Abstract
Cross-sectional age effects in normal control volunteers were investigated in 8 subcortical structures: lateral ventricles, thalamus, caudate, putamen, pallidum, hippocampus, amygdala and nucleus accumbens. Two hundred and twenty six control subjects, ranging in age from 19 to 85 years, were scanned on a 1.5T GE system (n = 184) or a 3.0T Siemens system (n = 42). Cranium-size adjusted subcortical structure volumes were estimated using FSL’s FIRST software, which is fully automated. Significant age effects were found for all volumes when the entire age range was analyzed, however the older subjects (60–85 years of age) showed a stronger correlation between age and structural volume for the ventricles, hippocampus, amygdala and accumbens than middle-aged (35–60 years of age) subjects. Middle-aged subjects were studied at both sites, and age effects in these groups were comparable, despite differences in magnet strength and acquisition systems. This agreement lends support to the validity of the image analysis tools and procedures used in the present study.
Keywords: subcortical, brain MRI, age, ventricles, thalamus, caudate, putamen, pallidum, hippocampus, amygdala, accumbens
1. Introduction
Knowledge of the effects of healthy aging on brain structures is necessary to help identify abnormal changes due to disease throughout the lifespan. Many cross-sectional and longitudinal studies have demonstrated age-related volume changes in the brain using MRI (Allen, et al. 2005, Bergfield, et al. 2010, Blatter, et al. 1995, Courchesne, et al. 2000, Fjell, et al. 2009, Fotenos, et al. 2005, Luft, et al. 1999, Mu, et al. 1999, Pfefferbaum, et al. 2010, Pieperhoff, et al. 2008, Raz, et al. 2010, 2001, 2005, 2006, Sullivan, et al. 1995, 2005, Walhovd, et al. 2005, Zimmerman, et al. 2006). A number of these studies have focused on subcortical structures, and age-related volume changes of the various subcortical structures have been identified.
Most studies examining the effects of normal aging on subcortical structures have utilized either manual or computer-assisted methods to delineate the structures (Allen, et al. 2005, Coffey, et al. 1998, Du, et al. 2006, Greenberg, et al. 2008, Gunning-Dixon, et al. 1998, Hasan, et al. 2008, Jernigan, et al. 2001, Krishnan, K.R., et al. 1990a, Liu, et al. 2003, Luft, et al. 1999, Mu, et al. 1999, Pruessner, et al. 2001, Raz, et al. 2005, 2003, Sullivan, et al. 1995, 2004, Van Der Werf, et al. 2001, Van Petten 2004). Automated methods have been employed in recent studies (Walhovd, et al. 2005, 2009), which have utilized FreeSurfer software (described in Fischl, et al. 2002, Han and Fischl 2007).
In the current study we utilize FIRST (FMRIB Image Registration and Segmentation Tool), a fully automated method within the FSL (FMRIB Software Library) suite of tools (Patenaude 2007, Smith, et al. 2004, Woolrich, et al. 2009). We have recently used the same method to segment subcortical structures and measure their volumes in a study comparing long-term abstinent alcoholics with non alcoholic controls (Sameti, et al. 2011, In Press). In the current report, we focus on age related differences in subcortical volume in non-alcoholic control samples. We compare data recorded in two middle-aged samples (35-60 years of age), one studied in San Francisco, CA on a 1.5T GE Signa system and the second studied in Honolulu, HI on a 3.0T Siemens Trio system. From the California site, we also compare the results from the middle-age sample to the results from younger adult (20–35 years of age) and healthy elderly (60–85 years of age) samples.
2. Methods and Materials
2.1. Participants
A total of 226 participants were recruited at the two sites by postings, mailings, newspaper advertisements, local internet sites and subject referrals. All participants were recruited as non-alcoholic controls (controls) for alcohol related studies at the sites, 184 controls were studied in the California site and 42 in Hawaii. The Hawaii subjects are completely separate from the California subjects. The California controls included 110 women and 74 men, ranging from 19 to 86 years of age (mean = 50.8). In California, the younger controls (age 19–35, n = 43), middle-aged (age 35–60, n = 71) and older (ages 60–86, n = 70) groups were recruited for different studies over a five-year period. These three groups of controls did not differ in years of education (averaging 16.3 years of education) or on the AMNART (Grober and Sliwinski 1991) estimated IQ, which averaged 127 across groups. The Hawaii controls consisted of 27 women and 15 men, ranging from 35 to 60 years of age (mean = 48.0 years). They were comparable to the middle-aged California controls on both age and years of education. The alcohol-related inclusion criterion for the all controls was a lifetime drinking average of less than 30 standard drinks per month, with no periods of drinking more than 60 drinks per month. A standard drink was defined as 12 oz. beer, 5 oz. wine, or 1.5 oz. liquor. Exclusion criteria for subjects of both sites were: 1) lifetime or current diagnosis of schizophrenia or schizophreniform disorder using the computerized Diagnostic Interview Schedule (c-DIS) (Bucholz, et al. 1991, Erdman, et al. 1992, Levitan, et al. 1991, Robins, et al. 1998), 2) history of lifetime or current drug abuse or dependence (other than nicotine or caffeine), 3) significant history of head trauma or cranial surgery, 4) history of significant neurological disease, 5) history of diabetes, stroke, or hypertension that required an emergent medical intervention, 6) laboratory evidence of hepatic disease, or 7) clinical evidence of Wernicke-Korsakoff syndrome.
All individuals participated in the following assessments: 1) psychiatric diagnoses and symptom counts were gathered using the c-DIS, 2) participants were interviewed on their lifetime drug and alcohol use using the timeline follow-back methodology (Skinner and Allen 1982, Skinner and Sheu 1982, Sobell and Sobell 1990, Sobell, et al. 1988), 3) medical histories were reviewed in an interview by a trained research associate, 4) blood was drawn to test liver functions, and 5) the Family Drinking Questionnaire was administered based on the methodology of Mann and colleagues (1985) and Stoltenberg and colleagues (1998). Approval for the study was obtained from a freestanding independent human subjects research review committee [Independent Review Consulting / E&I Review Services, LLC, Corte Madera, CA] and written informed consent was obtained from all research participants.
