Abstract
The nuclear–cytoplasmic protein transport is a critical process in cellular events. The identification of transport signals (nuclear localization signal and nuclear export signal) and their receptors has facilitated our understanding of this expanding field. Nuclear transport must be appropriately regulated to deliver proteins through the nuclear pore when their functions are required in the nucleus, and to export them into the cytoplasm when they are not needed in the nucleus. Altered nuclear transport processes have been observed in stressed cells, which would change gene expressions. Some viruses interfere with nuclear transport in host cells to evade immune defense. Moreover, certain transport factors negatively regulate nuclear protein transport in cells. Understanding the regulatory mechanisms of nuclear–cytoplasmic trafficking not only provides important information about cellular processes, but also is of use for developing specific inhibitors for transport pathways.
Introduction
In eukaryotic cells, the nuclear envelope separates the nucleus and the cytoplasm spatially and functionally. To maintain cell homeostasis, a variety of information is exchanged between the cytoplasm and the nucleus. Genomic information is transcribed to mRNAs in the nucleus and translated to proteins by ribosomes in the cytoplasm. Synthesized proteins are delivered to their target destinations where they function. Nuclear proteins, including structural proteins, transcription factors and other functional proteins must be delivered into the nucleus after their synthesis in the cytoplasm. The proteins that pass through nuclear pores contain a nuclear localization signal (NLS) and a nuclear export signal (NES) within their primary structure. Transport factors recognize and transport cargo proteins containing these signals through the nuclear pore. However, it is noteworthy that not all proteins that function in the nucleus translocate into the nucleus immediately after their synthesis. For example, in the resting state, signal transduction molecules predominantly exist in the cytoplasm, and when cells receive particular stimuli, such signaling molecules are activated and transported into the nucleus. After a certain period of time, in most cases, nuclear export factors return such signaling molecules to the cytoplasm through the nuclear pore. Quantitative analysis estimated that a single proliferating mammalian cell interchanges approximately 1 million macromolecules through nuclear pore within 1-s (Ribbeck & Görlich 2001; Cardarelli et al. 2012).
Many regulatory mechanisms concerning the translocation of cargo proteins through nuclear pores have been elucidated. Protein association/dissociation, phosphorylation/dephosphorylation or other modifications of cargo proteins can trigger the nuclear–cytoplasmic transport. Additionally, recent studies showed that other regulatory mechanisms control nuclear–cytoplasmic transport through transport factors and components of nuclear pore complexes (NPCs). Cellular stresses such as UV irradiation and heat shock have been shown to alter nuclear–cytoplasmic transport of proteins. In the field of virology, it has been shown that certain viruses inhibit nuclear–cytoplasmic protein transport specifically or globally in infected cells. Furthermore, some transport factors act as intrinsic negative regulators of certain nuclear proteins. In this review, we focus on the mechanisms and roles of negative regulation of nuclear protein transport, particularly the negative regulation exerted by transport factors themselves.
Fundamental mechanism of nuclear–cytoplasmic protein transport
To enter the nucleus, molecules must traverse the nuclear envelope via NPC. The NPC is composed of approximately 30 different protein components called nucleoporins (Nups) (Rout et al. 2000; Cronshaw et al. 2002). It has been proposed that the central channel of the NPC has a mesh-like structure that prevents nonkaryophilic molecules from passing through it freely (Frey et al. 2006; Lim et al. 2006). Indeed, small molecules such as ions, metabolites and proteins smaller than approximately 40 kDa are able to pass though the NPC diffusely. However, larger molecules require their specific carrier proteins to be transported from the cytoplasm to the nucleus or from the nucleus to the cytoplasm. NLS and NES have been identified in the primary sequences of proteins that are transported through the NPC (Jans et al. 2000; Kutay & Güttinger 2005). These signals are recognized by transport factors, called importins and exportins, to be transported into or out of the nucleus (Major et al. 2011).
A well-known NLS consists of one (monopartite) or two (bipartite) basic amino acid clusters (Table 1). Monopartite or bipartite basic-type NLSs have been identified in many nuclear proteins. Basic-type NLSs are generally recognized by importin α. Importin α transports a cargo protein containing the basic-type NLS in conjunction with importin β1, although importin α has the ability to enter the nucleus with or without its cargo protein in the absence of importin β1 (Miyamoto et al. 2002; Kotera et al. 2005). However, importin β family proteins can recognize some NLSs directly and transport them into the nucleus without importin α. A cargo–importin complex translocates into the nucleus through transient interaction with phenylalanine–glycine (FG) repeat containing Nups in the NPC (Pyhtila & Rexach 2003). After translocation into the nucleus, the binding of the GTP-bound form of Ran (Ran-GTP) to importin β induces a conformation change that dissociates the importin–cargo complex. The free cargo protein functions in the nucleus, and importins are returned into the cytoplasm by a specific recycling pathway for the next round of transport (Fig. 1).
Table 1.
Well-known transport signals (NLS and NES) and their specific inhibitors
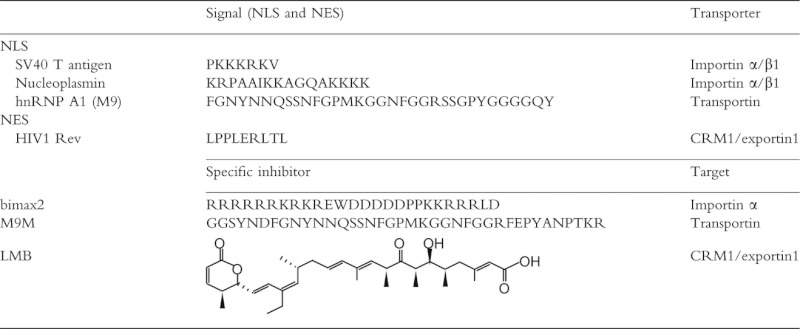 |
Figure 1.
