Abstract
Hydrocephalic hyh mutant mice undergo a programmed loss of the neuroepithelium/ependyma followed by a reaction of periventricular astrocytes, which form a new cell layer covering the denuded ventricular surface. We present a comparative morphological and functional study of the newly formed layer of astrocytes and the multiciliated ependyma of hyh mice. Transmission electron microscopy, immunocytochemistry for junction proteins (N-cadherin, connexin 43) and proteins involved in permeability (aquaporin 4) and endocytosis (caveolin-1, EEA1) were used. Horseradish peroxidase (HRP) and lanthanum nitrate were used to trace the intracellular and paracellular transport routes. The astrocyte layer shares several cytological features with the normal multiciliated ependyma, such as numerous microvilli projected into the ventricle, extensive cell–cell interdigitations and connexin 43-based gap junctions, suggesting that these astrocytes are coupled to play an unknown function as a cell layer. The ependyma and the astrocyte layers also share transport properties: (1) high expression of aquaporin 4, caveolin-1 and the endosome marker EEA1; (2) internalization into endocytic vesicles and early endosomes of HRP injected into the ventricle; (3) and a similar paracellular route of molecules moving between CSF, the subependymal neuropile and the pericapillary space, as shown by lanthanum nitrate and HRP. A parallel analysis performed in human hydrocephalic foetuses indicated that a similar phenomenon would occur in humans. We suggest that in foetal-onset hydrocephalus, the astrocyte assembly at the denuded ventricular walls functions as a CSF–brain barrier involved in water and solute transport, thus contributing to re-establish lost functions at the brain parenchyma–CSF interphase.
Electronic supplementary material
The online version of this article (doi:10.1007/s00401-012-0992-6) contains supplementary material, which is available to authorized users.
Keywords: Cerebrospinal fluid, Congenital hydrocephalus, Ependyma disruption, Astrocyte reaction, Barrier properties, Permeability, Transport, hyh mice, Human
Introduction
Congenital hydrocephalus is a developmental brain disorder. In the humans, its incidence is approximately 1–3 in every 1,000 live births. Dilatation of the brain ventricles and elevation of intraventricular pressure in rats with congenital or acquired hydrocephalus have harmful effects on the parenchyma and lead to oedema, oxidative stress [57], proteolytic damages in the white matter [15], cell death and reactive changes in glial cells [17]. Some of these alterations have also been reported in human chronic hydrocephalus [16]. Under these abnormal brain conditions, some mechanisms can be triggered to partially re-establish brain homoeostasis [31].
Astroglial reactions triggered by brain injuries are characterized by the hypertrophy and hyperplasia of astroglial cells. Astrocyte reactions have been reported to inhibit axonal regeneration [7], but have also been associated with the secretion of growth factors and trophic molecules [19, 24, 47, 49] such as NGF, IGF-I and bFGF that promote axonal re-growth [47]. It has been suggested that the astrocyte reaction initially protects the brain tissue and contributes to its functional recovery [22]. Therefore, the beneficial and detrimental functional consequences of these astroglial reactions are under debate [36, 58, 59]. The astrocyte reaction that occurs in the brain of hydrocephalic animals has been thought to be a harmful phenomenon, leading some authors to test the effects of anti-inflammatory drugs in rats that have been made hydrocephalic postnatally [35, 36].
There is a large body of evidence indicating that the neuroepithelium/ependyma lining the ventricular walls of the developing brain plays a key role in the onset and evolution of congenital hydrocephalus [1–3, 18, 26, 52, 55, 61, 65]. There are several ependymal cell lineages lining distinct regions of the ventricular walls. In the cerebral aqueduct of hydrocephalic hyh mice, different types of ependymal cells have been reported [65]. Some of these types disrupt, some remain unaffected and other ones proliferate [4, 65]. Recently, different types of ependymal cells have been described in the cerebral aqueduct of human foetuses [55]. Ependymal specializations also occur in other regions of the ventricular system [50]. Most of the different ependymal populations are multiciliated. Their cilia beat in a synchronized manner and thus contribute to the flow of the cerebrospinal fluid (CSF) [43, 68, 69]. Other functions have also been assigned to the multiciliated ependyma, including the regulation of the interaction between the ventricular CSF and the brain extracellular fluid, the clearance of metabolic substances and neurotransmitters and the mediation of adhesion of inflammatory cells [14, 65]. We have previously reported that foetal-onset hydrocephalus in mutant hyh mice and in human foetuses is associated with defects in the neuroepithelium/ependyma [18, 30, 43]. Such defects result in the loss of the neuroepithelium/ependyma and in a subsequent astroglial reaction that leads to the development of a new cell layer lining the denuded ventricular surface [18, 41, 65]. The functional significance of the new brain parenchyma/CSF interphase formed by reactive astrocytes is not known.
The present study has been designed to help elucidate the role of the periventricular astroglial reaction in congenital hydrocephalus. The study was performed on hyh (hydrocephalus with hop gait) [11] mutant mice, in which the onset and evolution of hydrocephalus resemble that of human congenital hydrocephalus. Two phases have been recognized in the development of congenital hydrocephalus in the hyh mutant mouse. During embryonic life, the neuroependyma disruption of the ventral fourth ventricle and cerebral aqueduct is followed by a moderate communicating hydrocephalus. During the first postnatal week, the dorsal wall of the cerebral aqueduct becomes disrupted triggering aqueduct obliteration and the onset of a severe hydrocephalus [65]. The investigation was carried out at the postnatal stages when hydrocephalus is severe and astrogliogenesis and astrocyte reaction are completed [41]. The study also analysed the astrocyte reaction occurring in the denuded ventricular walls of human hydrocephalic foetuses.
Materials and methods
Animals
Mutant hyh mice (hydrocephalus with hop gait, B6C3Fe-a/a-hyh/J strain) and their control littermate wild-type (wt) mice were used [11]. The hyh mouse carries a point mutation in the Napa gene that encodes α-Snap [13, 25], a protein involved in membrane fusion events. Mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA) and bred into two colonies, one at the Animal Experimentation Service of the University of Malaga and the other at the Medical School of the Austral University of Chile, Valdivia, Chile. The housing, handling, care and processing of the animals were conducted in accordance with the European and Spanish laws (DC 86/609/CEE and RD 1201/2005) and following the regulations approved by the council of the American Physiological Society. Wt and mutant hyh mice were identified by clinical inspection and genotyping [5]. The animals were anesthetized with intraperitoneally administered Dolethal (sodium pentobarbital; Vétoquinol, Lure, France; 0.2 mg/g bodyweight) and killed at the postnatal (P) ages detailed in Table 1.
Table 1.
Number of animals by age and genotype used in each experiment
| Experiment | Immunocytochemistry | Immunofluorescence | HRP tracing (light microscopy) | HRP tracing (electron microscopy) | Lanthanum tracing | Scanning electron microscopy |
|---|---|---|---|---|---|---|
| Animal postnatal age in days (number/genotype) | 10, 14, 20, 30 (4 wt and 4 hyh each age) | 6 (6 wt, 6 hyh), 14 (6 wt, 6 hyh), 20 (7 wt, 7 hyh), 30 (3 wt, 3 hyh) | 3, 8, 14, 30 (2 wt and 4 hyh each age) | 3 (2 each condition), 30 (2 wt, 4 hyh) | 3 (3 wt, 5 hyh), 6 (3 wt, 5 hyh), 10 (3 wt, 5 hyh), 20 (5 wt, 5 hyh), 30 (3 wt, 5 hyh) | 14 (6 hyh), 20 (10 hyh) |
Human foetuses
Paraffin sections of brains from 8 foetuses presenting communicating hydrocephalus and 15 control foetuses were used (for further information concerning this material see [18]).
Immunocytochemistry
Wt and hyh mice were transcardially perfused with Bouin fixative. The brain was dissected out and further fixed by immersion in Bouin fixative for 2 days. Serial paraffin sections were obtained and adjacent sections were incubated with a series of primary antibodies (Table 2). For further details, see Supplementary data.
Table 2.
Primary antibodies used
| Antibody (reference) | Source | Type | Dilution | Molecule/structure labelling |
|---|---|---|---|---|
| Aquaporin 4 (A5971) | Sigma | Rabbit polyclonal | 1:400 | Water channel protein |
| Caveolin-1 (N-20) | Santa Cruz Biotechnology, INC, San Diego, CA, USA | Rabbit polyclonal | 1:200 | Caveolae |
| Connexin 43 | a | Rabbit polyclonal | 1:750 | Gap junctions |
| EEA1 (PA1-063) | Affinity Bioreagents INC, Gonden, CO, USA | Rabbit polyclonal | 1:200 | Early endosome antigen 1 |
| GFAP (4650) | Biogenesis, Oxford, UK | Rabbit polyclonal | 1:250 | GFAP intermediate filaments |
| GFAP (4674) | Abcam, Cambridge, UK | Mouse monoclonal | 1:1,000 | GFAP intermediate filaments |
| GLUT1 | b | Rabbit polyclonal | 1:1,000 | Glucose transporter 1 |
| HRP | c | Rabbit polyclonal | 1:1,000 | Injected HRP |
| N-Cadherin (sc-8939) | Santa Cruz Biotechnology | Rabbit polyclonal | 1:50 | N-Cadherin (adherens junctions) |
| S100β (ab52642) | Abcam | Rabbit polyclonal | 1:200 | S100β |
| TGN46 (ab16059) | Abcam | Rabbit polyclonal | 1:500 | Trans-Golgi network |
| Tubulin βIV (T7941) | Sigma | Mouse monoclonal | 1:400 | Ependymal cilia |
| Tubulin βIV (ab11315) | Abcam | Mouse monoclonal | 1:100 | Ependymal cilia |
| Vimentin (V4630) | Sigma, St Louis, MO, USA | Goat polyclonal | 1:500 | Vimentin intermediate filaments |
aKindly provided by JC Sáez, Catholic University of Chile
bKindly provided by CI Ribas, Memorial Sloan-Kettering Cancer Center, NY, USA
cDeveloped at the Department of Cell Biology, Genetics and Physiology, University of Malaga, Spain
Single and double immunofluorescence and confocal microscopy
Wt and hyh mice (Table 1) were transcardially perfused with Bouin fixative or 4 % paraformaldehyde diluted in 0.1 M phosphate buffer (PB), pH 7.4. Bouin-fixed brains from P6 (6 days of age) and P30 mice were used to obtain paraffin sections that were hydrated and immunostained. Paraformaldehyde-fixed brains from P14 and P20 mice were used to obtain frozen sections that were immunostained with a free-floating section-staining protocol. In four P20 mice, the ventricular walls of the lateral ventricles were dissected out to obtain whole mounts for immunostaining. After incubation in the primary antibody (Table 2), appropriate fluorescent secondary antibodies were used.
