Abstract
Many Swedish infants carry Staphylococcus aureus in their intestinal microflora. The source of this colonization was investigated in 50 families. Infantile S. aureus strains were isolated from rectal swabs and stool samples at 3 days and at 1, 2, 4, and 8 weeks of age. The strains were identified by using the random amplified polymorphic DNA method and compared to strains from swab cultures of the mothers' hands, nipples, and nares and from the fathers' hands and nares. Maternal stool samples were also obtained at a later stage to compare infant and adult intestinal S. aureus colonization. Although 60% of 1-month-old children had S. aureus in the stools, this was true of only 24% of the mothers. The median population numbers in colonized individuals also differed: 106.8 CFU/g of feces among infants at 2 weeks of age versus 103.2 CFU/g of feces in the mothers. Of S. aureus strains in the stools of 3-day-old infants, 90% were identical to a parental skin strain. A total of 96% of infants whose parents were S. aureus skin carriers had S. aureus in their feces and 91% had the same strain as at least one of the parents. In comparison, only 37% of infants to S. aureus-negative parents had S. aureus in the stool samples. Thus, infantile intestinal S. aureus colonization was strongly associated with parental skin S. aureus carriage (P = 0.0001). These results suggest that S. aureus on parental skin establish readily in the infantile gut, perhaps due to poor competition from other gut bacteria.
The human gastrointestinal tract hosts a rich and complex microflora, which starts to establish directly after birth and continues to develop and change during life (3, 9, 19). It has been proposed that the hygienic life-style in modern Western countries has led to a changed intestinal microflora in infancy and that a decreased stimulation of the immune system by intestinal bacteria could be the cause of the high and rising incidence of allergy in Western industrialized countries (25). Thus, infants in Western countries are colonized by certain bacteria at a later stage and have a less diverse intestinal microflora compared to infants in developing countries (1, 2, 5).
We recently reported that >75% of Swedish infants born in the late 1990s have S. aureus in their stools at some time during their first year of life (15), and another recent Swedish study reported that 65% of 1-month-old infants had S. aureus in their stools (7). Moreover, individual S. aureus strains persist for months in the intestinal microflora of individual infants, and the S. aureus counts in stool samples are quite high (15). Although half of the S. aureus strains isolated from the infantile gut flora are toxin producers, they do not seem to negatively affect infant health (15).
The source of the S. aureus strains colonizing Swedish infant intestines is unknown. One possibility is that they derive from the skin flora of people in the environment. Between 20 and 70% of adult individuals carry S. aureus in the nose; some of these individuals are permanently colonized, and others are only transiently colonized (14, 20, 23). Nasal carriers commonly have S. aureus at other body sites as well (14, 20, 23). S. aureus could also be acquired during delivery when the infant is exposed to the vaginal and possibly the fecal flora of the mother. However, fewer than 10% of healthy women have S. aureus in the vagina (13, 16), and S. aureus has traditionally not been regarded as part of the bowel flora. If, however, intestinal colonization by S. aureus has increased in adult women in the last decades, this species could be passed on to the infant during a normal delivery.
The aim of the present study was to investigate the source of S. aureus strains colonizing Swedish newborn infants. S. aureus strains from infants' stools were identified by using RAPD (randomly amplified polymorphic DNA) assay and toxin production. These strains were compared to strains obtained from the parents' hands and anterior nares and from the mothers' nipples. We also sampled maternal feces to determine whether S. aureus has become more prevalent in the adult intestinal microflora today.
MATERIALS AND METHODS
Infants and parents.
The infants studied here were part of a prospective birth-cohort study designed to examine the relation between intestinal colonization pattern in infancy and later allergy development. Parents-to-be were recruited during visits to the prenatal clinic. Inclusion criteria were the ability to follow a quite demanding study protocol, and most parents were highly educated middle class ethnic Swedes. Many of the families contained at least one allergic parent, since these were more motivated to participate. Informed consent was obtained from the parents, and the Medical Ethics Committee of Göteborg University approved the study.
