Abstract
Aptamers are short, single-stranded nucleic acid sequences that are selected in vitro from large oligonucleotide libraries based on their high affinity to a target molecule. Hence, aptamers can be thought of as a nucleic acid analog to antibodies. However, several viewpoints hold that the potential of aptamers arises from interesting characteristics that are distinct from, or in some cases, superior to those of antibodies. This review summarizes the recent achievements in aptamer programs developed in our laboratory against basic and therapeutic protein targets. Through these studies, we became aware of the remarkable conformational plasticity and selectivity of RNA, on which the published report has not shed much light even though this is evidently a crucial feature for the strong specificity and affinity of RNA aptamers.
Introduction
The concept of using single-stranded nucleic acids (aptamers) as affinity molecules for protein or compound binding was initially described in 1990 (Ellington & Szostak 1990, 1992; Tuerk & Gold 1990). The concept is based on the ability of short oligonucleotides to fold, in the presence of a target, into unique three-dimensional (3D) structures that bind the target with high affinity and specificity. Aptamers are generated by a process known as systematic evolution of ligands by exponential enrichment (SELEX), which merges combinatorial chemistry with in vitro evolution from a complex library of randomized 1014−15 different sequences (Oguro et al. 2003; Klussmann 2006; Miyakawa et al. 2006, 2008; Ohuchi et al. 2006; Keefe & Schaub 2008). Importantly, aptamer targets can be small (e.g., chemical compounds) or large (e.g., proteins), and simple (e.g., purified proteins) or complex (e.g., protein complexes or cell surface receptors). Therefore, aptamers can be used as reagents for affinity purification (Romig et al. 1999; Blank et al. 2001; Srisawat & Engelke 2001) or as biosensor elements (reviewed in Mairal et al. 2008; Mok & Li 2008). Moreover, in December 2004, the US Food and Drug Administration (FDA) approved the first aptamer-based therapeutic, pegaptanib (Macugen), targeting vascular endothelial growth factor for the treatment of age-related macular degeneration (Ng et al. 2006; Zhou & Wang 2006).
A characteristic of RNA aptamers is the high potential to create a vast set of tertiary structures, which depend on the different primary sequences. Therefore, it is even likely that some RNA aptamers can fold into structures that resemble protein structures of interest. This idea arose in our previous studies of the structure–function relationship of translation factors, in which we discovered that translation factors mimic the shape of tRNA. One of them, a polypeptide release factor that is required for protein termination, encodes a tripeptide that serves as an ‘anticodon’ to decipher stop codons in mRNA (Ito et al. 2000; Nakamura et al. 2000). For over four decades, how protein synthesis terminates at stop codons was a long-standing puzzle. The discovery of the ‘peptide anticodon’ undoubtedly solved this persistent coding problem in the genetic code and emphasized a novel concept of molecular mimicry between protein and RNA (Nakamura & Ito 2011).
We speculate that RNA has high potential to create many different tertiary structures, much more than ever thought. The ‘RNA world’ hypothesis (Gesteland et al. 1999, 2006) provides the theoretical basis for the potential of RNA to create a variety of tertiary structures. Given this hypothesis, the origin of life was solely made of RNA as multifunctional biomaterials involved in genetic inheritance, cellular architecture and metabolisms; subsequently, the RNA world evolved into the modern ‘DNA/protein world’ by substituting many proteins for the RNA ancestors during the evolution. Therefore, we assume that molecular mimicry might have played an essential role for catalyzing the world transition from ‘RNA’ to ‘protein’. Most of such RNA ancestors have disappeared in the modern DNA/protein world, and we are probably looking at a few molecular fossils that have survived to date in the translation machinery, such as ribosome or tRNA. Nature must have evolved the ‘art’ of molecular mimicry between RNA and proteins using different protein architectures that are functionally active in a ribosome ‘machine’ (Nakamura & Ito 2003). This view reinforces the high potential of RNA for plasticity.
In this review, we present an overview of the structure and function of representative RNA aptamers raised against a variety of human proteins and sensor molecules in our laboratory. This will contribute to our basic understanding of the potential of RNA and the global applications of aptamers.
Conformational plasticity of RNA as exemplified by anti-IgG aptamer
Although the 3D structures of RNA aptamers are commonly solved by X-ray crystallography or NMR spectroscopy (Hermann & Patel 2000), only three high-resolution structures of RNA aptamers in complex with their targets were reported. These were RNA aptamers in complex with nuclear factor (NF)-κB solved at 2.45 Å (Huang et al. 2003), with bacteriophage MS2 capsid solved at 2.8 Å (Horn et al. 2004) and with thrombin solved at a resolution of 1.8 Å (Long et al. 2008; Fig. 1A–C). NF-κB and the bacteriophage MS2 capsid naturally bind to nucleic acids. The crystal structures of RNA aptamers in complex with the nucleic acid–binding domain of these two proteins reflect these properties by mimicking naturally occurring electrostatic interactions (Ghosh et al. 2004; Horn et al. 2004). The crystal structure of an RNA aptamer in a complex with thrombin, which is not a nucleic acid–binding protein, indicates that the aptamer binds to the positively charged surface of the protein that is naturally required for high-affinity heparin binding (Long et al. 2008). Thus, the crystal structures determined to date have suggested that RNA aptamers bind target proteins predominantly through electrostatic forces (Fig. 1A–C).
Figure 1.

Overall structure of known aptamer–protein complexes with electrostatic surface potential. The RNA aptamer is a yellow ball-and-stick model. (A) Aptamer–thrombin complex at 1.8-Å resolution (Long et al. 2008). (B) Aptamer–nuclear factor-κB complex at 2.45-Å resolution (Huang et al. 2003). (C) Aptamer–MS2 coat protein complex at 2.8-Å resolution (Horn et al. 2004). (D) Aptamer–Fc region of human IgG1 (hFc1) complex at 2.15-Å resolution (Nomura et al. 2010). ICM Pro (Molsoft, Inc.) produced images of the electrostatic surface potential using the default setting: The potential scale used was 5. Blue areas: positively charged; red areas: negatively charged.
We selected a 23-nucleotide, high-affinity RNA aptamer against the Fc region of human IgG1 (hFc1; Miyakawa et al. 2008). As hFc1 lacks a positively charged protein surface (Deisenhofer 1981), the selected aptamer was speculated to interact with hFc1 via nonelectrostatic forces. The aptamer exhibited remarkable specificity to human IgG and no cross-reactivity to IgGs from other animal species. The aptamer also required divalent cations for binding because the bound IgG was easily released with the addition of EDTA (Miyakawa et al. 2008). To investigate these remarkable properties, we solved the crystal structure of the aptamer–hFc1 complex at the resolution of 2.15 Å (Fig. 2A; Nomura et al. 2010).
Figure 2.
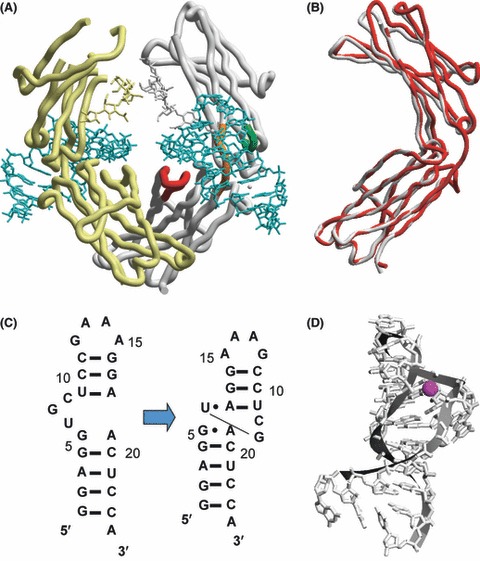
Structure of anti-hFc1 aptamer and the aptamer–hFc1 complex (Nomura et al. 2010). (A) The 2.15-Å crystal structure of a human IgG–aptamer complex. hFc1 backbone molecules are light yellow and gray, and bound aptamers are blue. Of the three regions colored red, orange and green in hFc1 (gray), a previous NMR study (Miyakawa et al. 2008) suggested that the aptamer binds the orange region, and the crystal structure confirms this prediction. (B) hFc1 conformations uncomplexed (gray) and in complex (red) with the aptamer. (C) M-fold-predicted secondary structure of anti-hFc1 aptamer (left) and its crystal structure in the complex (right). The global fold of the aptamer adapts a distorted hairpin structure with base flipping between U6 and G7. (D) Coordination sphere of Ca2+ (red sphere). Ca2+ is bound in a distorted octahedral coordination environment with the phosphate backbone and five water molecules (Nomura et al. 2010).
The solved structure showed several interesting features. First, the structure of the aptamer-bound hFc1 was superimposable upon the uncomplexed form of the hFc1 structure (Fig. 2B), indicating that the aptamer binding caused no significant structural changes to the backbone of hFc1. This, in turn, was indicative of the conformational plasticity of RNA to fit the target structure.
Second, the RNA structure in the aptamer–hFc1 complex diverged greatly from the secondary structure predicted by M-fold (Fig. 2C). Instead, the structure of the aptamer in complex formed a distorted hairpin structure with base flipping between U6 and G7, producing a GAAA tetraloop, an internal loop, and a terminal A-form helix (Fig. 2C). The internal loop formed by this distorted structure was crucial for binding to hFc1.
Third, the distorted structure was naturally unstable and required the presence of a hydrated calcium ion (Fig. 2D), found in the RNA major groove, that did not coordinate with protein ligands, but bound to nonbridging oxygen atoms of the G7 phosphates in the RNA. Therefore, Ca2+ may also help to maintain the distinct conformation of G7, which would be crucial for binding to hFc1. These structural features were consistent with the effect of EDTA, which chelates Ca2+, leading to loss of affinity by distorting the aptamer structure. Importantly, affinity was restored upon the addition of Ca2+ in the presence of hFc1 (Nomura et al. 2010). The reversible binding feature suggested reversible folding of the aptamer, achieved by the presence of the divalent cation and target hFc1.
Protein A affinity chromatography is currently the most frequently used procedure to purify humanized or chimeric antibodies (Fahrner et al. 2001; Ghose et al. 2005), but also requires an acidic elution step that can sometimes cause unexpected denaturation or inactivation of antibodies (Tsumoto et al. 2004; Ghose et al. 2005; Cromwell et al. 2006). Instead, bound IgGs can be easily released from the aptamer resin under neutral pH conditions using simple elution buffers containing EDTA (Miyakawa et al. 2008). Combined with the aptamer’s high specificity to hFc1, these purification advantages provide an alternative reagent for the mass purification of therapeutic antibodies (Miyakawa et al. 2008).
Fourth, and most importantly, unlike known RNA–protein interactions, which are generally stabilized by electrostatic forces, as described earlier, the aptamer bound to the neutral portion of the hFc1 surface (Fig. 1D) and the aptamer–hFc1 interaction was stabilized by multiple weak interactions such as hydrogen bonds and van der Waals forces (Nomura et al. 2010). For example, the stacking interaction between the aptamer’s G7 and tyrosine 373 (Tyr373) was crucial for the complex; therefore, the aptamer probably interacted through weaker forces supported by van der Waals contacts and hydrogen bonds (Nomura et al. 2010). The interaction between hFc1 and the aptamer covered 580 Å2 per Fc fragment (Fig. 2A), a surface area that is relatively small compared with that of other RNA aptamer interactions (c. 1000 Å2), but even so, it achieved remarkably strong affinity (Nomura et al. 2010). Therefore, it is likely that SELEX technology can select not only for molecules that interact through predominantly electrostatic forces (Hermann & Patel 2000; Huang et al. 2003; Horn et al. 2004), but also for high-specificity molecules that interact through weaker forces such as van der Waals contacts and hydrogen bonds. Together, these findings emphasize the excellent conformational plasticity and affinity of RNA molecules, suggesting that RNA aptamers may be applicable to a wider range of targets than previously thought.