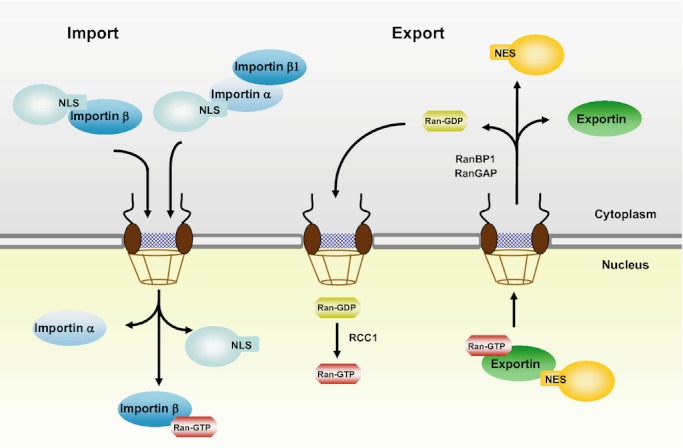
Active nuclear–cytoplasmic transport of proteins. The cargo proteins containing a nuclear localization signal (NLS) are recognized by importin βs alone or importin α/β1 heterodimer, whereas cargo proteins that possess a nuclear export signal (NES) bind to exportins in the presence of Ran-GTP. The transport complexes pass through nuclear pores by sequential and transient interaction of importin β with Nups within the NPC. Within the nucleus, Ran-GTP binding to importin β dissociates the import complex. Ran-GTP to Ran-GDP exchange facilitated by RanBP1 and RanGAP1 dissociates the nuclear export complex in the cytoplasm. Ran-GDP is converted to the GTP-bound form by RCC1 in the nucleus.
An NES-containing protein is exported from the nucleus into the cytoplasm. For nuclear protein export, the typical NES is characterized as a leucine-rich sequence that is recognized by CRM1/exportin 1 (Table 1). CRM1, a member of the importin β family, binds to Ran-GTP and forms a trimeric complex with the NES-containing cargo protein. This complex translocates through the NPC into the cytoplasm. In the cytoplasm, the conversion of Ran-GTP to Ran-GDP induces the dissociation of the export complex (Fig. 1). In addition to nuclear import, some members of the importin β family act as the NES receptor and transport the NES-containing proteins into the cytoplasm. The importin β family has at least 20 members and transports various proteins in and out of the nucleus. Interestingly, the importin β family is known to recognize protein regions with very diverse sequences, making it difficult to identify its consensus NLS or NES sequence. Low homology within the importin β family may be required to accommodate such divergent NLSs or NESs.
The directionality of protein transport is determined by the gradient of Ran-GTP, which regulates importin β family–cargo interaction. A higher concentration of Ran-GTP in the nucleus than in the cytoplasm has been reported. Ran-GTP induces the dissociation of the import complex and facilitates exportin–cargo binding in the nucleus. The cycling of Ran between GTP- and GDP-bound states is regulated by Ran GTPase-activating protein 1 (RanGAP1), Ran-binding protein 1 (RanBP1) and guanine nucleotide exchange factor RCC1 (Melchior & Gerace 1998; Fig. 1).
Cellular stresses affect the nuclear transport machinery
Cells are exposed to a variety of environmental stresses such as heat shock, UV irradiation and oxidative stress, but can respond to such stresses through various defense strategies. It has been reported that the classical nuclear import pathway is down-regulated by several stresses (Stochaj et al. 2000; Kodiha et al. 2004; Miyamoto et al. 2004). In the absence of stress, importins and Nups predominantly localize in the cytoplasm and at the nuclear envelope, respectively. After stress treatment, the intracellular ATP level decreases in the stressed cell and the Ran-GTP/Ran-GDP ratio is subsequently reduced, resulting in the cytoplasmic localization of Ran and the down-regulation of Ran-GTP-dependent nuclear export (Yasuda et al. 2006). Thus, the decrease in intracellular Ran-GTP induces the accumulation of the NLS receptor importin α and its export factor CAS (cellular apoptosis susceptibility gene) in the nucleus (Miyamoto et al. 2004; Kodiha et al. 2008a). In addition, oxidative stress by diethyl maleate (DEM) induces the formation of a high molecular mass complex containing importin α, Nup88 and Nup153 in the nucleus, resulting in the retention of importin α in the nucleus (Kodiha et al. 2008b). Nuclear importin α accumulated in response to the stresses is less mobile, indicating that importin α recycling from the nucleus to the cytoplasm is inhibited in the stressed cells. Consequently, the abnormal distribution of importin α results in a decrease in importin α/β1-dependent nuclear transport of proteins (Furuta et al. 2004).
The NPC exhibits an eightfold symmetry and has a characteristic three-dimensional architecture consisting of approximately 30 different nucleoporins (Nups) (Rout et al. 2000; Cronshaw et al. 2002; Lim et al. 2008; Fig. 2). This architecture is conserved from yeast to higher eukaryotes (Stoffler et al. 1999). Transport factors transiently interact with the tandem-repeated phenylalanine–glycine (FG repeats) in Nups to pass through the NPC (Pyhtila & Rexach 2003). Therefore, the appropriate composition of Nups is required for the proper function of the NPC. Cellular stress alters the localization and modification of certain nucleoporins (Fig. 2). Following oxidative stress, the amount of Nup358 is reduced at the nuclear envelope, and Nup98 is redistributed from the nuclear envelope to the nucleoli (Crampton et al. 2009). Furthermore, Nup62, Nup98 and Nup214 are phosphorylated, and O-linked N-acetylglucosamine modification is increased in Nup62 and Nup214 by stress treatment (Crampton et al. 2009). These modifications affect CRM1 binding to the NPC and are correlated with a decrease in nuclear protein export. Thus, not only importin α/β1- and CRM1-dependent pathways, but also other transport factor-mediated nuclear transport pathways would be impaired by stress treatment, indicating that cellular stress acts as a negative regulator of nuclear–cytoplasmic transport.
Figure 2.
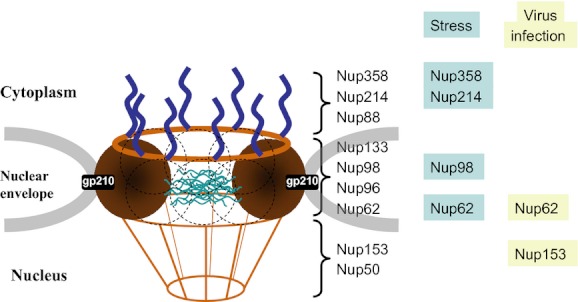
Schematic representation of the mammalian NPC. The relative position of various Nups is listed. The Nups altered by cellular stresses and virus infection are indicated in blue and yellow, respectively.
Viral strategies for their proliferation in host cells
In response to viral infection, signal transduction molecules translocate into the nucleus where they activate the transcription of antiviral genes. On the other hand, to replicate the virus particle in the host cell and to expand its infection to neighboring cells, the virus has various strategies to prevent the antiviral response of the host cell. One of the strategies is to inhibit the nuclear–cytoplasmic protein transport of the host cell. DNA viruses must use nuclear–cytoplasmic protein transport pathways because they replicate in the nucleus of host cells. In contrast, some RNA viruses, which do not use nuclear components of the host cell for their replication, develop strategies to evade host immune defenses by interfering with the nuclear–cytoplasmic protein transport.