The subjects of the present study consisted of families who were recruited during 2000 to 2002 and who were willing to participate in the extended sampling schedule. A total of 50 infants (20 girls and 30 boys) were included; 38 were born were born in the suburban Mölndal Hospital, 10 were born in the university clinic of Queen Silvia Children's Hospital in Göteborg, and 2 were born in Varberg Hospital (Varberg is a small town located 70 km from Göteborg). Six infants were delivered by caesarean section: four because of deteriorating fetal status, one because of maternal rheumatic disease, and one due to breech presentation. Mothers and infants stayed for 2 to 3 days (4 to 6 days after caesarean section) in the maternity ward, where rooming-in was practiced. All infants were breast-fed from birth, and 96% were still exclusively breast-fed by 2 months of age.
A total of 44% of the investigated parents reported previous or current allergic symptoms, most commonly rhinitis and/or conjunctivitis (64%), followed by asthma (18%) and eczema (8%). The most common allergens included pollens (69%), furred pets (39%), and foods (13%).
Collection and culture of samples from infants.
Rectal (n = 50) and nasal (n = 35) swabs were obtained from the infants 3 days after delivery. The swabs were streaked onto agar selective for staphylococci (Staphylococcus medium no. 110; Substrate Department, Bacteriological Laboratory, Sahlgrenska Hospital, Göteborg, Sweden), and the plates were incubated aerobically at 37°C for 2 days.
Quantitative cultures of all 50 infants' fecal flora were performed at 1, 2, 4, and 8 weeks of age. Feces was collected at home by the parents and put in a sterile petri dish, which was enclosed in a gas-tight cachet together with an anaerobic generator (Labfab; Becton Dickinson, Ljusne, Sweden), an anaerobic indicator (Oxoid, Hampshire, England), and a dampened piece of sponge. Samples were kept refrigerated until being transported to the laboratory, where they were processed within 24 h after collection. The samples were diluted serially in 10-fold steps in peptone-water and cultivated aerobically on Staphylococcus medium no. 110 at 37°C for 2 days. The level of detection was 330 (102.52) CFU/g of feces. Preliminary experiments showed that the procedure described above yielded similar staphylococcal counts as immediate processing of fecal samples.
Collection of samples from mothers and fathers.
Cotton-tipped swabs were obtained from all of the 50 mothers' and 39 of the fathers' anterior nares, the palms of their hands, and the mothers' breast nipples 3 days after delivery. Fecal samples were obtained from 37 of the mothers, 14 of whom were sampled 1 week after delivery and 23 of whom were sampled at a later stage. The samples were transported and processed in the same manner as those obtained from the children.
Identification of S. aureus and assessment of toxin production.
Colonies of different morphology growing on the staphylococcal agar were separately enumerated, subcultured on blood agar, Gram stained, and tested for catalase production. Isolates with a typical Gram-stained appearance and a positive catalase test were tested for coagulase production, and positive isolates were regarded as S. aureus.
To assess toxin production, S. aureus isolates were cultivated overnight in brain heart infusion or TS broth and tested for enterotoxins A, B, C, and D and toxic shock syndrome toxin 1 (SET-RPLA and TST-RPLA kits; Oxoid).
RAPD assay for typing of S. aureus strains.
RAPD assay was used for identification of different S. aureus strains. This method was described previously (15). In brief, bacterial DNA was isolated from an overnight brain heart infusion broth culture by using the modified Insta Gene protocol (Bio-Rad Hercules, Richmond, Calif.). Amplification of bacterial DNA was performed with 8 pmol of the primer 5′-AACGGTGACC-3′ (OPE-20, kit E; Operon Technologies), 10 ng of bacterial DNA, 0.2 mmol of deoxynucleoside triphosphate, and 3.5 mmol MgCl2. The mixture was heated to 80°C in a ThermoCycler (Perkin-Elmer Cetus model 480), whereafter 3 U of Taq polymerase was added, and the temperature was raised to 94°C for 120 s for denaturation. Thirty-five PCR cycles were run, each including incubation at 94°C for 70 s, at 33°C for 60 s, and at 72°C for 130 s. The PCR products were separated by polyacrylamide gel electrophoresis and silver stained (Plusone DNA silver staining kit; Pharmacia Biotech, Uppsala, Sweden). Isolates from one family were run together, and their band patterns were compared visually by the naked eye.