Therapeutic potential of RNA aptamers
On the basis of the conformational plasticity and targeting specificity of RNA, we developed aptamers against various therapeutic target proteins, including cytokines, growth factors, receptors and other regulatory proteins involved in transcription and translation (Oguro et al. 2003, 2009; Mori et al. 2004; Mochizuki et al. 2005; Sakamoto et al. 2005; Miyakawa et al. 2006, 2008; Tanaka et al. 2007; Ohuchi et al. 2008; Wang et al. 2008; Nakamura et al. 2009; Endo & Nakamura 2010; Hiep et al. 2010; Adachi et al. 2011; Ishiguro et al. 2011; Iwagawa et al. 2011). Of these, two therapeutic programs approaching clinical trials for autoimmune disorders are described later.
Therapeutic aptamer against interleukin-17A
Interleukin-17A (IL-17A) is a pro-inflammatory cytokine produced primarily by a subset of CD4+ T cells called Th17 cells, which represent a third subset of CD4+‘helper’ lymphocytes distinct from the classically described Th1 and Th2 populations (Korn et al. 2009; Miossec et al. 2009). The primary function of Th17 cells appears to be the clearance of pathogens that are not adequately handled by Th1 or Th2 cells. However, aberrant Th17 responses and IL-17A production have been implicated in a variety of autoimmune diseases and animal models, including rheumatoid arthritis (RA; Chabaud et al. 2000; Kirkham et al. 2006) and multiple sclerosis (MS; Matusevicius et al. 1999; Graber et al. 2008).
To control Th17-based autoimmune diseases, we selected RNA aptamers against human IL-17A (hIL-17A; Ishiguro et al. 2011). One such aptamer of 33 nucleotides’ (nts) length, Apt21-2 (Fig. 3A), bound not only to hIL-17A stably, but also to mouse IL-17A (mIL-17A). The dissociation constant (Kd) of Apt21-2 to hIL-17A and mIL-17A was estimated to be 48.5 and 701.3 pm, respectively (Ishiguro et al. 2011). Importantly, when examined with a sensor chip on which the extracellular domain of IL-17R was fused to Fc and immobilized via protein A, Apt21-2 blocked the binding of hIL-17A to its human receptor hIL-17R as well as of mIL-17A to its mouse receptor mIL-17R (Ishiguro et al. 2011). Consistent with this finding, Apt21-2 blocked IL-17A-dependent signaling and hampered phosphorylation of IκB (an NFκB inhibitor) and JNK (c-Jun N-terminal kinase; Emamaullee et al. 2009) proteins in normal human dermal fibroblasts (NHDF; Fig. 3B). Then, we examined the effect of Apt21-2 on the expression of IL-6, one of the cytokines induced by IL-17A, in NHDF cells after 24-hr incubation with hIL-17A. As expected, Apt21-2 definitively blocked the expression of IL-6 in NHDF cells in a dose-dependent manner (Fig. 3C). Of note is that under 1.3-nm hIL-17A conditions, Apt21-2 exhibited an IC50 range of 2–3 nm, whereas an available neutralizing anti-hIL-17A monoclonal antibody (mAb317; R&D Systems) had an IC50 of 200–300 nm (Fig. 3C). Apt21-2 also inhibited IL-6 production in mouse embryonic fibroblasts (MEF; Molet et al. 2001; Dong 2009) induced by mIL-17A, with an IC50 of 250–300 nm (Ishiguro et al. 2011). Therefore, the efficacy of Apt21-2 is two orders of magnitude greater in human cells than in mouse cells.
Figure 3.

Neutralizing anti-interleukin (IL)-17A aptamer (Ishiguro et al. 2011). (A) Secondary structure of Apt21-2, predicted by M-fold. Circles denote 2′-fluoro-modified pyrimidines. (B) Suppression of IL-17A-induced signaling pathways in normal human dermal fibroblasts (NHDF cells) by Apt21-2. NHDF cells were treated with human (h)IL-17A (40 ng/mL) with random RNA pool (control) or Apt21-2 RNA (30 nm) and analyzed by Western blotting using the indicated antibodies to detect phosphorylation levels. (C) IL-6 expression affected by Apt21-2 in NHDF cells. hIL-17A was preincubated with Apt21-2 or an anti-hIL-17A antibody at different concentrations and added to NHDF cell culture. After 24-h incubation, the amount of IL-6 secreted to the medium was assessed by ELISA.
Apt21-2 is composed of 13 ribose 2′-fluoropyrimidines (ribonuclease-resistant) and 20 unmodified purines (Fig. 3A). The pharmacokinetic property of Apt21-2 was further improved by chemical modifications with a 40-kDa polyethylene glycol (PEG) at the 5′ end and an inverted deoxythymidine (idT) at the 3′ end, giving rise to PEG21-2idT. Subsequently, the in vivo efficacy of PEG21-2idT was investigated in two mouse models of autoimmunity, experimental autoimmune encephalomyelitis (EAE; Lubberts et al. 2001) and glucose-6-phosphate isomerase (GPI)-induced RA (Koenders et al. 2005). EAE is a model for the human inflammatory demyelinating disease MS. C57BL/6 mice were immunized with myelin oligodendrocyte glycoprotein (MOG35–55) peptide in complete Freund’s adjuvant, and PEG21-2idT was administered intraperitoneally (i.p.; 0, 1, 3 and 10 mg/kg dosages) every other day. The appearance of EAE was significantly delayed in mice administered with 3 and 10 mg/kg PEG21-2idT, with incidence and symptoms reduced markedly in a dose-dependent manner (Fig. 4A). Mice were killed at day 25 and subjected to histological analysis. Consistent with clinical signs, typical foci of MNC infiltration and demyelination were observed in the white matter of the spinal cord of untreated mice, but these signs were not observed in most mice (8/10) administered with 10 mg/kg PEG21-2idT (Ishiguro et al. 2011).
Figure 4.

Attenuation of autoimmunity in mouse models by anti-IL-17A aptamer (Ishiguro et al. 2011). (A) Suppression of experimental autoimmune encephalitis (EAE) development by Apt21-2 (Ishiguro et al. 2011). Wild-type mice (n = 10 each) were immunized with myelin oligodendrocyte protein (MOG35–55) peptide in complete Freund’s adjuvant, and PEG21-2idT (0, 1, 3 and 10 mg/kg) was administered i.p. every other day after immunization. EAE clinical scores for vehicle and PEG21-2idT–administered mice. Values are the mean and SEM of 10 mice per group. (B) PEG21-2idT treatment suppresses development of glucose-6-phosphate isomerase (GPI)-induced arthritis. DBA/1 mice were immunized with 300 μg of mouse GPI, and the development of arthritis was monitored visually and scored on a scale of 0–2. Values are the mean and SEM of 10 mice per group.
Next, GPI-induced RA was induced by immunizing DBA/1 mice with recombinant mouse GPI (mGPI), and 2 PEG21-2idT efficacy tests were conducted. Doses of PEG21-2idT (0, 1, 3, 10 mg/kg) were administered i.p. to DBA/1 mice (n = 10) every other day after GPI immunization. PEG21-2idT resulted in significant improvement in the incidence of arthritis and the clinical scores of symptoms in a dose-dependent manner (Fig. 4B). We also injected PEG21-2idT (10 mg/kg, n = 10) i.p. every day from day 8 after GPI immunization, a time point at which RA was already established. Importantly, PEG21-2idT injection at day 8 significantly suppressed the progression of arthritis even after it had developed (Ishiguro et al. 2011). These results suggest that IL-17A blockade by PEG21-2idT has both protective and therapeutic activity against GPI-induced arthritis.
This study showed that i.p. administration of a PEGylated form of an anti-IL-17A RNA aptamer (PEG21-2idT) inhibits inflammatory lesions and neurological symptoms in EAE and RA mouse models. Although PEG21-2idT was generated against hIL-17, it also exhibited weaker affinity to mIL-17. The intriguing finding in this study relates to the fact that PEG21-2idT was 1–2 orders of magnitude less effective against mIL-17A than it was against hIL-17A, but even so, could exert therapeutic impact in EAE and RA mice. This strongly suggests that the currently generated anti-IL-17A aptamer has potent therapeutic potential for human autoimmune diseases. Such approaches are in progress toward clinical trials.
Rationalized selection of the aptamer specific to the IL-17A/F heterodimeric form
The IL-17 cytokine family is composed of six structurally related proteins (IL-17A, B, C, D, E and F). Of these, IL-17A and IL-17F are the most closely related to each other, sharing 55% amino acid sequence homology and four conserved cysteine residues at the C-terminal. These conserved cysteine residues participate in the formation of intermolecular disulfide bonds, leading to the formation of homodimeric (IL-17A/A, IL-17F/F) and heterodimeric (IL-17A/F) structures (Chang & Dong 2007; Wright et al. 2008). It has been reported that differentiated Th17 cells form IL-17A/F heterodimers in higher amounts than they do either homodimer, and distinct from IL-17F/F, IL-17A/A and IL-17A/F play primary roles in regulating airway inflammation (Liang et al. 2007; Wright et al. 2007). Furthermore, genetic analyses using IL-17A-deficient and/or IL-17F-deficient mice suggest both overlapping and specific functions for IL-17A and IL-17F (Wright et al. 2008; Korn et al. 2009). These studies also indicated that IL-17A might be a more important initiating factor than IL-17F in the EAE mouse model or in the development of allergic asthma, whereas both IL-17A and IL-17F contribute to chronic inflammation (Schnyder-Candrian et al. 2006; Graber et al. 2008). However, the physiological role of IL-17A/F is entirely unknown because of the lack of an experimental system or a reagent that specifically inhibits this heterodimeric form.
To date, the known anti-IL-17A antibodies react with both IL-17A/A and IL-17A/F dimers. Likewise, Apt21-2 bound to IL-17A/A and IL-17A/F, but not to IL-17F/F in a surface plasmon resonance (SPR) assay (Fig. 5A; Ishiguro et al. 2011). We aimed to create an experimental agent that would discriminate IL-17A/F heterodimers from IL-17A/A and IL-17F/F homodimers by SELEX. One such aptamer against human IL-17A/F, AptAF42, was isolated by repeated cycles of selection and counterselection against heterodimeric and homodimeric complexes, respectively. AptAF42 bound to IL-17A/F, but not to IL-17A/A or IL-17F/F (Fig. 5B) and blocked the binding of IL-17A/F, but not of IL-17A/A or IL-17F/F, to the IL-17 receptor in the SPR assay in vitro (Adachi et al. 2011). Thus, the optimized derivative, AptAF42d1, blocked cytokine GRO-α production induced by IL-17A/F, but not by IL-17A/A or IL-17F/F, in human cells (Fig. 5C). These findings demonstrate that RNA aptamers possess outstanding potential to probe a target structure and that combining selection and counterselection processes enables the selection of a desired aptamer to discriminate heterodimeric from homodimeric structures. Thus, AptAF42d1 is the first inhibitory tool specific to IL-17A/F, which might be applicable to an in vivo experiment to elucidate the physiological role of IL-17A/F independent of IL-17A/A and IL-17F/F.
Figure 5.

