Abstract
Allergic asthma is associated with excessive T helper type 2 (Th2) cells activation and airway hyperreactivity (AHR), implicated in the context of significant morbidity and mortality. Soluble ST2, a member of the interleukin (IL)-1 receptor family, has been shown to play a critical role in modulation of inflammatory disorders, yet the function of soluble ST2 in allergic inflammation remains unclear. In this study, we examined the possibility of regulating ovalbumin (OVA)-challenged airway inflammation by recombinant adenovirus-mediated sST2-Fc (Ad-sST2-Fc) gene transfer. Single intranasal administration of Ad-sST2-Fc before allergen challenge in OVA-immunized mice profoundly reduced serum immunoglobulin (Ig)E secretion, eosinophil infiltration and concentrations of IL-4, IL-5 and IL-13 in bronchoalveolar lavage fluid compared with administration of a control Ad vector. Histopathological examination of the lungs revealed that sST2-Fc over-expression markedly suppressed allergen-induced peribronchial inflammation and disruption of the alveolar architecture. Moreover, the beneficial effect of sST2-Fc in allergic lung inflammation is related to blocking the IL–33/ST2L signalling. Taken together, these results suggested that administration of Ad-sST2-Fc gene transfer may have therapeutic potential for the immunomodulatory treatment of OVA-mediated allergic pulmonary diseases.
Keywords: adenoviral vector, allergic inflammation, eosinophils, soluble ST2, Th2 cytokines
Introduction
Allergic asthma, a chronic airway inflammatory disease characterized by enhanced production of T helper type 2 (Th2) cytokines, pulmonary eosinophilia and airway hyperreactivity (AHR), is a leading cause of morbidity and mortality in children, adults and the elderly [1–3]. The severity of allergic asthma has been associated with the degree of airways eosinophilia, neutrophilia and Th2 cytokine-producing lymphocytes. Following the infiltration of inflammatory cells, large amounts of proinflammatory mediators, especially Th2 cytokines, are released and are involved in airway smooth muscle dysfunction. Th2 cytokines, such as interleukin (IL)-4, IL-5 and IL-13, all contribute to the regulation of pulmonary inflammation and mucus gland hyperplasia, as well as tissue remodelling [3–5]. Despite extensive investigation and diverse therapeutic trials, there are still few effective therapeutic strategies in preventing/reversing the severe airway inflammation.
The ST2 gene, a member of the IL-1 receptor family, was recognized originally as a late responsive gene in mouse fibroblasts [6]. The transcription of ST2 gene generated at least four distinct molecules, a transmembrane form (ST2L), a soluble form (sST2) and two variant forms (ST2V and ST2LV), by alterative splicing [7–9]. Recently, a specific ligand of ST2L, IL-33, has been identified and IL-33/ST2L signalling plays a crucial role in Th2-type immunopathological changes [10]. Soluble ST2 was known to be implicated in a variety of immune disorders in humans. Elevated levels of soluble ST2 have been observed in conditions such as septic shock, trauma and myocardial infarction, especially on acute exacerbation [11–13]. Meanwhile, several experimental studies have revealed that the induction of soluble ST2 by various inflammatory stimuli may confer protection against inflammatory damage. For example, pretreatment with soluble ST2 significantly alleviated the production of inflammatory cytokines in collagen-induced arthritis [14], and decreased inflammation and lethality in intestinal and hepatic ischaemia/reperfusion injury [15,16]. In addition, both anti-ST2 monoclonal antibodies (mAb) and soluble ST2 have been shown to exert a negative regulation of Th2-dominatant allergic diseases, such as fungal asthma, chronic allergic conjunctivitis and respiratory syncytial virus (RSV)-induced airway inflammation [17–19].
Given that soluble ST2 has been involved in modulation of immune function and inflammatory response, we hypothesized that high levels of local soluble ST2 could ameliorate ovalbumin (OVA)-induced allergic airway inflammation. Therefore, we constructed a recombinant replication-deficient adenovirus encoding soluble ST2-human immunoglobulin (Ig)G1 Fc (sST2-Fc), and studied its beneficial effect in a murine model of OVA-sensitized asthma. We report here that single intranasal delivery of Ad-sST2-Fc to OVA-sensitized mice reduces significantly the production of Th2 cytokines, bronchoalveolar lavage eosinophil infiltrates and histopathological changes in the lung. Moreover, the protective effect of sST2-Fc in allergic lung inflammation is related to blocking IL-33/ST2L signalling.