It is well known that interferon (IFN) induces antiviral activity. Studies of intracellular signaling by the IFN led to characterization of the Jak–Stat pathway. For example, in response to IFN-γ, interferon receptor–associated Janus kinase 1 (Jak1) and Jak2 are activated and phosphorylate signal transducer and activator of transcription 1 (Stat1; Levy & García-Sastre 2001). NPI-1/importin α2 binds to the tyrosine-phosphorylated Stat1 and transports it into the nucleus in conjunction with importin β1 (Sekimoto et al. 1997). After nuclear translocation, Stat1 activates the transcription of numerous genes including those encoding antiviral proteins (Levy & García-Sastre 2001). It has been shown that VP35 protein of Ebola virus (EBOV) has an ability to block the cellular production of IFN-α/β (Basler et al. 2000; Reid et al. 2005). Furthermore, EBOV also prevents cellular responses to IFN-α/β or INF-γ (Harcourt et al. 1999; Kash et al. 2006). The minor matrix protein VP24 of EBOV was found to bind to importin α2 and to prevent the Stat1 binding to importin α2, resulting in the cytoplasmic localization of phosphorylated Stat1 (Reid et al. 2006, 2007; Mateo et al. 2010; Fig. 3). Cytoplasmic Stat1 cannot activate transcription, and the cellular response to virus infection is therefore suppressed. Furthermore, it was found that protozoan parasites have similar negative regulatory activity against the nuclear transport of host cells. Leishmania donovani is parasitic in macrophages and evades the host cell's microbicidal properties (Kima 2007). Leishmania donovani amastigotes in the host macrophage inhibit the association of Stat1 with importin α2 in response to IFN-γ, causing reduced nuclear localization of activated Stat1 (Matte & Descoteaux 2010). In addition, although the precise mechanisms are unclear, it has been reported that infection by measles virus (MV-N), rotavirus and protozoan parasite Toxoplasma gondii also blocks the nuclear translocation of immunoresponsive proteins such as activated Stat1, Stat2 and NFκB (Lüder et al. 2001; Holloway et al. 2009; Takayama et al. 2012).
Figure 3.
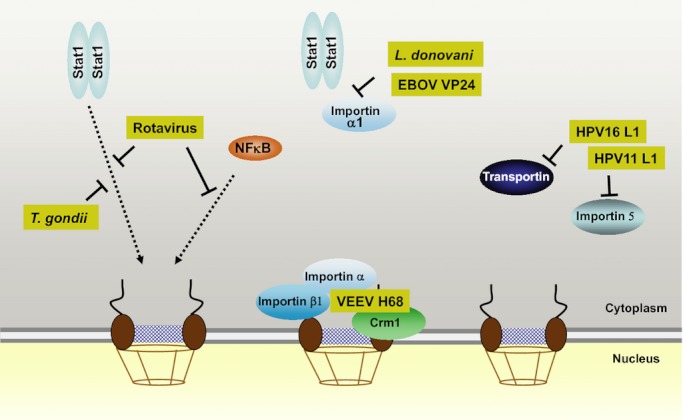
Some viruses and protozoans inhibit the nuclear–cytoplasmic protein trafficking of the host cell. The nuclear import of immune response factors such as Stat1 and NFκB is the target of certain viruses and protozoans. VEEV H68 inhibits both importin α/β1-mediated nuclear import and CRM1-dependent nuclear export pathways. Transportin- and importin 5-mediated nuclear imports are impaired by HPV L1 protein.
The L1 major capsid proteins of human papillomavirus (HPV) types 11 and 16 have been reported to inhibit the Kapβ2/transportin- and Kapβ3/importin 5-mediated pathways (Nelson et al. 2003l Fig. 3). The L1 protein strongly associates with transportin, and this complex cannot be dissociated by the addition of the M9 sequence, a well-known substrate for transportin (Pollard et al. 1996), as well as Ran-GTP, the role of which is to dissociate nuclear import complex. Kapβ3 is known to be a target for both HPV16 E5 oncoprotein and the nonstructural protein 5A (NA5A) of hepatitis C virus, and these viral proteins may also inhibit the Kapβ3-mediated transport pathway (Chung et al. 2000; Krawczyk et al. 2008).
Venezuelan equine encephalitis virus (VEEV) capsid protein has a unique inhibitory activity against the nuclear transport of the host cell (Atasheva et al. 2010). The short stretch containing both NLS and NES sequences inside the VEEV capsid protein, named H68, efficiently inhibits the cellular transcription of the full-length capsid protein (Atasheva et al. 2008). H68 shows a strong inhibitory activity toward the importin α/β1-dependent nuclear import pathway in vivo. In addition, it also prevents the nuclear export pathway mediated by CRM1 (Atasheva et al. 2010). Interestingly, the H68 sequence forms a tetrameric complex with importin α/β1 and CRM1, and this complex formation is essential for the inhibition of nuclear–cytoplasmic transport (Fig. 3). Indeed, the nonpathogenic type of VEEV contains mutations within this sequence, and the pathogenesis of this virus is closely correlated with this sequence. In addition, infection of rhinovirus induces degradation of Nup153 and p62, components of the NPC, resulting in the inhibition of nuclear protein import of the host cells (Gustin & Sarnow 2002; Fig. 2). It has also been reported that the matrix protein of vesicular stomatitis virus (VSV) inhibits bidirectional nuclear transport by interacting with the components of NPC or NPC-associated factors that participate in nuclear–cytoplasmic transport (Petersen et al. 2000).
Thus, inhibition of both the nuclear import of immune response molecules and the nuclear export of mRNAs for their target genes also impairs the ability of cells to defend against viral infection. Therefore, some virus proteins function as negative regulators of nuclear–cytoplasmic transport that allow the virus to escape the cellular immune response, raising the idea that these viral inhibitory activities might be a good target for drug discovery.
Post-translational modifications as negative regulators
In general, protein modifications are key regulators of protein function, and several modifications have been reported to affect the function of nuclear transport factors. Nitric oxide (NO) has been implicated in regulating the nuclear transport of a number of proteins modified by S-nitrosylation (Buckley et al. 2003; Hara et al. 2005; Qu et al. 2007). NO generated by nitric oxide synthase (NOS) reacts with intracellular glutathione to form nitrosoglutathione, which can interact with a susceptible cysteine residue of proteins, resulting in S-nitrosylation. S-nitrosylation modulates protein functions and is thought to be a major posttranslational regulation (Hess et al. 2005). Wang et al. (2009) found that CRM1/exportin 1 becomes S-nitrosylated after exposure to NO. Two cysteine residues located in the conserved region of CRM1 are reactive to NO. S-nitrosylation at either cysteine residue is sufficient for the repression of CRM1-dependent nuclear export (Wang et al. 2009; Fig. 4). In addition, screening of S-nitrosylated proteins in prostate epithelial cells identified importin β1 as an S-nitrosylated transport factor (Lam et al. 2010), although its effects on nuclear transport are still unclear.