Reactivity of anti-IL-17 aptamers to homo- or heterodimeric forms of IL-17A and IL-17F (Adachi et al. 2011). (A) Surface plasmon resonance (SPR) sensorgrams of Apt21-2 injected with homodimeric (IL-17A/A or IL-17F/F) and heterodimeric (IL-17A/F) protein complexes. Poly(A)-tailed Apt21-2 was immobilized to the sensor chip, and IL-17 proteins were injected. (B) SPR sensorgrams demonstrating the affinity of AptAF42 to IL-17A/F, IL-17A/A and IL-17F/F. Poly(A)-tailed AptAF42 was immobilized to the sensor chip, and IL-17 proteins were injected. (C) Suppression of GRO-α production in BJ cells by AptAF42d1. IL-17A/F, IL-17A/A and IL-17F/F were preincubated with the aptamers or N30 RNA (control) at the indicated molar ratios and added to BJ cells. After 6-h incubation in BJ cells, secreted GRO-α was analyzed by ELISA. The y-axis denotes the relative amount of GRO-α. The data represent the mean of three independent experiments, and standard deviations are indicated with error bars.
Therapeutic aptamer against midkine
Midkine (MK) is a heparin-binding growth factor and exerts pleiotropic effects, including cell proliferation, cell migration, angiogenesis and fibrinolysis in a variety of tissues (Muramatsu 2002). MK overexpression has been observed in a number of malignant tumors, Hodgkin’s disease and brain tumors (Muramatsu 2002). However, MK-deficient mice are reportedly resistant to ischemic renal injury (Sato et al. 2001) and neointima formation in atherosclerosis (Horiba et al. 2000). A recent study proposed that MK deficiency suppresses the development of an RA model by preventing inflammatory leukocyte migration and osteoclast differentiation (Maruyama et al. 2004). Furthermore, MK expression in the spinal cord is upregulated during the induction and progression phase of EAE (Liu et al. 1998; Hemmer et al. 2002). Although MS and EAE have been described as T-helper type 1 (TH1) cell–mediated autoimmune diseases, CD4+ CD25+ regulatory T (Treg) cells have recently received a great deal of attention as negative regulators of MS pathogenesis (Kohm et al. 2002; Baecher-Allan & Hafler 2004; Viglietta et al. 2004; Matarese et al. 2005). Treg cells regulate peripheral tolerance and autoimmunity, and abnormalities in Treg cell function may contribute to the development of autoimmune diseases (Sakaguchi 2004, 2005; Liu & Leung 2006). Thus, expansion of the Treg cell population could prevent autoimmune attacks such as gastritis, oophoritis, thyroiditis, inflammatory bowel disease and MS (Kohm et al. 2002; von Herrath & Harrison 2003; Mills 2004; Viglietta et al. 2004; Matarese et al. 2005). This is consistent with the finding that MK-deficient mice are resistant to MOG-induced EAE owing to an expansion of the Treg cell population in the peripheral lymph nodes (Wang et al. 2008).
On the basis of these reports, we isolated RNA aptamers against MK, reflecting a midline’s affinity to heparin; several high-affinity anti-MK aptamers were selected. One such derivative (MKapt) is 38 nts in length and has a Kd of 0.9 nm (Wang et al. 2008). MKapt was stabilized by substitutions of ribose 2′-fluoro, omethy, or deoxy nucleotides and modified with cholesterol and idT at the 5′ and 3′ ends, respectively (Ishikawa et al. 2008; Wang et al. 2008). To investigate the efficacy of the modified MKapt in the pathogenesis of EAE, we immunized C57BL/6 mice with MOG in complete Freund’s adjuvant, and the aptamer was administered i.p. (0 and 5 mg/kg doses) every other day. As shown in Fig. 6A, the appearance of EAE was significantly delayed in mice administered with 5 mg/kg of aptamer, with markedly reduced symptoms. The histological analysis of mice killed at day 28 was consistent with the clinical signs (Wang et al. 2008), demonstrating a clear correlation between the clinical and pathological features of EAE in untreated and aptamer-treated mice. Moreover, administration of the aptamer induced expansion of the Treg cell population (Fig. 6B; Wang et al. 2008). These findings suggest that MK is a suppressor of Treg cells and that MK blockade by an RNA aptamer may be a potent therapeutic strategy against autoimmune diseases, including MS (Fig. 6C).
Figure 6.
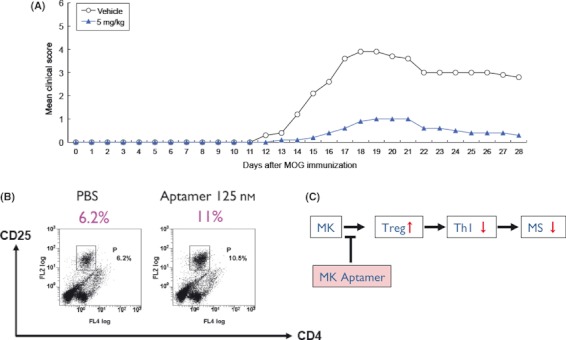
Anti-midkine (MK) aptamer and its therapeutic potential. (A) Clinical scores for wild-type EAE mice administered PBS (n = 5) or 5 mg/kg MKapt (n = 5) after the MOG injection. (B) Flow cytometric analysis of CD4+ CD25+ regulatory T (Treg) cell population expansion using the anti-MK RNA aptamer in vitro. (C) Predicted functional cascade of MK and the anti-MK aptamer.
Aptamer-based novel biosensor development
Anti-Cy3 aptamer
Aptamers have also been generated to dyes and fluorophores such as malachite green (Grate & Wilson 1999) and sulforhodamine B (Holeman et al. 1998; Wilson & Szostak 1998). These aptamers are composed of unmodified nucleotides and are thus applicable to sensitive, real-time detection of nucleic acid or small molecules by annealing to complementary sequences or binding to secondary aptamers to target molecules (Kolpashchikov 2003, 2005; Stojanovic & Kolpashchikov 2004). To reduce background fluorescence and increase detection specificity, Kolpashchikov (2005) designed a binary aptamer probe based on the malachite green aptamer (MGA). MGA is unique as it dramatically increases the fluorescence of the dye (Babendure et al. 2003). The MGA has a stem–loop structure containing internal bulged loops (Grate & Wilson 1999). These double-stranded sequences were separated into two single-strand sequences, each of which had no affinity to malachite green, and were tagged to sequences complementary to nucleic acid analytes. This probe is referred to as a binary MGA probe and provides immediate fluorescent response after hybridization to complementary nucleic acid analytes, thus offering easy and instant detection of specific DNA and RNA (Kolpashchikov 2005).
The choice of chromophore is crucially important for widespread practical application of aptamer probes to live cell imaging. Although malachite green has been successfully applied to the binary aptamer probe, it remains uncertain whether malachite green is the best choice for intracellular imaging. It is worth mentioning that malachite green very efficiently generates singlet oxygen upon irradiation and is used for targeted damage of mRNA constructs (Grate & Wilson 1999); therefore, it may also lead to undesirable consequences for the behavior of cells during the imaging process. Therefore, an alternative chromophore that is less toxic and better suited for live cell imaging is desirable.
On the basis of these considerations, we isolated an RNA aptamer against the cyanine dye Cy3 (Cy3apt), a widely used, membrane-permeant and nontoxic fluorophore (Endo & Nakamura 2010). The parental Cy3apt was 83 nts long and shortened to 49 nts length with increased affinity to Cy3 achieved by multiple base changes (Fig. 7A). The affinity of Cy3apt to Cy3 was examined by SPR using a Cy3-immobilized sensor chip injected with different concentrations of Cy3apt. The SPR signal plateaued immediately upon injection of Cy3apt RNA, and plateau levels increased in proportion to the amount of RNA injected, followed by rapid dissociation when the injection stopped (Fig. 7B). Interestingly, the fluorescence intensity of free Cy3 at 580 nm (50-nm bandpass) increased in proportion to the amount of Cy3apt RNA added, with no detectable change in the emission spectra (Endo & Nakamura 2010).
Figure 7.

Binary Cy3 aptamer probe composed of folded modules (Endo & Nakamura 2010). (A) Optimized structure of Cy3apt. Ten nucleotides (white letters in black boxes) represent substitutions from the original Cy3apt sequence to optimize affinity to Cy3. ‘Δ3′GCG’ denotes a 3-base deletion on the 3′ end. ‘Tri-reversion’ indicates three bases that reverted to the original. (B) SPR sensorgrams of Cy3apt binding to Cy3 immobilized on the sensor chip. The indicated concentrations of RNAs were injected at time 0 for 30 s at a flow rate of 10 μL/min. (C) Binary Cy3 aptamer probe to detect target oligonucleotides. Schematic representation of the target oligonucleotide and the binary aptamer probe (I-bin and II-bin). The target oligonucleotide T2 sequence is shown, and the variable linker sequences are boxed. M2-1 and M2-2 are single nucleotide mismatches introduced into T2. Target-binding sequences of the binary probe are depicted as lowercase letters. (D) Detection of target oligonucleotides using the binary probe as SPR signals. Target oligonucleotides (10 μm) with (closed box) or without (open box) the binary probe (16 μm) were subjected to SPR analysis.
The shortened derivative of Cy3apt is composed of two separate hairpin modules (Fig. 7A). Although each of these domains has no affinity to Cy3 separately, they exhibit affinity to Cy3 upon being properly arranged in a tertiary configuration. Each domain of the Cy3apt was separately used to construct a binary Cy3 aptamer probe. A heptanucleotide, corresponding to each half of a complementary sequence of a 14-nucleotide target sequence (T0 in Fig. 7C), was appended onto the 5′ terminus of one domain and the 3′ terminus of the other domain as a target-binding arm (Fig. 7C; I-bin and II-bin). In contrast to a preceding study (Kolpashchikov 2005), both of our binary probe elements folded into stem–loop structures and had no single-stranded extension except for the appended flanking sequences required for target recognition. The Cy3-binding activity of the binary probe was analyzed using SPR. As shown in Fig. 7D, the designed Cy3 aptamer probe alone did not bind to Cy3 in the absence of the target oligonucleotides. The aptamer probe bound to Cy3 when the target oligonucleotides were present (Fig. 7D). The binding efficiency varied depending on the nucleotide length of the central linker sequences in the target oligonucleotides T2, T4 and T6 (Fig. 7D). The best affinity in these experiments was observed with T4, which contained a tetranucleotide insert, suggesting that the orientation of the two probe elements was important in regenerating the tertiary structure so that it could bind to Cy3. When a single mismatch was introduced into each recognition site of the T2 target sequence (Fig. 7C; M2-1 and M2-2), the binary probe did not bind the target sequence (Fig. 7D), demonstrating single nucleotide discrimination. Unlike the other binary probes consisting of split primary sequences, this binary probe consisted of two folded modules and was referred to as a folded binary probe (Endo & Nakamura 2010).
Sequence-specific detection of nucleic acids is crucial to disease diagnosis, genome study and mRNA monitoring in living cells. Among the numerous nucleic acid analysis methods of particular interest are those that provide immediate visible or fluorescent response after hybridization to complementary nucleic acid analytes, thus offering easy and instant detection of specific DNA and RNA. The binary Cy3 aptamer probe generated in this study will facilitate this approach in view of the fact that it, for the first time, enabled us to deal with a pair of aptamers and Cy3, a commonly used, membrane-permeant, and nontoxic dye, for developing an in vitro and in vivo sensor system. As the selected aptamer is composed of unmodified (i.e., natural) nucleotides, the in vivo sensor system is designable by expressing the binary Cy3 aptamer in test cells and directing its cellular localization, if necessary, using a variety of expression vectors. Moreover, our binary aptamer probe is composed of distinctly folded modules and can be applied to monitor a tertiary RNA–RNA interaction.
