Abstract
Background
Serum dehydroepiandrosterone (DHEA) concentrations decrease ~80% between ages 25 and 75 yr. Aging also results in an increase in arterial stiffness, which is an independent predictor of cardiovascular disease (CVD) risk and mortality. Therefore, it is conceivable that DHEA replacement in older adults could reduce arterial stiffness. We sought to determine if DHEA replacement therapy in older adults reduces carotid augmentation index (AI) and carotid-femoral pulse wave velocity (PWV) as indices of arterial stiffness.
Methods
A randomized, double-blind trial was conducted to study the effects of 50 mg/d DHEA replacement on AI (n=92) and PWV (n=51) in women and men aged 65–75 yr. Inflammatory cytokines and sex hormones were measured in fasting serum.
Results
AI decreased in the DHEA group but not in the placebo group (difference between groups, −6±2 AI units, p=0.002). PWV also decreased (difference between groups, −3.5±1.0 m/sec, p=0.001); however, after adjusting for baseline values, the between-group comparison became non-significant (p=0.20). The reductions in AI and PWV were accompanied by decreases in inflammatory cytokines (TNFα and IL-6, p<0.05) and correlated with increases in serum DHEAS (r = −0.31 and −0.37, respectively, p<0.05). The reductions in AI also correlated with free testosterone index (r = −0.23, p=0.03).
Conclusion
DHEA replacement in elderly men and women improves indices of arterial stiffness. Arterial stiffness increases with age and is an independent risk factor for CVD. Therefore the improvements observed in the present study suggest that DHEA replacement might partly reverse arterial aging and reduce CVD risk.
Keywords: vasculature, augmentation index, aging
INTRODUCTION
Dehydroepiandrosterone (DHEA) and its sulfated form (DHEAS), which will be referred to together as DHEA, are present in a far higher concentration in plasma than any other steroid hormone in humans (Hornsby 1995). Adrenal production of DHEA begins during puberty and peaks at ~20 yr. At age ~25 yr, serum DHEA begins to decline rapidly, so that by age 75 yr DHEA level is ~80% lower than at 20 yr (Orentreich et al. 1984; Orentreich et al. 1992). This large decline in DHEA has led to interest in the possibility that development of DHEA deficiency may play a role in the deterioration in physiological and metabolic functions with aging, and in the development of aging-related disease processes. In support of this possibility, it has been reported that DHEA level is negatively correlated with mortality, and that lower levels of DHEA are associated with a higher risk of developing cardiovascular disease (CVD) in elderly people (Barrett-Connor, Khaw, and Yen 1986; Mazat et al. 2001; Berr et al. 1996).
Arterial stiffness increases with advancing age (Vaitkevicius et al. 1993; Hougaku et al. 2006), and elevated central arterial stiffness is a predictor of cardiovascular disease and all-cause mortality (Cohn 2006; Laurent et al. 2001; Sutton-Tyrrell et al. 2005). It has been reported that serum DHEA concentration is inversely associated with arterial stiffness (Fukui et al. 2007; Dockery et al. 2003b; Hougaku et al. 2006), and that DHEA has a number of effects that would be expected to prevent and reverse the stiffening of cardiovascular system tissues. These include inhibition of vascular smooth muscle cell proliferation, attenuation of collagen production by cardiac fibroblasts and reduction of left ventricular stiffness, activation of arterial endothelial cell nitric oxide synthase, increase in arterial endothelial cell proliferation and inhibition of arterial endothelial cell apoptosis, and inhibition of vascular inflammation (Williams et al. 2002b; Bonnet et al. 2009; Iwasaki et al. 2005; Alwardt et al. 2006; Liu and Dillon 2002; Williams et al. 2004; Liu et al. 2007b). In addition to being a sex hormone precursor, DHEA is an activator of peroxisome proliferator-activated receptor α (PPARα). DHEA, therefore, has anti-inflammatory and triglyceride lowering effects (Peters et al. 1996b; Poynter and Daynes 1998; LeFebvre et al. 2006; Staels and Fruchart 2005). Many of the effects of DHEA are similar to those of the fibrates, which are also PPARα activators (Han, Quon, and Koh 2005; Kasai et al. 2006; Tziomalos et al. 2009b; Ryan et al. 2007; Gizard et al. 2005).
While we were conducting a study on the effects of DHEA replacement on glucose tolerance in elderly men and women, a number of the papers, referred to above, were published reporting that DHEA (and fibrates) have effects that would be expected to reduce arterial stiffness. This information stimulated us to add the measurement of carotid artery augmentation index (AI) and carotid-femoral pulse wave velocity (PWV) to our study of the effects of DHEA replacement. In this paper, we report the response of these indices of arterial stiffness to 12 mo of DHEA replacement on the subgroup of participants on whom arterial stiffness measurements were made.
METHODS
Participants
Sedentary, nonsmoking, men and women, aged 65–75y were recruited from the Saint Louis metropolitan area. Screening tests included a medical history, physical examination, blood chemistry analysis, hematology, urinalysis, and electrocardiography. Candidates were excluded if they had evidence of chronic infection, a history or evidence of malignancy within the past 5 yr (other than innocuous skin cancer), unstable or occult CVD, advanced emphysema, advanced Parkinson’s disease, untreated severe hypertension, or diagnosed diabetes. Participants taking medications for dyslipidemia, hypertension, and thyroid dysfunction were required to maintain stable dosing regimens for six months prior to enrollment in the study. All participants gave their informed written consent to participate in the study, which was approved by the Human Research Protection Office at Washington University School of Medicine.
Intervention
Participants were randomized to 12 months of 50 mg/d DHEA or placebo. This DHEA dose was selected because it increases circulating DHEAS levels in older adults to those seen in young adults (Villareal and Holloszy 2004). All participants received multivitamin and calcium/vitamin D supplements and were advised to maintain their usual dietary and physical activity habits during the study. During monthly meetings with the participants, DHEA or placebo was dispensed, pill counts performed, and the participants were questioned about adverse events and changes in activity levels, diet, and medications.
Blood pressure and heart rate
Brachial artery blood pressure (BP) was measured (Dinamap 1846SX; Critikon Inc, Tampa, FL) in the left arm after the participant rested quietly for ≥5 minutes in the supine position. Heart rate was measured by palpation of the radial artery.
Augmentation index
Augmentation index was determined by using applanation tonometry (Millar Instruments, Inc., Model #TCB-500, Houston, TX) on the common carotid artery (Laurent et al. 2006). At least 20 digital pulse waves were recorded and analyzed with Windaq software (version 2.31, DATAQ Instruments, Inc., Akron, OH). The software was used to identify the maximum and minimum voltage on each wave form, with the difference corresponding to pulse pressure (PP). The software was also used to generate the second derivative of the pulse wave, which was used for the identification of the “shoulder” on the upstroke of the raw wave form. The difference between the peak voltage and the voltage at the shoulder was calculated to reflect augmentation pressure (AP). Augmentation index was calculated as AI = 100 × AP/PP for each of the 20+ waveforms and the resulting values were averaged. To ensure optimal data quality, the technician visually inspected the waveforms to ensure that the landmarks had been properly identified by the software and to omit waveforms that were of suboptimal quality due to artifacts or irregular heartbeats. When analyses were questionable (for example, large variation in AI values among waveforms), a second (blinded) technician re-analyzed the waveforms; when discrepancies between technicians occurred, the analyses were reviewed by both technicians together and if the differences could not be remediated, the data were excluded from the analyses for this report.