Figure 4.
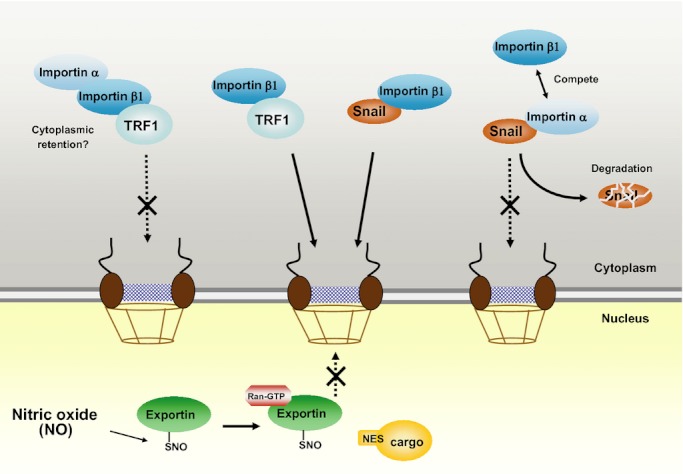
Importin α acts as a negative regulator for the nuclear import of certain proteins. TRF1 and Snail are transported into the nucleus by the importin β1-mediated pathway. In the presence of importin α, TRF1 forms a complex with importin α/β1 heterodimer by binding to importin β1, but this complex remains in the cytoplasm. In contrast, importin α competes with the binding of importin β1 to Snail zinc finger domain, resulting in the inefficient nuclear accumulation of Snail. S-nitrosylation of CRM1 by NO inhibits its binding to NES-containing cargos.
In addition to S-nitrosylation, the phosphorylation of transport machinery has been implicated to reduce nuclear–cytoplasmic protein transport (Kehlenbach & Gerace 2000). Importin α/β1- and transportin-mediated nuclear import, but not CRM1-dependent nuclear export, is decreased by treatment with phosphatase inhibitors (okadaic acid and microcystin). The cargo–importin α binding and the interaction of importin β1 with Nup358 and Nup153 were not affected by the phosphatase inhibitors. These findings suggest the possibility that other modifications might negatively control the transport factors or Nups.
Transport factors as negative regulators
Interestingly, it has been reported that importin α acts as an inhibitor of nuclear protein import in some cases. It is known that importin α acts as an adaptor between an NLS-containing cargo protein and importin β1. Although parathyroid hormone-related protein (PTHrP) and telomere repeat factor 1 (TRF1) are directly recognized and transported into the nucleus by importin β1 alone, PTHrP and TRF1 also form a complex with importin α/β1 heterodimer (Lam et al. 1999; Forwood & Jans 2002). Importin α does not directly associate with TRF1, whereas importin α/β1 complex sufficiently binds to TRF1 through importin β1. In contrast to other cargo proteins, this ternary complex is not active and is localized in the cytoplasm (Lam et al. 1999; Forwood & Jans 2002). The formation of similar transport-incompetent complexes containing importin α was reported in importin 7/importin β1-mediated nuclear transport of histone H1 (Jäkel et al. 1999). It is possible that importin α associates with cytoskeletal components to retain the transport complex in the cytoplasm (Forwood & Jans 2002).
Moreover, importin α directly inhibits the nuclear import of Snail, a zinc finger-containing transcription factor. The zinc finger domain of Snail functions as an NLS, and importin β1 directly recognizes this region and transports it into the nucleus (Yamasaki et al. 2005). Importin α also interacts with the Snail zinc finger domain and competes with the binding of importin β1, resulting in the reduced nuclear import of Snail and the induction of its proteasome-dependent degradation (Sekimoto et al. 2011; Fig. 4). Importin α acts as a negative regulator for the nuclear import of PTHrP, TRF1 and Snail, although the mechanisms are apparently distinct. Thus, the identification of increasing numbers of targets for the negative regulation mediated by importin α will help to understand more precisely how nuclear transport is inhibited by importin α and its biological significance.
Small molecules and peptides as negative regulators
Specific chemical inhibitors are powerful tools to study the biological functions of their target molecules. Many chemical compounds have been developed to inhibit specific molecules; in fact, they have contributed to unveiling the details of a variety of biological processes. A well-known inhibitor in the field of protein transport is leptomycin B (LMB), which was originally isolated from Streptomyces sp. as an antifungal agent (Hamamoto et al. 1983). LMB specifically inhibits a broad range of nuclear protein export pathways mediated by CRM1/exportin 1 (Fornerod et al. 1997; Fukuda et al. 1997; Kudo et al. 1997). It binds covalently to a cysteine residue located in the central conserved region of CRM1 and prevents the binding of CRM1 to proteins containing an NES (Kudo et al. 1999). Because of its specific ability to bind to CRM1, LMB is used to determine whether a target molecule is exported from the nucleus by CRM1. The myxobacterial cytotoxin, ratjadone, has been identified as a potent inhibitor of CRM1 (Köster et al. 2003). Like LMB, ratjadone covalently binds to the same cysteine residue of CRM1 and inhibits the cargo protein binding to CRM1 (Meissner et al. 2004). LMB and ratjadone are structurally related, and their inhibitory activities are similar (Köster et al. 2003). Prostaglandin 15-deoxy-D12,14-PGJ2 was also reported as an inhibitor of CRM1, although it requires a higher concentration (3 orders of magnitude greater than LMB) to block CRM1 (Hilliard et al. 2010). Although many chemical inhibitors have so far been developed to block CRM1/exportin 1-dependent nuclear export pathways (Daelemans et al. 2002; Van Neck et al. 2008; Monovich et al. 2009; Mutka et al. 2009), small molecule inhibitors for other exportins have not yet been identified.
Several small molecule inhibitors of the nuclear import pathway have been reported. Karyostatin 1A identified by screening for chemical compounds binds specifically to importin β1. It inhibits the interaction of importin β1 with Ran-GTP, resulting in the failure of the classical importin α/β1 pathway in vivo and in vitro (Hintersteiner et al. 2010). A small peptide mimetic inhibitor specific to the importin α/β1 pathway was also developed; however, its potency is low with an IC50 value of 106 μm (Ambrus et al. 2010). Furthermore, hematoxylin is known to be a pathway-specific inhibitor for the nuclear import of human immunodeficiency virus type 1 (HIV1) Vpr (Suzuki et al. 2009). In the presence of hematoxylin, the interaction between importin α and HIV1 Vpr NLS is inhibited in a dose-dependent manner, whereas nuclear import of classical NLS-containing cargos is not affected by hematoxylin (Suzuki et al. 2009). Although a micromolar concentration of hematoxylin is required to inhibit the HIV1 replication in macrophages, the discovery of specific inhibitors of viral proteins that affect nuclear transport hints at a new strategy for antiviral drug discovery.