Strategic selection of ‘RNA receptor’ to ‘RNA ligand’
In nature, many RNA molecules and motifs exhibit specific functions that require the formation of a specific 3D structure, rather than simply a linear carrier of genetic code information. The classical examples of such structural, protein-noncoding RNAs (ncRNAs) are tRNA and rRNA, which play key roles in the central dogma of molecular biology (Gesteland et al. 1999, 2006). In addition, several regulatory elements on mRNA, such as riboswitches and internal ribosome entry sites, also function via their specific 3D structures (Batey 2006). More recently, several structural ncRNAs have been discovered as specific modulators for intracellular proteins, and it is likely that a significant number of structural RNAs exist within the huge numbers of ncRNAs in higher eukaryote genomes (Mattick & Makunin 2006). Thus, the development of a novel tool to detect and control structural ncRNAs might greatly facilitate genome-encoded ncRNA research, as antibodies did for protein research (Sosnick & Pan 2003; Onoa & Tinoco 2004; Bokinsky & Zhuang 2005; Furtig et al. 2007).
To develop such RNA tools, we applied an artificial ligase ribozyme (Designed-and-Selected Ligase, DSL; Ikawa et al. 2002) to develop a selection system to generate novel RNA receptor motifs against a target RNA structure within a given structural context (Ohuchi et al. 2008). In this system, a GAAA tetraloop and its specific receptor motif (11-ntR) from an artificial RNA ligase ribozyme with modular architecture (the DSL ribozyme) were replaced with a target structure and random sequence, respectively (Fig. 8A). Motifs recognizing the target structure can be identified by in vitro selection based on the ribozyme activity. A model selection targeting a GAAA loop successfully identified motifs previously known as GAAA loop receptors (Ohuchi et al. 2008). In addition, a new selection targeting a C-loop motif also generated a novel motif, designated ‘C-loop receptor’, which interacts with this structure, although the C-loop is not considered an RNA–RNA interaction motif (Ohuchi et al. 2008).
Figure 8.

Selection of a novel class of RNA–RNA interaction motifs based on a ligase ribozyme with defined modular architecture (Ohuchi et al. 2008). (A) Secondary structures of the parental DSL-U5 ribozyme and its derived libraries. (a) The DSL-U5 ribozyme with the GAAA tetraloop/11-ntR pair essential for the ribozyme activity highlighted in gray. (b) The GAAA loop library with the target GAAA tetraloop and randomized nucleotides highlighted in gray. (c) The C-loop library with the target C-loop motif (C-50) with neighboring single base pairs and randomized nucleotides highlighted in gray. (B) Secondary structure of TectoRNA-derived, homodimer-forming constructs. The target C-loop and the C-loop receptor motifs are enclosed in gray boxes. (C) Autoradiogram of electrophoretic mobility shift assay of the [α-P32]-labeled TectoRNA derivative. Left–right: 0, 50, 100, 200, 400, 800 and 1600 nm of unlabeled RNA were added.
The interaction between the C-loop and the C-loop receptor was investigated by grafting them into TectoRNA, an artificial RNA architecture developed by Jaeger & Leontis (2000) and Atsumi et al. (2001), whose self-dimerization properties are suitable for examining the modularity of the selected motif (Fig. 8B). The electrophoretic mobility shift assay showed an apparent reduction in mobility in a concentration-dependent manner (Fig. 8C). The degree of the mobility change was consistent with a biphasic dimerization model with typical fast exchange kinetics, suggesting that the construct dimerized, as was the case for the original TectoRNA (Jaeger et al. 2001). Its Kd value, determined as the kinetic equilibrium, was 168 nm (Fig. 8C).
The physical interaction was further investigated by chemical footprinting using a reagent that cleaves the phosphate backbone of RNAs at non-base pairing, solvent-accessible sites (Ohuchi et al. 2008). Phosphates around the C-loop motif were cleaved under monomeric conditions but protected under dimeric conditions (Fig. 8B; positions marked blue), indicating that these phosphates, originally located at the surface of the RNA structure, became solvent-inaccessible upon dimerization. This observation supports the physical interaction between the C-loop and the C-loop receptor under dimeric conditions. In contrast, several residues in the C-loop receptor were cleaved efficiently under dimeric conditions but not under monomeric conditions (Fig. 8B; positions marked red). As there was no obvious sequence complementarity between these two sequences, the C-loop receptor is likely to recognize the C-loop motif by specific, non-Watson–Crick tertiary interactions.
Thus, we developed a selection system that enables the identification of novel RNA motifs that interact with a target RNA structure within a desired structural context. We believe that RNA motifs isolated via this selection system can be directly used for RNA engineering, such as the design of artificial RNA architectures (Jaeger & Chworos 2006) or novel molecular tools for desired target RNAs, including structured ncRNAs, regulatory mRNA elements, as well as RNA components of large, complicated ribonucleoprotein complexes such as the ribosome and the spliceosome.
Cell-based selection of aptamers specific to embryonic stem cell surface markers
Several hundred aptamers have been reported in the published report, and most of them are raised against purified proteins. However, recent development of cell-based SELEX procedures enabled us to isolate aptamers against cell surface molecules of unknown identity or proteins inappropriate for purification in fully active conformations (Morris et al. 1998; Ohuchi et al. 2006; Shangguan et al. 2006; Shamah et al. 2008). We have used this procedure to generate aptamers against cell surface markers of unknown identities on embryonic stem cells (ESCs; Iwagawa et al. 2011).
Embryonic stem cells are derived from the inner cell mass of blastocysts and able to differentiate into all three germ layers (Evans & Kaufman 1981; Martin 1981; Thomson et al. 1998). Under optimized culture conditions, ESCs remain in the undifferentiated state and self-renew indefinitely (Nunomura et al. 2005; Watanabe et al. 2007; Ying et al. 2008; Intoh et al. 2009; Shiraki et al. 2009; Pera & Tam 2010). Although extensive studies have been pursued to identity cell surface molecules on ESCs (Nunomura et al. 2005; Intoh et al. 2009) and to uncover the molecular mechanisms underlying the maintenance of the undifferentiated state and regulation of the differentiation process (Chambers & Tomlinson 2009; Young 2011), ESC-specific cell surface proteins, or markers, are not fully understood.
Aptamers were selected against intact, live mouse ESCs (mESCs) by SELEX with or without negative selection against fully differentiated A-9 cells (derived from the connective tissues of an adult mouse) from an RNA pool randomized to over 60 nts (N60) with 2′-fluoropyrimidine modifications to resist ribonucleases (Fig. 9A). The bound RNAs were released from the cell surface with a solution containing EDTA to chelate divalent cations. The formation of typical higher-order RNA structures often requires divalent cations, and thus, their elimination with EDTA is expected to inactivate most of the bound aptamers (Pyle 2002; Nomura et al. 2010). It is noteworthy that dead and damaged cells tend to adsorb nucleic acids nonspecifically (Raddatz et al. 2008), and the elimination of divalent cations rarely affects this nonspecific adsorption. Therefore, EDTA-mediated recovery of aptamers is an effective process to distinguish specific binders from nonspecific adsorbates. L2-2 was one of these aptamers (Fig. 9B).
Figure 9.
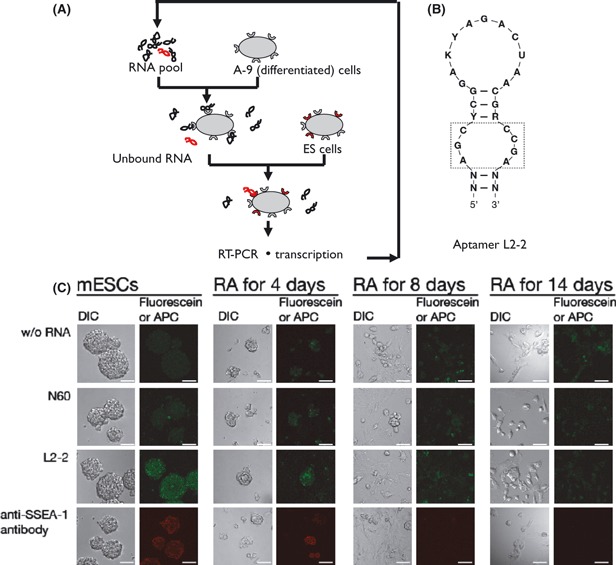
Cell-based selection of RNA aptamer against mouse embryonic stem cells (mESCs; Iwagawa et al. 2011). (A) SELEX schematic of live mESCs combined with counterselection against fully differentiated A-9 cells. (B) Consensus motif conserved in the anti-mESC aptamer L2-2. N, K, Y, and R indicate any 4, G or U, C or U, and A or G nucleotides, respectively. (C) Confocal fluorescence image of the L2-2 aptamer. mESCs before and after treatment for rheumatoid arthritis for 4, 8, or 14 days were stained with the indicated fluorescein-labeled RNA probes and APC-labeled antibodies. DIC images are also shown. Scale bar, 50 μm.
The binding specificity of [32P]-labeled L2-2 was examined against mESCs and five differentiated mouse cell lines: connective tissue (A-9 cells), embryonic fibroblast (NIH 3T3 cells), muscle tissues (C2C12 cells), liver cancer (Hepa 1–6 cells) and neuroblastoma (NB2a cells). The data indicated that this aptamer bound efficiently to mESCs, but failed or only weakly bound, if at all, to the differentiated mouse cell lines (Iwagawa et al. 2011). The loss of binding affinity of L2-2 for A-9 cells was consistent with the negative selection process against A-9. Several other mESC-specific aptamers were also isolated in these experiments (Iwagawa et al. 2011).
The mESC markers are known to be down-regulated during the course of differentiation (Nash et al. 2007; Spencer et al. 2007). Confocal microscopy imaging was carried out using fluorescein-labeled L2-2 and mESC-derived differentiated cells by treatment with retinoic acid for 4, 8 and 14 days. The same set of cells was also stained with an antibody against SSEA-1, a canonical mESC marker (Cui et al. 2004), as control. A set of fluorescence images showed that aptamer L2-2 as well as anti-SSEA-1 antibody bound to mESCs, whereas the N60 random RNA pool did not (Fig. 9C). Surface staining of the mESCs with the antibody was near absolute, but the staining intensity, or SSEA-1 expression level, differed among individual cells. Similarly, the aptamer staining intensity varied from cell to cell. However, the staining patterns of the antibody and the aptamer were completely different. The aptamer L2-2 preferentially bound to dot-like spots on cell–cell contact regions rather than on the whole cell surface (Fig. 9C). These findings suggest that the L2-2 target is localized on some microdomain structures on the cell–cell contact regions. During the course of retinoic acid–induced differentiation, the staining intensities of anti-SSEA-1 antibody and L2-2 aptamer exhibited different patterns and gradually decreased. The anti-SSEA-1 antibody signal was significantly weakened 4 days after retinoic acid addition and completely disappeared after 8 days (Fig. 9C). In contrast, the reduction of staining with L2-2 proceeded more slowly; a large proportion of the cells were stainable after 4 days, and half of the populations were stainable with the aptamer after 8 days. A few fractions could still be stained with the aptamer even after 14 days.