Adjacent sections from the series obtained from the brain of P6, P20 and P30 wt and hyh mice, and whole mounts from the lateral ventricles of P14 and P20 hyh mice were processed for double immunofluorescence. This procedure allowed the same brain regions to be analysed with a series of antibodies. For further details, see the Supplementary data.
Intracerebroventricular injections of horseradish peroxidase
Wt and hyh mice (Table 1) were anesthetized with 2,2,2-tribromoethanol (Sigma, 0.8 μg/g body weight). Wt and hyh mice were subperfused into the left lateral ventricle for 5 min with 1 μl (P3), 1.5 μl (P8), 2 μl (P14) and 2.5 μl (P30) of 3 % horseradish peroxidase (HRP) type IV (Sigma) in 0.9 % sodium chloride. The coordinates for injection in wt and hyh mice at different ages were previously calculated using injections with trypan blue. After the infusion, the needle remained at the injection site for an additional 15 min. The brains were processed to trace HRP at the light microscope. The brains were dissected out, fixed by immersion with Bouin fixative for 72 h, embedded in paraffin and serially sectioned. The sections were processed for the immunoperoxidase method using an anti-HRP antibody raised in rabbit in our laboratory. Adjacent sections were immunostained for GFAP.
To trace HRP with the electron microscope, wt and hyh mice at P3 and P30 (Table 1) were injected with the tracer into the left lateral ventricle, as described above. Five minutes after the injection, the animals were transcardially perfused with phosphate buffer containing 2 % paraformaldehyde and 2.5 % glutaraldehyde. Vibratome sections, 50-μm thick, were obtained and processed for the histochemical detection of HRP using DAB as the electron donor. The sections were postfixed in 1 % osmium tetroxide (Merck, Darmstadt, Germany) and flat embedded in Araldite 502. Ultrathin sections (60-nm thickness) were stained with lead citrate and studied under an electron microscope.
Lanthanum nitrate tracing at the electron microscope
To demonstrate the presence or absence of tight junctions in the ependymal and astrocyte barriers, lanthanum nitrate was used as a tracer under electron microscope [39, 48]. Wt and hyh mice (Table 1) were anesthetized and transcardially perfused with 2.5 % glutaraldehyde in 0.1 M cacodylate buffer, pH 7.4, and the brains were dissected out and immersed in fresh fixative for 1 h. After fixation, 2 % lanthanum nitrate in cacodylate buffer, pH 7.8, was delivered over 2 min into one of the lateral ventricles, the cerebral aqueduct or the fourth ventricle. The brains were further fixed in fresh fixative for 24 h at 4 °C. The walls of the cerebral aqueduct, the lateral, third and fourth ventricles, and the choroid plexus were dissected out and postfixed in 1 % osmium tetroxide in cacodylate buffer for 1 h at 4 °C. The tissue blocks were dehydrated and embedded in Araldite 502 (EMS, Hatfield, PA, USA). Ultrathin sections (60-nm thickness) were slightly stained with uranyl acetate and studied under an electron microscope (Philips CM100). The blood–brain barrier at the choroid plexus and the endothelial cells of the brain capillaries (presence of tight junctions) were used as controls.
Scanning electron microscopy
Killed hyh mice (Table 1) were used. Cold 2.5 % glutaraldehyde in phosphate buffer was injected into a lateral ventricle for 2 min. The brains were further fixed by immersion in the same fixative for 2 h at room temperature. Several areas of the ventricular cavities were dissected out and processed as previously described [30].
Data analysis
Morphometric, densitometric and image analyses were carried out to quantify several parameters. (1) The relative optic density of aquaporin 4 immunoreaction in ependymal cells, astrocyte cell bodies and perivascular endfeet of astrocytes was estimated. (2) The total area occupied by the early endosomal compartment (EEA-1 immunoreactivity) in ependymal cells or astrocytes was quantified. (3) The cell density of GFAP+ astrocytes at specific sites of the abnormal ventricles was estimated using whole mounts of ventricular walls of four hyh P20 mice processed for immunofluorescence for GFAP. (4) The degree of penetration of intraventricularly injected HRP into the brain parenchyma was estimated in sections immunostained using anti-HRP. See Supplementary data for a description of the procedures.
Results
In the description that follows, the morphological phenotype and the barrier properties of the multiciliated ependyma of wt mice will be compared with those of the multiciliated ependyma of hyh mice resisting denudation [41] and, of particular interest for the present study, with those of the astrocyte layer covering the denuded ventricular regions of the hyh mice. Most of this study were carried out at stages from P6 on, when the denudation process and the astrocyte reaction are completed [41] and a severe progressive hydrocephalus develops (Supplementary Fig. 1). After P6, most of the ventricular surface, with the exception of some specific sites, underwent denudation (Supplementary Fig. 2). The denudation sites resisting denudation are the circumventricular organs, the roof of the third ventricle, the roof of the middle region of the cerebral aqueduct and small patches consistently present at very specific sites of the aqueduct and lateral ventricles (Supplementary Fig. 2). All denuded areas presented periventricular astrocyte reactions. However, the description of the results will principally circumscribe to events occurring in the fourth and lateral ventricles. In hyh mice, the lateral ventricles are enormously expanded, whereas the fourth ventricle is not. However, both cavities undergo ependymal denudation and astrocyte reaction, indicating that such processes are associated with the genetic defect of these mice and not with the ventriculomegaly or increased intraventricular pressure. Therefore, it seemed relevant to compare the properties of the astrocytes lining the denuded areas in the fourth and lateral ventricles.
Cytoskeletal proteins in multiciliated ependyma and astrocytes covering the ventricular walls of wt and hyh mice
In mature wt mice, the multiciliated ependyma formed a single cell layer that was readily recognized by the expression of the S100β protein (Fig. 1a, e), tubulin βIV (Fig. 1f) and the intermediate filament protein vimentin (Fig. 1h). The ciliated ependyma did not express GFAP (Fig. 1g).
Fig. 1.
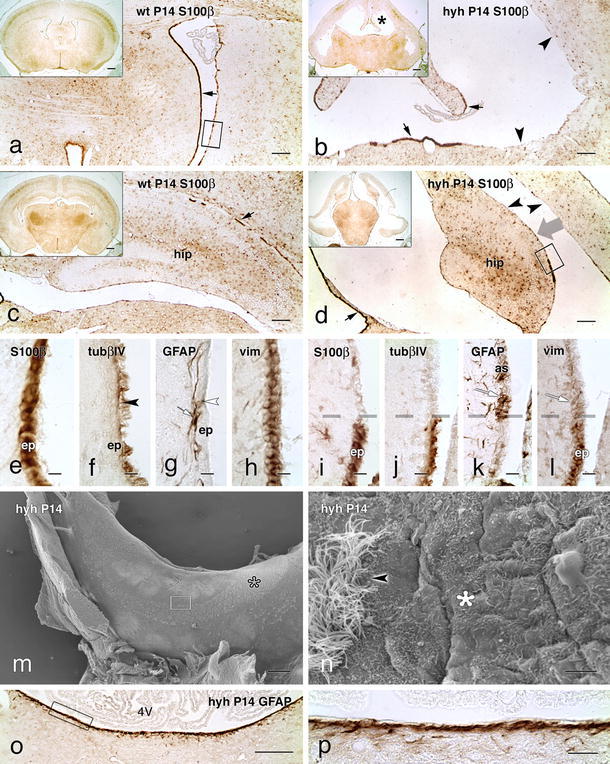
Expression of S100β and cytoskeleton proteins in the ependymal cells and astrocytes covering the ventricular surface of wt and hyh mice at P14. Frontal sections of the telencephalon and fourth ventricle (4V) of wt (a, c, e–h) and hyh (b, d, i–l, o, p) mice immunostained for S100β protein, tubulin βIV (tubβIV), GFAP and vimentin (vim). a–d The ependyma (ep) contains S100β protein (black arrows). In the hyh mice, the lateral ventricles are enlarged (black asterisk) and most of their surface is devoid of ependyma (black arrowheads). The thick grey arrow in d indicates the direction at which the area framed is shown under the scanning microscope as shown below in this panel. e–h Adjacent sections of an area similar to that framed in a. TubβIV is present in ependymal cilia (black arrowhead in f). In the wt mouse, GFAP+ astrocytes (as) lying in the subependymal (white arrow; in g) and ependymal (white arrowhead; in g) region are observed. i–l Adjacent sections of an area similar to that framed in d bordering the hippocampus. The broken line in i–l denotes the border between an ependymal patch resisting denudation (bottom) and the astrocyte layer covering the denuded surface (top). Reactive astrocytes are GFAP+ and vim+ (white arrows; in k, l). m, n Scanning electron microscopy of the hippocampus surface overlooking the lateral ventricle (for orientation, see thick arrow in d). A patch of ciliated ependyma partially covers the ventricle surface (open asterisk; in m). n A detailed view of the framed area in m, showing the border between the ependyma (arrowhead) and the astrocyte layer (asterisk). The area in the fourth ventricle in o is shown in p. A robust layer of GFAP+ astrocytes lines the floor of the denuded ventricle. Scale bars a–d 150 μm, e–l 10 μm, m 300 μm, n 5 μm, o 20 μm, p 200 μm; insets in a–d 500 μm
In mature hyh mice, most of the ventricular surface lacked ependyma due to its disruption during development (Fig. 1b, d) [29, 41]. Nevertheless, multiciliated ependymal cells that resisted denudation remained in situ as small patches that were consistently located at specific sites of the ventricular walls (Fig. 1b, d) [29, 41, 65]. In the areas lacking ependyma, astrocytes formed a glial layer that covered the denuded ventricular surface. These cells lacked cilia (Fig. 1j) and expressed the intermediate filament proteins GFAP (Fig. 1k, o, p) and vimentin (Fig. 1l).