Particular NLS or NES sequence peptides have been used to inhibit specific nuclear transport pathways. Peptide inhibitors are thought to have higher affinities for their targets than small molecule inhibitors, because they can interact with a larger contact area of their target molecules compared with small molecules. NFκB NLS-derived peptide inhibitors (SN50 and SN52) with cell-permeable sequence block the cargo–importin α association and prevent the expression of inflammatory genes by inhibiting NFκB pathways (Lin et al. 1995; Xu et al. 2008). Similar NLS- or NES-based inhibitors have been reported (Flint et al. 2005; Kaiser et al. 2009).
These peptides can act as competitors for specific transport pathways, but a high dosage is required for effective inhibition. Therefore, much effort has been made to design high-affinity peptide inhibitors specific to certain nuclear–cytoplasmic transport pathways. Bimax series of peptides are specific inhibitors of the classical importin α/β1 transport pathway (Kosugi et al. 2008). Two peptides named Bimax1 and Bimax2 were developed by systematic mutational analysis combined with activity-based profiling. These peptides bind to importin α very tightly with Kd values in the picomolar range, which is 100 times higher than the affinity of the SV40 NLS (Kosugi et al. 2008). Thus, Ran-GTP cannot dissociate the importin α–Bimax complex, resulting in the inhibition of nuclear import pathway mediated by classical importin α/β1.
In addition, a specific inhibitor of the importin β family member was also developed. The hnRNP A1 NLS, called M9, is recognized by transportin/Kapβ2 (Pollard et al. 1996). Structure-based design allowed the development of a high-affinity peptide specific to transportin, named M9M (Cansizoglu et al. 2007). It strongly binds to Kapβ2/transportin (200 times higher than original M9) and decreases the dissociation efficiency by Ran-GTP (Cansizoglu et al. 2007). These peptide inhibitors are expected to be used more broadly for studying the biological function of target transport factors.
Concluding remarks
Proper nuclear–cytoplasmic protein transport is essential to maintain cell homeostasis. While evidence for the negative regulation of nuclear–cytoplasmic protein trafficking has accumulated, the precise mechanisms and physiological significance remain unclear in most cases. However, it is interesting to know how a virus escapes from cellular immune response by inhibiting nuclear–cytoplasmic transport, because viral inhibitory factors will be good targets for antiviral drug. Furthermore, understanding whether only importin α functions as a negative regulator of nuclear protein transport, and precisely what its physiological significance is, will provide new insights into the nuclear–cytoplasmic transport field. In addition, spatiotemporal regulation of the function of nuclear proteins by specific inhibitors will provide a new strategy for regulating cell function and reveal new drug targets for a variety of diseases.
References
- Ambrus G, Whitby LR, Singer EL, Trott O, Choi E, Olson AJ, Boger DL, Gerace L. Small molecule peptidomimetic inhibitors of importin alpha/beta mediated nuclear transport. Bioorg. Med. Chem. 2010;18:7611–7620. doi: 10.1016/j.bmc.2010.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasheva S, Fish A, Fornerod M, Frolova EI. Venezuelan equine Encephalitis virus capsid protein forms a tetrameric complex with CRM1 and importin alpha/beta that obstructs nuclear pore complex function. J. Virol. 2010;84:4158–4171. doi: 10.1128/JVI.02554-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atasheva S, Garmashova N, Frolov I, Frolova E. Venezuelan equine encephalitis virus capsid protein inhibits nuclear import in Mammalian but not in mosquito cells. J. Virol. 2008;82:4028–4041. doi: 10.1128/JVI.02330-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basler CF, Wang X, Muhlberger E, Volchkov V, Paragas J, Klenk HD, Garcia-Sastre A, Palese P. The Ebola virus VP35 protein functions as a type I IFN antagonist. Proc. Natl Acad. Sci. USA. 2000;97:12289–12294. doi: 10.1073/pnas.220398297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley BJ, Marshall ZM, Whorton AR. Nitric oxide stimulates Nrf2 nuclear translocation in vascular endothelium. Biochem. Biophys. Res. Commun. 2003;307:973–979. doi: 10.1016/s0006-291x(03)01308-1. [DOI] [PubMed] [Google Scholar]
- Cansizoglu AE, Lee BJ, Zhang ZC, Fontoura BM, Chook YM. Structure-based design of a pathway-specific nuclear import inhibitor. Nat. Struct. Mol. Biol. 2007;14:452–454. doi: 10.1038/nsmb1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardarelli F, Tosti L, Serresi M, Beltram F, Bizzarri R. Fluorescent recovery after photobleaching (FRAP) analysis of nuclear export rates identifies intrinsic features of nucleocytoplasmic transport. J. Biol. Chem. 2012;287:5554–5561. doi: 10.1074/jbc.M111.304899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KM, Lee J, Kim JE, Song OK, Cho S, Lim J, Seedorf M, Hahm B, Jang SK. Nonstructural protein 5A of hepatitis C virus inhibits the function of karyopherin beta3. J. Virol. 2000;74:5233–5241. doi: 10.1128/jvi.74.11.5233-5241.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crampton N, Kodiha M, Shrivastava S, Umar R, Stochaj U. Oxidative stress inhibits nuclear protein export by multiple mechanisms that target FG nucleoporins and Crm1. Mol. Biol. Cell. 2009;20:5106–5116. doi: 10.1091/mbc.E09-05-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronshaw J, Krutchinsky A, Zhang W, Chait B, Matunis M. Proteomic analysis of the mammalian nuclear pore complex. J. Cell Biol. 2002;158:915–927. doi: 10.1083/jcb.200206106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daelemans D, Afonina E, Nilsson J, Werner G, Kjems J, De Clercq E, Pavlakis GN, Vandamme AM. A synthetic HIV-1 Rev inhibitor interfering with the CRM1-mediated nuclear export. Proc. Natl Acad. Sci. USA. 2002;99:14440–14445. doi: 10.1073/pnas.212285299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint SJ, Huang W, Goodhouse J, Kyin S. A peptide inhibitor of exportin1 blocks shuttling of the adenoviral E1B 55 kDa protein but not export of viral late mRNAs. Virology. 2005;337:7–17. doi: 10.1016/j.virol.2005.04.007. [DOI] [PubMed] [Google Scholar]