PFGE.
Some strains were also analyzed by pulsed-field gel electrophoresis (PFGE) to validate the results obtained by RAPD assay. PFGE was performed as previously described (18). Briefly, bacterial cells grown overnight were suspended in TEN buffer (100 mM Tris, 100 mM EDTA, 150 mM NaCl [pH 7.5]) spun down, resuspended in EC buffer with 1 mg of lysostaphin/ml, and cast in blocks of 2% low-melting-temperature agarose (SeaPlaque; AB In Vitro, Stockholm, Sweden). The blocks were treated with proteinase K for 1 h at 55°C, whereafter chromosomal DNA was cleaved with SmaI. PFGE was run at 6 V/cm for 23 h (GenePath System; Bio-Rad Laboratories, Sundbyberg, Sweden) by using an initial switch time of 5 s, a final switch time of 60 s, and an angle of 120°c. The gel was stained with ethidium bromide, visualized with UV light, and photographed.
Statistics.
Frequencies were compared by using the Fisher exact test. Bacterial population counts were compared by using Mann-Whitney's U-test.
RESULTS
Fecal S. aureus in infants and their mothers.
S. aureus were quantified in fecal samples obtained from 50 infants at 1, 2, 4, and 8 weeks of age and from 37 of the mothers 1 week or later after delivery. In the infants, colonization with S. aureus increased from 20% by day 3 (rectal swab culture) to 40% by 1 week, to 52% by 2 weeks, to 60% by 4 weeks, and to 64% by 8 weeks of age. Of the mothers, 24% had S. aureus in their feces. The infants' colonization frequency was significantly greater than the mothers' at 2, 4, and 8 weeks of age (P = 0.015, P = 0.0011, and P = 0.0004, respectively).
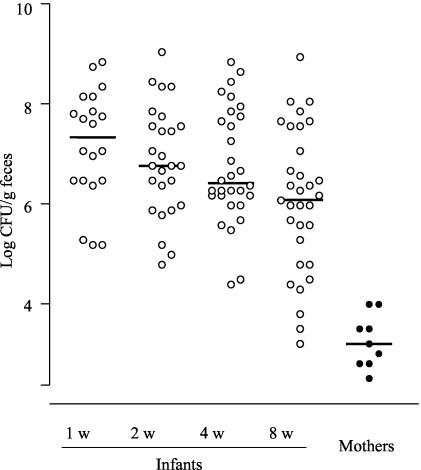
In colonized infants, the population counts of S. aureus were often >106 CFU/g of feces, whereas all nine colonized mothers had <104 CFU of S. aureus/g in their feces (Fig. 1). The differences in bacterial counts between infants and mothers were highly significant at all time points (P < 0.0001).
FIG. 1.
Fecal counts of S. aureus (log CFU/g of feces) in culture-positive infants (n = 34) and mothers (n = 9). The infants' stools were sampled at 1, 2, 4, and 8 weeks of age, whereas the mothers' stools were collected 1 week after delivery or later. Fecal samples were transported to the laboratory under anaerobic conditions and cultured quantitatively on staphylococcus agar. The limit of detection was 330 CFU/g of feces (2.52 log units). Each dot represents fecal S. aureus counts in one individual. The median is given for each time point.
Skin carriage of S. aureus.
Skin carriage of S. aureus was investigated in all 50 mothers and in 39 of the fathers. Samples were obtained from the hands and anterior nares and from the mothers' nipples. Samples were also obtained from the anterior nares of 35 of the infants.
