuchler T, Bestmann B, Rappat S, et al. Impact of psychotherapeutic support for patients with gastrointestinal cancer undergoing surgery: 10-year survival results of a randomized trial. J Clin Oncol. 2007;25:2702–2708. doi: 10.1200/JCO.2006.08.2883. [DOI] [PubMed] [Google Scholar]
- Luebbert K, Dahme B, Hasenbring M. The effectiveness of relaxation training in reducing treatment-related symptoms and improving emotional adjustment in acute non-surgical cancer treatment: a meta-analytical review. Psychooncology. 2001;10:490–502. doi: 10.1002/pon.537. [DOI] [PubMed] [Google Scholar]
- Lutgendorf SK, Degeest K, Sung CY, Arevalo JM, Penedo F, Lucci J, 3rd, et al. Depression, social support, and beta-adrenergic transcription control in human ovarian cancer. Brain Behav Immun. 2009;23:176–183. doi: 10.1016/j.bbi.2008.04.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, Sood A. Biobehavioral Factors and Cancer Progression: Physiological Pathways and Mechanisms. Invited review, Psychosomatic Medicine. 2011;73:724–730. doi: 10.1097/PSY.0b013e318235be76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, Sood A, Antoni MH. Host Factors and Cancer Progression: Biobehavioral Signaling Pathways and Interventions. Journal of Clinical Oncology. 2010;28:4094–4099. doi: 10.1200/JCO.2009.26.9357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf SK, Mullen-Houser E, Russell D, DeGeest K, Jacobsen G, Hart L, Bender D, Buekers T, Goodheart M, Antoni MH, Sood A, Lubaroff D. Preservation of Immune Function in Cervical Cancer Patients during chemoradiation using a Novel Integrative Approach. Brain, Behav Immun. 2010;24:1231–1240. doi: 10.1016/j.bbi.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor B, Antoni MH. Psychological intervention and health outcomes among women treated for breast cancer: a review of stress pathways and biological mediators. Brain Behav Immun. 2009;23:159–66. doi: 10.1016/j.bbi.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, Fok AK, Walker H, Lim A, Nicholls EF, et al. Low early-life social class leaves a biological residue manifested by decreased glucocorticoid and increased proinflammatory signaling. Proc Natl Acad Sci U S A. 2009;106:14716–14721. doi: 10.1073/pnas.0902971106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Cohen S, Ritchey AK. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychology. 2002;21:531–41. doi: 10.1037//0278-6133.21.6.531. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Sze J, Marin T, Arevalo JM, Doll R, et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64:266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Bowen D, Croyle R, Rowland J. Overview, current status, and future directions. In: Miller S, Bowen D, Croyle R, Rowland J, editors. Handbook of Cancer Control and Behavioral Science. American Psychological Association; Washington, DC: 2009. pp. 3–22. [Google Scholar]
- Nausheen B, Gidron Y, Peveler R, Moss-Morris R. Social support and cancer progression: a systematic review. J Psychosomatic Research. 2009;67:403–415. doi: 10.1016/j.jpsychores.2008.12.012. [DOI] [PubMed] [Google Scholar]
- Nelson E, Wenzel L, Osann K, Dogan-Ates A, Chatana N, Reina-Patton A, Laust A, Nashimoto K, Chicz-DeMet A, DuPont N, Monk B. Stress, immunity and cervical cancer: biobehavioral outcomes of a randomized clinical trial. Clinical Cancer Research. 2008;14(27):2111–2118. doi: 10.1158/1078-0432.CCR-07-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newell S, Sanson-Fisher R, Savolainen N. Systematic review of psychological therapies for cancer patients: Overview and recommendations for future research. J NCI. 2002;94:558–84. doi: 10.1093/jnci/94.8.558. [DOI] [PubMed] [Google Scholar]