- Fornerod M, Ohno M, Yoshida M, Mattaj IW. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. doi: 10.1016/s0092-8674(00)80371-2. [DOI] [PubMed] [Google Scholar]
- Forwood JK, Jans DA. Nuclear import pathway of the telomere elongation suppressor TRF1: inhibition by importin alpha. Biochemistry. 2002;41:9333–9340. doi: 10.1021/bi025548s. [DOI] [PubMed] [Google Scholar]
- Frey S, Richter RP, Görlich D. FG-rich repeats of nuclear pore proteins form a three-dimensional meshwork with hydrogel-like properties. Science. 2006;314:815–817. doi: 10.1126/science.1132516. [DOI] [PubMed] [Google Scholar]
- Fukuda M, Asano S, Nakamura T, Adachi M, Yoshida M, Yanagida M, Nishida E. CRM1 is responsible for intracellular transport mediated by the nuclear export signal. Nature. 1997;390:308–311. doi: 10.1038/36894. [DOI] [PubMed] [Google Scholar]
- Furuta M, Kose S, Koike M, Shimi T, Hiraoka Y, Yoneda Y, Haraguchi T, Imamoto N. Heat-shock induced nuclear retention and recycling inhibition of importin alpha. Genes Cells. 2004;9:429–441. doi: 10.1111/j.1356-9597.2004.00734.x. [DOI] [PubMed] [Google Scholar]
- Gustin KE, Sarnow P. Inhibition of nuclear import and alteration of nuclear pore complex composition by rhinovirus. J. Virol. 2002;76:8787–8796. doi: 10.1128/JVI.76.17.8787-8796.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamoto T, Gunji S, Tsuji H, Beppu T. Leptomycins A and B, new antifungal antibiotics. I. Taxonomy of the producing strain and their fermentation, purification and characterization. J. Antibiot. (Tokyo) 1983;36:639–645. doi: 10.7164/antibiotics.36.639. [DOI] [PubMed] [Google Scholar]
- Hara MR, Agrawal N, Kim SF, Cascio MB, Fujimuro M, Ozeki Y, Takahashi M, Cheah JH, Tankou SK, Hester LD, Ferris CD, Hayward SD, Snyder SH, Sawa A. S-nitrosylated GAPDH initiates apoptotic cell death by nuclear translocation following Siah1 binding. Nat. Cell Biol. 2005;7:665–674. doi: 10.1038/ncb1268. [DOI] [PubMed] [Google Scholar]
- Harcourt B, Sanchez A, Offermann M. Ebola virus selectively inhibits responses to interferons, but not to interleukin-1beta, in endothelial cells. J. Virol. 1999;73:3491–3496. doi: 10.1128/jvi.73.4.3491-3496.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DT, Matsumoto A, Kim SO, Marshall HE, Stamler JS. Protein S-nitrosylation: purview and parameters. Nat. Rev. Mol. Cell Biol. 2005;6:150–166. doi: 10.1038/nrm1569. [DOI] [PubMed] [Google Scholar]
- Hilliard M, Frohnert C, Spillner C, Marcone S, Nath A, Lampe T, Fitzgerald DJ, Kehlenbach RH. The anti-inflammatory prostaglandin 15-deoxy-delta(12,14)-PGJ2 inhibits CRM1-dependent nuclear protein export. J. Biol. Chem. 2010;285:22202–22210. doi: 10.1074/jbc.M110.131821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hintersteiner M, Ambrus G, Bednenko J, Schmied M, Knox AJ, Meisner NC, Gstach H, Seifert JM, Singer EL, Gerace L, Auer M. Identification of a small molecule inhibitor of importin beta mediated nuclear import by confocal on-bead screening of tagged one-bead one-compound libraries. ACS Chem. Biol. 2010;5:967–979. doi: 10.1021/cb100094k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway G, Truong TT, Coulson BS. Rotavirus antagonizes cellular antiviral responses by inhibiting the nuclear accumulation of STAT1, STAT2, and NF-κB. J. Virol. 2009;83:4942–4951. doi: 10.1128/JVI.01450-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jäkel S, Albig W, Kutay U, Bischoff FR, Schwamborn K, Doenecke D, Görlich D. The importin beta/importin 7 heterodimer is a functional nuclear import receptor for histone H1. EMBO J. 1999;18:2411–2423. doi: 10.1093/emboj/18.9.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jans DA, Xiao CY, Lam MH. Nuclear targeting signal recognition: a key control point in nuclear transport? Bioessays. 2000;22:532–544. doi: 10.1002/(SICI)1521-1878(200006)22:6<532::AID-BIES6>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Kaiser N, Lischka P, Wagenknecht N, Stamminger T. Inhibition of human cytomegalovirus replication via peptide aptamers directed against the nonconventional nuclear localization signal of the essential viral replication factor pUL84. J. Virol. 2009;83:11902–11913. doi: 10.1128/JVI.01378-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kash J, Mühlberger E, Carter V, Grosch M, Perwitasari O, Proll S, Thomas M, Weber F, Klenk H, Katze M. Global suppression of the host antiviral response by Ebola- and Marburgviruses: increased antagonism of the type I interferon response is associated with enhanced virulence. J. Virol. 2006;80:3009–3020. doi: 10.1128/JVI.80.6.3009-3020.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehlenbach RH, Gerace L. Phosphorylation of the nuclear transport machinery down-regulates nuclear protein import in vitro. J. Biol. Chem. 2000;275:17848–17856. doi: 10.1074/jbc.M001455200. [DOI] [PubMed] [Google Scholar]
- Kima PE. The amastigote forms of Leishmania are experts at exploiting host cell processes to establish infection and persist. Int. J. Parasitol. 2007;37:1087–1096. doi: 10.1016/j.ijpara.2007.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodiha M, Bański P, Ho-Wo-Cheong D, Stochaj U. Dissection of the molecular mechanisms that control the nuclear accumulation of transport factors importin-alpha and CAS in stressed cells. Cell. Mol. Life Sci. 2008a;65:1756–1767. doi: 10.1007/s00018-008-7588-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodiha M, Chu A, Matusiewicz N, Stochaj U. Multiple mechanisms promote the inhibition of classical nuclear import upon exposure to severe oxidative stress. Cell Death Diff. 2004;11:862–874. doi: 10.1038/sj.cdd.4401432. [DOI] [PubMed] [Google Scholar]