Approximately 40% of the parents had S. aureus on the skin, most often in the anterior nares. Quite a few mothers had S. aureus on their nipples, whereas few parents had S. aureus on their hands (Table 1). The S. aureus carriage rate was no higher in allergic than nonallergic parents: 31% of allergic parents and 46% of nonallergic parents were S. aureus positive.
TABLE 1.
Skin carriage of S. aureusa
| Subject group | n | % Positive individuals (n) at (site):
|
|||
|---|---|---|---|---|---|
| Nose | Nipple | Hand | Any site | ||
| Infants | 35 | 17 (6) | 17 (6) | ||
| Mothers | 50 | 30 (15) | 22 (11) | 8 (4) | 36 (18) |
| Fathers | 39 | 38 (15) | 8 (3) | 44 (17) | |
Infants, mothers, and fathers were sampled 3 days after delivery.
Relationship between parental and infantile S. aureus carriage.
At least one of the parents was S. aureus positive in 24 families. Infants in these families had S. aureus in their feces in all cases except one (96%). Both parents sampled were negative for S. aureus in 19 families. Seven infants from these families (37%) had S. aureus in the stools. Thus, infant fecal S. aureus carriage was strongly associated with parental skin carriage (23 of 24 versus 7 of 19 [P = 0.0001]).
Comparison of strain identity between S. aureus from infants and parents.
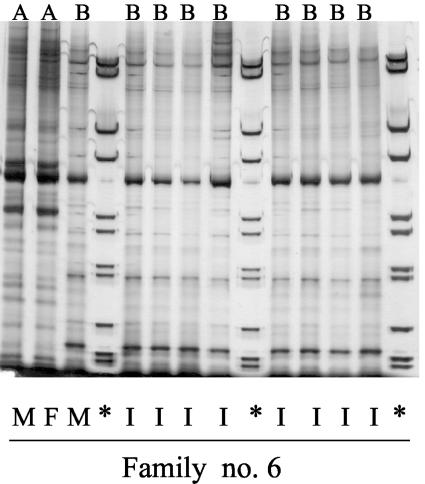
Figure 2 shows the RAPD pattern of S. aureus isolates from one family (family 6). The mother had two different strains in her nose designated “A” and “B” in the figure. The father also had the “A” strain in his nose, whereas the infant was consistently positive for the “B” strain in all stool samples.
FIG. 2.
RAPD patterns of S. aureus isolates from one family. The letters at the top indicate the strain identity (A or B). The origin of the isolates is indicated below: I, infant; M, mother; and F, father. ✽, Molecular weight standard.
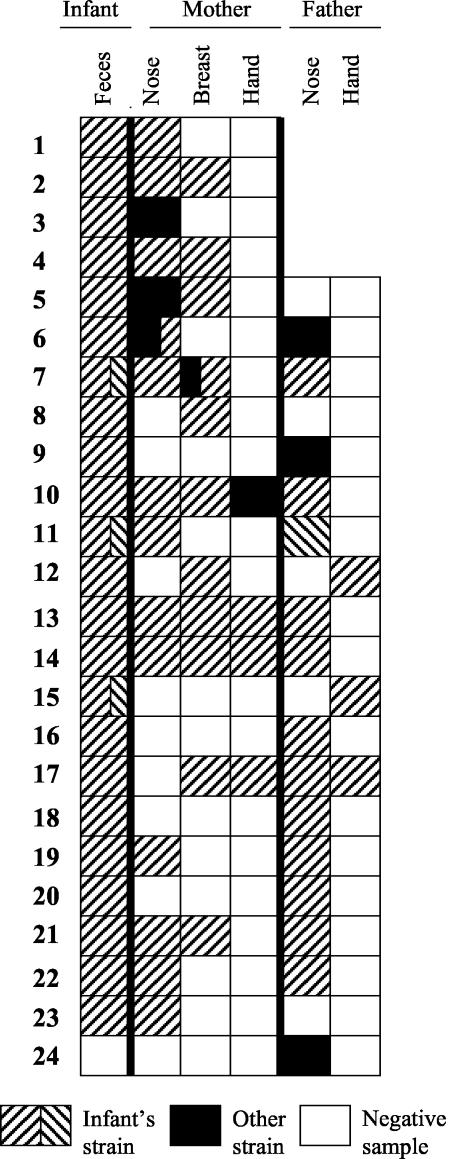
Figure 3 shows the degree of strain sharing within the 24 families in which at least one of the parents was an S. aureus carrier. The infant's strain in each family is represented by a hatched pattern, without any comparison between families. The fathers in the first four families were not sampled. In the 18 cases wherein the mother had S. aureus in the nose or on the nipple or hands, her child had the same strain in the stools in 17 cases. Fathers who were skin carriers (nose or hands) shared S. aureus strains with their child in 14 of 17 cases.