Materials and methods
Mice and reagents
Female BALB/c mice, 8–10 weeks of age, were purchased from the Center of Experimental Animals of Guangdong Province, and housed in an animal facility under pathogen-free conditions. All experiments including mice were approved by the Guangdong Pharmaceutical University Animal Care and Use Committee. Mouse recombinant proteins of soluble ST2 (sST2-Fc) and IL-33 (rIL-33) were prepared in our laboratory, as described previously [16,20].
Generation of recombinant adenoviral vectors
Recombinant adenovirus containing murine soluble ST2-human IgG1 Fc (Ad-sST2-Fc) was constructed using the AdEasy system, as we have described previously [21]. Briefly, the cDNA fragment encoding the mouse soluble ST2 was cloned from mouse spleen, and coupled to the Fc portion of human IgG1. The two sequences were then subcloned into the shuttle plasmid [pAdTrack-cytomegalovirus (CMV)]. After homologous recombination between the shuttle plasmids and an adenoviral backbone plasmid (pAdEasy-1) in Escherichia coli BJ5183, recombinant adenoviral vectors encoding sST2-Fc were prepared in HEK293 cells. After several rounds of passage, recombinant adenovirus was purified using two rounds of caesium chloride (CsCl) density gradient centrifugation. The purified virus was dialysed and stored at −80°C until needed. Viral titres were determined by using green fluorescent protein (GFP) assay in HEK293 cells. An adenovirus-expressing GFP (Ad-EGFP) was also constructed and used as a control vector.
OVA-induced airway inflammation
Mice were sensitized and challenged to ovalbumin (OVA; Sigma, St Louis, MO, USA) based on procedures described previously [22]. Briefly, mice were sensitized on days 1 and 14 by intraperitoneal injection of 20 µg OVA emulsified in 1 mg of aluminium hydroxide in a total volume of 200 µl. On days 25, 26 and 27 after initial sensitization, the mice were challenged for 30 min daily with an aerosol of 1% (wt/vol) OVA in saline (or with saline as a control) using an ultrasonic nebulizer (Yuyue, Jiangsu, China). Twenty-four hours after the last OVA challenge, the mice were prepared for the collection of blood, bronchoalveolar lavage fluid (BALF) and lung tissues.
Administration of Ad-sST2-Fc
Ad-sST2-Fc [5 × 108 plaque-forming units (pfu)/mice] was delivered intranasally into slightly anaesthetized mice 48 h before the first challenge with OVA. A mock virus (Ad-EGFP) or equivalent phosphate-buffered saline (PBS) was used as a control.
ST2-Fc protein assessment
The levels of ST2-Fc in the lung tissue were measured by enzyme-linked immunosorbent assay (ELISA), as described previously [16]. Briefly, a microtitre plate was coated overnight at 4°C with 100 µl of horse anti-human IgG (25 µg/ml; SIBP, Shanghai, China). Plates were washed and blocked with 10% fetal calf serum (FCS) for 3 h at 37°C. The supernatant of BALF was added to each well and incubated for 1 h at 37°C. Plates were then washed and incubated with horseradish peroxidase (HRP)-conjugated anti-human IgG (ZhongShan Biotechnology, Beijing, China) for 1 h at 37°C. After washing, plates were incubated with peroxidase substrate 3,3',5,5'-tetramethylbenzidine (TMB) for 15 min, and the optical density (OD450) was measured by a microplate reader.
Measurement of airway hyperreactivity
AHR was determined by changes in lung resistance in anaesthetized and tracheostomized mice in response to increasing concentrations of aerosolized methacholine (0–50 mg/ml) using a Buxco system, as described previously [23].
Flow cytometry
Splenic single-cell suspensions were prepared, followed by red blood cell lysis with ammonium chloride lysis buffer, and washed with PBS. Subsequently, anti-mouse CD16/CD32 antibody (BD Pharmingen, San Jose, CA, USA) was mixed with the splenocytes to block the Fc receptor for 5 min on ice. The cells were then incubated with anti-ST2 (R&D Systems) or isotype control antibody (rat IgG; BD Pharmingen) and stained with R-phycoerythrin-conjugated anti-rat IgG antibody (BD Pharmingen). Then cells were analysed by the fluorescence activated cell sorter (FACS)Calibur flow cytometer.