- Kodiha M, Tran D, Qian C, Morogan A, Presley JF, Brown CM, Stochaj U. Oxidative stress mislocalizes and retains transport factor importin-alpha and nucleoporins Nup153 and Nup88 in nuclei where they generate high molecular mass complexes. Biochim. Biophys. Acta. 2008b;1783:405–418. doi: 10.1016/j.bbamcr.2007.10.022. [DOI] [PubMed] [Google Scholar]
- Köster M, Lykke-Andersen S, Elnakady YA, Gerth K, Washausen P, Hofle G, Sasse F, Kjems J, Hauser H. Ratjadones inhibit nuclear export by blocking CRM1/exportin 1. Exp. Cell Res. 2003;286:321–331. doi: 10.1016/s0014-4827(03)00100-9. [DOI] [PubMed] [Google Scholar]
- Kosugi S, Hasebe M, Entani T, Takayama S, Tomita M, Yanagawa H. Design of peptide inhibitors for the importin alpha/beta nuclear import pathway by activity-based profiling. Chem. Biol. 2008;15:940–949. doi: 10.1016/j.chembiol.2008.07.019. [DOI] [PubMed] [Google Scholar]
- Kotera I, Sekimoto T, Miyamoto Y, Saiwaki T, Nagoshi E, Sakagami H, Kondo H, Yoneda Y. Importin alpha transports CaMKIV to the nucleus without utilizing importin beta. EMBO J. 2005;24:942–951. doi: 10.1038/sj.emboj.7600587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawczyk E, Hanover JA, Schlegel R, Suprynowicz FA. Karyopherin beta3: a new cellular target for the HPV-16 E5 oncoprotein. Biochem. Biophys. Res. Commun. 2008;371:684–688. doi: 10.1016/j.bbrc.2008.04.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Khochbin S, Nishi K, Kitano K, Yanagida M, Yoshida M, Horinouchi S. Molecular cloning and cell cycle-dependent expression of mammalian CRM1, a protein involved in nuclear export of proteins. J. Biol. Chem. 1997;272:29742–29751. doi: 10.1074/jbc.272.47.29742. [DOI] [PubMed] [Google Scholar]
- Kudo N, Matsumori N, Taoka H, Fujiwara D, Schreiner EP, Wolff B, Yoshida M, Horinouchi S. Leptomycin B inactivates CRM1/exportin 1 by covalent modification at a cysteine residue in the central conserved region. Proc. Natl Acad. Sci. USA. 1999;96:9112–9117. doi: 10.1073/pnas.96.16.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutay U, Güttinger S. Leucine-rich nuclear-export signals: born to be weak. Trends Cell Biol. 2005;15:121–124. doi: 10.1016/j.tcb.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Lam MH, Briggs LJ, Hu W, Martin TJ, Gillespie MT, Jans DA. Importin beta recognizes parathyroid hormone-related protein with high affinity and mediates its nuclear import in the absence of importin alpha. J. Biol. Chem. 1999;274:7391–7398. doi: 10.1074/jbc.274.11.7391. [DOI] [PubMed] [Google Scholar]
- Lam YW, Yuan Y, Isaac J, Babu CV, Meller J, Ho SM. Comprehensive identification and modified-site mapping of S-nitrosylated targets in prostate epithelial cells. PLoS ONE. 2010;5:e9075. doi: 10.1371/journal.pone.0009075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy D, García-Sastre A. The virus battles: IFN induction of the antiviral state and mechanisms of viral evasion. Cytokine Growth Factor Rev. 2001;12:143–156. doi: 10.1016/s1359-6101(00)00027-7. [DOI] [PubMed] [Google Scholar]
- Lim RY, Huang NP, Köser J, Deng J, Lau KH, Schwarz-Herion K, Fahrenkrog B, Aebi U. Flexible phenylalanine-glycine nucleoporins as entropic barriers to nucleocytoplasmic transport. Proc. Natl Acad. Sci. USA. 2006;103:9512–9517. doi: 10.1073/pnas.0603521103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim RY, Ullman KS, Fahrenkrog B. Biology and biophysics of the nuclear pore complex and its components. Int. Rev. Cell Mol. Biol. 2008;267:299–342. doi: 10.1016/S1937-6448(08)00632-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin YZ, Yao SY, Veach RA, Torgerson TR, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-kappa B by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J. Biol. Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- Lüder CG, Walter W, Beuerle B, Maeurer MJ, Gross U. Toxoplasma gondii down-regulates MHC class II gene expression and antigen presentation by murine macrophages via interference with nuclear translocation of STAT1alpha. Eur. J. Immunol. 2001;31:1475–1484. doi: 10.1002/1521-4141(200105)31:5<1475::AID-IMMU1475>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Major AT, Whiley PA, Loveland KL. Expression of nucleocytoplasmic transport machinery: clues to regulation of spermatogenic development. Biochim. Biophys. Acta. 2011;1813:1668–1688. doi: 10.1016/j.bbamcr.2011.03.008. [DOI] [PubMed] [Google Scholar]
- Mateo M, Reid SP, Leung LW, Basler CF, Volchkov VE. Ebolavirus VP24 binding to karyopherins is required for inhibition of interferon signaling. J. Virol. 2010;84:1169–1175. doi: 10.1128/JVI.01372-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matte C, Descoteaux A. Leishmania donovani amastigotes impair gamma interferon-induced STAT1alpha nuclear translocation by blocking the interaction between STAT1alpha and importin-alpha5. Infect. Immun. 2010;78:3736–3743. doi: 10.1128/IAI.00046-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner T, Krause E, Vinkemeier U. Ratjadone and leptomycin B block CRM1-dependent nuclear export by identical mechanisms. FEBS Lett. 2004;576:27–30. doi: 10.1016/j.febslet.2004.08.056. [DOI] [PubMed] [Google Scholar]
- Melchior F, Gerace L. Two-way trafficking with Ran. Trends Cell Biol. 1998;8:175–179. doi: 10.1016/s0962-8924(98)01252-5. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Hieda M, Harreman MT, Fukumoto M, Saiwaki T, Hodel AE, Corbett AH, Yoneda Y. Importin alpha can migrate into the nucleus in an importin beta- and Ran-independent manner. EMBO J. 2002;21:5833–5842. doi: 10.1093/emboj/cdf569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Saiwaki T, Yamashita J, Yasuda Y, Kotera I, Shibata S, Shigeta M, Hiraoka Y, Haraguchi T, Yoneda Y. Cellular stresses induce the nuclear accumulation of importin alpha and cause a conventional nuclear import block. J. Cell Biol. 2004;165:617–623. doi: 10.1083/jcb.200312008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monovich L, Koch KA, Burgis R, et al. Suppression of HDAC nuclear export and cardiomyocyte hypertrophy by novel irreversible inhibitors of CRM1. Biochim. Biophys. Acta. 2009;1789:422–431. doi: 10.1016/j.bbagrm.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Mutka SC, Yang WQ, Dong SD, Ward SL, Craig DA, Timmermans PB, Murli S. Identification of nuclear export inhibitors with potent anticancer activity in vivo. Cancer Res. 2009;69:510–517. doi: 10.1158/0008-5472.CAN-08-0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson LM, Rose RC, Moroianu J. The L1 major capsid protein of human papillomavirus type 11 interacts with Kap beta2 and Kap beta3 nuclear import receptors. Virology. 2003;306:162–169. doi: 10.1016/s0042-6822(02)00025-9. [DOI] [PubMed] [Google Scholar]