Given that anti-mESC aptamers could bind to artificially created, induced pluripotent stem cells (iPSCs; Takahashi & Yamanaka 2006; Takahashi et al. 2007), these should provide an opportunity for the isolation and purification of iPSCs to evade tumor formation upon transplantation of iPSCs and iPSC-derived cells (Kolossov et al. 2006). It has been showed that cell-binding aptamers can be used not only for molecular probes, but also for plating cell adhesion reagents on culture dishes, drug (including short interfering RNA)-delivery systems, etc. (Guo et al. 2006; McNamara et al. 2006; Fang & Tan 2010). Collectively, the anti-mESC aptamer might open the gateway to diverse applications in the fields of regenerative medicine and developmental biology.
Conclusions and perspectives
In this laboratory, RNA aptamers were selected against a variety of human proteins and a chemical reagent, and the key features were summarized in this review. Although many properties of the selected aptamers were similar to those of antibodies, the aptamers also exhibited superior features. Selected aptamers occasionally had a Kd on the picomolar scale, 100-times stronger affinity than that of normal antibody–antigen interactions. Certain aptamers are more than 50 nts long for specific and high-affinity binding to their target proteins. Occasionally, these proteins lack RNA recognition motifs or an intrinsic, strong affinity to RNA, and the high affinity of the aptamer is achieved through capture of the protein’s global conformation. In contrast, the anti-hFc1 aptamer achieved strong and specific binding to hFc1 mainly by van der Waals contacts and hydrogen bonds rather than via electrostatic forces, unlike most known RNA–protein interactions. These findings demonstrate that RNA has great potential to form a vast set of tertiary structures and to achieve high affinity: We refer to this property as ‘RNA plasticity’. This conformational plasticity and selectivity can be achieved by multiple interactions, which are applicable to many protein targets with low or no affinity to nucleic acids. These results provide us with a solid and promising basis for steps to create novel RNA molecules with distinct structures and with therapeutic potential superior to that of antibodies.
Therapeutic antibodies are being rapidly developed worldwide. In 2010, the US FDA approved 31 therapeutic antibodies, and the antibody therapeutics market is expected to achieve an excess of US$ 40 billion in 2010. Moving forward, aptamer therapeutics is not at a disadvantage. Several characteristics grant aptamers potential superior to that of therapeutic antibodies, including increased binding affinity, in vitro manipulation of activity and/or stability, less immunogenicity or toxicity, and scalable chemical production. In contrast to costly cell-based production of antibodies, the production costs of RNA aptamers will also be greatly reduced with the development of oligonucleotide-based therapies. Therefore, RNA aptamers offer a beneficial therapeutic approach for the treatment for diseases not only from a therapeutic standpoint, but also from the perspective of healthcare economics. Table 1 summarizes several aptamers that have undergone clinical trials. The observations that have been made in these trials will provide a better understanding of both the possibilities and limitations of aptamers as therapeutics. Some other applications of aptamers reported in the published report are listed in Table 2.
Table 1.
Aptamers in clinic
| Name | Company | Target | Indication | Phase | References |
|---|---|---|---|---|---|
| Macugen | Pfizer/Eyetech | VEGF | AMD | Approved | Ng et al. (2006) |
| AS1411 | Antisoma | Nucleolin | Cancer | Phase II | Mongelard & Bouvet (2010) and Bates et al. (2009) |
| REG1 | Regado | Factor IXa | ACS | Phase II | Rusconi et al. (2002) and Povsic et al. (2011) |
| ARC1779 | Archemix | vWF | TTP | Phase II | Jilma-Stohlawetz et al. (2011) |
| NU172 | ARCA | Thrombin | CABG | Phase II | Keefe et al. (2010) |
| E10030 | Ophthotech | PDGF | AMD | Phase II | Akiyama et al. (2006) |
| ARC1905 | Ophthotech | C5 | AMD | Phase I | Biesecker et al. (1999) |
| NOX-E36 | NOXXON | MCP-1 | DN | Phase I | Maasch et al. (2008) and Darisipudi et al. (2011) |
| NOX-A12 | NOXXON | SDF-1 | Cancer | Phase I | Darisipudi et al. (2011) and Duda et al. (2011) |
| NOX-H94 | NOXXON | Hepcidin | Anemia | Phase I | |
| BAX499/ARC19499 | Baxter/Archemix | TFPI | Hemophilia | Phase I | Waters et al. (2011) and Parunov et al. (2011) |
AMD, age-related macular degeneration; ACS, acute coronary syndrome. TTP, thrombotic thrombocytopenic purpura; CABG, coronary artery bypass grafting; DN, diabetic nephropathy.
Table 2.
Nontherapeutic applications of aptamers†
| Use | Target | Comment | References |
|---|---|---|---|
| In vivo imaging | Cancer cell | Activatable probe | Shi et al. (2011) |
| Delivery | PSMA | siRNA conjugate | Dassie et al. (2009) |
| gp120 | siRNA conjugate | Zhou et al. (2009) | |
| E-selectin | Liposome conjugate | Mann et al. (2011) | |
| PSMA | Nanoparticle | Farokhzad et al. (2006) | |
| Cell detection/separation | Cancer cell | Microfluidic device | Xu et al. (2009) |
| Live virus | Virus sensor | Labib et al. (2012) | |
| Cy3 | mRNA detection in live cells | Endo & Nakamura (2010) | |
| Somamer | 813 proteins | Disease diagnostic | Ostroff et al. (2010) and Gold et al. (2010) |
| Aptasensor | K+ | Beacon/FRET | Kim et al. (2012) |
| AMP | Electrochemical with [Ru(NH3)6]3+ | Shen et al. (2007) | |
| Thrombin | Electrochemical with Methylene blue | Xiao et al. (2005) | |
| IFN-γ | Electrochemical with DNAzyme | Zhang et al. (2012) | |
| IgE | Quartz crystal microbalance | Yao et al. (2010) | |
| Rabbit IgG | Immuno-aptamer PCR | Yoshida et al. (2009) | |
| Chromatography | l-selectin | Purification of l-selectin | Romig et al. (1999) |
| Human IgG-Fc | Therapeutic antibody, Fc-fusion protein | Miyakawa et al. (2008) |
There are many other excellent works that cannot be listed in this table.
To our great surprise, the completion of the Human Genome Project showed the existence of a large amount of ncRNAs. There are two classes of ncRNAs: one that includes antisense RNA and microRNA, and functions by sequence complementarity to target mRNA or DNA, whereas another functions independently of sequence complementarity by forming a functional 3D structure to target protein or macromolecules as an apparent equivalent to a protein. We believe that the former class of ncRNA is likely the tip of the ‘ncRNA iceberg’, regardless of it having received much attention. The latter class of RNAs, which may be referred to as ‘natural aptamers’, might play a crucial role in ncRNA function as well. Therefore, it is important to study both artificial and natural aptamer RNAs to consolidate the superior potential of RNA. The current study of RNA aptamers would be highly beneficial to a comprehensive understanding of genome-coded ncRNA function as well as to the development of RNA medicine.
Acknowledgments
We are grateful to all current and former coworkers and collaborators of our laboratory, Ribomic, Inc., Sosho, Inc., Chiba Institute of Technology, and Osaka University. This study was supported in part by a Core Research for Evolution Science and Technology (CREST) grant from the Japan Science and Technology Agency, and research grants from the Ministry of Education, Sports, Culture, Science, and Technology of Japan and the Ministry of Health, Labor, and Welfare.
References
- Adachi H, Ishiguro A, Hamada M, Sakota E, Asai K, Nakamura Y. Antagonistic RNA aptamer specific to a heterodimeric form of human interleukin-17A/F. Biochimie. 2011;93:1081–1088. doi: 10.1016/j.biochi.2011.04.003. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Kachi S, Silva RL, Umeda N, Hackett SF, McCauley D, McCauley T, Zoltoski A, Epstein DM, Campochiaro PA. Intraocular injection of an aptamer that binds PDGF-B: a potential treatment for proliferative retinopathies. J. Cell. Physiol. 2006;207:407–412. doi: 10.1002/jcp.20583. [DOI] [PubMed] [Google Scholar]
- Atsumi S, Ikawa Y, Shiraishi H, Inoue T. Design and development of a catalytic ribonucleoprotein. EMBO J. 2001;20:5453–5460. doi: 10.1093/emboj/20.19.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babendure JR, Adams SR, Tsien RY. Aptamers switch on fluorescence of triphenylmethane dyes. J. Am. Chem. Soc. 2003;125:14716–14717. doi: 10.1021/ja037994o. [DOI] [PubMed] [Google Scholar]
- Baecher-Allan C, Hafler DA. Suppressor T cells in human diseases. J. Exp. Med. 2004;200:273–276. doi: 10.1084/jem.20040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates PJ, Laber DA, Miller DM, Thomas SD, Trent JO. Discovery and development of the G-rich oligonucleotide AS1411 as a novel treatment for cancer. Exp. Mol. Pathol. 2009;86:151–164. doi: 10.1016/j.yexmp.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batey RT. Structures of regulatory elements in mRNAs. Curr. Opin. Struct. Biol. 2006;16:299–306. doi: 10.1016/j.sbi.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Biesecker G, Dihel L, Enney K, Bendele RA. Derivation of RNA aptamer inhibitors of human complement C5. Immunopharmacology. 1999;42:219–230. doi: 10.1016/s0162-3109(99)00020-x. [DOI] [PubMed] [Google Scholar]
- Blank M, Weinschenk T, Priemer M, Schluesener H. Systematic evolution of a DNA aptamer binding to rat brain tumor microvessels. selective targeting of endothelial regulatory protein pigpen. J. Biol. Chem. 2001;276:16464–16468. doi: 10.1074/jbc.M100347200. [DOI] [PubMed] [Google Scholar]
- Bokinsky G, Zhuang X. Single-molecule RNA folding. Acc. Chem. Res. 2005;38:566–573. doi: 10.1021/ar040142o. [DOI] [PubMed] [Google Scholar]
- Chabaud M, Garnero P, Dayer JM, Guerne PA, Fossiez F, Miossec P. Contribution of interleukin-17 to synovium matrix destruction in rheumatoid arthritis. Cytokine. 2000;12:1092–1099. doi: 10.1006/cyto.2000.0681. [DOI] [PubMed] [Google Scholar]