2.2. Image acquisition
All MRIs for California controls were collected on a 1.5T GE Signa Infinity with the LX platform (GE Medical Systems, Waukesha, WI) located at the Pacific Campus of the California Pacific Medical Center in San Francisco. For each subject, we acquired a transaxial T1-weighted Spoiled Gradient image (TR = 35 ms, TE = 5 ms, acquisition matrix = 256 × 256) with 124 axial slices at 1.3 mm thickness and a Fluid Attenuated Inversion Recovery (FLAIR) image (TR = 8800 ms, TE = 144.7 ms, inversion time = 2200 ms, acquisition matrix = 256 × 256) with 29 axial slices at 5 mm thickness.
MRIs of the Hawaii controls were collected on a 3.0T Siemens Trio Tim platform, located at the Queens Medical Center in Honolulu, HI. For each subject, we acquired a sagittal T1-weighted (MPRAGE) image (TR = 2200 ms, TE = 4.1 ms, TI = 1000 ms, acquisition matrix = 256 × 256) with 160 slices at 1.0 mm thickness and a Fluid Attenuated Inversion Recovery (FLAIR) image (TR = 9100 ms, TE = 83 ms, TI = 2500 ms, acquisition matrix = 204 × 230) with 44 slices at 3 mm thickness. A neuroradiologist read all MRI scans. All scans were free from abnormalities other than white matter signal hyperintensities (WMSH).
2.3. Image analysis
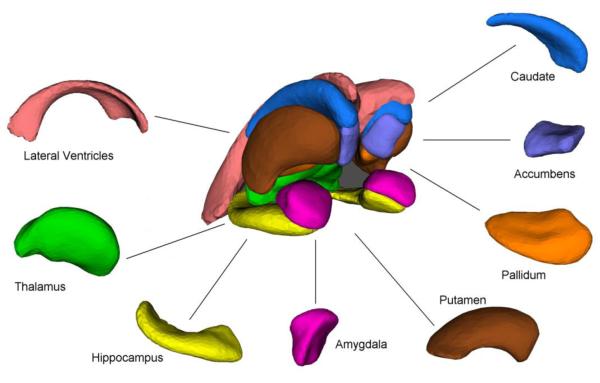
We studied 16 of the 17 subcortical brain structures which are extracted by FSL’s FIRST (FMRIB Image Registration and Segmentation Tool) (Patenaude 2007), a method that has been used successfully in a number of recent investigations (Angstrom, et al. 2004, Corthorn, et al. 1997, Figueroa Cave 1997, Markov, et al. 1997, Rinehart, et al. 1997, Sameti, et al. 2011). The brainstem was excluded because the shape model used in FIRST extended beyond the inferior boundary of the image for the California subjects. The inferior boundary was prescribed at the time of acquisition. The following structures (shown in Figure 1) were extracted and their volumes were measured for all 226 T1-weighted MR images: left and right lateral ventricles, left and right thalamus, left and right caudate, left and right putamen, left and right pallidum, left and right hippocampus, left and right amygdala, and left and right nucleus accumbens.
Figure 1.
Sixteen brain subcortical structures (left and right) examined in this study.
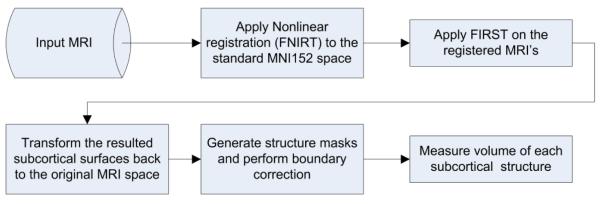
Image analysis was performed using FSL, version 4.1 (Oxford, UK). FIRST is the FSL tool for segmentation of subcortical structures. When boundaries of the delineated structures were visually inspected, we observed boundary underestimation of lateral ventricles in MRIs of subjects with ventricles significantly larger than those in the MNI152 standard template (which occurs as a natural result of age-related atrophy). To ensure a more accurate segmentation of the lateral ventricles, MRIs were first registered MNI152 standard space template using FSL’s FNIRT (FMRIB’s Nonlinear Image Registration Tool). The warped (registered) MRIs were then processed through FIRST to extract the surface mesh of each of the 16 subcortical structures. The surfaces in the warped space were then transformed back (un-warped) to the original MRI space. The un-warped surfaces of the segmented structures were then filled and boundary corrected using the FSL tool “first_utils” (preventing voxel overlap between structures). Figure 2 shows the processing steps we used for this segmentation. Boundaries of each delineated structure were visually inspected for gross errors. For each of the 16 extracted structures, the volume is measured in cubic millimeters. Cranium size estimation (an estimate of premorbid brain size) was also performed using FSL’s SIENAX (Structural Image Evaluation, using Normalisation, of Atrophy) tool.
Figure 2.
Flowchart of the steps for subcortical structure segmentation and volume measurement when a non-linear registration is utilized.
2.4. Statistical analyses
The General Linear Model (GLM) (SPSS Inc. 2009) was first used to determine whether subcortical volumes were correlated with the cranium size index. Since all 16 subcortical volumes were significantly positively correlated with the cranium size, we used linear regression to adjust each structure volume for the cranium size index, calculated by FSL’s SIENAX for each subject. We have previously shown that the FSL cranium size index is an excellent surrogate for the intracranial vault volume (Fein, et al. 2004). For each subcortical structure, we then added the left and right volumes, resulting in 8 measures per subject. These cranium size adjusted volumes were used in all subsequent analyses.