Pulse wave velocity
Pulse wave velocity was determined by transcutaneous Doppler flow measurements (Model 806-CB, Parks Medical Electronics, Inc., Aloha, OR) at the right common carotid artery and the right femoral artery (Laurent et al. 2006). Twenty Doppler wave forms were recorded (Windaq software, version 2.31, DATAQ Instruments, Inc., Akron, OH) at the two sites simultaneously. Pulse transit time was determined as the difference in pulse arrival times for the carotid and femoral sites and was based on foot-to-foot comparisons of wave forms from the two sites, with the foot being identified as the peak on the second derivative of the pulse wave. The distances between the aorta and the carotid site and the aorta and the femoral site were measured over the skin using the second intercostal space as a landmark for the aorta; the difference between these distances was considered propagation distance (Karamanoglu 2003). PWV for each carotid-femoral pair of waveforms was calculated as propagation distance in meters divided by transit time in seconds. The average of the 20 waveforms was used to reflect the PWV for one test. Quality control procedures were identical to those described above for the AI method.
Blood analyses
In the morning after an overnight fast, blood was collected from an arm vein; serum was isolated by using centrifugation. The serum samples were stored at −20° C for later batch analyses. Commercially available ELISA assay kits (Quantikine High Sensitive, R&D Systems, Minneapolis, MN) were used to quantify serum concentrations of interleukin-6 (IL-6) and tumor necrosis factor α (TNFα). Sex hormone binding globulin (SHBG), total testosterone, and DHEAS were measured using chemiluminescent assays (Immulite 2000, Diagnostic Products Corporation, San Diego, CA); total estradiol was measured using an ultra-sensitive radioimmunoassay (Diagnostic Systems Laboratories, Webster TX). Free testosterone index was calculated as total testosterone / SHBG, where the units for testosterone are nmol/L and for SHBG are nmol/L. Free estradiol index was calculated as (total estradiol / 1000) / SHBG, where the units for estradiol are pmol/L and for SHBG are nmol/L. White blood cells, lymphocytes, and lipids were measured in plasma by the medical center’s CLIA-certified clinical laboratory.
Height and weight
Body weight and height were measured in the morning, after an overnight fast, while the participant was wearing only underwear and a hospital gown. Body mass index (BMI) was calculated (kg/m2).
Physical activity and energy intake
Habitual physical activity levels were evaluated by using a questionnaire that focuses on habitual exercise and non-exercise physical activity performed during the prior three months (The Aerobics Center Longitudinal Study Physical Activity Questionnaire 1997). Energy intake was evaluated by having the participants record 4-day food diaries, which were analyzed by the study dietitian using computerized nutrient analysis (Nutrition Data System for Research, versions 4.05, 4.06, and 5.0, Nutrition Coordination Center, University of Minnesota, Minneapolis, MN). Prior to the diary recording period, participants received detailed instructions from the dietitian on how to measure and record all foods, beverages, and supplements consumed. After the recording period, the dietitian reviewed the diary and queried the participant, as needed to clarify any incomplete or ambiguous entries in the diary.
Statistical Analyses
Comparisons of baseline characteristics between the DHEA and placebo groups were performed using independent t-tests, chi-square tests, and Fisher’s exact tests. Outcomes were analyzed with analysis of covariance (ANCOVA), in which the independent variable was study group, the dependent variable was the change in the outcome (i.e. final value minus baseline value), and the covariate was the baseline value of the outcome. Additional ANCOVAs were performed in which a sex by study group interaction term was included to evaluate the equality of responses to DHEA in men and women. Paired t-tests were used for within group comparisons of baseline and one year data. Spearman correlations were performed on data from both groups combined and were used to identify associations between variables. Data are presented as arithmetic means ± SE unless noted otherwise. Significance was accepted at p≤0.05 and all tests were two-tailed. Analyses were conducted with SAS for Windows XP Pro (version 9.2, SAS Institute, Cary, NC).
RESULTS
Participants
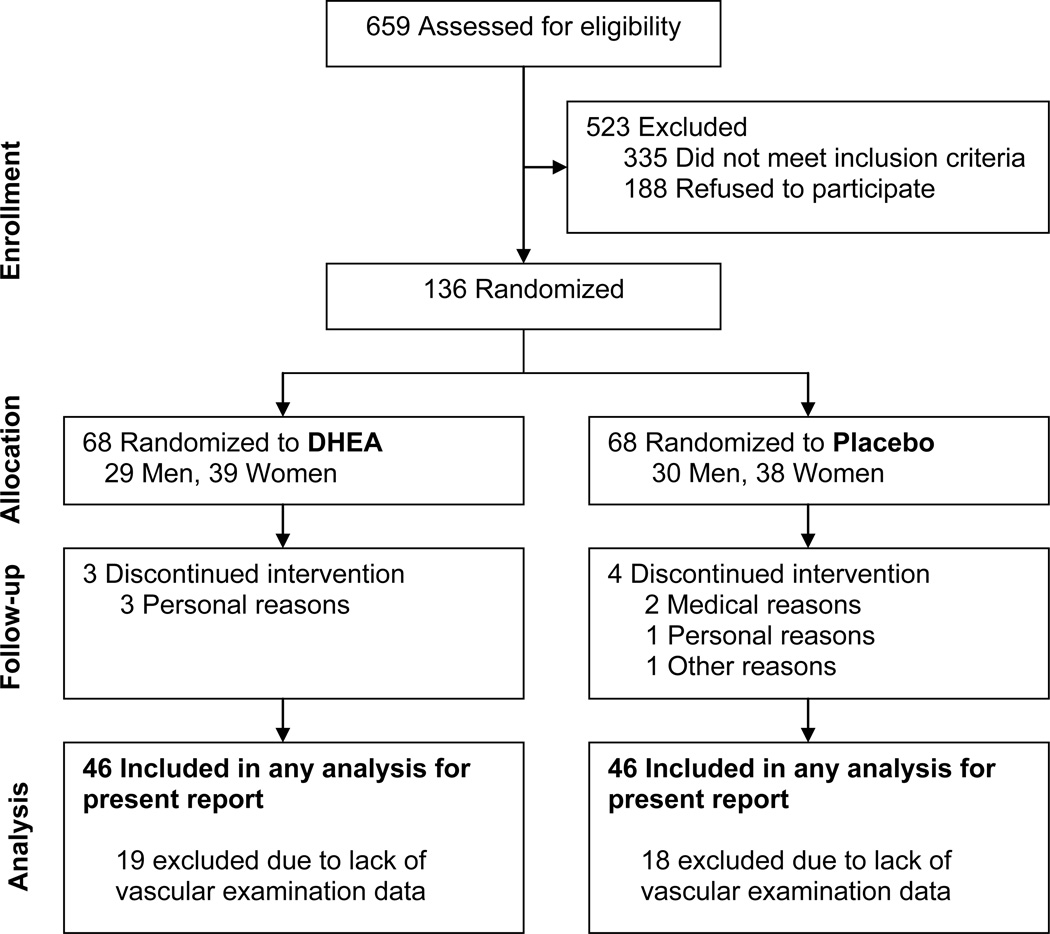
Among the 659 volunteers who inquired about the study, 335 did not meet the inclusion criteria and 188 were not interested in participating. The remaining 136 subjects were enrolled and randomized; among these, 7 dropped out before completing the study (Figure 1). As mentioned above, the AI and PWV measures were phased-in after the larger study had begun. Therefore, data are not available for all participants. Additionally, some data are missing because of the presence of a carotid bruit (contraindication for the vascular examination) or because the acquired pulse wave forms were not suitable for analysis. Therefore, the sample size for the present report was 92 subjects (n=46 for each group) (Figure 1), except for PWV, the sample was 51 subjects (DHEA, n=27; placebo, n=24).