- Chambers I, Tomlinson S. The transcriptional foundation of pluripotency. Development. 2009;136:2311–2322. doi: 10.1242/dev.024398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SH, Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17:435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- Cromwell MEM, Hilario E, Jacobson F. Protein aggregation and bioprocessing. AAPS J. 2006;8:E572–E579. doi: 10.1208/aapsj080366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui L, Johkura K, Yue F, Ogiwara N, Okouchi Y, Asanuma K, Sasaki K. Spatial distribution and initial changes of SSEA-1 and other cell adhesion-related molecules on mouse embryonic stem cells before and during differentiation. J. Histochem. Cytochem. 2004;52:1447–1457. doi: 10.1369/jhc.3A6241.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darisipudi MN, Kulkarni OP, Sayyed SG, Ryu M, Migliorini A, Sagrinati C, Parente E, Vater A, Eulberg D, Klussmann S, Romagnani P, Anders HJ. Dual blockade of the homeostatic chemokine CXCL12 and the proinflammatory chemokine CCL2 has additive protective effects on diabetic kidney disease. Am. J. Pathol. 2011;179:116–124. doi: 10.1016/j.ajpath.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dassie JP, Liu X-Y, Thomas GS, Whitaker RM, Thiel KW, Stockdale KR, Meyerholz DK. Systemic administration of optimized aptamer-siRNA chimeras promotes regression of PSMA-expressing tumors. Nat. Biotech. 2009;27:839–846. doi: 10.1038/nbt.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deisenhofer J. Crystallographic refinement and atomic models of a human Fc fragment and its complex with fragment B of protein A from Staphylococcus aureus at 2.9- and 2.8-A resolution. Biochemistry. 1981;20:2361–2370. [PubMed] [Google Scholar]
- Dong C. Differentiation and function of pro-inflammatory Th17 cells. Microbes Infect. 2009;11:584–588. doi: 10.1016/j.micinf.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duda DG, Kozin SV, Kirkpatrick ND, Xu L, Fukumura D, Jain RK. CXCL12 (SDF1α)-CXCR4/CXCR7 pathway inhibition: an emerging sensitizer for anticancer therapies? Clin. Cancer Res. 2011;17:2074–2080. doi: 10.1158/1078-0432.CCR-10-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–822. doi: 10.1038/346818a0. [DOI] [PubMed] [Google Scholar]
- Ellington AD, Szostak JW. Selection in vitro of single stranded DNA molecules that fold into specific ligand-binding structures. Nature. 1992;355:850–852. doi: 10.1038/355850a0. [DOI] [PubMed] [Google Scholar]
- Emamaullee JA, Davis J, Merani S, Toso C, Elliott JF, Thiesen A, Shapiro AM. Inhibition of Th17 cells regulates autoimmune diabetes in NOD mice. Diabetes. 2009;58:1302–1311. doi: 10.2337/db08-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo K, Nakamura Y. A binary Cy3 aptamer probe composed of folded modules. Anal. Biochem. 2010;400:103–109. doi: 10.1016/j.ab.2010.01.015. [DOI] [PubMed] [Google Scholar]
- Evans M, Kaufman M. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–156. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- Fahrner RL, Knudsen HL, Basey CD, Galan W, Feuerhelm D, Vanderlaan M, Blank GS. Industrial purification of pharmaceutical antibodies: development, operation, and validation of chromatography processes. Biotechnol. Genet. Eng. Rev. 2001;18:301–327. doi: 10.1080/02648725.2001.10648017. [DOI] [PubMed] [Google Scholar]
- Fang X, Tan W. Aptamers generated from cell-SELEX for molecular medicine: a chemical biology approach. Acc. Chem. Res. 2010;43:48–57. doi: 10.1021/ar900101s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhzad OC, Cheng J, Teply BA, Sherifi I, Jon S, Kantoff PW, Richie JP, Langer R. Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc. Natl Acad. Sci. USA. 2006;103:6315–6320. doi: 10.1073/pnas.0601755103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürtig B, Buck J, Manoharan V, Bermel W, Jäschke A, Wenter P, Pitsch S, Schwalbe H. Time-resolved NMR studies of RNA folding. Biopolymers. 2007;86:360–383. doi: 10.1002/bip.20761. [DOI] [PubMed] [Google Scholar]
- Gesteland RF, Cech TR, Atkins JF, editors. The RNA World. New York: Cold Spring Harbor Laboratory Press; 1999. [Google Scholar]
- Gesteland RF, Cech TR, Atkins JF. The RNA World. 3rd edn. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press; 2006. [Google Scholar]
- Ghose S, Allen M, Hubbard B, Brooks C, Cramer SM. Antibody variable region interactions with Protein A: implications for the development of generic purification processes. Biotechnol. Bioeng. 2005;92:665–673. doi: 10.1002/bit.20729. [DOI] [PubMed] [Google Scholar]
- Ghosh G, Huang DB, Huxford T. Molecular mimicry of the NF-kappaB DNA target site by a selected RNA aptamer. Curr. Opin. Struct. Biol. 2004;14:21–27. doi: 10.1016/j.sbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Gold L, Ayers D, Bertino J, et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE. 2010;5:e1504. doi: 10.1371/journal.pone.0015004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graber JJ, Allie SR, Mullen KM, Jones MV, Wang T, Krishnan C, Kaplin AI, Nath A, Kerr DA, Calabresi PA. Interleukin-17 in transverse myelitis and multiple sclerosis. J. Neuroimmunol. 2008;196:124–132. doi: 10.1016/j.jneuroim.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Grate D, Wilson C. Laser-mediated, site-specific inactivation of RNA transcripts. Proc. Natl Acad. Sci. USA. 1999;96:6131–6136. doi: 10.1073/pnas.96.11.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo KT, Schäfer R, Paul A, Gerber A, Ziemer G, Wendel HP. A new technique for the isolation and surface immobilization of mesenchymal stem cells from whole bone marrow using high-specific DNA aptamers. Stem Cells. 2006;24:2220–2231. doi: 10.1634/stemcells.2006-0015. [DOI] [PubMed] [Google Scholar]
- Hemmer B, Archelos JJ, Hartung HP. New concepts in the immunopathogenesis of multiple sclerosis. Nat. Rev. Neurosci. 2002;3:291–301. doi: 10.1038/nrn784. [DOI] [PubMed] [Google Scholar]
- Hermann T, Patel DJ. Adaptive recognition by nucleic acid aptamers. Science. 2000;287:820–825. doi: 10.1126/science.287.5454.820. [DOI] [PubMed] [Google Scholar]
- von Herrath MG, Harrison LC. Antigen-induced regulatory T cells in autoimmunity. Nat. Rev. Immunol. 2003;3:223–232. doi: 10.1038/nri1029. [DOI] [PubMed] [Google Scholar]
- Hiep HM, Saito M, Nakamura Y, Tamiya E. RNA aptamer-based optical nanostructured sensor for highly sensitive and label-free detection of antigen-antibody reactions. Anal. Bioanal. Chem. 2010;396:2575–2581. doi: 10.1007/s00216-010-3488-z. [DOI] [PubMed] [Google Scholar]
- Holeman LA, Robinson SL, Szostak JW, Wilson C. Isolation and characterization of fluorophore-binding RNA aptamers. Fold Design. 1998;3:423–431. doi: 10.1016/S1359-0278(98)00059-5. [DOI] [PubMed] [Google Scholar]
- Horiba M, Kadomatsu K, Nakamura E, Muramatsu H, Ikematsu S, Sakuma S, Hayashi K, Yuzawa Y, Matsuo S, Kuzuya M, Kaname T, Hirai M, Saito H, Muramatsu T. Neointima formation in a restenosis model is suppressed in midkine-deficient mice. J. Clin. Invest. 2000;105:489–495. doi: 10.1172/JCI7208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn WT, Maire AC, Nicola J, Adams CJ, Liljas L, Phillips SE, Stockley PG. The crystal structure of a high affinity RNA stem-loop complexed with the bacteriophage MS2 capsid: further challenges in the modeling of ligand–RNA interactions. RNA. 2004;10:1776–1782. doi: 10.1261/rna.7710304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang DB, Vu D, Cassiday LA, Zimmerman JM, Maher LJ, 3rd, Ghosh G. Crystal structure of NF-kappaB (p50)2 complexed to a high-affinity RNA aptamer. Proc. Natl Acad. Sci. USA. 2003;100:9268–9273. doi: 10.1073/pnas.1632011100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikawa Y, Fukada K, Watanabe S, Shiraishi H, Inoue T. Design, construction, and analysis of a novel class of self-folding RNA. Structure. 2002;10:527–534. doi: 10.1016/s0969-2126(02)00739-6. [DOI] [PubMed] [Google Scholar]
- Intoh A, Kurisaki A, Yamanaka Y, Hirano H, Fukuda H, Sugino H, Asashima M. Proteomic analysis of membrane proteins expressed specifically in pluripotent murine embryonic stem cells. Proteomics. 2009;9:126–137. doi: 10.1002/pmic.200800496. [DOI] [PubMed] [Google Scholar]
- Ishiguro A, Akiyama T, Adachi H, Inoue J, Nakamura Y. Therapeutic potential of anti-interleukin-17A aptamer: suppression of IL-17A signaling and attenuation of autoimmunity in mouse models. Arthritis Rheum. 2011;63:455–466. doi: 10.1002/art.30108. [DOI] [PubMed] [Google Scholar]
- Ishikawa Y, Fujiwara M, Nakamura Y. Therapeutic application of RNA aptamer. Inflam. Immunol. (Jpn.) 2008;16:627–633. [Google Scholar]
- Ito K, Uno M, Nakamura Y. A tripeptide ‘anticodon’ deciphers stop codons in messenger RNA. Nature. 2000;403:680–684. doi: 10.1038/35001115. [DOI] [PubMed] [Google Scholar]
- Iwagawa T, Ohuchi SP, Watanabe S, Nakamura Y. Selection of RNA aptamers against mouse embryonic stem cells. Biochimie. 2011;94:250–257. doi: 10.1016/j.biochi.2011.10.017. [DOI] [PubMed] [Google Scholar]
- Jaeger L, Chworos A. The architectonics of programmable RNA and DNA nanostructures. Curr. Opin. Struct. Biol. 2006;16:531–543. doi: 10.1016/j.sbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Jaeger L, Leontis NB. Tecto-RNA: one-dimensional self-assembly through tertiary interactions. Angew. Chem. Int. Ed. Engl. 2000;39:2521–2524. doi: 10.1002/1521-3773(20000717)39:14<2521::aid-anie2521>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Jaeger L, Westhof E, Leontis NB. TectoRNA: modular assembly units for the construction of RNA nano-objects. Nucleic Acids Res. 2001;29:455–463. doi: 10.1093/nar/29.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jilma-Stohlawetz P, Gilbert JC, Gorczyca ME, Knöbl P, Jilma B. A dose ranging phase I/II trial of the von Willebrand factor inhibiting aptamer ARC1779 in patients with congenital thrombotic thrombocytopenic purpura. Thromb. Haemost. 2011;106:539–547. doi: 10.1160/TH11-02-0069. [DOI] [PubMed] [Google Scholar]
- Keefe AD, Pai S, Ellington A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010;9:537–550. doi: 10.1038/nrd3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefe AD, Schaub RG. Aptamers as candidate therapeutics for cardiovascular indications. Curr. Opin. Pharmacol. 2008;8:147–152. doi: 10.1016/j.coph.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Kim B, Jung IH, Kang M, Shim HK, Woo HY. Cationic conjugated polyelectrolytes-triggered conformational change of molecular beacon aptamer for highly sensitive and selective potassium ion detection. J. Am. Chem. Soc. 2012;134:3133–3138. doi: 10.1021/ja210360v. [DOI] [PubMed] [Google Scholar]
- Kirkham BW, Lassere MN, Edmonds JP, Juhasz KM, Bird PA, Lee CS, Shnier R, Portek IJ. Synovial membrane cytokine expression is predictive of joint damage progression in rheumatoid arthritis: a two-year prospective study (the DAMAGE study cohort) Arthritis Rheum. 2006;54:1122–1131. doi: 10.1002/art.21749. [DOI] [PubMed] [Google Scholar]