First, we ran GLM analysis on the entire California controls, for both raw volumes and cranium adjusted volumes, with the expectation that the cranium size adjusted values would remove gender differences in the structure volumes and would increase the effect size of age effects as a result of removing extraneous variance. Curve fitting analysis was also performed to determine the best model of age related differences in subcortical volumes. Then we divided the California subjects into younger (under 35 years of age), middle-aged (35–60 years of age) and older (above 60 years of age) subgroups. We used the middle-aged group as the standard, and performed GLM analysis to determine whether age effects in younger or older groups differed from those in the middle-aged group. Finally, GLM was conducted to assess the repeatability of age effects across sites (California vs. Hawaii), with the analysis limited to the 35 to 60 year age range which was sampled at both sites.
3. Results
3.1. Subcortical volumes and age in California controls
All eight subcortical structures showed significant volume differences related to subject age. This is consistent with findings from previous brain imaging studies (Behets, et al. 1997, Figueroa, et al. 1997, Hantouche, et al. 2003, Helal, et al. 1997, Raz, et al. 2010, Tarres, et al. 1997, Walhovd, et al. 2009). Table 1 indicates the mean volume for each structure, separated by gender, and effect sizes resulted from GLM analysis. Gender differences in subcortical volumes are present for all structures in the raw data, but the cranium size adjustments effectively remove these differences, showing that they are a consequence of larger cranium sizes in men compared to women. Note also that the age effect sizes are larger after cranium size adjustment, since it removes extraneous variance due to inter-subject differences in head size.
Table 1. Average subcortical structure volumes (raw and adjusted for cranium size) in California controls with age and gender effect sizes.
| SUBCORTICAL STRUCTURE |
Raw Volumes | Cranium Size Adjusted Volumes | ||||||
|---|---|---|---|---|---|---|---|---|
| Mean Volume | Effect Size (%) (Partial eta square) |
Mean Volume | Effect Size (%) (Partial eta square) |
|||||
| Female (n = 110) |
Male (n = 74) |
Age | Gender | Female (n = 110) |
Male (n = 74) |
Age | Gender | |
| Lateral Ventricles | 20623 | 23446 | 43.4** | 8.2** | 22132 | 21201 | 45.5** | 0.7 |
| Thalamus | 15418 | 16882 | 20.3** | 15.5** | 15953 | 16087 | 35.2** | 0.3 |
| Caudate | 6466 | 7119 | 2.3* | 2.7* | 6971 | 6816 | 3.7** | 1.5.094 |
| Putamen | 9170 | 9942 | 43.4** | 8.1** | 9427 | 9560 | 52.9** | 0.6 |
| Pallidum | 3282 | 3494 | 8.3** | 4.5** | 3367 | 3367 | 10.8** | 0.3 |
| Hippocampus | 7138 | 7494 | 13.5** | 2.8* | 7303 | 7249 | 17.5** | 1.2 |
| Amygdala | 2712 | 2942 | 3.7** | 10.1** | 2785 | 2834 | 5.2** | 0.2 |
| Nucleus Accumbens | 916 | 1002 | 37.4** | 2.1.052 | 948 | 954 | 42.5** | 1.3 |
All volumes are reported in cubic millimeters.
Effect is significant: p ≤ 0.05
p ≤ 0.01.
Trends are reported as superscripted p levels.
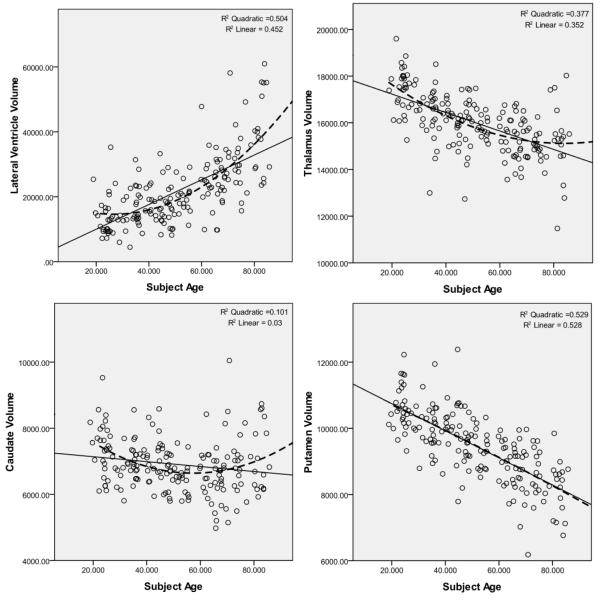
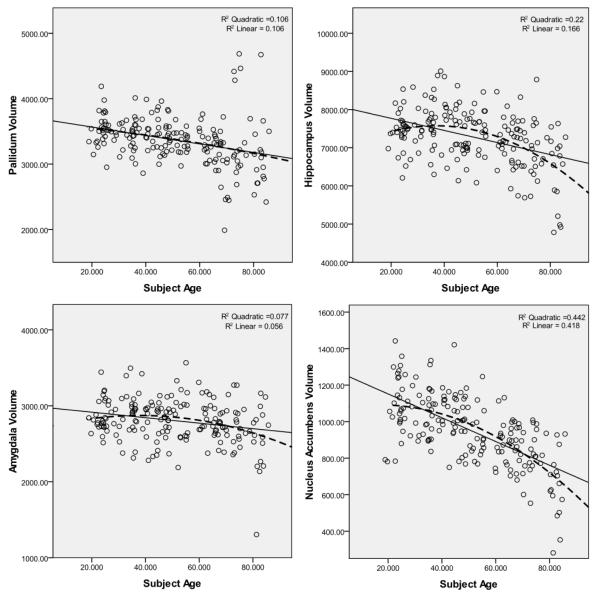
Figure 3 shows the scatter plots for all subcortical volumes vs. age. Linear and quadratic curves are also fitted into each plot with their corresponding R2 values. In all structures, the quadratic model was a better fit when compared to linear, with the exception of putamen and pallidum, for which the linear model was virtually identical to the quadratic one.
Figure 3.