FIG. 3.
S. aureus strain sharing pattern within the 24 families in which at least one of the parents was a carrier of S. aureus. The fathers of the first four families were not sampled. Strains were not compared between families. Strain identity was assessed by RAPD assay and confirmed by toxin analysis.
The most common colonization pattern was that all family members carried the same strain (nine families). Four infants shared strains only with their mother, and four shared strains only with their father. In one family the infant shared one strain with the mother and another with the father. Only in a one case was the child's strain found in neither of the parents.
Six infants were delivered by caesarean section. Four lacked S. aureus in the stools, and their parents were S. aureus negative. The fifth had S. aureus-negative parents but harbored S. aureus in his feces from the second week. The sixth was colonized already from day 3 with a S. aureus strain identical to one isolated from his mother's breast.
Most infants (30 of 34) who had S. aureus in their stools were stably colonized by a single strain. Ten infants were colonized already by day 3; nine of these shared the strain with a parent. In children first colonized by 2 weeks or later, 54% of the strains were identical to the one found in a parent 3 days after delivery.
Of the six infants who were nasal carriers of S. aureus at 3 days of age and also had S. aureus in the stools any time during the first month of life, five had the same strain in the feces and the nasal cavity, whereas one had different strains at the two sites. This child (Fig. 3, infant 5) had the same strain in the stools as his mother had on her breast; the strain in the infant's nose was the same as that found in the mother's nose.
Validation of strain identification.
Forty-three percent of S. aureus strains isolated from the infants produced one or more toxins. (SEC, 29%; toxic shock syndrome toxin 1, 14%; SEA, 10%). In all cases in which a parent and an infant carried the same strain, as judged by their RAPD patterns, the strain produced the same toxin(s) whether isolated from parent or child.
When RAPD patterns of 24 families were compared across families, one clone appeared in six families, and three other clones each appeared in two or three families. This pattern was also seen with PFGE (data not shown). The infants in families carrying the same clone were in several cases born in different hospitals, suggesting a widespread occurrence of certain S. aureus clones or strains. Nevertheless, most strains gave unique RAPD patterns. The almost obligate strain sharing within families could therefore not be explained by a limited resolution of the RAPD method or a global occurrence of these strains.
DISCUSSION
We recently reported that S. aureus has become a major colonizer of the of Swedish infants (15). The present study was initiated to determine the sources of the S. aureus strains colonizing the intestinal tracts of Swedish infants.
S. aureus reached average counts of >106 CFU/g in infant's stools, which is higher than reported in studies from the 1980s (17, 22). In contrast, only a minority of the investigated mothers had S. aureus in their feces, and their population counts did not exceed 104 CFU/g feces, suggesting that S. aureus is no more common in the adult intestinal flora today than it was 20 years ago (8, 10). We therefore regard contamination from maternal fecal or vaginal flora during delivery as a minor source of the S. aureus strains found in the infants' bowel flora.