Figure 1.
Consort diagram indicating sample sizes at each stage during the study. DHEA, dehydroepiandrosterone.
On average, the participants were 70 years of age with BMI in the overweight range (Table 1). The demographic and baseline characteristics did not differ between groups. The percentage of participants who were taking medications or vitamin supplements that could affect the study outcomes was similar between groups. Furthermore, the percentage of participants with prior diagnoses of hypertension or CVD was similar in the two groups.
Table 1.
Subject Characteristics
| DHEA | Placebo | Between Group | ||
|---|---|---|---|---|
| (n = 46) | (n = 46) | P value | ||
| Sex | ||||
| Men | 20 (43%) | 22 (48%) | ||
| Women | 26 (57%) | 24 (52%) | 0.68 | |
| Race | ||||
| African American/Black | 1 (2%) | 2 (4%) | ||
| White | 45 (98%) | 44 (96%) | 0.56 | |
| Education | ||||
| < College degree | 24 (52%) | 19 (41%) | ||
| College degree | 12 (26%) | 13 (28%) | ||
| Graduate School | 10 (22%) | 14 (30%) | 0.52 | |
| Age, yr | 70 ± 3 | 70 ± 3 | 0.95 | |
| Weight, kg | ||||
| Men | 87.9 ± 14.6 | 83.4 ± 12.6 | 0.29 | |
| Women | 71.4 ± 16.6 | 76.1 ± 18.9 | 0.36 | |
| BMI, kg/m2 | 27.7 ± 5.5 | 27.8 ± 5.3 | 0.95 | |
| Medication use | ||||
| Anti-dyslipidemic | 16 (35%) | 24 (52%) | 0.09 | |
| Antihypertensive | 23 (50%) | 23 (50%) | 1.00 | |
| Multivitamin | 28 (61%) | 33 (72%) | 0.27 | |
| Vitamin C and/or E | 23 (50 %) | 18 (39 %) | 0.29 | |
| Self-reported diagnoses | ||||
| Cardiovascular disease | 5 (10%) | 6 (13%) | 0.75 | |
| Hypertension | 20 (43%) | 18 (39 %) | 0.67 | |
Values are means ± SD or n (% of participants). P-values are for independent t-tests for quantitative data and chi-square tests or fisher’s exact tests for counts. BMI, body mass index.
Compliance and Safety
Pill compliance has been reported previously (Weiss et al. 2009) and was 94.4±0.4% in the DHEA group and 95.6±0.4% in the placebo group. Circulating DHEAS increased from 59±5 μg/dL to 333±20 μg/dL (p<0.0001) in the DHEA group and did not change in the placebo group (baseline: 56±8 μg/dL, 1 year: 46±5 μg/dL; p=0.09; p<0.0001 vs. DHEA group).
As reported in greater detail previously for a larger sample (n=136) (Weiss et al. 2009), a total of 12 serious adverse events and 124 minor side effects were documented; the frequencies of these did not differ between groups. Serum prostate specific antigen concentrations in men did not change in either group during intervention nor were there differences between groups. Based on mammograms and pap smears, no breast cancer or cervical abnormalities were identified in women.
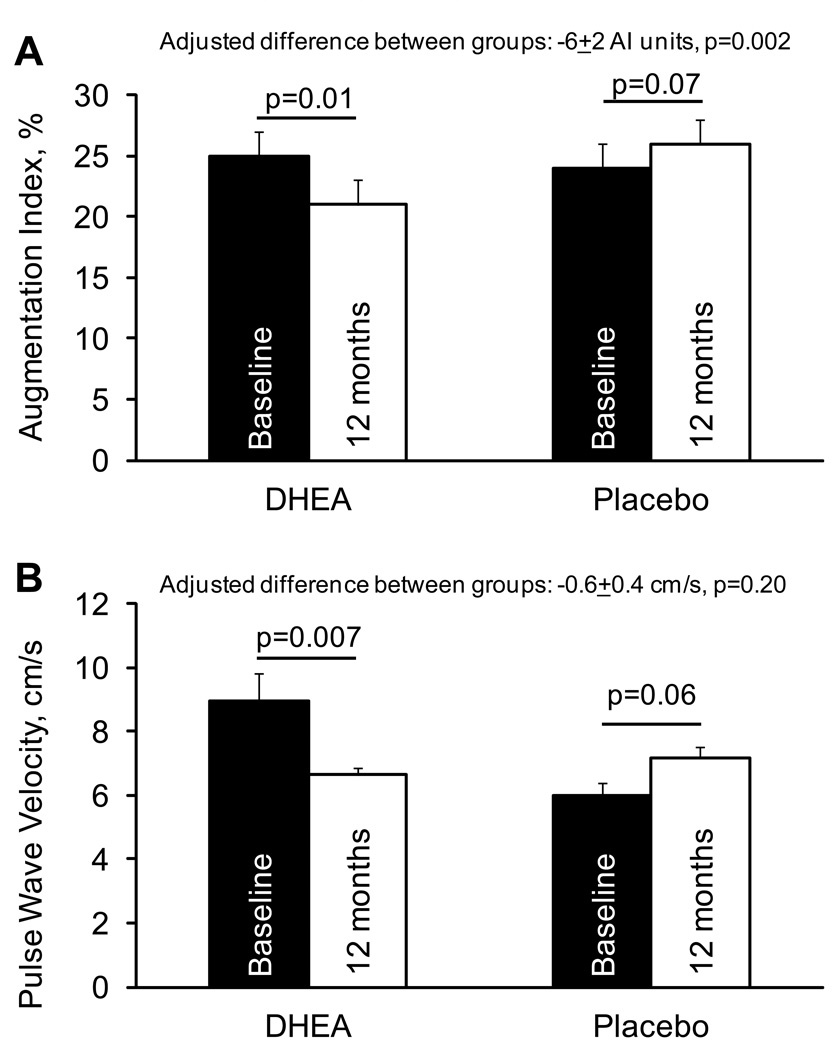
Augmentation index
AI decreased in the DHEA group and tended to increase in the placebo group (Figure 2). These findings were not affected by the inclusion of blood pressure or heart rate as covariates. Likewise, adjustment of the AI data to a standardized heart rate of 75 beats/min (based on the inverse relationship between AI and HR of 4.8 AI units per 10 beats/min (Wilkinson et al. 2000; Wilkinson et al. 2002)) did not alter the significance of the results. Exclusion of 10 participants in the DHEA group and 9 participants in the placebo group who started, stopped, or had dose changes in blood pressure medications during the intervention did not change the statistical significance of the results (between-group comparison, p=0.004). The DHEA-associated improvements in AI did not differ (p=0.58) between men (−7.4 ± 2.8 AI units, p=0.01) and women (−5.3 ± 2.6 AI units, p=0.05).
Figure 2.
Changes in augmentation index and pulse wave velocity in response to 12 months of 50 mg/d DHEA supplementation or placebo. Adjusted differences reflect the comparison of the changes the DHEA and placebo groups after adjusting for baseline values. Sample size for the AI data are n=46 in each group. For the PWV data, the sample size was DHEA, n=27 and placebo, n=24. The unadjusted between-group comparison of PWV results was significant (p=0.001).