Subcortical volumes (in mm3) vs. age in California controls. Linear and quadratic regression curves with their associated R2 values are displayed on each plot.
GLM analysis on the middle-aged California subjects (35 to 60 years of age) showed significant age effect for the caudate (F1,68 = 16.850, p < 0.001, effect size 19.9% of variance), putamen (F1,68 = 8.168, p < 0.01, effect size 10.7% of variance) and accumbens (F1,68 = 15.419, p < 0.001, effect size 18.5% of variance) volumes, with a trend toward an effect for the thalamus (F1,68 = 3.629, p < 0.061, effect size 5.1% of variance). There was also a significant gender effect on caudate volume (F1,68 = 10.125, p < 0.01, effect size 13.0% of variance). Parameter estimates of the age-related linear regression coefficient, computed from the middle-aged sample for each subcortical structure, were applied to all subjects, regardless of age. We then examined age effects on these adjusted values in the younger (below 35) and older (above 60) subject groups separately. The results would highlight age effects in the younger or older groups that differed from the age effects in the middle-aged group. For the younger group, only the thalamus showed a negative correlation with age (F1,40 = 6.483, p < 0.05, effect size 13.9% of variance). For the older group, there were effects for the ventricles (F1,67 = 17.915, p < 0.001, effect size 21.1% of variance), caudate (F1,67 = 21.638, p < 0.001, effect size 24.4% of variance) and amygdala (F1,67 = 9.969, p < 0.01, effect size 13.0% of variance) and a trend toward an effect for the hippocampus (F1,67 = 3.257, p < 0.076, effect size 4.6% of variance). In the older group, the correlation between lateral ventricle volume and age is 19.1% more per decade than the middle-age group correlation. The changes to the age correlate for the amygdala and hippocampus were 5.9% and 3.2% per decade, respectively. However, the older group caudate age-volume correlation is 9.6% per decade more than the younger group.
Based on the lack of differences between the younger and middle-aged subjects, we combined those groups in California calculate age related change rates for each subcortical volume. Table 2 shows the age-related difference in California controls for all 8 subcortical structures. These percentages are calculated based on regression lines modeling subcortical volume changes vs. age, for subjects younger than 60 years of age (n = 114) and subjects who were 60 or older (n = 70).
Table 2. Age-related difference in volume per decade for subjects younger and older than 60 years of age.
| SUBCORTICAL STRUCTURE |
Volume difference per decade (%) | |
|---|---|---|
| Under 60 (n = 114) |
60 & Above (n = 70) |
|
| Lateral Ventricles | 13.13** | 34.31** |
| Thalamus | −2.99** | −0.83 |
| Caudate | −3.30** | 4.55.053 |
| Putamen | −3.48** | −4.20** |
| Pallidum | −1.21061 | −1.28 |
| Hippocampus | −0.08 | −4.91** |
| Amygdala | −0.03 | −4.24* |
| Nucleus Accumbens | −4.27** | −11.80** |
Effect is significant: p ≤ 0.05
p ≤ 0.01.
Trends are reported as superscripted p levels.
3.2. Subcortical volumes in middle-age - repeatability of results across sites and scanners
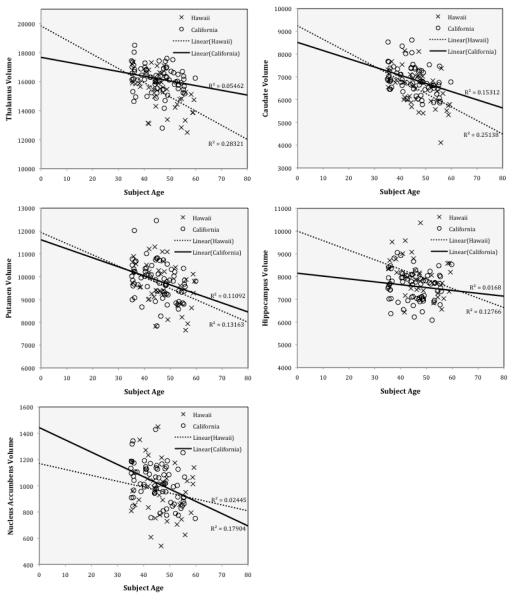
Middle-aged data were re-analyzed with data from both California and Hawaii included. Note that the MRI’s for the two sites were acquired on two magnets from different manufacturers and with different field strengths. Table 3 shows the result of the analysis of this data. There was no significant site effect or site by age interaction for any of the subcortical structure volumes. Thalamus, caudate, putamen, hippocampus and accumbens continued to show a significant are-related decrease. However, age did not have a significant effect on ventricles, pallidum and amygdala volumes for these middle age subjects. Figure 4 shows the scatter plots for subcortical volumes vs. age with significant age-related decreases for the middle-age subjects only. The California and Hawaii subjects are marked separately. There is reasonable agreement between cohorts for the thalamus, caudate, putamen, hippocampus and accumbens. This is consistent with the findings of Gunning-Dixon et al. (1998), Jernigan et al. (2001), Luft et al. (1999) and Raz et al. (2003), on pallidum and also with Doty et al. (2008), Jernigan et al. (2001) and Pruessner et al. (2001) on amygdala.
Table 3. Average subcortical structure volumes for California and Hawaii controls of the same age range (35 to 60 years).
| SUBCORTICAL STRUCTURE |
California Controls Mean Volume (n = 71) |
Hawaii Controls Mean Volume (n = 42) |
Effect Size (%) (Partial eta square |
||
|---|---|---|---|---|---|
| Age | Site | Site × Age |
|||
| Lateral Ventricles | 18395 | 16295 | 1.3 | 1.0 | 1.5 |
| Thalamus | 16183 | 15261 | 14.5** | 1.6 | 3.3.056 |
| Caudate | 6863 | 6536 | 4.3** | 0.0 | 0.1 |
| Putamen | 9819 | 9632 | 10.2** | 0.0 | 0.0 |
| Pallidum | 3458 | 3659 | 0.2 | 0.5 | 1.0 |
| Hippocampus | 7563 | 8029 | 6.7** | 1.5 | 0.3 |
| Amygdala | 2852 | 2894 | 0.1 | 1.1 | 0.6 |
| Nucleus Accumbens | 1009 | 961 | 9.7** | 1.7 | 1.4 |
All volumes are reported in cubic millimeters.