The data presented here instead suggest that the main source of these strains is the skin flora of the parents. Indeed, 90% of S. aureus strains found in infants' feces 3 days after delivery and 75% of the strains occurring up to 1 month of age were identical to a strain present in the parental skin flora 3 days after delivery. We regard it highly unlikely that the direction of spread was the reverse: from infant to parent. S. aureus colonized only 20% of 3-day-old infants, but the colonization rate rose steadily to reach 64% in 2-month-old infants. This confirms our earlier findings of maximal intestinal S. aureus colonization between 2 and 6 months of age, suggesting that most strains derive from the home environment and not from the hospital (15). In Sweden, rooming-in and short hospital stays after delivery is practiced, which minimizes colonization of infants by hospital bacteria (6). In accordance with this, there was no indication that infants born in one hospital shared the same strains. Most families had their unique strains, although a few strains seemed to be shared by several families.
S. aureus may spread from mother to infant through breast-feeding. The breast is especially prone to become colonized by S. aureus during lactation (26), probably due to increased dampness. In accordance, we detected S. aureus on the mother's nipples almost as often as in her nares (22% versus 30%), and an infant delivered by caesarean section had an S. aureus in his feces already by day 3 that was identical to a strain found on his mother's nipples. Intestinal colonization with S. aureus is also known to be more common in breast-fed than in bottle-fed infants (4, 17, 21). The very high rate of breast-feeding in Sweden (27) may contribute to the pronounced S. aureus colonization of Swedish infants. However, fathers also evidently transferred S. aureus to their children. We found four families in which only the father and the infant shared strains, while most commonly the mother, father, and infant shared the same strain. This suggests that spread occurs also during general care-taking. In Sweden, fathers are paid 10 days off from work when their babies are born to take part in their care.
One-third of the infants of S. aureus-negative parents were colonized by S. aureus. These infants may have acquired their strains from siblings or other people in the environment. However, we could not demonstrate any higher S. aureus colonization rate in children who had elder siblings, compared to firstborn children (unpublished observations). We took samples from the parents only on one occasion, at 3 days after delivery. Parents who were S. aureus negative then might have become positive later on and then transferred S. aureus to their child, since many people are only intermittent nasal carriers of S. aureus (14, 20, 23).
We do not believe that the frequent colonization by S. aureus in the infants studied here was due to exposure to particularly high doses of S. aureus. The 34% parental nasal S. aureus carriage reported here fits well with the 20 to 70% reported in other studies (14, 20, 23). Traumatized skin is especially prone to being colonized by S. aureus as seen, for example, in atopic dermatitis (11). However, although many of the parents in the study were atopic, they were no more often S. aureus carriers than the nonatopic parents. Furthermore, their infants were no more often colonized by S. aureus than the infants of nonatopic parents (15; unpublished observations).
Instead, we propose that staphylococci become more easily established in the infantile gut today due to lack of competition. An established intestinal microflora with a multitude of facultative and anaerobic species exerts a strong pressure against new bacteria, a phenomenon termed “colonization resistance” (24). Therefore, adults, who have a much more complex microflora than newborn infants, have lower S. aureus population counts. In accordance with this, we have observed that infants delivered by caesarean section have higher S. aureus counts by 6 months of age than infants delivered vaginally (unpublished observations). Infants delivered by caesarean section are delayed in their acquisition of a complex intestinal microflora (12). Furthermore, we have observed that infants from families who have pets have less S. aureus compared to other infants (unpublished observations). Pets may be a source of microbial exposure and may also be a marker of a less “microphobic” life-style. We propose that the generally low microbial exposure in characterizing contemporary Western countries has allowed S. aureus to establish and expand in the infantile intestinal microflora, although it is not optimally suited for this niche.
Acknowledgments
We thank Birgitta Åberg for the excellent help with recruiting and interviewing the parents and Eva Ågren, Jolanta Bonislawska, and Ingela Kinell for expert technical assistance. We also thank Christina Welinder-Ohlsson, Eva Kjellin, and Kerstin Florén-Johansson at the Bacteriological Laboratory, Sahlgrenska University Hospital, for PFGE analyses.