Stimulation of splenocytes
Splenocytes were cultured at 4 × 107 cells/well in six-well plates and stimulated with 200 µg/ml OVA for 48 h. The OVA-stimulated cells were washed and resuspended with serum-free RPMI-1640 medium at 2·5 × 106 cells/well in 24-well plates. After incubation for 15 h, sST2-Fc was added to a final concentration of 500 ng/ml for 1 h. The cells were then treated with 20 ng/ml rIL-33 for 48 h. The culture supernatant was harvested and stored at −80°C until assay.
Real-time quantitative reverse transcription–polymerase chain reaction (RT–PCR)
Total RNA was extracted from the lung tissues using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. After removal of potentially contaminating DNA with DNase I (Invitrogen), cDNA was synthesized using a first-strand cDNA synthesis kit (MBI Fermentas Inc., Burlington, ON, Canada). The expression of the gene was quantified by real-time PCR with SYBR Green quantitative PCR (qPCR) assays (Applied Biosystems). The results were shown as relative expression standardized with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA content. The nucleotide sequences of primers used were as follows: ST2 forward primer, common to the long (ST2L) and short (sST2) isoforms, 5'-CCATAAGGCTGAGAAGGAAA-3', ST2L reverse primer 5'-AACAAAGTACTCCACAGAGT-3' and sST2 reverse primer 5'-TTGATCATGATGGATTCCCT-3'; IL-33, forward 5'-CCTGCCTCCCTGAGTACATACA-3' and reverse 5′- CTTCTTCCCATCCACACCGT-3'; and GAPDH, forward 5'-TTCACCACCATGGAGAAGGC-3' and reverse 5'-GGCATGGACTGTGGTCATGA-3'.
Cytokine measurement
The concentrations of IL-4, IL-5, IL-13 and IFN-γ in mouse BALF and culture supernatants were quantified with ELISA kits (eBioscience, San Diego, CA, USA), according to the manufacturer's protocol.
IgE assay
Levels of total IgE in serum were detected by ELISA using paired antibodies according to the manufacturer's instructions (BD Biosciences). Levels of serum OVA-specific IgE were measured by ELISA, as described previously [24]. Briefly, a microtitre plate was coated with OVA (50 µg/ml) and blocked with blocking buffer (PBS containing 0·5% Tween 20). Mouse sera were incubated in the antigen-coated wells and bound IgE was determined with biotin-labelled anti-mouse IgE (Pharmingen). Diluted streptavidin–peroxidase conjugate was added, the bound enzyme was detected with substrate TMB and the OD450 was measured. OVA-specific IgE levels were calculated from the pooled standard serum generated from OVA-sensitized BALB/c mice in our laboratory and were assigned the arbitrary values (units per millilitre).
Histology
Histopathological evaluation was performed on animals that were not subjected to BAL. Lungs were inflated and fixed with 10% buffered formalin. Samples were embedded in paraffin, and tissue sections were then stained with haematoxylin and eosin (H&E) and examined under light microscope.
Statistical analysis
Values for all measurements are expressed as the mean ± standard error of the mean (s.e.m.). Results were analysed using Student's t-test or one way analysis of variance (anova). Differences were considered to be statistically significant when P < 0·05.
Results
Expression of ST2 and IL-33 mRNAs in the lung of sensitized mice with allergen challenge
It has been known that IL-33, in an ST2-dependent manner, plays a critical role in context of a mouse model of asthma-like lung inflammation. To test whether soluble ST2, as a decoy receptor for IL-33, was involved in the regulation of allergic airway inflammation, we first examined the expression of ST2 and IL-33 genes in the lungs of sensitized mice on OVA challenge. BALB/c mice were immunized twice with OVA in aluminium hydroxide, and then triple-challenged with OVA at a 24-h interval. Using real-time PCR, we found that mRNA levels of both IL-33 and its membrane-bound receptor ST2 (ST2L) were considerably up-regulated in lung tissue of OVA-challenged mice when compared with these of saline-challenged mice (Fig. 1a). Conversely, in the presence of immunization with OVA and instilled OVA challenge, little, if any, expression of soluble ST2 (sST2) mRNA was detected in the lung tissues (Fig. 1). Therefore, these data suggest that expression as well as regulation of soluble ST2 in lung tissue may be absent or minimal in this model.