Effect is significant: p ≤ 0.05
p ≤ 0.01.
Trends are reported as superscripted p levels
Figure 4.
Subcortical volumes (in mm2) vs age in California controls and Hawaii controls. Linear regression curves with their associated R2 values are displayed on each plot.
4. Discussion
For all subcortical volumes, age effects were observed. In the California sample, all structures showed a negative correlation with age, except for the lateral ventricles, which correlate positively with age. However, when subject age range was limited to the middle age group (35–60 years of age), and the data from the California and Hawaii cohorts were analyzed together, 5 subcortical structures showed reduced volume as an age-related difference: thalamus, caudate, putamen, hippocampus and nucleus accumbens, and none showed a volume increase. These effects were comparably present in both cohorts (i.e., there was no significant site by age interaction), indicating the consistency of the findings across cohorts and of the image analysis methodology across GE and Siemens MRI acquisition systems at different field strengths.
The age-related increase in lateral ventricle volume is in agreement with decades of findings (e.g. Coffey, et al. 1998, Jernigan, et al. 2001, Sullivan, et al. 1995, Walhovd, et al. 2005, 2009). We found the relationship between volume and age per decade is 2.6 times greater in the subjects over 60 years of age than the middle-age and younger groups. This is consistent with the literature, including Walhovd and colleagues (2005, 2009), who reported a higher rate of lateral ventricles volume increase in elderly subjects compared to the middle-age and younger subjects.
The thalamus was found to have smaller volumes with increasing age in several studies ((Pieperhoff, et al. 2008, Sullivan, et al. 2004, Van Der Werf, et al. 2001, Walhovd, et al. 2005, 2009); however, Jernigan and colleagues (1991, 2001) did not report such an age effect. We found the age effect on thalamus volume is of 3% per decade for the middle-age group with no difference in younger subjects but with a decreased age effect in the older group to less than 1% (which is a statistically non-significant rate of decline in the elderly sample). This is in agreement with findings of one study (Van Der Werf, et al. 2001), which reported a smaller rate of thalamic volume reduction in elderly compared to middle-age samples.
Caudate volume was consistently found to decline with age in several cross-sectional (Greenberg, et al. 2008, Gunning-Dixon, et al. 1998, Hasan, et al. 2008, Jernigan, et al. 2001, Krishnan, K. R., et al. 1990b, Walhovd, et al. 2005) and longitudinal studies ((Raz, et al. 2005, 2003). We found the same overall effect for the entire California sample; however when subjects were divided into separate age groups, the caudate volume is greater in older subjects. This is consistent with the findings of Walhovd and colleagues (2009) in their analysis of 883 subjects from 5 different samples which also showed caudate volume increases for subjects between 60 and 95 years of age. A possible confounding factor contributing to this volume increase may be the presence of periventricular white matter signal hyperintensities (PWMSH) that could have been misclassified as caudate. PWMSH are common in elderly subjects and in T1 images are often difficult to distinguish from grey matter. The authors believe this phenomenon is responsible for the counter-intuitive increase in caudate volume.
Putamen volume showed a consistent decrease in age-related volume throughout the range studied (19–85 years). This is in agreement with other findings (Greenberg, et al. 2008, Gunning-Dixon, et al. 1998, Raz, et al. 2003, Walhovd, et al. 2005, 2009). In contrast, Jernigan and colleagues (2001) did not find age effects on the lenticular nuclei which includes the globus pallidus and putamen; the aggregation of these two structures may have contributed to their failure to find an age effect.
Age changes in pallidum volume were less consistently reported in the literature than for the other structures mentioned above. No age effect was found in several studies (Gunning-Dixon, et al. 1998, Jernigan, et al. 2001, Luft, et al. 1999, Raz, et al. 2003), while Walhovd and colleagues reported a trend (2005), and a significant negative association with age (2009). Our results also showed less of an age effect on the pallidum than on other structures. In fact, the age effect was only observed when the entire California sample (n = 184) was included in the analysis.
Many studies have focused on the hippocampus and its volume changes with age. The majority of these studies reported volume reductions with age (Allen, et al. 2005, Greenberg, et al. 2008, Jernigan, et al. 2001, Mu, et al. 1999, Pruessner, et al. 2001, Raz, et al. 2010, Walhovd, et al. 2005, 2009), while others found no age-related hippocampal volume changes (Du, et al. 2006, Liu, et al. 2003, Sullivan, et al. 1995, 2005, Van Petten 2004). We found hippocampus volume to correlate negatively with age in both middle-age (California and Hawaii cohorts combined) and older subject groups, with the correlation considerably stronger in the elderly subjects.
For the amygdala, a negative correlation between volume and age was found when all California subjects were analyzed together; however, this effect was not present when younger or middle-age groups (both in California and Hawaii) were examined separately. This is consistent with a number of studies that found amygdala age-related volume reduction in elderly samples (Allen, et al. 2005, Mu, et al. 1999, Walhovd, et al. 2005, 2009), but not in middle-age samples (Doty, et al. 2008, Jernigan, et al. 2001, Pruessner, et al. 2001).
Finally, our finding of age related volume differences in the accumbens across all age groups is consistent with the three previous reports in the literature (Jernigan, et al. 2001, Walhovd, et al. 2005, 2009). We found the rate strength of the negative age-related correlation for accumbens volume in the older group is 2.7 times that of the middle-age and younger groups.