The study was supported by grants from the Swedish Foundation for Health Care Sciences and Allergy Research (grants 2001/0037 A2002 064 and VS1999-0017), the European Commission (QLK4-2000-00538), the Swedish Medical Research Council (grant K2002-06X-14017-02B), the Göteborg Freemasons' Orphanage Foundation, the Asthma and Allergy Foundation, the Wilhelm and Martina Lundgren Foundation, and the Petrus and Augusta Hedlund Foundation.
REFERENCES
- 1.Adlerberth, I., B. Carlsson, P. de Man, F. Jalil, S. R. Khan, P. Larsson, L. Mellander, C. Svanborg, A. E. Wold, and L. Å. Hanson. 1991. Intestinal colonization with Enterobacteriaceae in Pakistani and Swedish hospital-delivered infants. Acta Paediatr. Scand. 80:602-610. [DOI] [PubMed] [Google Scholar]
- 2.Adlerberth, I., F. Jalil, B. Carlsson, L. Mellander, L. Å. Hanson, P. Larsson, K. Khalil, and A. E. Wold. 1998. High turnover rate of Escherichia coli strains in the intestinal flora of infants in Pakistan. Epidemiol. Infect. 121:587-598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adlerberth, I., L. Å. Hansson, and A. E. Wold. 1999. The ontogeny of the intestinal flora, p. 279-292. In I. R. Sanderson, and W. A. Walker (ed.), Development of the gastrointestinal tract. B. C. Decker Publishing, Hamilton, Ontario, Canada.
- 4.Balmer, S. E., and B. A. Wharton. 1989. Diet and fecal flora in the newborn: breastmilk and infant formula. Arch. Dis. Child. 64:1672-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bennet, R., M. Eriksson, N. Tafari, and C. E. Nord. 1991. Intestinal bacteria of newborn Ethiopian infants in relation to antibiotic treatment and colonization by pathogenic gram-negative bacteria. Scand. J. Infect. Dis. 23:63-69. [DOI] [PubMed] [Google Scholar]
- 6.Bettelheim, K., B. Peddie, and A. Chereshsky. 1983. The ecology of Escherichia coli in a maternity ward in Christchurch, New Zealand. Zentbl. Bakteriol. Mikrobiol. Hyg. B 178:389-393. [PubMed] [Google Scholar]
- 7.Bjorksten, B., E. Sepp, K. Julge, T. Voor, and M. Mikelsaar. 2001. Allergy development and the intestinal microflora during the first year of life. J. Allergy Clin. Immunol. 108:516-520. [DOI] [PubMed] [Google Scholar]
- 8.Crossley, K., and J. Solliday. 1980. Comparison of rectal swabs and stool cultures for the detection of gastrointestinal carriage of Staphylococcus aureus. J. Clin. Microbiol. 11:433-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis-Pegler, R. B., C. Crabtree, and H. P. Lambert. 1975. The fecal flora of children in the United Kingdom. J. Hyg. 75:135-142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Finegold, S. M., V. L. Sutter, and G. E. Mathisen. 1983. Normal indigenous intestinal flora, p. 3. In D. J. Hentges (ed.), Human intestinal microflora in health and disease. Academic Press, Ltd., London, England.