Pulse wave velocity
PWV decreased in the DHEA group and tended to increase in the placebo group (Figure 2), resulting in a significant difference between groups (−3.5±1.0 m/sec, p=0.001). However, after accounting for a substantial difference in baseline values between groups, the between-group comparison became a weak, non-significant (p=0.20) trend. These findings were not affected by the inclusion of blood pressure or heart rate as covariates (data not shown). Furthermore, the results were not affected by exclusion of 8 participants in the DHEA group and 6 participants in the placebo group who started, stopped, or had dose changes in blood pressure medications during the intervention. The responses for men (−0.9 ± 0.6 m/s, DHEA vs. placebo, p=0.14) and women (−0.3 ± 0.6 m/s, p=0.59) did not differ significantly (p=0.44).
BMI and body weight
There were tendencies for reductions in BMI and body weight with DHEA supplementation (Table 2). As reported in a previous paper from this study (Weiss et al. 2011), these effects appear to be specific to men (differences between DHEA and placebo groups: BMI, −0.9 ± 0.3 kg/m2, p = 0.002; body weight: −2.3 ± 0.7 kg, p = 0.003), with no effect seen in women (BMI, 0.0 ± 0.3 kg/m2, p = 0.99; body weight: 0.0 ± 0.7 kg, p = 0.96) (group by sex interaction, both p<0.05).
Table 2.
Effect of 12 months of DHEA supplementation or placebo on cardiovascular disease risk factors, physical activity levels, and energy intake.
| DHEA | Placebo | Adjusted Difference Between Groups |
Between Group P value |
|
|---|---|---|---|---|
| BMI, kg/m2 | ||||
| Baseline | 27.7 ± 0.8 | 27.8 ± 0.8 | ||
| 12 months | 27.8 ± 0.8 | 28.3 ± 0.8 | ||
| Change | 0.1 ± 0.1 | 0.5 ± 0.1 | −0.4 ± 0.2 | 0.07 |
| Within group P value | 0.58 | 0.002 | ||
| Body weight, kg | ||||
| Baseline | 78.6 ± 2.6 | 79.6 ± 2.4 | ||
| 12 months | 78.5 ± 2.5 | 80.5 ± 2.5 | ||
| Change | −0.1 ± 0.4 | 0.9 ± 0.4 | −1.0 ± 0.5 | 0.06 |
| Within group P value | 0.84 | 0.02 | ||
| Systolic BP, mmHg | ||||
| Baseline | 126 ± 2 | 132 ± 3 | ||
| 12 months | 128 ± 2 | 132 ± 2 | ||
| Change | 1 ± 2 | 0 ± 2 | 0 ± 2 | 0.89 |
| Within group P value | 0.53 | 0.79 | ||
| Diastolic BP, mmHg | ||||
| Baseline | 68 ± 1 | 71 ± 1 | ||
| 12 months | 70 ± 1 | 72 ± 1 | ||
| Change | 2 ± 1 | 1 ± 1 | 0 ± 1 | 0.81 |
| Within group P value | 0.06 | 0.25 | ||
| Heart rate, beats/min | ||||
| Baseline | 64 ± 1 | 63 ± 1 | ||
| 12 months | 64 ± 1 | 66 ± 1 | ||
| Change | 0 ± 1 | 3 ± 1 | −3 ± 2 | 0.10 |
| Within group P value | 0.76 | 0.03 | ||
| Triglycerides, mg/dL | ||||
| Baseline | 112 ± 10 | 102 ± 9 | ||
| 12 months | 99 ± 9 | 105 ± 10 | ||
| Change | −14 ± 5 | 3 ± 5 | −15 ± 6 | 0.03 |
| Within group P value | 0.006 | 0.56 | ||
| Total cholesterol, mg/dL | ||||
| Baseline | 189 ± 5 | 184 ± 6 | ||
| 12 months | 180 ± 5 | 182 ± 7 | ||
| Change | −9 ± 4 | −3 ± 4 | −5 ± 5 | 0.33 |
| Within group P value | 0.02 | 0.54 | ||
| LDL-cholesterol, mg/dL | ||||
| Baseline | 109 ± 5 | 103 ± 6 | ||
| 12 months | 104 ± 5 | 101 ± 7 | ||
| Change | −4 ± 3 | −2 ± 3 | −3 ± 4 | 0.54 |
| Within group P value | 0.13 | 0.15 | ||
| HDL-cholesterol, mg/dL | ||||
| Baseline | 58 ± 3 | 58 ± 3 | ||
| 12 months | 56 ± 3 | 59 ± 4 | ||
| Change | −2 ± 2 | 1 ± 2 | −3 ± 2 | 0.19 |
| Within group P value | 0.34 | 0.37 | ||
| Physical Activity, kcal/day | ||||
| Baseline | 453 ± 46 | 511 ± 58 | ||
| 12 months | 459 ± 55 | 431 ± 45 | ||
| Change | −2 ± 35 | −71 ± 36 | 68 ± 50 | 0.18 |
| Within group P value | 0.95 | 0.05 | ||
| Energy intake, kcal/day | ||||
| Baseline | 2226 ± 66 | 2183 ± 79 | ||
| 12 months | 2093 ± 66 | 2190 ± 81 | ||
| Change | −40 ± 50 | 94 ± 50 | −133 ± 72 | 0.07 |
| Within group P value | 0.43 | 0.07 | ||
Body mass, plasma lipid, and energy intake data have been reported previously for a larger sample (Weiss et al. 2011). Values are arithmetic means ± SE except for mean differences between groups, which have been adjusted for baseline values. Between-group P values reflect the between-group comparison change-scores from ANCOVAs that included baseline values as the covariate. Within-group P values are from paired t-tests. Lipid data do not include participants who had changes in lipid medications during the study.
Blood pressure and heart rate
The DHEA and placebo groups did not differ with respect to the 1-year changes in supine resting systolic or diastolic blood pressure, or resting heart rate (Table 2). However, as compared to men, women tended to have more favorable responses to DHEA replacement, with respect to improvements in systolic BP (women: −7 ± 3 mmHg; men: 5 ± 4 mmHg; p=0.02 for men vs. women), diastolic BP (women: −2 ± 2 mmHg; men, 4 ± 2 mmHg; p=0.06 for men vs. women), and resting HR (women: −4 ± 1 beats/min; men, 0 ± 2 beats/min; p=0.09 for men vs. women).
Results from seated bilateral brachial pressures measured in triplicate were available on a subset of study participants (n=20 in each group). As was the case for supine blood pressures, there was no difference in BP changes between the DHEA and placebo groups. However, the differences observed between men and women, with respect to DHEA-induced changes in systolic and diastolic BP, became non-significant (p=0.44 and p=0.14, respectively).
Plasma lipids
As reported previously (Weiss et al. 2011), DHEA replacement resulted in lower plasma triglyceride concentrations and did not alter total or LDL-cholesterol levels (Table 2). Furthermore, although DHEA did not alter HDL-cholesterol levels in the group as a whole (Table 2), women experienced a reduction in HDL-cholesterol (DHEA: −6+2 mg/dL, placebo, 4±2 mg/dL, p=0.0007 DHEA vs. placebo).
Physical activity levels, and dietary energy intake
There was no difference between the DHEA and placebo groups with respect to changes in habitual physical activity levels or dietary energy intake (Table 2).