Our findings are consistent with that of many other studies, in particular, Walhovd and colleagues (2009) who studied the largest sample among all age related studies of subcortical structures and incorporated results from FreeSurfer’s ASEG (subcortical segmentation) (Fischl, et al. 2002). Both FIRST and ASEG were trained from manual segmentations provided by the CMA (Center for Morphometric Analysis), providing the same definitions of each structure. The two automated methods do differ in methodology. FIRST uses a surface based shape/appearance model approach, where intensities are sample normal to the surface. FreeSurfer uses an integrated volumetric registration and segmentation, incorporating voxel-wise shape and intensity priors. In addition, the consistency of the findings between our two cohorts with MRIs acquired at two separate sites utilizing different magnets (a 1.5T GE and a 3.0T Siemens) confirms the reliability of the delineation method. Furthermore, the agreement between this study which used FIRST and previous studies using other segmentation methods (e.g. FreeSurfer or manual methods) adds to the limited, but growing number of studies demonstrating the accuracy and reliability of FIRST as a structural imaging tool. The results of the study demonstrate increased age related differences in the older group (60 years of age and older) for lateral ventricles, putamen, hippocampus, amygdala and nucleus accumbens and validate the hypothesis that brain changes accelerate with increasing age.
Acknowledgements
This work was supported by the National Institutes for Health, NIH Grants #5R01AA016944 and #5R01AA013659
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The manuscript submitted reports research which was conducted in accordance with the relevant guidelines for ethical research and the materials enclosed have not been published or submitted for review in any other publications and none of the authors have any commercial affiliation or consultant role that could be construed as a conflict of interest.
References
- Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: the major lobes and a parcellation of the temporal region. Neurobiology of Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. discussion 1279-1282. [DOI] [PubMed] [Google Scholar]
- Angstrom J, Larsson T, Hansson GC, Karlsson KA, Henry S. Default biosynthesis pathway for blood group-related glycolipids in human small intestine as defined by structural identification of linear and branched glycosylceramides in a group O Le(a-b-) nonsecretor. Glycobiology. 2004;14:1–12. doi: 10.1093/glycob/cwh003. [DOI] [PubMed] [Google Scholar]
- Behets FM, Brathwaite A, Bennett L, Douglas KG, Dallabetta GA, Figueroa JP, Collaborative Working Group on Decentralized Syphilis Screening The decentralization of syphilis screening for improved care in Jamaican public clinics. American Journal of Public Health. 1997;87:1019–1021. doi: 10.2105/ajph.87.6.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergfield KL, Hanson KD, Chen K, Teipel SJ, Hampel H, Rapoport SI, Moeller JR, Alexander GE. Age-related networks of regional covariance in MRI gray matter: reproducible multivariate patterns in healthy aging. NeuroImage. 2010;49:1750–1759. doi: 10.1016/j.neuroimage.2009.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatter DD, Bigler ED, Gale SD, Johnson SC, Anderson CV, Burnett BM, Parker N, Kurth S, Horn SD. Quantitative volumetric analysis of brain MR: normative database spanning 5 decades of life. American Journal of Neuroradiology. 1995;16:241–251. [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Robins LN, Shayka JJ, Przybeck TR, Helzer JE, Goldring E, Klein MH, Greist JH, Erdman HP, Skare SS. Performance of two forms of a computer psychiatric screening interview: version I of the DISSI. Psychiatry Research. 1991;25:117–129. doi: 10.1016/0022-3956(91)90005-u. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Lucke JF, Saxton JA, Ratcliff G, Unitas LJ, Billig B, Bryan RN. Sex differences in brain aging: a quantitative magnetic resonance imaging study. Archives of Neurology. 1998;55:169–179. doi: 10.1001/archneur.55.2.169. [DOI] [PubMed] [Google Scholar]
- Corthorn J, Figueroa C, Valdes G. Estrogen and luminal stimulation of rat uterine kallikrein. Biology of Reproduction. 1997;56:1432–1438. doi: 10.1095/biolreprod56.6.1432. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Chisum HJ, Townsend J, Cowles A, Covington J, Egaas B, Harwood M, Hinds S, Press GA. Normal brain development and aging: quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- Doty TJ, Payne ME, Steffens DC, Beyer JL, Krishnan KR, LaBar KS. Age-dependent reduction of amygdala volume in bipolar disorder. Psychiatry Research. 2008;163:84–94. doi: 10.1016/j.pscychresns.2007.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du AT, Schuff N, Chao LL, Kornak J, Jagust WJ, Kramer JH, Reed BR, Miller BL, Norman D, Chui HC, Weiner MW. Age effects on atrophy rates of entorhinal cortex and hippocampus. Neurobiology of Aging. 2006;27:733–740. doi: 10.1016/j.neurobiolaging.2005.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdman HP, Klein MH, Greist JH, Skare SS, Husted JJ, Robins LN, Helzer JE, Goldring E, Hamburger M, Miller JP. A comparison of two computer-administered versions of the NIMH Diagnostic Interview Schedule. Psychiatry Research. 1992;26:85–95. doi: 10.1016/0022-3956(92)90019-k. [DOI] [PubMed] [Google Scholar]
- Fein G, Di Sclafani V, Taylor C, Moon K, Barakos J, Tran H, Landman B, Shumway R. Controlling for premorbid brain size in imaging studies: T1-derived cranium scaling factor vs. T2-derived intracranial vault volume. Psychiatry Research. 2004;131:169–176. doi: 10.1016/j.pscychresns.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Figueroa Cave G. El trastorno de angustia y la enfermedad de Freud [Anxiety disorder and Freud’s disease] Revista médica de Chile. 1997;125:363–370. [PubMed] [Google Scholar]