- Petersen J, Her L, Varvel V, Lund E, Dahlberg J. The matrix protein of vesicular stomatitis virus inhibits nucleocytoplasmic transport when it is in the nucleus and associated with nuclear pore complexes. Mol. Cell. Biol. 2000;20:8590–8601. doi: 10.1128/mcb.20.22.8590-8601.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard VW, Michael WM, Nakielny S, Siomi MC, Wang F, Dreyfuss G. A novel receptor-mediated nuclear protein import pathway. Cell. 1996;86:985–994. doi: 10.1016/s0092-8674(00)80173-7. [DOI] [PubMed] [Google Scholar]
- Pyhtila B, Rexach M. A gradient of affinity for the karyopherin Kap95p along the yeast nuclear pore complex. J. Biol. Chem. 2003;278:42699–42709. doi: 10.1074/jbc.M307135200. [DOI] [PubMed] [Google Scholar]
- Qu J, Liu GH, Huang B, Chen C. Nitric oxide controls nuclear export of APE1/Ref-1 through S-nitrosation of cysteines 93 and 310. Nucleic Acids Res. 2007;35:2522–2532. doi: 10.1093/nar/gkl1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid SP, Cardenas WB, Basler CF. Homo-oligomerization facilitates the interferon-antagonist activity of the ebolavirus VP35 protein. Virology. 2005;341:179–189. doi: 10.1016/j.virol.2005.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid SP, Leung LW, Hartman AL, Martinez O, Shaw ML, Carbonnelle C, Volchkov VE, Nichol ST, Basler CF. Ebola virus VP24 binds karyopherin alpha1 and blocks STAT1 nuclear accumulation. J. Virol. 2006;80:5156–5167. doi: 10.1128/JVI.02349-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid SP, Valmas C, Martinez O, Sanchez FM, Basler CF. Ebola virus VP24 proteins inhibit the interaction of NPI-1 subfamily karyopherin alpha proteins with activated STAT1. J. Virol. 2007;81:13469–13477. doi: 10.1128/JVI.01097-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbeck K, Görlich D. Kinetic analysis of translocation through nuclear pore complexes. EMBO J. 2001;20:1320–1330. doi: 10.1093/emboj/20.6.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rout M, Aitchison J, Suprapto A, Hjertaas K, Zhao Y, Chait B. The yeast nuclear pore complex: composition, architecture, and transport mechanism. J. Cell Biol. 2000;148:636–651. doi: 10.1083/jcb.148.4.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimoto T, Imamoto N, Nakajima K, Hirano T, Yoneda Y. Extracellular signal-dependent nuclear import of Stat1 is mediated by nuclear pore-targeting complex formation with NPI-1, but not Rch1. EMBO J. 1997;16:7067–7077. doi: 10.1093/emboj/16.23.7067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekimoto T, Miyamoto Y, Arai S, Yoneda Y. Importin alpha protein acts as a negative regulator for Snail protein nuclear import. J. Biol. Chem. 2011;286:15126–15131. doi: 10.1074/jbc.M110.213579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stochaj U, Rassadi R, Chiu J. Stress-mediated inhibition of the classical nuclear protein import pathway and nuclear accumulation of the small GTPase Gsp1p. FASEB J. 2000;14:2130–2132. doi: 10.1096/fj.99-0751fje. [DOI] [PubMed] [Google Scholar]
- Stoffler D, Fahrenkrog B, Aebi U. The nuclear pore complex: from molecular architecture to functional dynamics. Curr. Opin. Cell Biol. 1999;11:391–401. doi: 10.1016/S0955-0674(99)80055-6. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Yamamoto N, Nonaka M, Hashimoto Y, Matsuda G, Takeshima SN, Matsuyama M, Igarashi T, Miura T, Tanaka R, Kato S, Aida Y. Inhibition of human immunodeficiency virus type 1 (HIV-1) nuclear import via Vpr-Importin alpha interactions as a novel HIV-1 therapy. Biochem. Biophys. Res. Commun. 2009;380:838–843. doi: 10.1016/j.bbrc.2009.01.180. [DOI] [PubMed] [Google Scholar]
- Takayama I, Sato H, Watanabe A, Omi-Furutani M, Sugai A, Kanki K, Yoneda M, Kai C. The nucleocapsid protein of measles virus blocks host interferon response. Virology. 2012;424:45–55. doi: 10.1016/j.virol.2011.12.011. [DOI] [PubMed] [Google Scholar]
- Van Neck T, Pannecouque C, Vanstreels E, Stevens M, Dehaen W, Daelemans D. Inhibition of the CRM1-mediated nucleocytoplasmic transport by N-azolylacrylates: structure-activity relationship and mechanism of action. Bioorg. Med. Chem. 2008;16:9487–9497. doi: 10.1016/j.bmc.2008.09.051. [DOI] [PubMed] [Google Scholar]
- Wang P, Liu GH, Wu K, Qu J, Huang B, Zhang X, Zhou X, Gerace L, Chen C. Repression of classical nuclear export by S-nitrosylation of CRM1. J. Cell Sci. 2009;122:3772–3779. doi: 10.1242/jcs.057026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Fang F, St Clair DK, Sompol P, Josson S, St Clair WH. SN52, a novel nuclear factor-kappaB inhibitor, blocks nuclear import of RelB:p52 dimer and sensitizes prostate cancer cells to ionizing radiation. Mol. Cancer Ther. 2008;7:2367–2376. doi: 10.1158/1535-7163.MCT-08-0238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H, Sekimoto T, Ohkubo T, Douchi T, Nagata Y, Ozawa M, Yoneda Y. Zinc finger domain of Snail functions as a nuclear localization signal for importin beta-mediated nuclear import pathway. Genes Cells. 2005;10:455–464. doi: 10.1111/j.1365-2443.2005.00850.x. [DOI] [PubMed] [Google Scholar]
- Yasuda Y, Miyamoto Y, Saiwaki T, Yoneda Y. Mechanism of the stress-induced collapse of the Ran distribution. Exp. Cell Res. 2006;312:512–520. doi: 10.1016/j.yexcr.2005.11.017. [DOI] [PubMed] [Google Scholar]