Sex hormones
Sex hormone data have been presented previously for a larger group of subjects from this trial (Weiss et al. 2009) and are being presented here to assist in the interpretation of AI and PWV data. For both men and women, serum testosterone concentrations increased in the DHEA group but not in the placebo group. However, the between group comparison of these responses was only significant for women (Table 3). Free testosterone index, estradiol, and free estradiol index increased in men and women in the DHEA group but not in the placebo group; the between-group comparisons of responses were all significant for men and women (Table 3).
Table 3.
Circulating hormones in response to 12 months of DHEA supplementation or placebo
| Men |
Women |
|||||||
|---|---|---|---|---|---|---|---|---|
| DHEA (n=20) |
Placebo (n=22) |
Between-Group |
DHEA (n=26) |
Placebo (n=24) |
Between-Group |
|||
| Difference | P | Difference | P | |||||
| Tot. testosterone, ng/dL | ||||||||
| Baseline | 426 ± 30 | 426 ± 38 | 24 ± 1 | 22 ± 1 | ||||
| 12 months | 496 ± 32 | 453 ± 32 | 54 ± 5 | 24 ± 2 | ||||
| Change | 70 ± 19 | 28 ± 25 | 43 ± 29 | 0.15 | 30 ± 4 | 2 ± 2 | 28 ± 5 | <0.0001 |
| Within group P value | 0.001 | 0.28 | <0.0001 | 0.27 | ||||
| Free testosterone index × 10−2 | ||||||||
| Baseline | 43.9 ± 2.6 | 40.7 ± 3.0 | 2.2 ± 0.2 | 2.2 ± 0.2 | ||||
| 12 months | 54.6 ± 3.3 | 39.7 ± 2.6 | 5.7 ± 0.6 | 2.4 ± 0.3 | ||||
| Change | 10.7 ± 2.4 | −1.0 ± 2.0 | 12.5 ± 3.0 | 0.0002 | 3.5 ± 0.5 | 0.2 ± 0.2 | 3.3 ± 0.6 | <0.0001 |
| Within group P value | 0.0003 | 0.63 | <0.0001 | 0.38 | ||||
| Total estradiol, pg/mL | ||||||||
| Baseline | 18.5 ± 1.3 | 15.9 ± 0.9 | 10.0 ± 1.0 | 11.3 ± 0.8 | ||||
| 12 months | 22.2 ± 1.2 | 14.6 ± 0.8 | 16.2 ± 0.8 | 10.4 ± 0.6 | ||||
| Change | 3.7 ± 1.2 | −1.3 ± 0.8 | 6.3 ± 1.3 | <0.0001 | 6.2 ± 1.1 | −0.9 ± 0.5 | 6.3 ± 0.9 | <0.0001 |
| Within group P value | 0.008 | 0.14 | <0.0001 | 0.08 | ||||
| Free estradiol index × 10−4 | ||||||||
| Baseline | 21.7 ± 2.2 | 17.7 ± 1.6 | 10.0 ± 1.3 | 13.0 ± 1.8 | ||||
| 12 months | 27.2 ± 2.4 | 15.0 ± 1.2 | 19.0 ± 2.1 | 11.5 ± 1.5 | ||||
| Change | 5.5 ± 1.7 | −2.7 ± 1.0 | 9.3 ± 1.9 | <0.0001 | 9.0 ± 1.6 | −1.5 ± 0.7 | 10.1 ± 1.8 | <0.0001 |
| Within group P value | 0.004 | 0.02 | <0.0001 | 0.04 | ||||
| SHBG, nmol/L | ||||||||
| Baseline | 34.4 ± 2.1 | 37.0 ± 2.7 | 42.3 ± 2.6 | 40.3 ± 3.2 | ||||
| 12 months | 32.9 ± 2.6 | 39.7 ± 2.8 | 35.9 ± 2.4 | 42.5 ± 3.6 | ||||
| Change | −1.5 ± 1.0 | 2.8 ± 1.0 | −4.2 ± 1.5 | 0.007 | −6.4 ± 1.4 | 2.1 ± 1.3 | −8.3 ± 1.9 | <0.0001 |
| Within group P value | 0.15 | 0.01 | 0.0001 | 0.11 | ||||
Values are arithmetic means ± SE except for mean differences between groups which have been adjusted for baseline values. Between-group P values reflect the between-group comparison change-scores from ANCOVAs that included baseline values as the covariate. Within-group P values are from paired t-tests. SHBG, sex hormone binding globulin. To convert testosterone to SI units (nmol/L) divide by 28.82; to convert estradiol to SI units (pmol/L) divide by 0.27.
Inflammatory markers and white blood cells
As compared to the placebo group, the DHEA group had favorable changes in serum TNFα and IL-6 concentrations (Table 4). The effect on TNFα was attributed to a modest decrease in TNFα in the DHEA group while TNFα tended to increase in the placebo group. The effect on IL-6 was attributed to a significant decrease in the DHEA group while the placebo group experienced a significant increase. TNFα and IL-6 data from this study have been reported previously (Weiss et al. 2011) but are reported here for this subgroup of participants because of their importance in vascular health. There were no differences between groups for white blood cell or lymphocyte counts (Table 4). There were no differences between men and women, with respect to the DHEA-induced changes in inflammatory markers or white blood cells (all p>0.48 for sex by group interactions).
Table 4.
Circulating inflammatory markers and white blood cells in response to 12 months of DHEA supplementation or placebo
| DHEA | Placebo | Adjusted Difference Between Groups |
Between Group P value |
|
|---|---|---|---|---|
| TNFα, pg/mL | ||||
| Baseline | 1.45 ± 0.15 | 1.22 ± 0.15 | ||
| 12 months | 1.21 ± 0.10 | 1.61 ± 0.26 | ||
| Change | −0.24 ± 0.09 | 0.38 ± 0.22 | −0.56 ± 0.23 | 0.02 |
| Within group P value | 0.02 | 0.09 | ||
| IL-6, pg/mL | ||||
| Baseline | 2.61 ± 0.24 | 2.41 ± 0.17 | ||
| 12 months | 2.21 ± 0.16 | 2.90 ± 0.20 | ||
| Change | −0.40 ± 0.15 | 0.49 ± 0.19 | −0.80 ± 0.20 | 0.0001 |
| Within group P value | 0.01 | 0.01 | ||
| WBC, k/cumm | ||||
| Baseline | 5.7 ± 0.2 | 5.3 ± 0.2 | ||
| 12 months | 5.8 ± 0.2 | 5.6 ± 0.2 | ||
| Change | 0.1 ± 0.2 | 0.3 ± 0.1 | −0.1 ± 0.2 | 0.77 |
| Within group P value | 0.65 | 0.04 | ||
| Lymphocytes, k/cumm | ||||
| Baseline | 1.55 ± 0.07 | 1.48 ± 0.05 | ||
| 12 months | 1.57 ± 0.07 | 1.58 ± 0.06 | ||
| Change | 0.02 ± 0.04 | 0.11 ± 0.04 | −0.07 ± 0.06 | 0.20 |
| Within group P value | 0.67 | 0.02 | ||
Values are arithmetic means ± SE except for mean differences between groups which have been adjusted for baseline values. Between-group P values reflect the between-group comparison change-scores from ANCOVAs that included baseline values as the covariate. Within-group P values are from paired t-tests. TNFα, tumor necrosis factor α; IL-6, interleukin-6; WBC, white blood cells.