- Klussmann S. The Aptamer Handbook. Weinheim: Wiley-VCH Verlag GmbH & Co. KGaA; 2006. [Google Scholar]
- Koenders MI, Lubberts E, Oppers-Walgreen B, van den Bersselaar L, Helsen MM, Di Padova FE, Boots AM, Gram H, Joosten LA, van den Berg WB. Blocking of interleukin-17 during reactivation of experimental arthritis prevents joint inflammation and bone erosion by decreasing RANKL and interleukin-1. Am. J. Pathol. 2005;167:141–149. doi: 10.1016/S0002-9440(10)62961-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohm AP, Carpentier PA, Anger HA, Miller SD. Cutting edge: CD4+ CD25+ regulatory T cells suppress antigen-specific autoreactive immune responses and central nervous system inflammation during active experimental autoimmune encephalomyelitis. J. Immunol. 2002;169:4712–4716. doi: 10.4049/jimmunol.169.9.4712. [DOI] [PubMed] [Google Scholar]
- Kolossov E, Bostani T, Roell W, et al. Engraftment of engineered ES cell-derived cardiomyocytes but not BM cells restores contractile function to the infarcted myocardium. J. Exp. Med. 2006;203:2315–2327. doi: 10.1084/jem.20061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolpashchikov DM. Superselective labelling of proteins: approaches and techniques. J. Biomol. Struct. Dyn. 2003;21:55–64. doi: 10.1080/07391102.2003.10506905. [DOI] [PubMed] [Google Scholar]
- Kolpashchikov DM. Binary malachite green aptamer for fluorescent detection of nucleic acids. J. Am. Chem. Soc. 2005;127:12442–12443. doi: 10.1021/ja0529788. [DOI] [PubMed] [Google Scholar]
- Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- Labib M, Zamay AS, Muharemagic D, Chechik AV, Bell JC, Berezovski MV. Aptamer-based viability impedimetric sensor for viruses. Anal. Chem. 2012;84:2548–2556. doi: 10.1021/ac203412m. [DOI] [PubMed] [Google Scholar]
- Liang SC, Long AJ, Bennett F, Whitters MJ, Karim R, Collins M, Goldman SJ, Dunussi-Joannopoulos K, Williams CM, Wright JF, Fouser LA. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J. Immunol. 2007;179:7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- Liu H, Leung BP. CD4+ CD25+ regulatory T cells in health and disease. Clin. Exp. Pharmacol. Physiol. 2006;33:519–524. doi: 10.1111/j.1440-1681.2006.04401.x. [DOI] [PubMed] [Google Scholar]
- Liu X, Mashour GA, Webster HF, Kurtz A. Basic FGF and FGF receptor 1 are expressed in microglia during experimental autoimmune encephalomyelitis: temporally distinct expression of midkine and pleiotrophin. Glia. 1998;24:390–397. doi: 10.1002/(sici)1098-1136(199812)24:4<390::aid-glia4>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Long SB, Long MB, White RR, Sullenger BA. Crystal structure of an RNA aptamer bound to thrombin. RNA. 2008;14:2504–2512. doi: 10.1261/rna.1239308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubberts E, Joosten LA, Oppers B, van den Bersselaar L, Coenen-de Roo CJ, Kolls JK, Schwarzenberger P, van de Loo FA, van den Berg WB. IL-1-independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J. Immunol. 2001;167:1004–1013. doi: 10.4049/jimmunol.167.2.1004. [DOI] [PubMed] [Google Scholar]
- Maasch C, Buchner K, Eulberg D, Vonhoff S, Klussmann S. Physicochemical stability of NOX-E36, a 40mer L-RNA (Spiegelmer) for therapeutic applications. Nucleic Acids Symp. Ser. 2008;52:61–62. doi: 10.1093/nass/nrn031. [DOI] [PubMed] [Google Scholar]
- Mairal T, Ozalp VC, Lozano Sánchez P, Mir M, Katakis I, O’Sullivan CK. Aptamers: molecular tools for analytical applications. Anal. Bioanal. Chem. 2008;390:989–1007. doi: 10.1007/s00216-007-1346-4. [DOI] [PubMed] [Google Scholar]
- Mann AP, Bhavane RC, Somasunderam A, Liz Montalvo-Ortiz B, Ghaghada KB, Volk D, Nieves-Alicea R, Suh KS, Ferrari M, Annapragada A, Gorenstein DG, Tanaka T. Thioaptamer conjugated liposomes for tumor vasculature targeting. Oncotarget. 2011;2:298–304. doi: 10.18632/oncotarget.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin G. Isolation of a pluripotent cell line from early mouse embryos cultured in medium conditioned by teratocarcinoma stem cells. Proc. Natl Acad. Sci. USA. 1981;78:7634–7638. doi: 10.1073/pnas.78.12.7634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama K, Muramatsu H, Ishiguro N, Muramatsu T. Midkine, a heparin-binding growth factor, is fundamentally involved in the pathogenesis of rheumatoid arthritis. Arthritis Rheum. 2004;50:1420–1429. doi: 10.1002/art.20175. [DOI] [PubMed] [Google Scholar]
- Matarese G, Carrieri PB, La Cava A, Perna F, Sanna V, De Rosa V, Aufiero D, Fontana S, Zappacosta S. Leptin increase in multiple sclerosis associates with reduced number of CD4(+)CD25+ regulatory T cells. Proc. Natl Acad. Sci. USA. 2005;102:5150–5155. doi: 10.1073/pnas.0408995102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattick JS, Makunin IV. Non-coding RNA. Hum. Mol. Genet. 2006;15:R17–R29. doi: 10.1093/hmg/ddl046. [DOI] [PubMed] [Google Scholar]
- Matusevicius D, Kivisakk P, He B, Kostulas N, Ozenci V, Fredrikson S, Link H. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult. Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]
- McNamara JO, 2nd, Andrechek ER, Wang Y, Viles KD, Rempel RE, Gilboa E, Sullenger BA, Giangrande PH. Cell type-specific delivery of siRNAs with aptamer-siRNA chimeras. Nat. Biotechnol. 2006;24:1005–1015. doi: 10.1038/nbt1223. [DOI] [PubMed] [Google Scholar]
- Mills KH. Regulatory T cells: friend or foe in immunity to infection? Nat. Rev. Immunol. 2004;4:841–855. doi: 10.1038/nri1485. [DOI] [PubMed] [Google Scholar]
- Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N. Engl. J. Med. 2009;361:888–898. doi: 10.1056/NEJMra0707449. [DOI] [PubMed] [Google Scholar]
- Miyakawa S, Nomura Y, Sakamoto T, Yamaguchi Y, Kato K, Yamazaki S, Nakamura Y. Structural and molecular basis for hyperspecificity of RNA aptamer to human immunoglobulin G. RNA. 2008;14:1154–1163. doi: 10.1261/rna.1005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyakawa S, Oguro A, Ohtsu T, Imataka H, Sonenberg N, Nakamura Y. RNA aptamers to mammalian initiation factor 4G inhibit cap-dependent translation by blocking the formation of initiation factor complexes. RNA. 2006;12:1825–1834. doi: 10.1261/rna.2169406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki K, Oguro A, Ohtsu T, Sonenberg N, Nakamura Y. High affinity RNA for mammalian initiation factor 4E interferes with mRNA-cap binding and inhibits translation. RNA. 2005;11:77–89. doi: 10.1261/rna.7108205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok W, Li Y. Recent progress in nucleic acid aptamer-base biosensors and bioassays. Sensors. 2008;8:7050–7084. doi: 10.3390/s8117050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molet S, Hamid Q, Davoine F, Nutku E, Taha R, Pagé N, Olivenstein R, Elias J, Chakir J. IL-17 is increased in asthmatic airways and induces human bronchial fibroblasts to produce cytokines. J. Allergy Clin. Immunol. 2001;108:430–438. doi: 10.1067/mai.2001.117929. [DOI] [PubMed] [Google Scholar]
- Mongelard F, Bouvet P. AS-1411, a guanosine-rich oligonucleotide aptamer targeting nucleolin for the potential treatment of cancer, including acute myeloid leukemia. Curr. Opin. Mol. Ther. 2010;12:107–114. [PubMed] [Google Scholar]
- Mori T, Oguro A, Ohtsu T, Nakamura Y. RNA aptamers selected against the receptor activator of NF-kappaB acquire general affinity to proteins of the tumor necrosis factor receptor family. Nucleic Acids Res. 2004;32:6120–6128. doi: 10.1093/nar/gkh949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris K, Jensen K, Julin C, Weil M, Gold L. High affinity ligands from in vitro selection: complex targets. Proc. Natl Acad. Sci. USA. 1998;95:2902–2907. doi: 10.1073/pnas.95.6.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu T. Midkine and pleiotrophin: two related proteins involved in development, survival, inflammation and tumorigenesis. J. Biochem. (Tokyo) 2002;132:359–371. doi: 10.1093/oxfordjournals.jbchem.a003231. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Endo K, Adachi H, Ishiguro A. RNA aptamers to translational components. In: Hershey JWB, editor. Progress in Molecular Biology and Translational Science, Vol. 90, Translational Control in Health and Disease. Academic Press/Elsevier; 2009. pp. 369–395. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Ito K. Making sense of mimic in translation termination. Trends Biochem. Sci. 2003;28:99–105. doi: 10.1016/S0968-0004(03)00006-9. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Ito K. tRNA mimicry in translation termination and beyond. Wiley Interdiscip. Rev. RNA. 2011;2:647–668. doi: 10.1002/wrna.81. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Ito K, Ehrenberg M. Mimicry grasps reality in translation termination. Cell. 2000;101:349–352. doi: 10.1016/s0092-8674(00)80845-4. [DOI] [PubMed] [Google Scholar]
- Nash R, Neves L, Faast R, Pierce M, Dalton S. The lectin Dolichos biflorus agglutinin recognizes glycan epitopes on the surface of murine embryonic stem cells: a new tool for characterizing pluripotent cells and early differentiation. Stem Cells. 2007;25:974–982. doi: 10.1634/stemcells.2006-0224. [DOI] [PubMed] [Google Scholar]
- Ng EW, Shima DT, Calias P, Cunningham ET, Jr, Guyer DR, Adamis AP. Pegaptanib, a targeted anti-VEGF aptamer for ocular vascular disease. Nat. Rev. Drug. Discov. 2006;5:123–132. doi: 10.1038/nrd1955. [DOI] [PubMed] [Google Scholar]
- Nomura Y, Sugiyama S, Sakamoto T, et al. Conformational plasticity of RNA for target recognition as revealed by the 2.15 Å crystal structure of a human IgG-aptamer complex. Nucleic Acids Res. 2010;38:7822–7829. doi: 10.1093/nar/gkq615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura K, Nagano K, Itagaki C, Taoka M, Okamura N, Yamauchi Y, Sugano S, Takahashi N, Izumi T, Isobe T. Cell surface labeling and mass spectrometry reveal diversity of cell surface markers and signaling molecules expressed in undifferentiated mouse embryonic stem cells. Mol. Cell. Proteomics. 2005;4:1968–1976. doi: 10.1074/mcp.M500216-MCP200. [DOI] [PubMed] [Google Scholar]
- Oguro A, Ohtsu T, Nakamura Y. Aptamer-based biosensor for mammalian initiation factor eIF4A. Anal. Biochem. 2009;388:102–107. doi: 10.1016/j.ab.2009.01.046. [DOI] [PubMed] [Google Scholar]
- Oguro A, Otsu T, Svitkin YV, Sonenberg N, Nakamura Y. RNA aptamers to initiation factor 4A helicase hinder cap-dependent translation by blocking ATP hydrolysis. RNA. 2003;9:394–407. doi: 10.1261/rna.2161303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohuchi S, Ohtsu T, Nakamura Y. Selection of RNA aptamers against recombinant transforming growth factor-beta type III receptor displayed on cell surface. Biochimie. 2006;88:897–904. doi: 10.1016/j.biochi.2006.02.004. [DOI] [PubMed] [Google Scholar]