- Figueroa CD, Novoa U, Valdes G, Corthorn J, Muller-Esterl W. Localization of the bradykinin B2 receptor in uterus, bladder and Madin-Darby canine kidney cells. Immunopharmacology. 1997;36:127–133. doi: 10.1016/s0162-3109(97)00011-8. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fjell AM, Walhovd KB, Fennema-Notestine C, McEvoy LK, Hagler DJ, Holland D, Brewer JB, Dale AM. One-year brain atrophy evident in healthy aging. Journal of Neuroscience. 2009;29:15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fotenos AF, Snyder AZ, Girton LE, Morris JC, Buckner RL. Normative estimates of cross-sectional and longitudinal brain volume decline in aging and AD. Neurology. 2005;64:1032–1039. doi: 10.1212/01.WNL.0000154530.72969.11. [DOI] [PubMed] [Google Scholar]
- Greenberg DL, Messer DF, Payne ME, Macfall JR, Provenzale JM, Steffens DC, Krishnan RR. Aging, gender, and the elderly adult brain: an examination of analytical strategies. Neurobiology of Aging. 2008;29:290–302. doi: 10.1016/j.neurobiolaging.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grober E, Sliwinski M. Development and validation of a model for estimating premorbid verbal intelligence in the elderly. Clinical & Experimental Neuropsychology. 1991;13:933–949. doi: 10.1080/01688639108405109. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Head D, McQuain J, Acker JD, Raz N. Differential aging of the human striatum: a prospective MR imaging study. American Journal of Neuroradiology. 1998;19:1501–1507. [PMC free article] [PubMed] [Google Scholar]
- Han X, Fischl B. Atlas renormalization for improved brain MR image segmentation across scanner platforms. IEEE Transactions on Medical Imaging. 2007;26:479–486. doi: 10.1109/TMI.2007.893282. [DOI] [PubMed] [Google Scholar]
- Hantouche EG, Angst J, Demonfaucon C, Perugi G, Lancrenon S, Akiskal HS. Cyclothymic OCD: a distinct form? Journal of Affective Disorders. 2003;75:1–10. doi: 10.1016/s0165-0327(02)00461-5. [DOI] [PubMed] [Google Scholar]
- Hasan KM, Halphen C, Boska MD, Narayana PA. Diffusion tensor metrics, T2 relaxation, and volumetry of the naturally aging human caudate nuclei in healthy young and middle-aged adults: possible implications for the neurobiology of human brain aging and disease. Magnetic Resonance in Medicine. 2008;59:7–13. doi: 10.1002/mrm.21434. [DOI] [PubMed] [Google Scholar]
- Helal M, Black T, Lockhart J, Figueroa TE. The Hickman peel-away sheath: alternative for pediatric percutaneous nephrolithotomy. Journal of Endourology. 1997;11:171–172. doi: 10.1089/end.1997.11.171. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Berhow MT, Sowell ER, Foster DS, Hesselink JR. Cerebral structure on MRI, Part I: Localization of age-related changes. Biological Psychiatry. 1991;29:55–67. doi: 10.1016/0006-3223(91)90210-d. [DOI] [PubMed] [Google Scholar]
- Jernigan TL, Archibald SL, Fennema-Notestine C, Gamst AC, Stout JC, Bonner J, Hesselink JR. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiology of Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Husain MM, McDonald WM, Doraiswamy PM, Figiel GS, Boyko OB, Ellinwood EH, Nemeroff CB. In vivo stereological assessment of caudate volume in man: effect of normal aging. Life Sci. 1990a;47:1325–1329. doi: 10.1016/0024-3205(90)90196-x. [DOI] [PubMed] [Google Scholar]
- Krishnan KR, Husain MM, McDonald WM, Doraiswamy PM, Figiel GS, Boyko OB, Ellinwood EH, Nemeroff CB. In vivo stereological assessment of caudate volume in man: effect of normal aging. Life Sciences. 1990b;47:1325–1329. doi: 10.1016/0024-3205(90)90196-x. [DOI] [PubMed] [Google Scholar]
- Levitan RD, Blouin AG, Navarro JR, Hill J. Validity of the computerized DIS for diagnosing psychiatric inpatients. Canadian Journal of Psychiatry. 1991;36:728–731. [PubMed] [Google Scholar]
- Liu RS, Lemieux L, Bell GS, Sisodiya SM, Shorvon SD, Sander JW, Duncan JS. A longitudinal study of brain morphometrics using quantitative magnetic resonance imaging and difference image analysis. NeuroImage. 2003;20:22–33. doi: 10.1016/s1053-8119(03)00219-2. [DOI] [PubMed] [Google Scholar]
- Luft AR, Skalej M, Schulz JB, Welte D, Kolb R, Burk K, Klockgether T, Voight K. Patterns of age-related shrinkage in cerebellum and brainstem observed in vivo using three-dimensional MRI volumetry. Cerebral Cortex. 1999;9:712–721. doi: 10.1093/cercor/9.7.712. [DOI] [PubMed] [Google Scholar]
- Mann RE, Sobell LC, Sobell MB, Pavan D. Reliability of a family tree questionnaire for assessing family history of alcohol problems. Drug and Alcohol Dependence. 1985;15:61–67. doi: 10.1016/0376-8716(85)90030-4. [DOI] [PubMed] [Google Scholar]
- Markov AK, Brumley MA, Figueroa A, Skelton TN, Lehan PH. Hemodynamic effects of fructose 1,6-diphosphate in patients with normal and impaired left ventricular function. American Heart Journal. 1997;133:541–549. doi: 10.1016/s0002-8703(97)70149-2. [DOI] [PubMed] [Google Scholar]
- Mu Q, Xie J, Wen Z, Weng Y, Shuyun Z. A quantitative MR study of the hippocampal formation, the amygdala, and the temporal horn of the lateral ventricle in healthy subjects 40 to 90 years of age. American Journal of Neuroradiology. 1999;20:207–211. [PMC free article] [PubMed] [Google Scholar]