Junction proteins in multiciliated ependyma and astrocytes lining the ventricular walls of wt and hyh mice
The junction protein N-cadherin was present at the lateral plasma membrane forming a continuous belt around the apical cell pole of the multiciliated ependymal cells of wt and hyh mice (Fig. 2d). However, the layer of reactive astrocytes covering the denuded areas did not express N-cadherin (Fig. 2e).
Fig. 2.
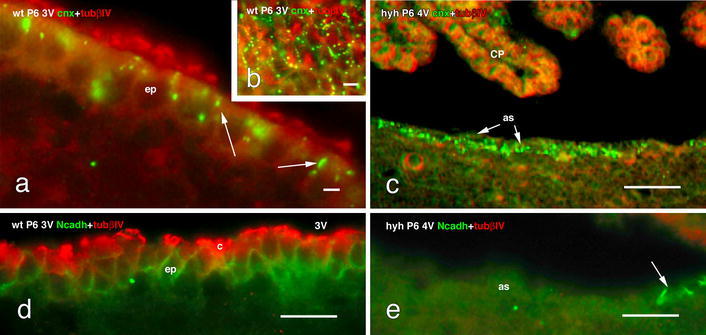
Expression of junction proteins in the cell lining the floor of the fourth ventricle of wt and hyh mice at P6. a–c Double immunofluorescence for tubulin βIV (tubβIV, red) and connexin 43 (cnx, green). a The multiciliated ependyma (ep) in wt mice present cnx+ spots localized preferentially at the apical cell pole of ependymal cells (arrows). b Tangential section through the apical cell poles of the ependyma showing the distribution of cnx as dots in the lateral plasma membrane. c The astrocyte layer (as) lining the denuded ventricle in the hyh mouse expresses cnx (arrow) but not tubβIV. d, e Double immunofluorescence for tubβIV (red) and N-cadherin (Ncadh, green). d The ependyma in wt mice shows the belt-like distribution of Ncadh. e The astrocytes lining the denuded ventricles do not express Ncadh. Arrow points to a few ependymal cells remaining in situ and expressing Ncadh. 3V third ventricle, 4V fourth ventricle, c cilia, CP choroid plexus. Scale bars a 6 μm, b 8 μm, c 40 μm, d 12 μm, e 12 μm
The multiciliated ependymal cells of wt and hyh mice express connexin 43. This junction protein appeared as supranuclear granules and slender patches (possibly connexons) at the lateral plasma membrane (Fig. 2a, b). In the reactive astrocytes layer, connexin 43 appeared as granules throughout the cytoplasm (Fig. 2c). The older astrocyte layer of P30 mice apparently expressed more connexin 43 than did the P6 mice.
Molecules implicated in transport mechanisms in multiciliated ependyma and astrocytes covering the ventricular walls of wt and hyh mice
In wt mice, the water channel protein aquaporin 4 was detected in the latero-basal cell domains, and less intensively in the apical domain, of the ependymal cells (Fig. 3a) and in the perivascular endfeet of the astrocytes. Aquaporin 4 was not detected in the cell bodies of the astrocytes. In contrast, in the hyh mouse, aquaporin 4 was found throughout the cell body and in the processes of the astrocytes covering the ependymal-denuded areas (Fig. 3c; Supplementary Fig. 3), as well as in the perivascular endfeet of the parenchymal astrocytes (Fig. 3b). Densitometric analysis showed that in hyh mice, the reactive astrocytes covering the ependyma-denuded surfaces presented a small but significant increase in immunoreactive aquaporin 4 with respect to the ependyma of wt mice (Fig. 3d).
Fig. 3.
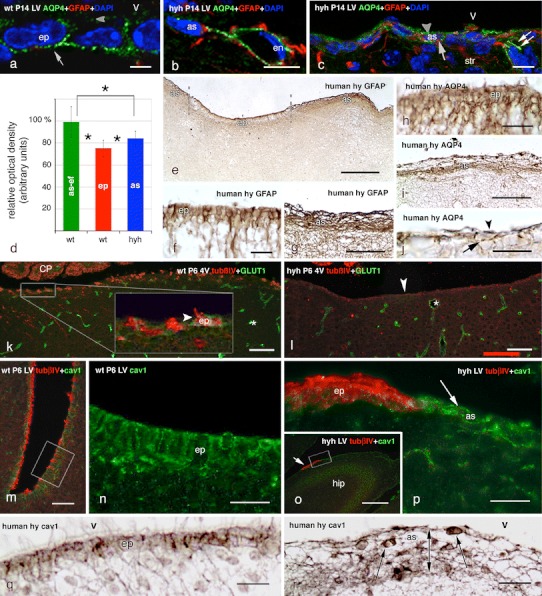
Expression of aquaporin 4, the glucose transporter 1 (GLUT1) and caveolin-1 in the ependyma of wt mice and in the astrocyte layer that covers the ependymal-denuded surface in hyh mice and a human hydrocephalic foetus. a Immunolabelling for aquaporin 4 (AQP4, green) in the ependyma (ep) of the lateral ventricle (LV) of a wt mouse; DAPI counterstaining (blue). The water channel is mainly located in the baso-lateral plasma membrane domain (arrow), but there is also a weak reaction at the apical domain (arrowhead). b, c Latero-medial wall of a hyh mouse with double immunofluorescence for GFAP (red), AQP4 (green) and DAPI counterstaining (blue). AQP4 immunoreaction is present in a perivascular astrocyte (as) and its endfeet surrounding endothelial (en) cells (b). Reactive astrocytes present AQP4 at the apical (arrowhead) and basal (arrow) cytoplasm and in their cell processes (double arrow) (c). d Optical density of the immunoreaction for AQP4 was recorded at (1) the ependyma (ep) of the latero-medial wall of the lateral ventricles of wt mice; (2) the cell layer of reactive astrocytes lining denuded areas of the latero-medial wall of the lateral ventricles of hyh mice (as); (3) the perivascular endfeet of the astrocytes (as-ef) of wt mice. Data represent the mean and standard deviation from four wt and four hyh mice (3–4 sections each mouse; four neighbour areas from each section). Data are expressed as relative percentage of the values obtained in each section where the mean of as-ef in wt mice was considered to be 100 %. *Correlation analysis showed significant differences (p < 0.001, Student’s t test). e–j Lateral ventricle of a 40-week-old human hydrocephalic foetus. Immunostaining for GFAP (e–g) and AQP4 (h–j). e Low power view showing ependyma not yet disrupted (ep) and denuded areas lined by a layer of GFAP+ astrocytes (as). These two regions are shown at higher magnification in f and g. The ependyma (h) and the astrocyte layer (i, j) express AQP4. k, l Fourth ventricle (4V) with double immunofluorescence for tubulin βIV (tubβIV, red) and GLUT1 (green). Endothelial cells (asterisks) are reactive to GLUT1. k The inset shows a weak reaction for GLUT1 in the ependyma lining the floor of the fourth ventricle of a P6 wt mouse. l The astrocyte layer lining the denuded floor of the fourth ventricle of hyh mice does not express GLUT1. m Lateral ventricle of a wt P6 mouse. Double immunofluorescence for tubβIV (red) and caveolin-1 (cav1, green). The framed area is shown in n using only the cav1 channel. n Detailed view of area framed in m, showing the strong expression of cav1 in the multiciliated ependyma of the lateral ventricle. o Wall of the lateral ventricle close to the hippocampus (hip) of a P6 hyh mouse. Double immunofluorescence for tubβIV (red) and cav1 (green). The walls are denuded with the exception of a tubβIV+ resistant ependymal patch (arrow). The area framed is shown in p. p Astrocytes lining the denuded areas of the lateral ventricle strongly express cav1 (arrow). The patch of ependyma is strongly labelled for tubβIV (red), whereas the astrocyte layer is not. q, r Lateral ventricle of a 40-week-old hydrocephalic human foetus. Section adjacent to that shown in f and g, immunostained for cav1. The cell body of ependymal cells contains immunoreactive granules (q). The cell body (arrows) and processes of the astrocytes forming the thick cell layer lining denuded areas (double-ended arrow) are immunoreactive (r). CP choroid plexus, V ventricle lumen. Scale bars a 5 μm, b, c 10 μm, e 200 μm, f, h, j 20 μm, g 50 μm, i 50 μm, k, o 100 μm, l 80 μm, m 40 μm, n, p 20 μm
The lateral ventricles of human hydrocephalic foetuses displayed large areas of ependymal denudation that clearly contrasted with those still lined by ciliated ependymal cells (cf. [18]; Fig. 3e–g). The denuded areas were covered by a layer of astrocyte cell bodies and processes (Fig. 3g). In the ependyma, aquaporin 4 outlined the cell profile (Fig. 3h). In the astrocytes, the water channel was present in the cell body and the processes (Fig. 3i, j).
The glucose transporter GLUT1 was weakly expressed in the multiciliated ependyma lining the fourth ventricle of P6 wt mice (Fig. 3k). It was not detectable in the ependyma of P30 wt mice or in the astrocytes covering the ependyma-denuded areas (Fig. 3l). In contrast, the immunoreaction was strong in the endothelial cells of wt and hyh mice (Fig. 3k, l).
The protein caveolin-1 labelled caveolae in a dotted pattern at the plasma membrane and cytoplasm of the multiciliated ependyma of mice (Fig. 3m, n) and of human foetuses (Fig. 3q). Caveolin-1 was detected throughout the cell body and the processes of astrocytes covering the ependyma-denuded areas in hyh mice (Fig. 3o, p) and in human hydrocephalic foetuses (Fig. 3r). This finding is in contrast with the poor or absent immunoreaction found in parenchymal astrocytes.