Fig. 1.
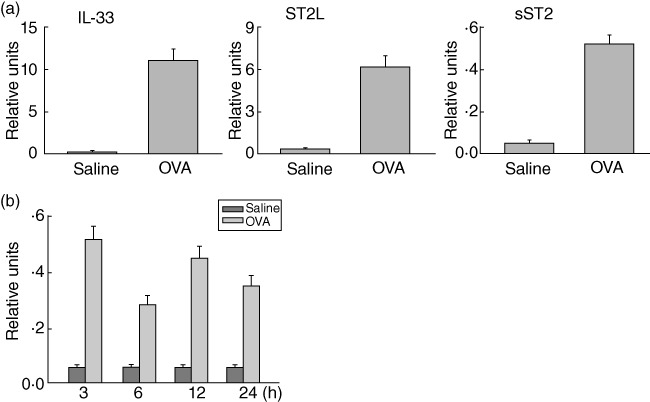
Expression of ST2 and interleukin (IL)-33 mRNAs in the lungs of sensitized mice with ovalbumin (OVA) challenge. (a) Real-time polymerase chain reaction (PCR) for IL-33, membrane ST2 (ST2L) or soluble ST2 (sST2) was performed on pooled mRNA from the lung tissue of saline- or OVA-challenged BALB/C mice at 3 h. Results presented are normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) expression. (b) Total RNA was prepared from the lung tissue of saline- or OVA-challenged mice at the indicated time. The levels of soluble ST2 mRNA were determined as described in (a). Data are presented as the mean ± standard error of the mean (n = 6 in each group).
Exogenous expression of soluble ST2 in the mouse lung after Ad-sST2-Fc administration
To elucidate further the significance of soluble ST2 up-regulation during allergen-induced airway inflammation, we detected the expression of recombinant soluble ST2 (sST2-Fc) by transgene delivery in the mouse lung. Anaesthetized female BALB/c mice were instilled a single dose intranasally of Ad-sST2-Fc (5 × 108 pfu/mice) and harvested at various time-points. As shown in Fig. 2, sST2-Fc protein expression in BALF started to increase at day 1 and increased maximally at day 2. Sustained up-regulation was still found at day 9. In contrast, there was no detectable sST2-Fc protein in the non-transfected mice, nor was any sST2-Fc detected in the control virus, Ad-EGFP-treated group. Of note, at the dose of 5 × 108 pfu, there was no evidence of vascular congestion and hypersecretion of mucous in bronchioles as shown by lung H&E staining (data not shown).
Fig. 2.
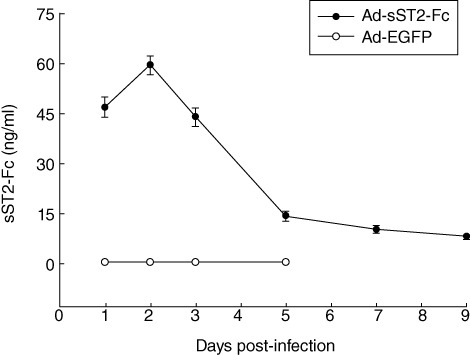
Expression of recombinant adenovirus-mediated soluble ST2 (Ad-sST2-Fc) in vivo. Ad-sST2-Fc was delivered intranasally to BALB/c mice at a dose of 5 × 108 plaque-forming units (pfu)/mice. Time–course of sST2-Fc protein levels in bronchoalveolar lavage fluid (BALF) was qualified by enzyme-linked immunosorbent assay (ELISA). Data are presented as the mean ± standard error of the mean (n = 6 in each group).
sST2-Fc over-expression alleviates OVA-induced airway inflammation
After demonstrating the feasibility of increasing sST2-Fc levels by airway administration of Ad-sST2-Fc, we continued to observe the protective effect of sST2-Fc against OVA-induced airway inflammation. In accordance with previous reports [25], intranasal OVA challenge resulted in remarkable proinflammatory alterations characterized by inflammatory cell infiltration in the lung interstitium around the airway and pulmonary blood vessels (Fig. 3a). These findings were ascertained by blinded pathological examination and were supported by clarifying the cellular compositions in the BALF when compared with the negative control group (Fig. 3b). By contrast, pretreatment of mice with Ad-sST2-Fc led to drastic decline in the allergic airway inflammation with eosinophil influx in the lung and BALF that followed OVA challenge (Fig. 3a,b). Meanwhile, sST2-Fc over-expression distinctly suppressed the development of AHR induced by OVA challenge (Fig. 3c). As expected, Ad-EGFP pretreatment did not attenuate these pathological changes after OVA challenge.