- Patnaik JL, Byers T, DiGuiseppi C, Denberg TD, Dabelea D. The influence of comorbidities on overall survival among older women diagnosed with breast cancer. J NCI. 2011;103:1101–1111. doi: 10.1093/jnci/djr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pall SK, Hurria A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. J Clinical Oncology. 2010;28:4086–4093. doi: 10.1200/JCO.2009.27.0579. [DOI] [PubMed] [Google Scholar]
- Phillips KM, Antoni MH, Lechner SC, Blomberg BB, Llabre M, Avisar E, Gluck S, Dehragopian R, Carver C. Stress management intervention reduces serum cortisol and increases relaxation during treatment for non-metastatic breast cancer. Psychosomatic Medicine. 2008;70:1044–1049. doi: 10.1097/PSY.0b013e318186fb27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KM, Antoni MH, Carver CS, Lechner SC, Penedo FJ, McCullough ME, Glück S, DerHagopian R, Blomberg BB. Stress management skills and reductions in serum cortisol across the year after surgery for non-metastatic breast cancer. Cognitive Therapy and Research. 2011;35:595–600. [Google Scholar]
- Pierce BL, Ballard-Barbash R, Bernstein L, Baumgartner RN, Neuhouser ML, Wener MH, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–3444. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinquart M, Duberstein P. Associations of social networks with cancer mortality: a meta-analysis. Critical Reviews in Oncology and Hematology. 2010;75:122–137. doi: 10.1016/j.critrevonc.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savard J, Simard S, Ivers H, Morin CM. Randomized study on the efficacy of cognitive-behavioral therapy for insomnia secondary to breast cancer, part II: Immunologic effects. Journal of Clinical Oncology. 2005;23:6097–6106. doi: 10.1200/JCO.2005.12.513. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Chang-Claude J, Vrieling A, Heinz J, Flesch-Janys D, Steindorf K. Fatigue and quality of life in breast cancer survivors: temporal courses and long-term pattern. J Cancer Survivorship. 2012;6:11–19. doi: 10.1007/s11764-011-0197-3. [DOI] [PubMed] [Google Scholar]
- Schneiderman N, Antoni MH, Penedo F, Ironson G. Psychosocial and Behavioral Interventions in the Treatment of Physical Illnesses and Disease Processes. In: Steptoe A, editor. Handbook of Behavioral Medicine: Methods and Applications. N.Y: Springer; 2010. pp. 989–1007. [Google Scholar]
- Sephton S, Spiegel D. Circadian disruption in cancer: a neuroendocrine-immune pathway from stress to disease? Brain, Behavior, and Immunity. 2003;17:321–28. doi: 10.1016/s0889-1591(03)00078-3. [DOI] [PubMed] [Google Scholar]
- Spencer S, Lehman J, Wynings C, Arena P, Carver CS, Antoni MH, Derhagopian R, Ironson G, Love N. Concerns about breast cancer and relations to psychosocial well-being in a multi-ethnic sample of early stage patients. Health Psychology. 1999;18:159–169. doi: 10.1037//0278-6133.18.2.159. [DOI] [PubMed] [Google Scholar]
- Sestak I, Distler W, Forbes JF, et al. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: An exploratory analysis from the ATAC trial. J Clin Oncol. 2010;28:3411–3415. doi: 10.1200/JCO.2009.27.2021. [DOI] [PubMed] [Google Scholar]
- Sephton S, Sapolsky RM, Kraemer HC, Spiegel D. Early mortality in metastatic breast cancer patients with absent of abnormal diurnal cortisol rhythms. J Nat Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- Spiegel D. Mind matters in cancer survival. JAMA. 2011;305:502–503. doi: 10.1001/jama.2011.69. [DOI] [PubMed] [Google Scholar]
- Spiegel D, Bloom JR, Kraemer HC, Gottheil E. Effect of psychosocial treatment on survival of patients with metastatic breast cancer. Lancet. 1989;2:888–891. doi: 10.1016/s0140-6736(89)91551-1. [DOI] [PubMed] [Google Scholar]
- Spiegel D, Butler LD, Giese-Davis J, Koopman C, Miller E, DiMiceli S, et al. Effects of Supportive-Expressive Group Therapy on Survival of Patients with Metastatic Breast Cancer: A Randomized Prospective Trial. Cancer. 2007;110:1130. doi: 10.1002/cncr.22890. [DOI] [PubMed] [Google Scholar]