Endocytosis in multiciliated ependyma and in the astrocyte layer lining the ependyma-denuded regions of hyh mice
Early endosomes, immunodetected by the presence of the EEA1 antigen, were present in the apical juxtanuclear domain of the multiciliated ependymal cells of wt and hyh mice (Fig. 4a, d). The trans-Golgi network was detected in the supranuclear region below the layer of the early endosomes (Fig. 4b). HRP administered in vivo into the lateral ventricle of wt and hyh mice was traced with the electron microscope. In both the normal ependyma of wt mice and the denudation-resistant ependyma of hyh mice, HRP was visualized within small endocytic vesicles and in large irregular compartments that corresponded to early endosomes (Fig. 4c; Supplementary Fig. 4).
Fig. 4.
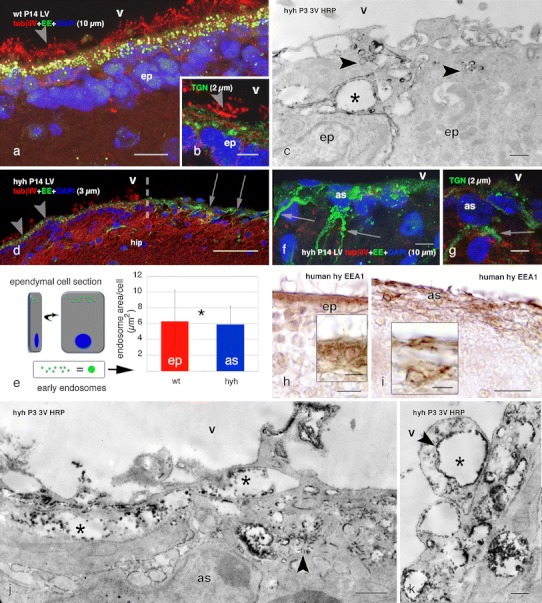
Endocytosis of HRP injected in vivo into the lateral ventricle of wt and hyh mice, and location of endocytic vesicles, early endosomes and the Golgi apparatus. Lateral ventricle of wt (a, b) and hyh (d, f, g) mice, at P14. Confocal laser microscopy of immunolabelling for the EEA1 antigen of early endosomes (EE, green in a, d, f), the trans-Golgi network (TGN, green in b, g) and tubulin βIV (tubβIV, red). DAPI nuclear staining (blue). Z-projections comprising confocal planes for different thicknesses (between brackets). Numerous EE are present in the apical pole (arrowheads in a, d) of the ependymal cells (ep) in wt and hyh mice. The broken line in d shows the border between a patch of intact ependyma (arrowheads) and the astrocyte layer covering an ependyma-denuded surface (arrows) in the hippocampus (hip). The TGN in ependymal cells is located juxtanuclear (arrowhead in b). In the astrocytes (as) covering the ependyma-denuded surface of hyh mice (arrows in d), abundant EE and TGN are present in the cell bodies and processes (arrows in f and g). e Total area occupied by EEA1-reactive EE per ependymal cell of wt mice and per reactive astrocyte (as) of hyh mice in confocal laser cuts of frozen sections (explanation on the left side of the figure). Data represent the mean and standard deviation obtained from sections corresponding to four wt and four hyh mice (4 sections each mouse). *Correlation analysis did not show a significant difference (p = 0.695, Student’s t test). c, j, k Ultrastructural detection in the third ventricle (3V) wall of HRP injected into a lateral ventricle at P3 in wt mice (c) and hyh mice (h, i). In the apical pole of ependymal cells and in astrocytes, HRP is located within endocytic vesicles (arrowheads) and large EE (asterisks). h, i Lateral ventricle of a 40-week-old human hydrocephalic foetus. Section adjacent to that shown in Fig. 3f, g, immunostained for EEA1. The cell body of ependymal cells (ep) contains immunoreactive granules (h). The cell body and processes of the astrocytes forming the thick cells layer lining denuded areas are immunoreactive (i). LV lateral ventricle, str striatum, V ventricle lumen. Scale bars a 10 μm, b, g 5 μm, c, j 400 nm, d 50 μm, f 7 μm, h, i 20 μm; Insets in h, i 10 μm, k 200 nm
At variance with the parenchymal astrocytes, the astrocytes covering the ependyma-denuded areas of hyh mice displayed numerous EEA1-positive endosomes scattered throughout the cell body and processes (Fig. 4d, f). In these astrocytes, the cytoplasm area occupied by the early endosomes was not significantly different from that of the ependymal cells of wt mice (Fig. 4e); the trans-Golgi network was located in a cytoplasmic region that was close to the ventricle (Supplementary Fig. 5) and along the processes (Fig. 4g). HRP administered in vivo to hyh mice was incorporated into small endocytic vesicles and early endosomes of astrocytes covering denuded areas (Fig. 4j, k; Supplementary Fig. 4).
The ependyma of the lateral ventricles of human hydrocephalic foetuses displayed a strong EEA1 immunoreactivity in the supranuclear cytoplasm (Fig. 4h). The cell body and processes of the astrocytes lining the adjacent denuded areas were strongly reactive with anti-EEA1 (Fig. 4i).
Paracellular routes of transport in ependyma of wt mice and astrocytes covering ependyma-denuded areas in hyh mice
On electron microscope, the lateral plasma membranes of neighbouring ependymal cells were found to be extensively interdigitated (Fig. 5a; Supplementary Fig. 4) and joined together by adherents and gap junctions; tight junctions were missing. Lanthanum applied to the ventricular surface penetrated through the labyrinth of extracellular spaces, filled the intercellular space of the underlying neuropile and labelled the basement membrane of the capillaries and the intercellular space of the endothelium up to the tight junctions joining the endothelial cells (Supplementary Fig. 5). Lanthanum tracing further supported the absence of tight junctions at the multiciliated ependymal lining.
Fig. 5.
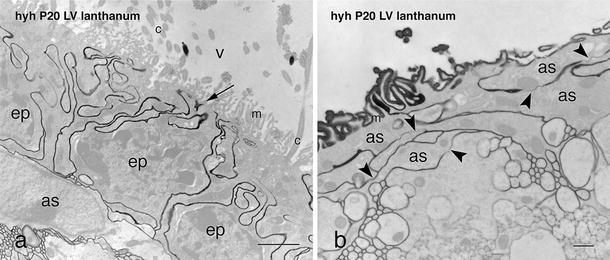
Ultrastructural detection of lanthanum nitrate applied into the lateral ventricle of a P20 hyh mouse. a Lanthanum penetrates from the ventricular lumen (V, arrow) towards the brain parenchyma through the winding extracellular spaces of the denudation-resistant, ciliated ependyma (ep). b In the astrocytic layer (as) lining the denuded ventricular surface of a hyh mouse, the tracer penetrates through the extracellular spaces and bypasses the gap junctions joining the astrocytes (arrowheads). c cilia, LV lateral ventricle, m microvilli. Scale bars a 2 μm, b 500 nm
Electron microscope analysis revealed that astrocytes projected into the ventricle numerous, irregularly shaped, microvilli; cilia were missing (Figs. 1m, n, 5b). These cells displayed numerous sheet-like processes that interdigitated extensively with those of adjacent cells, forming a dense subventricular network of astrocyte processes (Fig. 5b; see Supplementary Fig. 5) that was readily visualized with GFAP immunocytochemistry (Fig. 1k, p). The surface astrocytes were joined together by gap junctions (Fig. 5b) and lacked zonula adherens and tight junctions (Fig. 5b; Supplementary Figs. 4 and 5).
In hyh mice, the astrocyte layer covering the denuded brain ventricles showed regional differences. The astrocytes formed a cell layer with varying degrees of tightness that ranged from loose to compact. In the latero-medial wall of the lateral ventricles lining the hippocampus and striatum (Figs. 1k, 6a, b, g), the third ventricle, the ventral wall of the cerebral aqueduct and the fourth ventricle (Fig. 1o, p), the denuded surface was lined by densely packed astrocytes. In other denuded areas, such as those of the dorsal and external walls of the lateral ventricles, reactive astrocytes were arranged as a loose cell layer (Fig. 6c–e). The different degrees of cell density of the astrocyte assembled at the denuded areas became most evident in whole mount preparations of different ventricular regions immunostained for GFAP (Fig. 6a–d) and used for a densitometric analysis (Fig. 6f). This study led to identify three types of astrocyte arrangements: (1) compact, characterized by a continuous layer of tightly packed astrocytes (Figs. 1o, p, 2c, 4b, 6a, b); (2) loose, recognized by a continuous layer of astrocytes separated by wide intercellular spaces (Fig. 6c–e); (3) very loose, characterized by a discontinuous layer of astrocytes (Fig. 6c, inset).
Fig. 6.