Fig. 3.
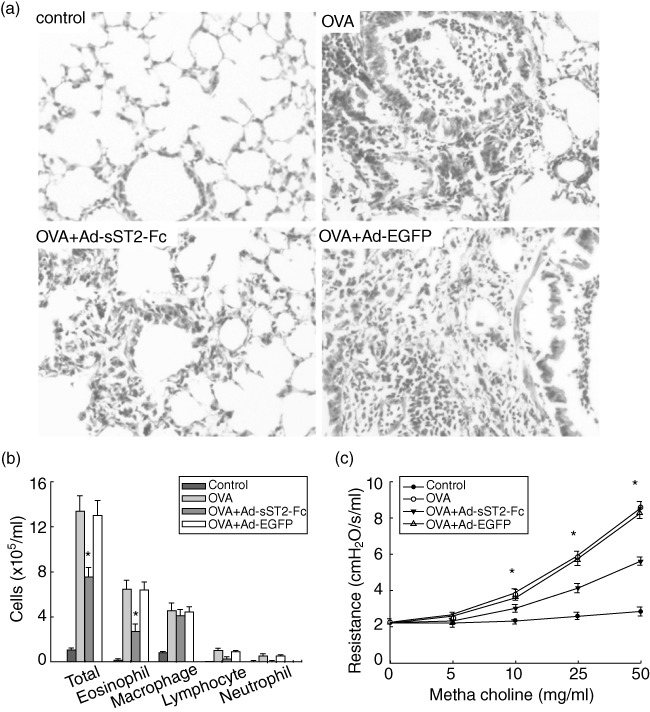
Alleviation of allergic lung inflammation and airway hyperresponsiveness (AHR) in ovalbumin (OVA)-challenged mice after administration of Ad-sST2-Fc. (a) Lung sections were stained with haematoxylin and eosin for measurement of inflammatory cells around the airways. Data revealed a different extent of cellular infiltration of the peri-airway region at 24 h after the last OVA challenge. Original magnification: ×200. (b) Bronchoalveolar lavage fluid (BALF) was collected and cell differentiation was determined at 24 h after the last OVA challenge. (c) AHR was assessed by measuring lung resistance in response to increasing concentrations of methacholine (0–50 mg/ml). Data are presented as the mean ± standard error of the mean (n = 6 in each group). *P < 0·05 versus the OVA group.
sST2-Fc suppresses the production of local Th2 cytokines in OVA-challenged mice
Next, we investigate the effect of sST2-Fc on the production of Th2-associated cytokines such as IL-4, IL-5 and IL-13, which is known to be involved in the pathophysiology of allergic pulmonary inflammation. The concentrations of BALF Th2 cytokines were measured by ELISA 24 h after the last challenge. As shown in Fig. 4, mice immunized with OVA revealed an increase in IL-4, IL-5 and IL-13 release when compared with a control group. In contrast, mice that pretreated with Ad-sST2-Fc markedly inhibited the levels of these cytokines following OVA challenge. Additionally, the level of Th1 cytokine IFN-γ in BALF showed no difference among the various groups. Again, pretreatment with Ad-EGFP had no inhibitory effect on the production of all these Th2 cytokines.
Fig. 4.