Correlations
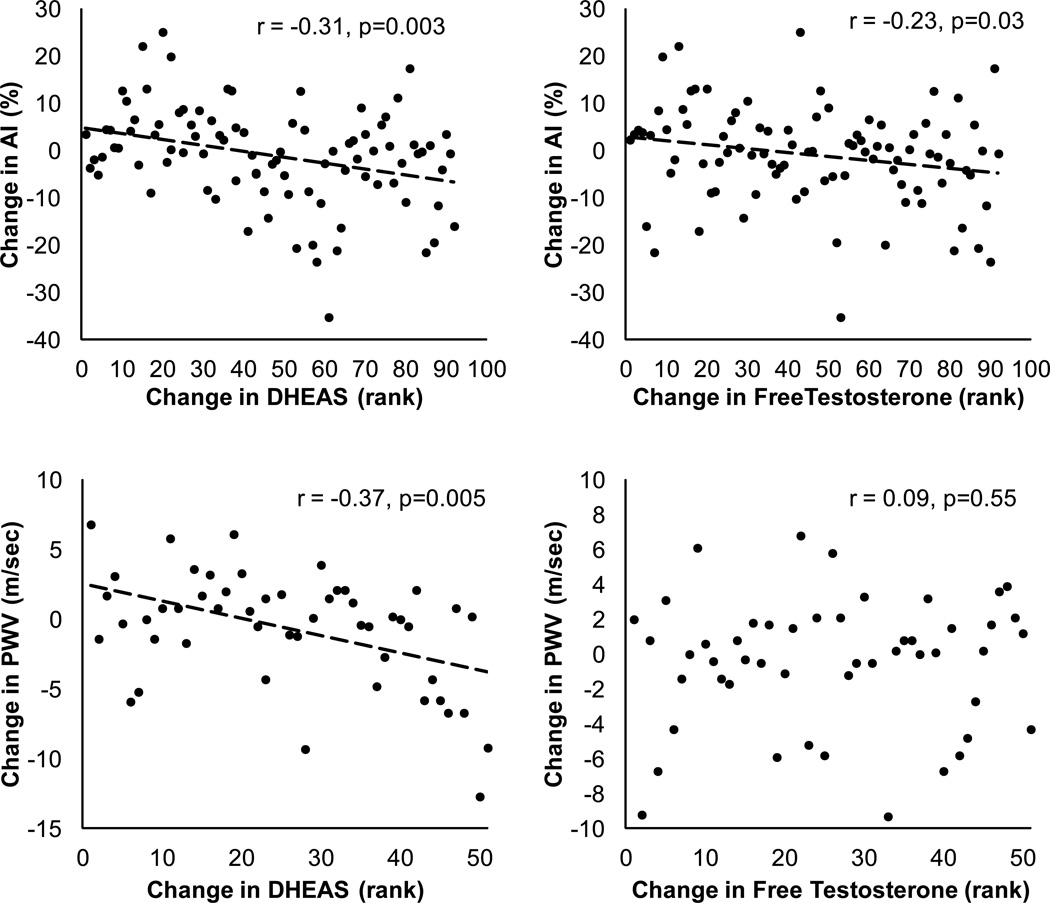
Decreases in AI were correlated with increases in serum DHEAS (r = −0.31, p=0.003) and increases in total (r = −0.26, p=0.01) and free testosterone index (r = −0.23, p=0.03). Reductions in AI also tended to correlate with reductions in SHBG (r = 0.18, p=0.08), IL-6 (r = 0.21, p=0.06) and TNFα (r = 0.19, p=0.07). Changes in AI were not correlated with changes in estradiol or free estradiol index, BMI or body weight, plasma lipids, or with changes in dietary energy intake or habitual physical activity levels (r = −0.07 to 0.18, p>0.05).
Decreases in pulse wave velocity were correlated with increases in serum DHEAS concentrations (r = −0.37, p=0.005) and tended to correlate with decreases in SHBG (r = 0.25, p=0.07) and decreases in triglycerides (r = 0.25, p=0.09; excludes subjects who had changes in lipid medications), but were not associated with changes in other lipids or with changes in sex hormones, inflammatory cytokines, body mass or BMI, energy intake, or physical activity (r = −0.19 to 0.18, p>0.05).
DISCUSSION
The purpose of this study was to evaluate the hypothesis that DHEA replacement improves arterial elasticity in elderly men and women. This hypothesis was based on evidence that DHEA, like fibrates (Tziomalos et al. 2009a), is a PPARα activator (Peters et al. 1996a; Tamasi et al. 2008) and mediates a number of adaptations, similar to those induced by fibrates (Liu et al. 2007a; Williams et al. 2002a), that would be expected to improve cardiovascular elasticity. Our finding that DHEA replacement reduced augmentation index and pulse wave velocity in elderly men and women supports this hypothesis. Altman et al. (Altman et al. 2008) have reported that inhibition of PPARα prevents an anti-inflammatory effect of DHEA on human aortic endothelial cells. This finding and our observation of decreases in IL-6 and in plasma triglyceride levels (Weiss et al. 2011) are in keeping with the possibility that the effects of DHEA observed in this study were, at least partially mediated by activation of PPARα.
An additional mechanism that may have contributed to the decrease in arterial stiffness in response to DHEA replacement is the increase in free testosterone. This increase, which was modest in the men but large, in relative terms, in the women, correlated with the reduction in augmentation index. Physiological levels of testosterone appear to be beneficial for vascular health, as evidenced by the finding that patients undergoing androgen suppression therapy for prostate cancer and hypogonandal men have greater arterial stiffness than age-matched controls (Dockery et al. 2003a). Testosterone replacement reverses this increase in arterial stiffness (Yaron et al. 2009).
The 24% decrease in augmentation index that we observed with DHEA supplementation is large in the context of arterial aging. Based on a 0.30 percentage point per year increase in AI during adulthood (Vaitkevicius et al. 1993), the DHEA-induced reduction in augmentation index is equivalent in magnitude to a 20 year reversal of arterial aging. Furthermore, based on a meta-analysis which shows that every 10 percentage point increase in AI corresponds with a ~36% greater risk for cardiovascular and all-cause mortality during a 4 yr follow-up period (Vlachopoulos et al. 2010), the reductions in AI that we observed would be expected to correspond with a 17% reduction in mortality risk (Vlachopoulos, Aznaouridis, and Stefanadis 2010).
To our knowledge, no other studies have evaluated the effects of DHEA supplementation on indices of arterial stiffness in older adults. However, in middle-aged patients with low levels of DHEA due to primary or secondary adrenal insufficiency, it has been reported that DHEA supplementation does not alter augmentation index or aortic pulse wave velocity (Rice et al. 2009). It is possible that the shorter-term, 12-week supplementation period did not allow sufficient time for the vascular remodeling that may be important for reductions in arterial stiffness. Furthermore, the pathogenesis and secondary effects of adrenal insufficiency are distinct from the hormonal changes that occur during normal aging; therefore conditions present in patients with adrenal insufficiency may have precluded DHEA-related changes in arterial stiffness.
The subject sample used in this study was heterogeneous with respect to medical history, medications, and sex, which might be viewed as a limitation. Some participants had diagnosed cardiovascular disease (12%), were taking anti-hypertensive medications (52%), or were taking anti-dyslipidemic medications (~43%). Although our study was not powered to perform subanalyses after excluding subjects with CVD or those on blood pressure or lipid medications, exclusion of each of these subgroups did not alter the significance of the results (data not shown), despite the smaller sample sizes for these analyses. Furthermore, the responses to DHEA replacement therapy in men and women did not differ; however, this finding should be interpreted cautiously, as the sample sizes were not optimal for testing hypotheses about sex differences. Taken together, these subanalyses and sex comparisons indicate that our findings are robust and can be generalized to a fairly homogeneous population of elderly men and women.