- Stanton AL. Psychosocial concerns and interventions for cancer survivors. J Clinical Oncol. 2006;24:5132–5173. doi: 10.1200/JCO.2006.06.8775. [DOI] [PubMed] [Google Scholar]
- Stark J, Avitsur R, Padgett DA, Sheridan JF. Social stress induces glucocorticoid resistance in macrophages. American J Physiol Reg Int Comp Physiol. 2001;280:R1799–R1805. doi: 10.1152/ajpregu.2001.280.6.R1799. [DOI] [PubMed] [Google Scholar]
- Steginga S, Lynch B, Hawkes A, Dunn J, Aitken J. Antecedents of domain-specific quality of life after colorectal cancer. Psychooncology. 2009;18:216–220. doi: 10.1002/pon.1388. [DOI] [PubMed] [Google Scholar]
- Stein K, Syrjala K, Andrykowski M. Physical and psychological long-term and late effects of cancer. Cancer. 2008;112:2577–2592. doi: 10.1002/cncr.23448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med. 2010;363:733–42. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- Talley A, Molix L, Schlegel RJ, Bettencourt A. The influence of breast cancer survivors’ perceived partner social support and need satisfaction on depressive symptoms: A longitudinal analysis. Psychology & Health. 2010;25:433–449. doi: 10.1080/08870440802582682. [DOI] [PubMed] [Google Scholar]
- Thomson C, Thompson PA, Wright-Bea J, Nardi E, Frey GR, Stopeck A. Metabolic syndrome and elevated C-reactive protein in breast cancer survivors on adjuvant hormone therapy. Journal of Women’s Health. 2009;18:2041–2047. doi: 10.1089/jwh.2009.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton L, Andersen B, Scholer T, Carson W. A psychological intervention reduces inflammatory markers by alleviating depressive symptoms: secondary analysis of a randomized controlled trial. Psychosomatic Medicine. 2009;71:715–724. doi: 10.1097/PSY.0b013e3181b0545c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton L, Andersen B, Carson W. Immune, endocrine, and behavioral precursors to breast cancer recurrence: a case-control analysis. Cancer Immunol Immunother. 2008;57:1471–1481. doi: 10.1007/s00262-008-0485-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pompe G, Antoni MH, Heijen C. The effects of surgical stress and psychological stress on the immune function of operative cancer patients. Psychology and Health. 1998;13:1015–1026. [Google Scholar]
- Vitaliano P, Scanlon J, Zhang J, Savage M, Hirsch I, Siegler I. A path model of chronic stress, the metabolic syndrome, and coronary heart disease. Psychosomatic Med. 2002;64:418–435. doi: 10.1097/00006842-200205000-00006. [DOI] [PubMed] [Google Scholar]
- Wenzel L, DeAlba I, Habbal R, Kluhsman B, Fairclough D, Krebs L, Anton-Culver H, Berkowitz R, Aziz N. Quality of life in long-term cervical cancer survivors. Gynecological Oncology. 2005;97:310–317. doi: 10.1016/j.ygyno.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Wimberly SR, Carver CS, Laurenceau JP, Harris SD, Antoni MH. Perceived Partner Reactions to Diagnosis and Treatment of Breast Cancer: Impact on Psychosocial and Psychosexual Adjustment. Journal of Consulting and Clinical Psychology. 2005;73:300–311. doi: 10.1037/0022-006X.73.2.300. [DOI] [PubMed] [Google Scholar]
- Witek-Janusek L, Albuquerque K, Chroniak KR, Chroniak C, Durazo-Arvizu R, Mathews H. Effect of mindfulness-based stress reduction on immune function, quality of life and coping in women newly diagnosed with early stage breast cancer. Brain Behav Immun. 2008;22:969–981. doi: 10.1016/j.bbi.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward WA, Storm EA, Tucker SL, McNeese MD. Changes in the 2003 AJCC staging for breast cancer dramatically affect stage specific survival. Journal of Clinical Oncology. 2003;21:3244–3248. doi: 10.1200/JCO.2003.03.052. [DOI] [PubMed] [Google Scholar]