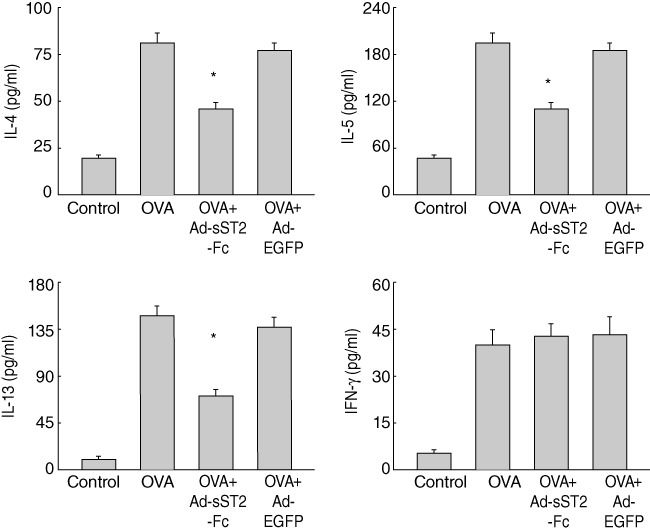
Inhibition of bronchoalveolar lavage fluid (BALF) T helper type 2 (Th2) cytokine expression after treatment with Ad-sST2-Fc. The levels of interleukin (IL)-4, IL-5, IL-13 and interferon (IFN)-γ in BALF were measured at 24 h after the last ovalbumin (OVA) challenge. Data are presented as the mean ± standard error of the mean (n = 6 in each group). *P < 0·05 versus the OVA group.
sST2-Fc decreases IgE level in serum of OVA-challenged mice
The concentration of serum IgE is associated closely with the severity of asthma, especially in children [26]. To determine the effects of soluble ST2 on the expression of IgE in allergen-challenged mice, we measured total and OVA-specific IgE levels in mice that were immunized with OVA or unimmunized. As shown in Fig. 5, serum levels of total and OVA-specific IgE were enhanced significantly in OVA-challenged mice relative to values in the negative control group. Administration of Ad-sST2-Fc, but not Ad-EGFP, evidently reduced the concentration of total IgE and OVA-specific IgE in serum.
Fig. 5.
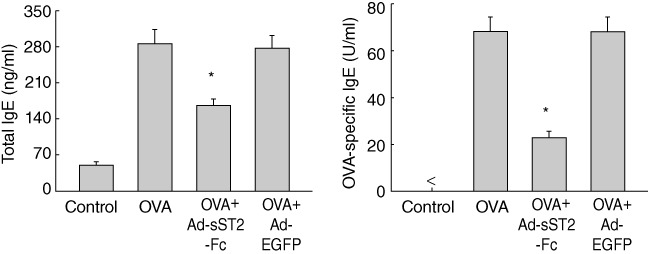
Reduction of serum total and ovalbumin (OVA)-specific immunoglobulin (Ig)E after delivery of Ad-sST2-Fc. The levels of serum IgE were determined at 24 h after the last OVA challenge. Data are presented as the mean ± standard error of the mean (n = 6 in each group). *P < 0·05 versus the OVA group.
sST2-Fc abrogates Th2 cytokine production from IL-33-stimulated splenocytes of asthmatic mice
Recent studies have suggested that IL-33 is implicated in the development of Th2-type immunity [20]. To observe the effects of soluble ST2 on the biological activity of IL-33, we analysed the production of Th2 cytokines from IL-33-treated splenocytes of asthmatic mice. We first detected the expression of IL-33 receptor (ST2L) on splenocytes surface using flow cytometry. As shown in Fig. 6a, compared with saline-treated mice ST2L expression was elevated significantly on the surface of splenocytes prepared from OVA-challenged mice. Subsequently, we demonstrated the effects of soluble ST2 on the production of Th1 and Th2 cytokines from IL-33-stimulated splenocytes. The cells were stimulated with OVA for activation of lymphocytes. After treatment with sST2-Fc for 1 h, cells were cultured with or without IL-33, and the concentrations of IL-4, IL-5 and IFN-γ in the supernatants were measured by ELISA at 48 h. The results showed that stimulation with IL-33 increased specifically the production of IL-4 and IL-5 from splenocytes of OVA-challenged mice, whereas the levels of both cytokines were abrogated by pretreatment with sST2-Fc (Fig. 6b). However, the production of IFN-γ was increased according to the reduction of Th2 cytokine production in splenocytes of OVA-challenged mice.
Fig. 6.