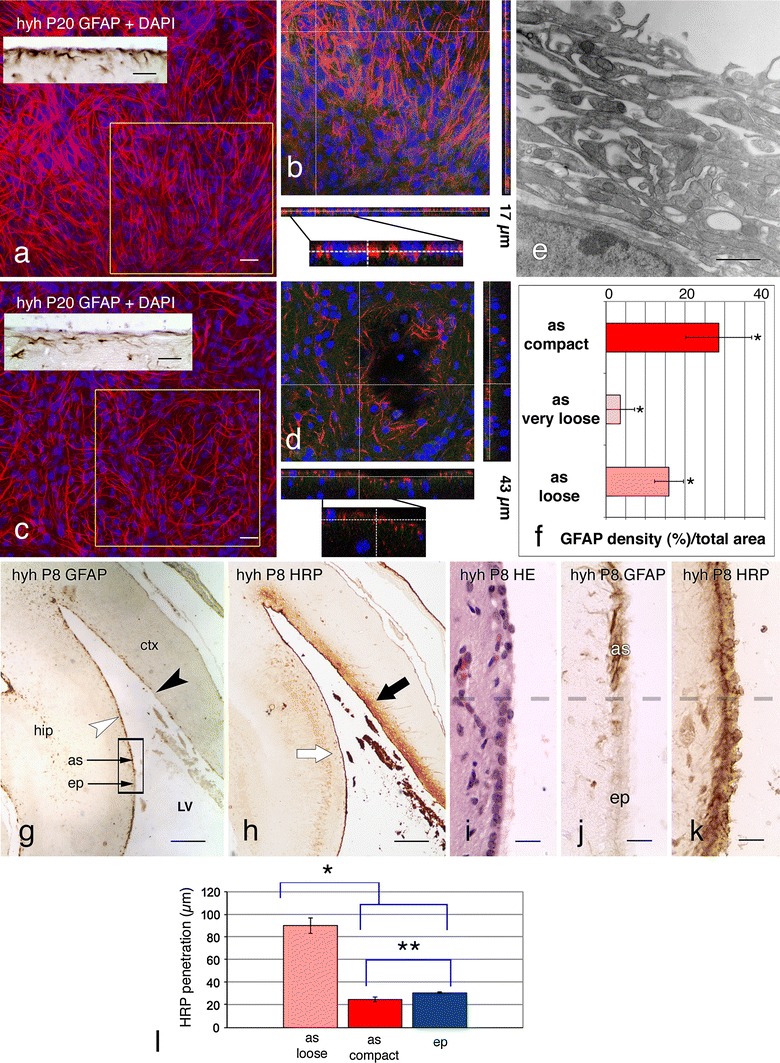
Tightness and permeability of the layer of astrocytes lining different denuded regions of the lateral ventricle of hyh mice. a–d Hyh mice at P20. Surface views obtained from whole mount preparations of ventricular walls processed for GFAP (red) immunofluorescence and DAPI nuclear staining. The astrocyte cell density in the latero-medial wall of the lateral ventricle (a, b) is much higher than that of the latero-external wall of the lateral ventricle (c, d; for orientation, see g). b, d 1 μm confocal planes of the areas framed in a and b displaying pseudo 3D reconstructions (17 μm in b, 43 μm in d) bring out the differential arrangement of the astrocytes. Insets in a, c immunocytochemistry for GFAP in sections of similar areas. e Ultrastructure of the latero-external wall of a lateral ventricle of a hyh mouse at P20 showing the loose organization of astrocytes. f Measurement of astrocyte (as) cell densities using whole mount preparations of regions of the lateral ventricle displaying compact (latero-medial wall, see a), loose (see c) or very loose (latero-external walls) arrangements of astrocytes. Data represent mean and standard deviation of the percentage of the area occupied by the GFAP-immunoreactive profiles with respect to the total area. Data were collected from four hyh mice, four with whole mounts/mouse/each location. *Correlation analysis showed significant differences between the three types of astrocyte organization (p < 0.001, Student’s t test). g Frontal section through the telencephalon of a hyh mouse at P8 immunostained for GFAP. The latero-medial wall of the lateral ventricle is covered by an astrocytic layer (as; white arrowhead) and a few patches of ependyma (ep). The area framed is shown in i–k. The latero-external wall of the lateral ventricle contains a discontinuous astrocytic layer and scattered ependymal cells (black arrowhead). h Adjacent section to that shown in g, immunostained using anti-HRP to visualize the HRP injected in vivo into a lateral ventricle. In the latero-medial wall of the ventricle, the tracer is incorporated equally by the layer of astrocytes and the patch of ependyma (white arrow). In the latero-external wall of the ventricle, HRP penetrates deeply into the brain parenchyma (black arrow). i–k Adjacent serial sections through an area similar to that framed in g, including a patch of ependyma lying close to a denuded area lined by an astrocytic layer. The broken line points to the border between both areas. Haematoxylin–eosin staining (i), anti-GFAP (j) and anti-HRP (k) immunolabelling. The astrocyte layer and the ependymal patch incorporate HRP following a similar pattern. l Measurement of the penetration of intraventricularly injected HRP into the brain parenchyma at three different regions of the lateral ventricles: with ependyma (j, k), compact layer of reactive astrocyte (white arrow/arrowhead in g, h) and loose layer of astrocytes (black arrow/arrowhead in g, h). Data represent mean and standard deviations from four hyh P8 mice (4 measurements in 3–4 sections from each mouse). *The correlation analysis showed a significant difference between the degree of penetration of HRP through the loose astrocyte layer and that of the other two regions (p < 0.001, Student’s t test). **There was not a significant difference between the data from the penetration through the astrocyte compact organization and the ependyma in the hippocampus of hyh mice (p = 1.119, Student’s t test). ctx cerebral cortex, hip hippocampus, LV lateral ventricle. Scale bars a, c insets in a and c 20 μm, e 1 μm, g, h 200 μm, i–k 20 μm
The barrier property, in terms of paracellular permeability, for these different types of astrocyte arrangements located at distinct regions of the ventricular system was tested by in vivo administration of HRP. Five minutes after HRP injection into a lateral ventricle of hyh mice, the tracer was incorporated by the dense astrocytic layer and penetrated about 20 μm into the underlying neuropile, resembling the barrier property of the neighbouring patch of multiciliated ependyma (Fig. 6h–l). In the areas that displayed loosely arranged astrocytes, HRP penetrated about 90 μm into the brain parenchyma (Fig. 6g, h), indicating a rather free movement of HRP at this level.
Discussion
In hyh hydrocephalic mice, there is a programme of neuroepithelium/ependyma denudation starting early in foetal life and ending by the end of the first postnatal week; the missing ependyma is replaced by a layer of astrocytes forming a new interface between the CSF and the brain parenchyma [29, 41, 65]. This phenomenon has also been described in human hydrocephalic foetuses [18, 53, 55]. The present study, carried out in hyh mice and human cases, has revealed that the new surface layer of astrocytes shares some phenotypic and functional features with the ependyma [summarized in Table 3], suggesting that such a unique astrocyte reaction may represent an attempt to re-establish some lost functions at the brain parenchyma–CSF interface.
Table 3.
Summary of results displaying the presence (+) or absence (−) of the structural and functional markers in the hyh mouse
| Cytoskeleton | Gap junctions (connexin 43) | Adherens junctions (N-cadherin) | Microvilli | Glucose transport (GLUT1) | Water transport (aquaporin 4) | Endocytosis/transcytosis (caveolae containing caveolin 1) | Endocytosis/transcytosis (endosomes containing EEA1 antigen | Endocytosis/transcytosis (HRP uptake) | Absence of tight junctions/paracellular permeability (lanthanum tracing) | |
|---|---|---|---|---|---|---|---|---|---|---|
| Ependyma | (GFAP)a Vimentin | + | + | + | ±b | + | + | + | + | + |
| Cell layer of reactive astrocytes | GFAP, vimentin | + | – | + | – | + | + | + | + | + |
Findings in bold have been also obtained in human foetuses
aAt variance with mouse ependyma, the ependyma of human foetus is reactive with anti-GFAP
bGLUT1 is only present in immature ependymal cells
The astrocytes covering the denuded ventricular walls of hydrocephalic hyh mice form a new cell layer with a cell organization that resembles the ependyma
The astrocytes found at the denuded ventricular walls form a new cell layer that in several aspects resembles the ependyma; i.e. it expresses vimentin, lacks tight junctions, displays connexin 43-based gap junctions, projects numerous microvilli to the ventricle and displays numerous lateral interdigitations that result in a winding intercellular space (Fig. 7). The existence of gap junctions between ependymal cells has been widely demonstrated [6, 8, 9, 21, 28], and they are believed to play a role in the synchronization of cilia beating [50, 55]. Their functional significance in the astrocyte layer is unknown; they could be associated with electrical and metabolic activities, the determination of cell phenotype [45], or the clearance of cytotoxic molecules and the spreading of neuroprotective factors that takes place in brain injuries, ischaemia and hypoxia [27, 33, 37, 51, 58, 60]. Whatever the functional significance of the gap junctions connecting the astrocytes covering the denuded ventricular surface, it may be suggested that these cells are coupled to play a function as a CSF–brain barrier involved in water and solute transport (Fig. 7).
Fig. 7.
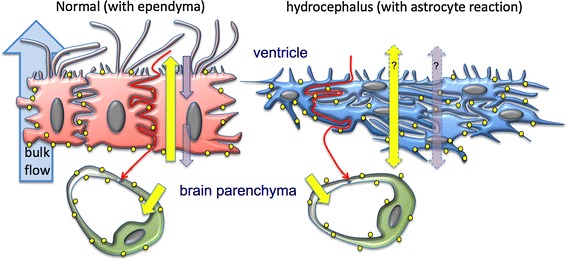
Schematic representation of the transcellular and paracellular transport mechanisms that would operate at the ependyma and the layer of reactive astrocytes. In the ependymal cells of wt mice (left), most of the aquaporin 4 (yellow dots) is located at the basolateral domains (some is found at the apical domain), suggesting that the ependyma transports water from the brain parenchyma (bottom) towards the ventricular CSF (upper) (yellow arrow across the ependyma). There are pinocytic processes and transcytosis directed in the opposite direction through this barrier (purple arrow). In hyh mouse (right), a layer of reactive astrocytes cover the ependyma-denuded surfaces. These cells express aquaporin 4 throughout the cell body and processes (yellow dots) and could be involved in water transport from or to the CSF (double-headed yellow arrow). The pinocytic processes in the astrocytes could also operate in both directions (double head purple arrows). In addition, as in the normal situation, parenchymal astrocytes together with the endothelial cells would be involved in an aquaporin 4-mediated transport towards the brain capillaries. Both barriers would transport molecules from the CSF to the brain parenchyma through a paracellular route (winding red arrows)
The ependyma and the astrocyte cell layer present active endocytic mechanisms
Ependymal cells incorporate CSF proteins into the pinocytic-lysosome pathway [14]. The multiciliated ependyma of wt mice and the denudation-resistant ependyma of hyh mice express caveolin-1, which is in agreement with the expression of EEA1 antigen, a reliable marker of early endosomes, in these cells (present report). Early endosomes are dynamic cell compartments that are involved in endocytosis and sorting mechanisms [32, 67]. Caveolin-1 is a protein that is present in caveolae, structures that play a role in endocytosis and transcytosis [20, 23]; caveolin is also present in both the ependyma [44, 50, present report] and the reactive astrocytes [present study]. Although most cargo that is endocytosed via caveolae is fluid, certain compounds enter into caveolae via specific receptors [62]. The presence of caveolae in the ependyma is in agreement with the known capacity of ependymal cells to incorporate tracer molecules present in the CSF [8, 10]. In the reactive astrocytes, caveolae can play a similar role, which is in agreement with the existence of endocytosis and sorting mechanisms [32, 67], which have also been experimentally tested in the present study by injecting HRP into the CSF of living mice. At variance with the ependyma, the non-polarized distribution of caveolae and endosomes in the reactive astrocytes indicate that they can incorporate fluid and substances not only from the ventricle but also from the parenchymal fluid (Fig. 7).