- Patenaude BM. Department of Clinical Neurology. Vol. 236. University of Oxford; Oxford: 2007. Bayesian Statistical Models of Shape and Appearence for Subcortical Brain Segmentation. [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Rohlfing T, Sullivan EV. Diffusion tensor imaging of deep gray matter brain structures: effects of age and iron concentration. Neurobiology of Aging. 2010;31:482–493. doi: 10.1016/j.neurobiolaging.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieperhoff P, Homke L, Schneider F, Habel U, Shah NJ, Zilles K, Amunts K. Deformation field morphometry reveals age-related structural differences between the brains of adults up to 51 years. Journal of Neuroscience. 2008;28:828–842. doi: 10.1523/JNEUROSCI.3732-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Collins DL, Pruessner M, Evans AC. Age and gender predict volume decline in the anterior and posterior hippocampus in early adulthood. Journal of Neuroscience. 2001;21:194–200. doi: 10.1523/JNEUROSCI.21-01-00194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Williamson A, Acker JD. Age and sex differences in the cerebellum and the ventral pons: a prospective MR study of healthy adults. American Journal of Neuroradiology. 2001;22:1161–1167. [PMC free article] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Kennedy KM, Dahle C, Head D, Acker JD. Differential age-related changes in the regional metencephalic volumes in humans: a 5-year follow-up. Neuroscience Letters. 2003;349:163–166. doi: 10.1016/s0304-3940(03)00820-6. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cerebral Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neuroscience & Biobehavioral Reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz N, Ghisletta P, Rodrigue KM, Kennedy KM, Lindenberger U. Trajectories of brain aging in middle-aged and older adults: regional and individual differences. NeuroImage. 2010;51:501–511. doi: 10.1016/j.neuroimage.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinehart J, Keville L, Clayton S, Figueroa JA. Corticosteroids alter hematopoiesis in vitro by enhancing human monocyte secretion of granulocyte colony-stimulating factor. Experimental Hematology. 1997;25:405–412. [PubMed] [Google Scholar]
- Robins LN, Cottler L, Buckholz K, Compton W. The Diagnostic Interview Schedule for DSM-IV. Washington University School of Medicine; St. Louis, MO: 1998. [Google Scholar]
- Sameti M, Smith S, Patenaude B, Fein G. Subcortical Volumes in Long Term Abstinent Alcoholics: Associations With Psychiatric Comorbidity. Alcoholism: Clinical and Experimental Research. 2011;35:1067–1080. doi: 10.1111/j.1530-0277.2011.01440.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. Journal of Abnormal Psychology. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Sheu WJ. Reliability of alcohol use indices. The Lifetime Drinking History and the MAST. Journal of Studies on Alcohol. 1982;43:1157–1170. doi: 10.15288/jsa.1982.43.1157. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB, Riley DM, Schuller R, Pavan DS, Cancilla A, Klajner F, Leo GI. The reliability of alcohol abusers’ self-reports of drinking and life events that occurred in the distant past. Studies of Alcohol. 1988;49:225–232. doi: 10.15288/jsa.1988.49.225. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Self-reports issues in alcohol abuse: State of the art and future directions. Behaviorial Assessment. 1990;12:77–90. [Google Scholar]
- SPSS Inc. SPSS Statistics 18.0, Rel.18.0.0. SPSS Inc.; Chicago: 2009. [Google Scholar]
- Stoltenberg SF, Mudd SA, Blow FC, Hill EM. Evaluating measures of family history of alcoholism: density versus dichotomy. Addiction. 1998;93:1511–1520. doi: 10.1046/j.1360-0443.1998.931015117.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Age-related decline in MRI volumes of temporal lobe gray matter but not hippocampus. Neurobiology of Aging. 1995;16:591–606. doi: 10.1016/0197-4580(95)00074-o. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rosenbloom M, Serventi KL, Pfefferbaum A. Effects of age and sex on volumes of the thalamus, pons, and cortex. Neurobiology of Aging. 2004;25:185–192. doi: 10.1016/s0197-4580(03)00044-7. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Pfefferbaum A. Preservation of hippocampal volume throughout adulthood in healthy men and women. Neurobiology of Aging. 2005;26:1093–1098. doi: 10.1016/j.neurobiolaging.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Tarres MC, Martinez SM, Montenegro SM, Figueroa NS, D’Ottavio AE, Picena JC. Influence of gonadectomy in eSS diabetic rats. Rev Esp Fisiol. 1997;53:211–216. [PubMed] [Google Scholar]
- Van Der Werf YD, Tisserand DJ, Visser PJ, Hofman PA, Vuurman E, Uylings HB, Jolles J. Thalamic volume predicts performance on tests of cognitive speed and decreases in healthy aging. A magnetic resonance imaging-based volumetric analysis. Brain Res Cogn Brain Res. 2001;11:377–385. doi: 10.1016/s0926-6410(01)00010-6. [DOI] [PubMed] [Google Scholar]
- Van Petten C. Relationship between hippocampal volume and memory ability in healthy individuals across the lifespan: review and meta-analysis. Neuropsychologia. 2004;42:1394–1413. doi: 10.1016/j.neuropsychologia.2004.04.006. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM, Reinvang I, Lundervold A, Dale AM, Eilertsen DE, Quinn BT, Salat D, Makris N, Fischl B. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiology of Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 1275-1268. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, Agartz I, Salat DH, Greve DN, Fischl B, Dale AM, Fjell AM. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiology of Aging. 2009;32:916–932. doi: 10.1016/j.neurobiolaging.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, Beckmann C, Jenkinson M, Smith SM. Bayesian analysis of neuroimaging data in FSL. NeuroImage. 2009;45:S173–186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
- Zimmerman ME, Brickman AM, Paul RH, Grieve SM, Tate DF, Gunstad J, Cohen RA, Aloia MS, Williams LM, Clark CR, Whitford TJ, Gordon E. The relationship between frontal gray matter volume and cognition varies across the healthy adult lifespan. American Journal of Geriatric Psychiatry. 2006;14:823–833. doi: 10.1097/01.JGP.0000238502.40963.ac. [DOI] [PubMed] [Google Scholar]