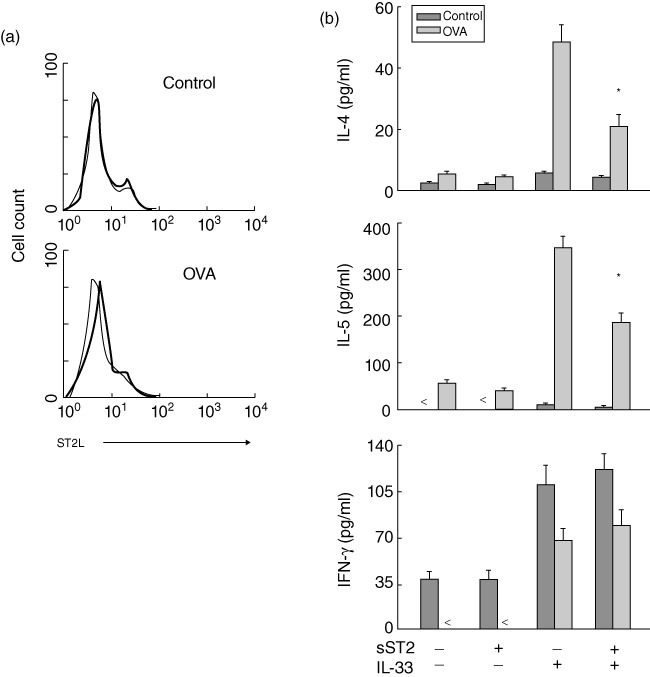
Effects of soluble ST2 on the production of T helper type 2 (Th2) cytokines from interleukin (IL)-33-stimulated splenocytes. (a) Expression of IL-33 receptor (ST2L) on the surface of splenocytes. The cells were prepared from saline- and ovalbumin (OVA)-challenged mice at 24 h and analysed for ST2L expression by flow cytometry. The thin lines indicated staining with isotype-matched control antibody. The data are representative of three separate experiments and showing similar results. (b) Suppression of Th2 cytokine production from IL-33-stimulated splenocytes. The splenocytes were prepared from saline- and OVA-challenged mice and restimulated with OVA. The cells then treated with sST2-Fc in the presence or absence of IL-33. After 48 h, the supernatant was monitored for IL-4, IL-5 and interferon (IFN)-γ by enzyme-linked immunosorbent assay. Data are presented as the mean ± standard error of the mean (n = 6 in each group). *P < 0·05, IL-33 alone versus ST2 plus IL-33 in splenocytes of OVA/OVA mice.
Discussion
This study has examined the immunomodulatory effect of soluble ST2, a member of the IL-1R superfamily, on allergic respiratory diseases. We found that high levels of local soluble ST2 induced by Ad-sST2-Fc exert essential negative regulation of OVA-mediated inflammation and confer a beneficial outcome of allergic asthma.
Several studies have documented that soluble form of cytokine receptors may act as positive or negative regulators in the expression of cytokines and growth factors. The soluble form of type II IL-1 receptor (sIL-1RII), IL-4 receptor α-chain (sIL-4Rα) and IL-22 receptor α-chain 2 (sIL-22Rα2) play antagonistic roles on IL-1, IL-4 and IL-22 signallings, respectively [27–29]. Conversely, the soluble IL-6 receptor α-chain (sIL-6Rα) plays agonistic roles on IL-6 signalling and modulates the production of chemokines [30]. Soluble forms of cytokine receptors are generated by several mechanisms including alternative splicing of pre-mRNA and proteolytic cleavage of receptors [31]. In the case of ST2 protein, soluble ST2 is produced by alternative splicing of pre-mRNA, and its amino acid sequence is mainly consistent with that of the extracellular domain of ST2L [7,8]. The interaction of IL-33 with its receptor ST2L contributes to immune response from Th1-to-Th2 switch and the release of Th2 cytokines through a signalling cascade that involves the activation of nuclear factor (NF)-κB and mitogen-activated protein (MAP) kinases [32]. Intriguingly, soluble ST2, as a decoy receptor for IL-33, has been shown to block IL-33/ST2L signalling pathway [33]. Consistent with these findings, our results demonstrated that the inhibitory effect of soluble ST2 in OVA-sensitized allergic asthma may be due at least partly to its ability to trap IL-33 and prevent it from binding to ST2L.
Serum soluble ST2 level was increased transiently in mice subjected to allergen-induced inflammatory responses and in human patients with acute asthma, suggesting a close correlation of soluble ST2 with the severity of allergic pulmonary disorders [34]. In the present study we observed that, unlike the expression of IL-33 and its membrane-bound receptor ST2, little increase of soluble ST2 mRNA was detected in the lung tissues after allergen exposure. Subsequently, to evaluate the effect of high levels of local soluble ST2 in OVA-mediated allergic asthma, we employed an Ad vector system for soluble ST2 gene transfer. Replication-deficient Ad are adopted as gene delivery vectors for the genetic treatment of a variety of diseases, especially in the transfer of exogenous genes to airway epithelial cells in vivo[35–37]. Our results showed that single intranasal administration of Ad-sST2-Fc could induce prominently enhanced levels of sST2-Fc expression within the murine lung, even 9 days after virus administration. Together with our recent studies [21], these data apparently demonstrate that adenovirus-based vectors can be used to induce high levels of sST2-Fc expression within the lung.