Role of the expression of the water channel protein aquaporin 4 in the ependyma and in the new astrocyte cell barrier
Aquaporin 4 is a water channel with marked prevalence in periventricular areas [63]. In the ependymal cells, it is located in the latero-basal domains [46, present report] and, less extensively, in the apical domain (present report). In parenchymal astrocytes, it is mostly found in the vascular endfeet [46, present report]. At variance, reactive astrocytes lining the denuded ependyma in hydrocephalic mice and human foetuses overexpress aquaporin 4, and this protein is found throughout the cell body and processes (Fig. 7). The presence of aquaporin 4 in parenchymal reactive astrocytes has been proposed to be involved in the water entry to astrocytes in initial stages of oedema formation [38] to re-establish the osmotic equilibrium [64]. The periventricular reaction found in the present report, both in mice and humans, could represent an attempt to re-establish the equilibrium between the ventricular and parenchymal fluids, or to help CSF transport from ventricles to brain capillaries (Fig. 7). Ependymal aquaporin 4 has been proposed to play a protective role in hydrocephalus by allowing for the transependymal re-absorption of CSF into brain capillaries [56]. The presence of aquaporin 4 in the apical plasma membrane of ependymal cells supports this possibility. It appears that aquaporin 4 may have a relevant role in hydrocephalus and be a useful therapeutic target [34, 54; reviewed by 40, 42, 56].
The astrocyte layer and the ependyma present similar paracellular routes of transport
The multiciliated ependyma is an epithelial-like layer interposed between the CSF and the brain parenchyma. Although it does not behave like a tight barrier [8, 9], it seems to regulate the transport of ions, small molecules and water [12, 66]. Tight junctions are absent or poorly developed in the mature multiciliated ependyma [8, 12]. Lanthanum nitrate injected into the ventricle of wt and hyh mice further proves that the extracellular spaces of both, the ependyma and astrocyte cell layer, are not sealed (Fig. 7). Furthermore, the ependymal and glial barriers responded similarly to the in vivo intraventricular injection of HRP; the tracer moved through the intercellular space of both barriers and penetrated only a few micrometres into the underlying neuropile, suggesting that both barriers somehow limited the amount of HRP moving from the CSF to the brain parenchyma. This possibility is supported by the finding that HRP penetrated deeply into the brain parenchyma in the regions of the ventricular walls with a poorly developed astrocyte cell layer.
Different and distinct regions of the denuded ventricular walls trigger different astrocyte reactions that lead to glial layers with different degrees of cell density and tightness. What are the signals mediating these different responses? In hyh mice, ependymal denudation takes place at prenatal stages prior to detectable hydrocephalus [30]. Therefore, intraventricular pressure or expanding ventricles cannot be considered to be responsible for the absence of ependyma in these mice. Similarly, the periventricular astrocyte reaction appears shortly after denudation, when astrogliogenesis takes place but at a stage when ventriculomegaly is just starting to develop. Furthermore, the most robust astrocyte layer is that lining the denuded floor of the fourth ventricle, a cavity displaying no dilatation. The mechanism underlying the formation of the astrocyte layer replacing the lost ependyma is not known. Is the actual absence of the ependyma, or the direct exposure of the neuropile to the CSF, or a mechanical effect of the expanding ventricles involved? Are there different physiopathological consequences for brain regions protected by a new and compact layer of astrocytes and those close to a ventricular wall lined by a loose and highly permeable layer of astrocytes? These are open questions for future investigations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Figure 1. Hyh mouse at P6, beginning to develop a severe hydrocephalus. Frontal paraffin sections stained with haematoxylin-eosin at rostro-caudal levels from a to f. Abbreviations: 3V, third ventricle, 4V, fourth ventricle; CA, cerebral aqueduct; VL, lateral ventricle. Scale bars: a-f, 500 µm (TIFF 18455 kb)
Supplementary Figure 2. Schematic representation of the ventricles of a hyh mouse at P14 with full severe hydrocephalus. Frontal (a) and sagittal (b) views. Black thick lines show the ependyma resisting denudation. Abbreviations: 3V, third ventricle; 4V, fourth ventricle; CA, cerebral aqueduct; Ceb, cerebellum; DREA, denudation resistant ependyma of the aqueduct (see reference [19]); Hb, habenula; HRc, habenular recess; PRc, pineal recess; ME, median eminence (TIFF 13881 kb)
Supplementary Figure 3. Expression of aquaporin 4 and the EEA1 antigen in the astrocyte layer covering the cerebral aqueduct and the fourth ventricle of hyh mice, at P6 and P20. (a, b) Immunolabelling for aquaporin 4 in the cerebral aqueduct (a) and fourth ventricle (b) of a hyh mouse at P6. Brain capillaries (arrows) are labelled in addition to the reactive astrocytes. (c, d) Immunolabelling for the early endosomal antigen EEA1 (green) and GFAP (red) in the reactive astrocytes covering the cerebral aqueduct (c) and fourth ventricle (d) of a hyh mouse at P20. DAPI nuclear staining (blue). Abbreviations: 4V, fourth ventricle; AQP4, aquaporin 4; CA, cerebral aqueduct; CP, choroid plexus; EE, early endosomes. Scale bars: a, b, 50 µm; c, d, 10 µm (TIFF 26806 kb)
Supplementary Figure 4. Fourth ventricle of wt and hyh mice at P20 and P30 after in vivo administration of HRP into a lateral ventricle and in vitro tracing with lanthanum nitrate. (a) Wt mouse. Lanthanum applied into the ventricle passed through the thin interwoven extracellular spaces of the ependyma lining the floor of the fourth ventricle (arrowheads). (b) Wt mouse. HRP is incorporated into early endosomes located at the apical pole of the ependyma (large arrow) and into the intercellular space (small arrows). (c) The denuded floor of the fourth ventricle of hyh mice is covered by a layer of densely packed reactive astrocytes. (d) Hyh mouse. Lanthanum applied into the ventricle passed through the thin interwoven extracellular spaces of the astrocyte layer (arrow). Abbreviations: CP, choroid plexus. Scale bars: a, 1 µm; b, d, 2 µm; c, 30 µm (TIFF 8258 kb)
Supplementary Figure 5. Ultrastructural detection of lanthanum nitrate applied into the lateral ventricle of a hyh mouse, at P20. (a-c; c is a detailed view of the area framed in b) Lanthanum penetrates from the ventricular lumen towards the brain parenchyma through the winding extracellular spaces of the astrocytic layer (as) lining the denuded ventricular surface (black arrows). Lanthanum reached the intercellular space of the neuropile and the pericapillary basement membrane, a transport pathway similar to that of the areas lined by ependyma. Tight junctions present in the endothelial cells (en; in b) prevent the extracellular progression of the tracer (white arrows; in b, c). (d) Tight junctions present in the choroid plexus ependyma also prevent the extracellular progression of the tracer (white arrow). Asterisk: intermediate filament bundles of astrocytes. Abbreviations: CP, Choroid plexus; g, Golgi apparatus dictiosomes; LV, lateral ventricle; V, intercellular space open to the ventricular lumen. Scale bars: a, 500 nm; b, 1 µm; b, 30 µm; d, 200 nm (TIFF 13478 kb)
Acknowledgments
The authors wish to thank Dr. Conrad Johanson for critical reading of the manuscript and David Navas, Gregorio Martín, Adolfo Martínez and Manuela Vega from the University of Malaga (Spain), Raquel Ruiz from CESUR (Malaga, Spain) and Genaro Alvial from Austral University of Chile for their valuable technical support. Supported by Grants PS09/0376 from Instituto de Salud Carlos III (Spain) and PCI2006-A/-0669 from Ministerio de Educación y Ciencia (Spain) to AJJ, and 1070241 and 1111018 from Fondecyt (Chile) to EMR.