- Ohuchi SP, Ikawa Y, Nakamura Y. Selection of a novel class of RNA–RNA interaction motifs based on the ligase ribozyme with defined modular architecture. Nucleic Acids Res. 2008;36:3600–3607. doi: 10.1093/nar/gkn206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onoa B, Tinoco I., Jr RNA folding and unfolding. Curr. Opin. Struct. Biol. 2004;14:374–379. doi: 10.1016/j.sbi.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Ostroff RM, Bigbee WL, Franklin W, Gold L, Mehan M, Miller YE, Pass HI, Rom WN, Siegfried JM, Stewart A, Walker JJ, Weissfeld JL, Williams S, Zichi D, Brody EN. Unlocking biomarker discovery: large scale application of aptamer proteomic technology for early detection of lung cancer. PLoS ONE. 2010;5:e1503. doi: 10.1371/journal.pone.0015003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parunov LA, Fadeeva OA, Balandina AN, Soshitova NP, Kopylov KG, Kumskova MA, Gilbert JC, Schaub RG, McGinness KE, Ataullakhanov FI, Panteleev MA. Improvement of spatial fibrin formation by the anti-TFPI aptamer BAX499: changing clot size by targeting extrinsic pathway initiation. J. Thromb. Haemost. 2011;9:1825–1834. doi: 10.1111/j.1538-7836.2011.04412.x. [DOI] [PubMed] [Google Scholar]
- Pera MF, Tam PP. Extrinsic regulation of pluripotent stem cells. Nature. 2010;465:713–720. doi: 10.1038/nature09228. [DOI] [PubMed] [Google Scholar]
- Povsic TJ, Cohen MG, Chan MY, Zelenkofske SL, Wargin WA, Harrington RA, Alexander JH, Rusconi CP, Becker RC. Dose selection for a direct and selective factor IXa inhibitor and its complementary reversal agent: translating pharmacokinetic and pharmacodynamic properties of the REG1 system to clinical trial design. J. Thromb. Thrombolysis. 2011;32:21–31. doi: 10.1007/s11239-011-0588-3. [DOI] [PubMed] [Google Scholar]
- Pyle A. Metal ions in the structure and function of RNA. J. Biol. Inorg. Chem. 2002;7:679–690. doi: 10.1007/s00775-002-0387-6. [DOI] [PubMed] [Google Scholar]
- Raddatz M, Dolf A, Endl E, Knolle P, Famulok M, Mayer G. Enrichment of cell-targeting and population-specific aptamers by fluorescence-activated cell sorting. Angew. Chem. Int. Ed. Engl. 2008;47:5190–5193. doi: 10.1002/anie.200800216. [DOI] [PubMed] [Google Scholar]
- Romig TS, Bell C, Drolet DW. Aptamer affinity chromatography: combinatorial chemistry applied to protein purification. J. Chromatogr. B Biomed. Sci. Appl. 1999;731:275–284. [PubMed] [Google Scholar]
- Rusconi CP, Scardino E, Layzer J. RNA aptamers as reversible antagonists of coagulation factor IXa. Nature. 2002;419:90–94. doi: 10.1038/nature00963. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising CD4+ regulatory t cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- Sakaguchi S. Naturally arising Foxp3-expressing CD25+ CD4+ regulatory T cells in immunological tolerance to self and non-self. Nat. Immunol. 2005;6:345–352. doi: 10.1038/ni1178. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Oguro A, Kawai G, et al. NMR structures of double loops of an RNA aptamer against mammalian initiation factor 4A. Nucleic Acids Res. 2005;33:745–754. doi: 10.1093/nar/gki222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato W, Kadomatsu K, Yuzawa Y, Muramatsu H, Hotta N, Matsuo S, Muramatsu T. Midkine is involved in neutrophil infiltration into the tubulointerstitium in ischemic renal injury. J. Immunol. 2001;167:3463–3469. doi: 10.4049/jimmunol.167.6.3463. [DOI] [PubMed] [Google Scholar]
- Schnyder-Candrian S, Togbe D, Couillin I, Mercier I, Brombacher F, Quesniaux V, Fossiez F, Ryffel B, Schnyder B. Interleukin-17 is a negative regulator of established allergic asthma. J. Exp. Med. 2006;203:2715–2725. doi: 10.1084/jem.20061401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamah S, Healy J, Cload S. Complex target SELEX. Acc. Chem. Res. 2008;41:130–138. doi: 10.1021/ar700142z. [DOI] [PubMed] [Google Scholar]
- Shangguan D, Li Y, Tang Z, Cao ZC, Chen HW, Mallikaratchy P, Sefah K, Yang CJ, Tan W. Aptamers evolved from live cells as effective molecular probes for cancer study. Proc. Natl Acad. Sci. USA. 2006;103:11838–11843. doi: 10.1073/pnas.0602615103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Chen Z, Li Y, Jing P, Xie S, He S, He P, Shao Y. A chronocoulometric aptamer sensor for adenosine monophosphate. Chem. Commun. 2007;2007:2169–2171. doi: 10.1039/b618909a. [DOI] [PubMed] [Google Scholar]
- Shi H, He X, Wang K, et al. Activatable aptamer probe for contrast-enhanced in vivo cancer imaging based on cell membrane protein-triggered conformation alteration. Proc. Natl Acad. Sci. USA. 2011;108:3900–3905. doi: 10.1073/pnas.1016197108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiraki N, Higuchi Y, Harada S, Umeda K, Isagawa T, Aburatani H, Kume K, Kume S. Differentiation and characterization of embryonic stem cells into three germ layers. Biochem. Biophys. Res. Commun. 2009;381:694–699. doi: 10.1016/j.bbrc.2009.02.120. [DOI] [PubMed] [Google Scholar]
- Sosnick TR, Pan T. RNA folding: models and perspectives. Curr. Opin. Struct. Biol. 2003;13:309–316. doi: 10.1016/s0959-440x(03)00066-6. [DOI] [PubMed] [Google Scholar]
- Spencer HL, Eastham AM, Merry CL, Southgate TD, Perez-Campo F, Soncin F, Ritson S, Kemler R, Stern PL, Ward CM. E-cadherin inhibits cell surface localization of the pro-migratory 5T4 oncofetal antigen in mouse embryonic stem cells. Mol. Biol. Cell. 2007;18:2838–2851. doi: 10.1091/mbc.E06-09-0875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srisawat C, Engelke DR. Streptavidin aptamers: affinity tags for the study of RNAs and ribonucleoproteins. RNA. 2001;7:632–641. doi: 10.1017/s135583820100245x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojanovic MN, Kolpashchikov DM. Modular aptameric sensors. J. Am. Chem. Soc. 2004;126:9266–9270. doi: 10.1021/ja032013t. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Akagi K, Nakamura Y, Kozu T. RNA aptamers targeting the C-terminal of KRAS oncoprotein generated by an improved SELEX with isothermal RNA amplification. Oligonucleotides. 2007;17:12–21. doi: 10.1089/oli.2006.0035R1. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Tsumoto K, Umetsu M, Kumagai I, Ejima D, Philo JS, Arakawa T. Role of arginine in protein refolding, solubilization, and purification. Biotechnol. Prog. 2004;20:1301–1308. doi: 10.1021/bp0498793. [DOI] [PubMed] [Google Scholar]
- Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–510. doi: 10.1126/science.2200121. [DOI] [PubMed] [Google Scholar]
- Viglietta V, Baecher-Allan C, Weiner HL, Hafler DA. Loss of functional suppression by CD4+ CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Takeuchi H, Sonobe Y, Jin S, Mizuno T, Miyakawa S, Fujiwara M, Nakamura Y, Kato T, Muramatsu H, Muramatsu T, Suzumura A. Inhibition of midkine alleviates experimental autoimmune encephalomyelitis through the expansion of regulatory T cell population. Proc. Natl Acad. Sci. USA. 2008;105:3915–3920. doi: 10.1073/pnas.0709592105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, Takahashi JB, Nishikawa S, Nishikawa S, Muguruma K, Sasai Y. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Waters EK, Genga RM, Schwartz MC, Nelson JA, Schaub RG, Olson KA, Kurz JC, McGinness KE. Aptamer ARC19499 mediates a procoagulant hemostatic effect by inhibiting tissue factor pathway inhibitor. Blood. 2011;117:5514–5522. doi: 10.1182/blood-2010-10-311936. [DOI] [PubMed] [Google Scholar]
- Wilson C, Szostak JW. Isolation of a fluorophore-specific DNA aptamer with weak redox activity. Chem. Biol. 1998;5:609–617. doi: 10.1016/s1074-5521(98)90289-7. [DOI] [PubMed] [Google Scholar]
- Wright JF, Bennett F, Li B, Brooks J, Luxenberg DP, Whitters MJ, Tomkinson KN, Fitz LJ, Wolfman NM, Collins M, Dunussi-Joannopoulos K, Chatterjee-Kishore M, Carreno BM. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J. Immunol. 2008;181:2799–2805. doi: 10.4049/jimmunol.181.4.2799. [DOI] [PubMed] [Google Scholar]
- Wright JF, Guo Y, Quazi A, Luxenberg DP, Bennett F, Ross JF, Qiu Y, Whitters MJ, Tomkinson KN, Dunussi-Joannopoulos K, Carreno BM, Collins M, Wolfman NM. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J. Biol. Chem. 2007;282:13447–13455. doi: 10.1074/jbc.M700499200. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Piorek BD, Plaxco KW, Heeger AJ. A reagentless signal-on architecture for electron, aptamer-based sensors via target-induced strand displacement. J. Am. Chem. Soc. 2005;127:17990–17991. doi: 10.1021/ja056555h. [DOI] [PubMed] [Google Scholar]
- Xu Y, Phillips JA, Yan J, Li Q, Fan ZH, Tan W. Aptamer-based microfluidic device for enrichment, sorting, and detection of multiple cancer cells. Anal. Chem. 2009;81:7436–7442. doi: 10.1021/ac9012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao C, Zhu T, Qi Y, Zhao Y, Xia H, Fu W. Development of a quartz microbalance biosensor with aptamers as bio-recognition element. Sensors. 2010;10:5859–5871. doi: 10.3390/s100605859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying QL, Wray J, Nichols J, Batlle-Morera L, Doble B, Woodgett J, Cohen P, Smith A. The ground state of embryonic stem cell self-renewal. Nature. 2008;453:519–523. doi: 10.1038/nature06968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y, Horii K, Sakai N, Masuda H, Furuichi M, Waga I. Antibody-specific aptamer-based PCR analysis for sensitive protein detection. Anal. Bioanal. Chem. 2009;395:1089–1096. doi: 10.1007/s00216-009-3041-0. [DOI] [PubMed] [Google Scholar]
- Young RA. Control of the embryonic stem cell state. Cell. 2011;144:940–954. doi: 10.1016/j.cell.2011.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Jiang B, Xiang Y, Chai Y, Yuan R. Label-free and amplified electrochemical detection of cytokine based on hairpin aptamer and catalytic DNAzyme. Analyst. 2012;137:1020–1023. doi: 10.1039/c2an15962g. [DOI] [PubMed] [Google Scholar]
- Zhou B, Wang B. Pegaptanib for the treatment of age-related macular degeneration. Exp. Eye Res. 2006;83:615–619. doi: 10.1016/j.exer.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Zhou J, Swiderski P, Li H, Zhang J, Neff CP, Akkina R, Rossi JJ. Selection, characterization and application of new RNA HIV gp120 aptamers for facile delivery of Dicer substrate siRNAs into HIV infected cells. Nucleic Acids Res. 2009;37:3094–3109. doi: 10.1093/nar/gkp185. [DOI] [PMC free article] [PubMed] [Google Scholar]