While the results from the pulse wave velocity data support the hypothesis that DHEA supplementation reduces arterial stiffness, this finding has limitations. First, because PWV measures were added later in the study, the sample size was small (~half of that for AI), thereby resulting in less statistical power. Additionally, by chance, there was a large baseline difference in PWV between the DHEA and placebo groups, thereby making the interpretation of results difficult. Nonetheless, there was a clear significant improvement in PWV in the DHEA group and this improvement was statistically greater than the change observed in the placebo group (p=0.001). Only after accounting for the baseline differences in baseline values did the between-group statistical test become non-significant (p=0.20).
In conclusion, DHEA replacement in elderly men and women reduces arterial stiffness. Arterial stiffness increases with age and is an independent risk factor for CVD. Therefore the improvement in arterial elasticity suggests that DHEA replacement might partly reverse arterial aging and reduce CVD risk in older men and women.
Figure 3.
Associations between changes in indices of arterial stiffness and changes in serum DHEAS concentrations and free testosterone index. Correlation analyses were performed by using Spearman rank correlations because the data were not normally distributed. DHEAS and free testosterone index data were rank-transformed for the figure with high ranks corresponding to large increases in DHEA or free testosterone index.
ACKNOWLEGEMENTS
We are grateful to the study participants for their cooperation and to the staff of the Applied Physiology Laboratory and the Intensive Research Unit at Washington University School of Medicine for their skilled assistance.
Grant Support: This research was supported by NIH Research Grant AG020076, NIH General Clinical Research Center RR00036, NIH National Center for Research Resources RR024992, and NIH Clinical Nutrition Research Unit DK56341. EPW was supported by NIH Institutional National Research Service Grant AG00078 and Research Grant DK080886.
References
- 1.The Aerobics Center Longitudinal Study Physical Activity Questionnaire. Med Sci Sports Exerc. 1997;29(6 supplement):10–14. [Google Scholar]
- 2.Altman R, et al. Inhibition of vascular inflammation by dehydroepiandrosterone sulfate in human aortic endothelial cells: roles of PPARalpha and NF-kappaB. Vascul.Pharmacol. 2008;48(2–3):76–84. doi: 10.1016/j.vph.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alwardt CM, et al. Comparative effects of dehydroepiandrosterone sulfate on ventricular diastolic function with young and aged female mice. Am.J.Physiol:Regul.Integr.Comp.Physiol. 2006;290:R251–R256. doi: 10.1152/ajpregu.00272.2005. [DOI] [PubMed] [Google Scholar]
- 4.Barrett-Connor E, Khaw K-T, Yen SSC. A prospective study of dehydroepiandrosterone sulfate, mortality, and cardiovascular disease. N.Eng.J.Med. 1986;315:1519–1524. doi: 10.1056/NEJM198612113152405. [DOI] [PubMed] [Google Scholar]
- 5.Berr C, et al. Relationships of dehydroepiandrosterone sulfate in the elderly with functional, psychological, and mental status, and short-term mortality: A french community-based study. Proc.Natl.Acad.Sci.USA. 1996;93:13410–13415. doi: 10.1073/pnas.93.23.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonnet S, et al. Dehydroepiandrosterone reverses systemic vascular remodeling through the inhibition of the Akt/GSK3-2/NFAT axis. Circulation. 2009;120:1231–1240. doi: 10.1161/CIRCULATIONAHA.109.848911. [DOI] [PubMed] [Google Scholar]
- 7.Cohn JN. Arterial stiffness, vascular disease, and risk of cardiovascular events. Circulation. 2006;113(5):601–603. doi: 10.1161/CIRCULATIONAHA.105.600866. [DOI] [PubMed] [Google Scholar]
- 8.Dockery F, et al. Testosterone suppression in men with prostate cancer leads to an increase in arterial stiffness and hyperinsulinaemia. Clin Sci (Lond) 2003a;104:195–201. doi: 10.1042/CS20020209. [DOI] [PubMed] [Google Scholar]
- 9.Dockery F, et al. The relationship between androgens and arterial stiffness in older men. J Am Geriatr.Soc. 2003b;51(11):1627–1632. doi: 10.1046/j.1532-5415.2003.51515.x. [DOI] [PubMed] [Google Scholar]
- 10.Fukui M, et al. Relationship between low serum endogenous androgen concentrations and arterial stiffness in men with type 2 diabetes mellitus. Metabolism. 2007;56(9):1167–1173. doi: 10.1016/j.metabol.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 11.Gizard F, et al. PPARα inhibits vascular smooth muscle cell proliferation underlying intimal hyperplasia by inducing the tumor suppressor p16INK4A. J.Clin.Invest. 2005;115:3228–3238. doi: 10.1172/JCI22756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Han SH, Quon MJ, Koh KK. Beneficial vascular and metabolic effects of peroxisome proliferator-activated receptorα activators. Hypertension. 2005;46:1086–1092. doi: 10.1161/01.HYP.0000187900.36455.4c. [DOI] [PubMed] [Google Scholar]
- 13.Hornsby PJ. Biosynthesis of DHEAS by the human adrenal cortex and its age-related decline. Ann.N.Y.Acad.Sci. 1995;774:29–46. doi: 10.1111/j.1749-6632.1995.tb17370.x. [DOI] [PubMed] [Google Scholar]
- 14.Hougaku H, et al. Relationship between androgenic hormones and arterial stiffness, based on longitudinal hormone measurements. AJP - Endocrinology and Metabolism. 2006;290(2):E234–E242. doi: 10.1152/ajpendo.00059.2005. [DOI] [PubMed] [Google Scholar]
- 15.Iwasaki T, et al. Marked attenuation of production of collagen type I from cardiac fibroblasts by dehydroepiandrosterone. Am.J.Physiol:Endocrin.Metab. 2005;288:E1222–E1228. doi: 10.1152/ajpendo.00370.2004. [DOI] [PubMed] [Google Scholar]
- 16.Karamanoglu M. Errors in estimating propagation distances in pulse wave velocity. Hypertension. 2003;41(6):e8. doi: 10.1161/01.HYP.0000070980.67512.77. [DOI] [PubMed] [Google Scholar]
- 17.Kasai T, et al. Efficacy of peroxisome proliferative activated receptor (PPAR)-alpha ligands, fenofibrate, on intimal hyperplasia and constrictive remodeling after coronary angioplasty in porcine models. Atherosclerosis. 2006;188:274–280. doi: 10.1016/j.atherosclerosis.2005.10.047. [DOI] [PubMed] [Google Scholar]
- 18.Laurent S, et al. Aortic stiffness is an independent predictor of all-cause and cardiovascular mortality in hypertensive patients. Hypertension. 2001;37(5):1236–1241. doi: 10.1161/01.hyp.37.5.1236. [DOI] [PubMed] [Google Scholar]
- 19.Laurent S, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur.Heart J. 2006;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 20.LeFebvre P, et al. Sorting out the roles of PPARα in energy metabolism and vascular homeostasis. J.Clin.Invest. 2006;116:571–580. doi: 10.1172/JCI27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu D, Dillon JS. Dehydroepiandrosterone activates endothelial cell nitric-oxide synthase by a specific plasma membrane receptor coupled to Gαi2,3. J.Biol.Chem. 2002;277:21379–21388. doi: 10.1074/jbc.M200491200. [DOI] [PubMed] [Google Scholar]