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
References
- 1.Abouhamed M, Grobe K, San IV, et al. Myosin IXa regulates epithelial differentiation and its deficiency results in hydrocephalus. Mol Biol Cell. 2009;20:5074–5085. doi: 10.1091/mbc.E09-04-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baas D, Meiniel A, Benadiba C, et al. A deficiency in RFX3 causes hydrocephalus associated with abnormal differentiation of ependymal cells. Eur J Neurosci. 2006;24:1020–1030. doi: 10.1111/j.1460-9568.2006.05002.x. [DOI] [PubMed] [Google Scholar]
- 3.Banizs B, Pike MM, Millican CL, et al. Dysfunctional cilia lead to altered ependyma and choroid plexus function, and result in the formation of hydrocephalus. Development. 2005;132:5329–5339. doi: 10.1242/dev.02153. [DOI] [PubMed] [Google Scholar]
- 4.Bátiz LF, Jiménez A, Guerra M, et al. New ependymal cells are originated postnatally in discrete regions of the mouse brain and support ventricular enlargement in hydrocephalus. Acta Neuropathol. 2011;121:721–735. doi: 10.1007/s00401-011-0799-x. [DOI] [PubMed] [Google Scholar]
- 5.Bátiz LF, Roales-Buján R, Rodríguez-Pérez LM, et al. A simple PCR-based genotyping method for M105I mutation of alpha-SNAP enhances the study of early pathological changes in hyh phenotype. Mol Cell Probes. 2009;23:281–290. doi: 10.1016/j.mcp.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Bouillé C, Mesnil M, Barriere H, Gabrion J. Gap junctional intercellular communication between cultured ependymal cells, revealed by lucifer yellow CH transfer and freeze-fracture. Glia. 1991;4:25–36. doi: 10.1002/glia.440040104. [DOI] [PubMed] [Google Scholar]
- 7.Bovolenta P, Wandosell F, Nieto-Sampedro M. CNS glial scar tissue: a source of molecules which inhibit central neurite outgrowth. Prog Brain Res. 1992;94:367–379. doi: 10.1016/S0079-6123(08)61765-3. [DOI] [PubMed] [Google Scholar]
- 8.Brightman MW. The distribution within the brain of ferritin injected into cerebrospinal fluid compartments. I. Ependymal distribution. J Cell Biol. 1965;26:99–123. doi: 10.1083/jcb.26.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brightman MW. The distribution within the brain of ferritin injected into cerebrospinal fluid compartments. II. Parenchymal distribution. Am J Anat. 1965;117:193–220. doi: 10.1002/aja.1001170204. [DOI] [PubMed] [Google Scholar]
- 10.Brightman MW. The intracerebral movement of proteins injected into blood and cerebrospinal fluid of mice. Prog Brain Res. 1968;29:19–40. doi: 10.1016/S0079-6123(08)64147-3. [DOI] [PubMed] [Google Scholar]
- 11.Bronson RT, Lane PW. Hydrocephalus with hop gait (hyh): a new mutation on chromosome 7 in the mouse. Dev Brain Res. 1990;54:131–136. doi: 10.1016/0165-3806(90)90073-8. [DOI] [PubMed] [Google Scholar]
- 12.Bruni JE. Ependymal development, proliferation, and functions: a review. Microsc Res Tech. 1998;41:2–13. doi: 10.1002/(SICI)1097-0029(19980401)41:1<2::AID-JEMT2>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 13.Chae TH, Kim S, Marz KE, et al. The hyh mutation uncovers roles for alpha Snap in apical protein localization and control of neural cell fate. Nat Genet. 2004;36:264–270. doi: 10.1038/ng1302. [DOI] [PubMed] [Google Scholar]
- 14.Del Bigio MR. Ependymal reactions to injury. A review. J Neuropathol Exp Neurol. 1995;54:405–406. doi: 10.1097/00005072-199505000-00014. [DOI] [PubMed] [Google Scholar]
- 15.Del Bigio MR. Calcium-mediated proteolytic damage in white matter of hydrocephalic rats? J Neuropathol Exp Neurol. 2000;59:946–954. doi: 10.1093/jnen/59.11.946. [DOI] [PubMed] [Google Scholar]
- 16.Del Bigio MR, Wilson MJ, Enno T. Chronic hydrocephalus in rats and humans: white matter loss and behavior changes. Ann Neurol. 2003;53:337–346. doi: 10.1002/ana.10453. [DOI] [PubMed] [Google Scholar]
- 17.Del Bigio MR, Zhang YW. Cell death, axonal damage, and cell birth in the immature rat brain following induction of hydrocephalus. Exp Neurol. 1998;154:157–169. doi: 10.1006/exnr.1998.6922. [DOI] [PubMed] [Google Scholar]
- 18.Domínguez-Pinos MD, Páez P, Jiménez AJ, et al. Ependymal denudation and alterations of the subventricular zone occur in human fetuses with a moderate communicating hydrocephalus. J Neuropathol Exp Neurol. 2005;64:595–604. doi: 10.1097/01.jnen.0000171648.86718.bb. [DOI] [PubMed] [Google Scholar]
- 19.Eddleston M, Mucke L. Molecular profile of reactive astrocytes: implications for their role in neurologic disease. Neuroscience. 1993;54:15–36. doi: 10.1016/0306-4522(93)90380-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Frank PG, Woodman SE, Park DS, Lisanti MP. Caveolin, caveolae, and edothelial cell function. Arterioscler Thromb Vasc Biol. 2003;23:1161–1168. doi: 10.1161/01.ATV.0000070546.16946.3A. [DOI] [PubMed] [Google Scholar]
- 21.Gabrion JB, Herbuté S, Bouillé C, et al. Ependymal and choroidal cells in culture: characterization and functional differentiation. Microsc Res Tech. 1998;41:124–157. doi: 10.1002/(SICI)1097-0029(19980415)41:2<124::AID-JEMT3>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 22.Giaume C, Kirchhoff F, Matute C, et al. Glia: the fulcrum of brain diseases. Cell Death Differ. 2007;14:1324–1335. doi: 10.1038/sj.cdd.4402144. [DOI] [PubMed] [Google Scholar]
- 23.Gosens R, Mutawe M, Martin S, et al. Caveolae and caveolins in the respiratory system. Curr Mol Med. 2008;8:741–753. doi: 10.2174/156652408786733720. [DOI] [PubMed] [Google Scholar]
- 24.Hatten ME, Liem RK, Shelanski ML, et al. Astroglia in CNS injury. Glia. 1991;4:233–243. doi: 10.1002/glia.440040215. [DOI] [PubMed] [Google Scholar]
- 25.Hong HK, Chakravarti A, Takahashi JS. The gene for soluble N-ethylmaleimide sensitive factor attachment protein alpha is mutated in hydrocephaly with hop gait (hyh) mice. Proc Natl Acad Sci USA. 2004;101(6):1748–1753. doi: 10.1073/pnas.0308268100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ibañez-Tallon I, Pagenstecher A, Fliegauf M, et al. Dysfunction of axonemal dynein heavy chain Mdnah5 inhibits ependymal flow and reveals a novel mechanism for hydrocephalus formation. Hum Mol Genet. 2004;13:2133–2141. doi: 10.1093/hmg/ddh219. [DOI] [PubMed] [Google Scholar]
- 27.Jäderstad J, Brismar H, Herlenius E. Hypoxic preconditioning increases gap-junctional graft and host communication. NeuroReport. 2010;21:1126–1132. doi: 10.1097/WNR.0b013e328340a77b. [DOI] [PubMed] [Google Scholar]
- 28.Jarvis CR, Andrew RD. Correlated electrophysiology and morphology of the ependyma in rat hypothalamus. J Neurosci. 1988;8:3691–3702. doi: 10.1523/JNEUROSCI.08-10-03691.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiménez AJ, García-Verdugo JM, González CA, et al. Disruption of the neurogenic niche in the subventricular zone of postnatal hydrocephalic hyh mice. J Neuropathol Exp Neurol. 2009;68:1006–1020. doi: 10.1097/NEN.0b013e3181b44a5a. [DOI] [PubMed] [Google Scholar]
- 30.Jiménez AJ, Tomé M, Páez P, et al. A programmed ependymal denudation precedes congenital hydrocephalus in the hyh mutant mouse. J Neuropathol Exp Neurol. 2001;60:1105–1119. doi: 10.1093/jnen/60.11.1105. [DOI] [PubMed] [Google Scholar]
- 31.Johanson CE, Duncan JA, 3rd, Klinge PM, et al. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jovic M, Sharma M, Rahajeng J, Caplan S. The early endosome: a busy sorting station for proteins at the crossroads. Histol Histopathol. 2010;25:99–112. doi: 10.14670/hh-25.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin JH, Weigel H, Cotrina ML, et al. Gap-junction-mediated propagation and amplification of cell injury. Nat Neurosci. 1998;1:494–500. doi: 10.1038/2210. [DOI] [PubMed] [Google Scholar]
- 34.Mao X, Enno TL, Del Bigio MR. Aquaporin 4 changes in rat brain with severe hydrocephalus. Eur J Neurosci. 2006;23:2929–2936. doi: 10.1111/j.1460-9568.2006.04829.x. [DOI] [PubMed] [Google Scholar]
- 35.McAllister JP, Miller JM. Minocycline inhibits glial proliferation in the H-Tx rat model of congenital hydrocephalus. Cerebrospinal Fluid Res. 2010;7:7. doi: 10.1186/1743-8454-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miller JM, McAllister JP. Reduction of astrogliosis and microgliosis by cerebrospinal fluid shunting in experimental hydrocephalus. Cerebrospinal Fluid Res. 2007;4:5. doi: 10.1186/1743-8454-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nakase T, Yoshida Y, Nagata K. Enhanced connexin 43 immunoreactivity in penumbral areas in the human brain following ischemia. Glia. 2006;54:369–375. doi: 10.1002/glia.20399. [DOI] [PubMed] [Google Scholar]
- 38.Nase G, Helm PJ, Enger R, Ottersen OP. Water entry into astrocytes during brain edema formation. Glia. 2008;56:895–902. doi: 10.1002/glia.20664. [DOI] [PubMed] [Google Scholar]
- 39.Nilsson H, Dragomir A, Ahlander A, et al. A modified technique for the impregnation of lanthanum tracer to study the integrity of tight junctions on cells grown on a permeable substrate. Microsc Res Tech. 2006;69:776–783. doi: 10.1002/jemt.20347. [DOI] [PubMed] [Google Scholar]
- 40.Owler BK, Pitham T, Wang D. Aquaporins: relevance to cerebrospinal fluid physiology and therapeutic potential in hydrocephalus. Cerebrospinal Fluid Res. 2010;7:15. doi: 10.1186/1743-8454-7-S1-S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Páez P, Bátiz LF, Roales-Buján R, et al. Patterned neuropathologic events occurring in hyh congenital hydrocephalic mutant mice. J Neuropathol Exp Neurol. 2007;66:1082–1092. doi: 10.1097/nen.0b013e31815c1952. [DOI] [PubMed] [Google Scholar]
- 42.Paul L, Madan M, Rammling M, et al. Expression of aquaporin 1 and 4 in congenital hydrocephalus rat model. Neurosurgery. 2011;68:462–473. doi: 10.1227/NEU.0b013e3182011860. [DOI] [PubMed] [Google Scholar]