- 11.Goodyear, H. M., P. J. Watson, S. A. Egan, E. H. Price, P. A. Kenny, and J. L. Harper. 1993. Skin microflora of atopic eczema in first time hospital attenders. Clin. Exp. Dermatol. 18:300-304. [DOI] [PubMed] [Google Scholar]
- 12.Gronlund, M. M., O. P. Lehtonen, E. Eerola, and P. Kero. 1999. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J. Pediatr. Gastroenterol. Nutr. 28:19-25. [DOI] [PubMed] [Google Scholar]
- 13.Guinan, M. E., B. B. Dan, R. J. Guidotti, A. L. Reingold, G. P. Schmid, E. J. Bettoli, J. G. Lossick, K. N. Shands, M. A. Kramer, N. T. Hargrett, R. L. Anderson, and C. V. Broome. 1982. Vaginal colonization with Staphylococcus aureus in healthy women: a review of four studies. Ann. Intern. Med. 96:944-947. [DOI] [PubMed] [Google Scholar]
- 14.Kluytmans, J., A. van Belkum, and H. Verbrugh. 1997. Nasal carriage of Staphylococcus aureus: epidemiology, underlying mechanisms, and associated risks. Clin. Microbiol. Rev. 10:505-520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lindberg, E., F. Nowrouzian, I. Adlerberth, and A. E. Wold. 2000. Long-time persistence of superantigen-producing Staphylococcus aureus strains in the intestinal microflora of healthy infants. Pediatr. Res. 48:741-747. [DOI] [PubMed] [Google Scholar]
- 16.Linnemann, C. C., Jr., J. L. Staneck, S. Hornstein, T. P. Barden, J. L. Rauh, P. F. Bonventre, C. R. Buncher, and A. Beiting. 1982. The epidemiology of genital colonization with Staphylococcus aureus. Ann. Intern. Med. 96:940-944. [DOI] [PubMed] [Google Scholar]
- 17.Lundequist, B., C. E. Nord, and J. Winberg. 1985. The composition of the fecal microflora in breastfed and bottlefed infants from birth to 8 weeks. Acta Paediatr. Scand. 74:54-58. [DOI] [PubMed] [Google Scholar]
- 18.Maslow, J. N., A. M. Slutsky, and R. D. Arbeit. 1993. Application of pulsed-field gel electrophoresis to molecular epidemiology, p. 563-572. In D. Persing, T. Smith, F. Tenover, and T. White (ed.), Diagnostic molecular microbiology, principles and applications. American Society for Microbiology, Washington, D.C.
- 19.Midtvedt, A.-C. 1994. Ph.D. thesis. Stockholm University, Stockholm, Sweden.
- 20.Nouwen, J. L., A. van Belkum, and H. A. Verbrugh. 2001. Determinants of Staphylococcus aureus nasal carriage. Neth. J. Med. 59:126-133. [DOI] [PubMed] [Google Scholar]
- 21.Simhon, A., J. R. Douglas, B. S. Drasar, and J. F. Soothill. 1982. Effect of feeding on infants' fecal flora. Arch. Dis. Child. 57:54-58. [PMC free article] [PubMed] [Google Scholar]
- 22.Stark, P. L., and A. Lee. 1982. The microbial ecology of the large bowel of breast-fed and formula-fed infants during the first year of life. J. Med. Microbiol. 15:189-203. [DOI] [PubMed] [Google Scholar]
- 23.Vandenbergh, M. F., and H. A. Verbrugh. 1999. Carriage of Staphylococcus aureus: epidemiology and clinical relevance. J. Lab. Clin. Med. 133:525-534. [DOI] [PubMed] [Google Scholar]
- 24.van der Waaij, D. 1979. The colonization resistance of the digestive tract in man and animals. Zentbl. Bakteriol. (Suppl. 7) 7:155-161.
- 25.Wold, A. E. 1998. The hygiene hypothesis revised: is the rising frequency of allergy due to changes in the intestinal flora? Allergy 53:20-25. [DOI] [PubMed] [Google Scholar]
- 26.Zdrazilek, J., P. Petras, H. Sramova, V. Subertova, and L. Maskova. 1988. Staphylococcus aureus at a maternity ward. I. Colonization of mothers and neonates and survival of various S. aureus types in colonized individuals. J. Hyg. Epidemiol. Microbiol. Immunol. 32:49-57. [PubMed] [Google Scholar]
- 27.Zetterstrom, R. 1999. Breastfeeding and infant-mother interaction. Acta Paediatr. Suppl. 88:1-6. [DOI] [PubMed] [Google Scholar]