- 22.Liu D, et al. Dehydroepiandrosterone protects vascular endothelial cells against apoptosis through a Galphai protein-dependent activation of phosphatidylinositol 3-kinase/Akt and regulation of antiapoptotic Bcl-2 expression. Endocrinology. 2007a;148(7):3068–3076. doi: 10.1210/en.2006-1378. [DOI] [PubMed] [Google Scholar]
- 23.Liu D, et al. Dehydroepiandrosterone protects vascular endothelial cells against apoptosis through a Gαi protein-dependent activation of phosphatidylinositol 3-kinase/Akt and regulation of antiapoptotic Bcl-2 expression. Endo. 2007b;148:3068–3076. doi: 10.1210/en.2006-1378. [DOI] [PubMed] [Google Scholar]
- 24.Mazat L, et al. Prospective measurements of dehydroepiandrosterone sulfate in a cohort of elderly subjects: relationship to gender, subjective health, smoking habits, and 10-year mortality. Proc.Natl.Acad.Sci.USA. 2001;98:8145–8150. doi: 10.1073/pnas.121177998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Orentreich N, et al. Age changes and sex differences in serum dehydroepiandrosterone sulfate concentrations throughout adulthood. J.Clin.Endocrinol.Metab. 1984;59:551–555. doi: 10.1210/jcem-59-3-551. [DOI] [PubMed] [Google Scholar]
- 26.Orentreich N, et al. Long-term longitudinal measurements of plasma dehydroepiandrosterone sulfate in normal men. J.Clin.Endocrinol.Metab. 1992;75:1002–1004. doi: 10.1210/jcem.75.4.1400863. [DOI] [PubMed] [Google Scholar]
- 27.Peters JM, et al. Peroxisome proliferator-activated receptor alpha required for gene induction by dehydroepiandrosterone-3 beta-sulfate. Mol.Pharmacol. 1996a;50(1):67–74. [PubMed] [Google Scholar]
- 28.Peters JM, et al. Peroxisome proliferator-activated receptor α required for gene induction by dehydroepiandrosterone-3b-sulfate. Mol.Pharmacol. 1996b;50:67–74. [PubMed] [Google Scholar]
- 29.Poynter ME, Daynes RA. Peroxisome proliferator-activated receptor α activation modulates cellular redox status, represses nuclear factor-kB signaling, and reduces inflammatory cytokine production in aging. J.Biol.Chem. 1998;273:32833–32841. doi: 10.1074/jbc.273.49.32833. [DOI] [PubMed] [Google Scholar]
- 30.Rice SP, et al. Effects of dehydroepiandrosterone replacement on vascular function in primary and secondary adrenal insufficiency: a randomized crossover trial. Journal of Clinical Endocrinology Metabolism. 2009;94(6):1966–1972. doi: 10.1210/jc.2008-2636. [DOI] [PubMed] [Google Scholar]
- 31.Ryan KE, et al. Fenofibrate and pioglitazone improve endothelial function and reduce arterial stiffness in obese glucose tolerant men. Atherosclerosis. 2007;194:e123–e130. doi: 10.1016/j.atherosclerosis.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 32.Staels B, Fruchart J-C. Therapeutic roles of peroxisome proliferator-activated receptor agonists. Diabetes. 2005;54:2460–2470. doi: 10.2337/diabetes.54.8.2460. [DOI] [PubMed] [Google Scholar]
- 33.Sutton-Tyrrell K, et al. Elevated aortic pulse wave velocity, a marker of arterial stiffness, predicts cardiovascular events in well-functioning older adults. Circulation. 2005;111(25):3384–3390. doi: 10.1161/CIRCULATIONAHA.104.483628. [DOI] [PubMed] [Google Scholar]
- 34.Tamasi V, et al. Modulation of receptor phosphorylation contributes to activation of peroxisome proliferator activated receptor alpha by dehydroepiandrosterone and other peroxisome proliferators. Mol.Pharmacol. 2008;73(3):968–976. doi: 10.1124/mol.107.036780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tziomalos K, et al. Anti-inflammatory effects of fibrates: an overview. Curr Med Chem. 2009a;16(6):676–684. doi: 10.2174/092986709787458416. [DOI] [PubMed] [Google Scholar]
- 36.Tziomalos K, et al. Anti-inflammatory effects of fibrates: an overview. Curr Med Chem. 2009b;16:676–684. doi: 10.2174/092986709787458416. [DOI] [PubMed] [Google Scholar]
- 37.Vaitkevicius PV, et al. Effects of age and aerobic capacity on arterial stiffness in healthy adults. Circulation. 1993;88(4 Pt 1):1456–1462. doi: 10.1161/01.cir.88.4.1456. [DOI] [PubMed] [Google Scholar]
- 38.Villareal DT, Holloszy JO. Effect of DHEA on abdominal fat and insulin action in elderly women and men: a randomized controlled trial. JAMA. 2004;292(18):2243–2248. doi: 10.1001/jama.292.18.2243. [DOI] [PubMed] [Google Scholar]
- 39.Vlachopoulos C, et al. Prediction of cardiovascular events and all-cause mortality with central haemodynamics: a systematic review and meta-analysis. Eur.Heart J. 2010;31(15):1865–1871. doi: 10.1093/eurheartj/ehq024. [DOI] [PubMed] [Google Scholar]
- 40.Vlachopoulos CK, Aznaouridis KC, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll.Cardiol. 2010;55(13):1318–1327. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 41.Weiss EP, et al. Dehydroepiandrosterone replacement therapy in older adults: 1- and 2-y effects on bone. American Journal of Clinical Nutrition. 2009;89(5):1459–1467. doi: 10.3945/ajcn.2008.27265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Weiss EP, et al. Dehydroepiandrosterone (DHEA) replacement decreases insulin resistance and lowers inflammatory cytokines in aging humans. Aging (Albany.NY) 2011;3(5):533–542. doi: 10.18632/aging.100327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wilkinson IB, et al. The influence of heart rate on augmentation index and central arterial pressure in humans. J.Physiol. 2000;525(Pt 1):263–270. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wilkinson IB, et al. Heart rate dependency of pulse pressure amplification and arterial stiffness. Am.J.Hypertens. 2002;15(1 Pt 1):24–30. doi: 10.1016/s0895-7061(01)02252-x. [DOI] [PubMed] [Google Scholar]
- 45.Williams MR, et al. Dehydroepiandrosterone inhibits human vascular smooth muscle cell proliferation independent of ARs and ERs. J.Clin.Endocrinol.Metab. 2002b;87:176–181. doi: 10.1210/jcem.87.1.8161. [DOI] [PubMed] [Google Scholar]
- 46.Williams MR, et al. Dehydroepiandrosterone inhibits human vascular smooth muscle cell proliferation independent of ARs and ERs. Journal of Clinical Endocrinology Metabolism. 2002a;87(1):176–181. doi: 10.1210/jcem.87.1.8161. [DOI] [PubMed] [Google Scholar]
- 47.Williams MRI, et al. Dehydroepiandrosterone increases endothelial cell proliferation in vitro and improves endothelial function in vivo by mechanisms independent of androgen and estrogen receptors. J.Clin.Endocrinol.Metab. 2004;89:4708–4715. doi: 10.1210/jc.2003-031560. [DOI] [PubMed] [Google Scholar]
- 48.Yaron M, et al. Effect of testosterone replacement therapy on arterial stiffness in older hypogonadal men. Eur J Endocrinol. 2009;160:839–846. doi: 10.1530/EJE-09-0052. [DOI] [PubMed] [Google Scholar]