- 43.Pérez-Fígares JM, Jiménez AJ, Rodríguez EM, et al. Subcommissural organ, cerebrospinal fluid circulation, and hydrocephalus. Microsc Res Tech. 2001;52:591–607. doi: 10.1002/1097-0029(20010301)52:5<591::AID-JEMT1043>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 44.Peruzzo B, Pastor FE, Blázquez JL, et al. Polarized endocytosis and transcytosis in the hypothalamic tanycytes of the rat. Cell Tissue Res. 2004;317:147–164. doi: 10.1007/s00441-004-0899-1. [DOI] [PubMed] [Google Scholar]
- 45.Ransom BR, Ye ZC. Gap junctions and hemichannels. In: Ketternmann H, Ransom BR, editors. Neuroglia. New York: Oxford University Press; 2005. pp. 177–189. [Google Scholar]
- 46.Rash JE, Yasumura T, Hudson CS, et al. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc Natl Acad Sci USA. 1998;95:11981–11986. doi: 10.1073/pnas.95.20.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renault-Mihara F, Okada S, Shibata S, et al. Spinal cord injury: emerging beneficial role of reactive astrocytes migration. Int J Biochem Cell Biol. 2008;40:1649–1653. doi: 10.1016/j.biocel.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 48.Revel JP, Karnovsky MJ. Hexagonal array of subunits in intercellular junctions of the mouse heart and liver. J Cell Biol. 1967;33:C7–C12. doi: 10.1083/jcb.33.3.C7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ridet JL, Malhotra SK, Privat A, et al. Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci. 1997;20:570–577. doi: 10.1016/S0166-2236(97)01139-9. [DOI] [PubMed] [Google Scholar]
- 50.Rodríguez EM, Blázquez JL, Guerra M. The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: The former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides. 2010;31:757–776. doi: 10.1016/j.peptides.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 51.Rouach N, Avignone E, Même W, et al. Gap junctions and connexin expression in the normal and pathological central nervous system. Biol Cell. 2002;94:457–475. doi: 10.1016/S0248-4900(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 52.Sakakibara S, Nakamura Y, Yoshida T, et al. RNA-binding protein Musashi family: roles for CNS stem cells and a subpopulation of ependymal cells revealed by targeted disruption and antisense ablation. Proc Natl Acad Sci USA. 2002;99:15194–15199. doi: 10.1073/pnas.232087499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sarnat HB. Ependymal reactions to injury. A review. J Neuropathol Exp Neurol. 1995;54:1–15. doi: 10.1097/00005072-199501000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Shen XQ, Miyajima M, Ogino I, et al. Expression of the water-channel protein aquaporin 4 in the H-Tx rat: possible compensatory role in spontaneously arrested hydrocephalus. J Neurosurg. 2006;105:459–464. doi: 10.3171/ped.2006.105.6.459. [DOI] [PubMed] [Google Scholar]
- 55.Sival DA, Guerra M, den Dunnen WF, et al. Neuroependymal denudation is in progress in full-term human foetal spina bifida aperta. Brain Pathol. 2011;21:163–179. doi: 10.1111/j.1750-3639.2010.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Skjolding AD, Rowland IJ, Søgaard LV, et al. Hydrocephalus induces dynamic spatiotemporal regulation of aquaporin-4 expression in the rat brain. Cerebrospinal Fluid Res. 2010;7:20. doi: 10.1186/1743-8454-7-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Socci DJ, Bjugstad KB, Jones HC, et al. Evidence that oxidative stress is associated with the pathophysiology of inherited hydrocephalus in the H-Tx rat model. Exp Neurol. 1999;155:109–117. doi: 10.1006/exnr.1998.6969. [DOI] [PubMed] [Google Scholar]
- 58.Sofroniew MV. Reactive astrocytes in neural repair and protection. Neuroscientist. 2005;11:400–407. doi: 10.1177/1073858405278321. [DOI] [PubMed] [Google Scholar]
- 59.Sofroniew MV, Vinters HV. Astrocytes: biology and pathology. Acta Neuropathol. 2010;119:7–35. doi: 10.1007/s00401-009-0619-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Talhouk RS, Zeinieh MP, Mikati MA, El-Sabban ME. Gap junctional intercellular communication in hypoxia–ischemia-induced neuronal injury. Prog Neurobiol. 2008;84:57–76. doi: 10.1016/j.pneurobio.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 61.Tissir F, Qu Y, Montcouquiol M, et al. Lack of cadherins Celsr2 and Celsr3 impairs ependymal ciliogenesis, leading to fatal hydrocephalus. Nat Neurosci. 2010;13:700–707. doi: 10.1038/nn.2555. [DOI] [PubMed] [Google Scholar]
- 62.Tuma PL, Hubbard AL. Transcytosis: crossing cellular barriers. Physiol Rev. 2003;83:871–932. doi: 10.1152/physrev.00001.2003. [DOI] [PubMed] [Google Scholar]
- 63.Venero JL, Vizuete ML, Machado A, Cano J. Aquaporins in the central nervous system. Prog Neurobiol. 2001;63:321–336. doi: 10.1016/S0301-0082(00)00035-6. [DOI] [PubMed] [Google Scholar]
- 64.Vizuete ML, Venero JL, Vargas C, et al. Differential upregulation of aquaporin-4 mRNA expression in reactive astrocytes after brain injury: potential role in brain edema. Neurobiol Dis. 1999;6:245–258. doi: 10.1006/nbdi.1999.0246. [DOI] [PubMed] [Google Scholar]
- 65.Wagner C, Batiz LF, Rodríguez S, et al. Cellular mechanisms involved in the stenosis and obliteration of the cerebral aqueduct of hyh mutant mice developing congenital hydrocephalus. J Neuropathol Exp Neurol. 2003;62:1019–1040. doi: 10.1093/jnen/62.10.1019. [DOI] [PubMed] [Google Scholar]
- 66.Wang HW, Amin MS, El-Shahat E, et al. Effects of central sodium on epithelial sodium channels in rat brain. Am J Physiol Regul Integr Comp Physiol. 2010;299:R222–R233. doi: 10.1152/ajpregu.00834.2009. [DOI] [PubMed] [Google Scholar]
- 67.Weisz OA, Rodriguez-Boulan E. Apical trafficking in epithelial cells: signals, clusters and motors. J Cell Sci. 2009;122:4253–4266. doi: 10.1242/jcs.032615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Worthington WC, Jr, Cathcart RS. Ependymal cilia: distribution and activity in the adult human brain. Science. 1963;139:221–222. doi: 10.1126/science.139.3551.221. [DOI] [PubMed] [Google Scholar]
- 69.Yamadori T, Nara K. The directions of ciliary beat on the wall of the lateral ventricle and the currents of the cerebrospinal fluid in the brain ventricles. Scan Electron Microsc. 1979;3:335–340. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Hyh mouse at P6, beginning to develop a severe hydrocephalus. Frontal paraffin sections stained with haematoxylin-eosin at rostro-caudal levels from a to f. Abbreviations: 3V, third ventricle, 4V, fourth ventricle; CA, cerebral aqueduct; VL, lateral ventricle. Scale bars: a-f, 500 µm (TIFF 18455 kb)
Supplementary Figure 2. Schematic representation of the ventricles of a hyh mouse at P14 with full severe hydrocephalus. Frontal (a) and sagittal (b) views. Black thick lines show the ependyma resisting denudation. Abbreviations: 3V, third ventricle; 4V, fourth ventricle; CA, cerebral aqueduct; Ceb, cerebellum; DREA, denudation resistant ependyma of the aqueduct (see reference [19]); Hb, habenula; HRc, habenular recess; PRc, pineal recess; ME, median eminence (TIFF 13881 kb)
Supplementary Figure 3. Expression of aquaporin 4 and the EEA1 antigen in the astrocyte layer covering the cerebral aqueduct and the fourth ventricle of hyh mice, at P6 and P20. (a, b) Immunolabelling for aquaporin 4 in the cerebral aqueduct (a) and fourth ventricle (b) of a hyh mouse at P6. Brain capillaries (arrows) are labelled in addition to the reactive astrocytes. (c, d) Immunolabelling for the early endosomal antigen EEA1 (green) and GFAP (red) in the reactive astrocytes covering the cerebral aqueduct (c) and fourth ventricle (d) of a hyh mouse at P20. DAPI nuclear staining (blue). Abbreviations: 4V, fourth ventricle; AQP4, aquaporin 4; CA, cerebral aqueduct; CP, choroid plexus; EE, early endosomes. Scale bars: a, b, 50 µm; c, d, 10 µm (TIFF 26806 kb)
Supplementary Figure 4. Fourth ventricle of wt and hyh mice at P20 and P30 after in vivo administration of HRP into a lateral ventricle and in vitro tracing with lanthanum nitrate. (a) Wt mouse. Lanthanum applied into the ventricle passed through the thin interwoven extracellular spaces of the ependyma lining the floor of the fourth ventricle (arrowheads). (b) Wt mouse. HRP is incorporated into early endosomes located at the apical pole of the ependyma (large arrow) and into the intercellular space (small arrows). (c) The denuded floor of the fourth ventricle of hyh mice is covered by a layer of densely packed reactive astrocytes. (d) Hyh mouse. Lanthanum applied into the ventricle passed through the thin interwoven extracellular spaces of the astrocyte layer (arrow). Abbreviations: CP, choroid plexus. Scale bars: a, 1 µm; b, d, 2 µm; c, 30 µm (TIFF 8258 kb)
Supplementary Figure 5. Ultrastructural detection of lanthanum nitrate applied into the lateral ventricle of a hyh mouse, at P20. (a-c; c is a detailed view of the area framed in b) Lanthanum penetrates from the ventricular lumen towards the brain parenchyma through the winding extracellular spaces of the astrocytic layer (as) lining the denuded ventricular surface (black arrows). Lanthanum reached the intercellular space of the neuropile and the pericapillary basement membrane, a transport pathway similar to that of the areas lined by ependyma. Tight junctions present in the endothelial cells (en; in b) prevent the extracellular progression of the tracer (white arrows; in b, c). (d) Tight junctions present in the choroid plexus ependyma also prevent the extracellular progression of the tracer (white arrow). Asterisk: intermediate filament bundles of astrocytes. Abbreviations: CP, Choroid plexus; g, Golgi apparatus dictiosomes; LV, lateral ventricle; V, intercellular space open to the ventricular lumen. Scale bars: a, 500 nm; b, 1 µm; b, 30 µm; d, 200 nm (TIFF 13478 kb)