Recently, the beneficial effect of soluble ST2 on allergic diseases has been investigated intensively. Inhibition of ST2L in OVA-exposed Th2 recipient mice with either soluble ST2 or anti-ST2L mAb led to a decrease in allergic airway inflammation by preventing the influx of eosinophils and other inflammatory cells [38]. Hayakawa et al. [33] found that inhibition of IL-33 signalling, using soluble ST2 or anti-IL-33 mAb, effectively abrogates the activation of NF-κB and the production of Th2 cytokines following allergen exposure in the experimental asthma setting. Consistent with these findings, our results showed that administration of Ad-sST2-Fc locally in OVA-sensitized murine asthma model markedly ameliorated allergic inflammation such as AHR, eosinophilia in BALF. Histological examination also revealed that high levels of local sST2-Fc are capable of lessening OVA-mediated peribronchial inflammation. Besides, Ad-sST2-Fc treated mice exhibited strikingly reduced levels of Th2 cytokines, such as IL-4, IL-5 and IL-13 in BALF, compared with control mice. Suzuki et al. [35] reported that replication-deficient Ad can result in enhanced expression of IFN-γ in BALF and subsequent suppression of allergic responses, but suggested that there could be potentially promoted efficacy of recombinant Ad with transgene-expressing antagonizing molecule for host Th2-mediated reactions. However, other studies have not shown such an immune response to Ad-mock [36,37]. In accordance with the latter studies, our results presented no evidence of an IFN-γ response in Ad-EGFP-treated control mice.
IL-33, as a specific ligand for ST2, has been known to be expressed abundantly in the lungs of mice with OVA-induced airway inflammation, and alveolar macrophages are one of the cell types that express IL-33 [39]. Our results are consistent with previous reports which showed that soluble ST2 inhibited the binding of IL-33 to ST2L-positive cells and negatively modulated the production of Th2 cytokines through IL-33 signalling in allergic airway inflammation. Although the definite molecular mechanisms of soluble ST2 in regulation of Th1/Th2 responses in vivo remain to be clarified further, our findings are in agreement with other reports indicating that soluble ST2 can degrade not only IL-33/ST2L-mediated non-specific inflammatory responses but also the development of antigen-specific allergic inflammation [23,33].
In conclusion, these results demonstrate that soluble ST2 exerts anti-inflammatory and protective function in OVA-induced allergic airway inflammation. Treatment with recombinant adenovirus encoding sST2-Fc in vivo markedly reduced the production of Th2-associated cytokines, the severity of AHR and histopathological damage in the lung. Furthermore, the mechanism of action of sST2-Fc is associated closely with blockade of the IL-33/ST2L signalling cascades. These data suggest that local soluble ST2 may mediate a negative feedback modulation of Th2-predominant allergic airway inflammation, and that the intranasal administration of Ad-sST2-Fc has therapeutic potential for the treatment of allergic asthma.
Acknowledgments
The project was sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry [no. (2011)1139] and PhD Research Foundation of Guangdong Pharmaceutical University (2007JCX06).
Disclosure
The authors declare no conflicts of interest.
References
- 1.Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–81. doi: 10.1146/annurev.immunol.17.1.255. [DOI] [PubMed] [Google Scholar]
- 2.Humbles AA, Lloyd CM, McMillan SJ, et al. A critical role for eosinophils in allergic airways remodeling. Science. 2004;305:1776–9. doi: 10.1126/science.1100283. [DOI] [PubMed] [Google Scholar]
- 3.Umetsu DT, DeKruyff RH. TH1 and TH2 CD4+ cells in human allergic diseases. J Allergy Clin Immunol. 1997;100:1–6. doi: 10.1016/s0091-6749(97)70186-6. [DOI] [PubMed] [Google Scholar]
- 4.Robinson DS, Hamid Q, Ying S, et al. Predominant TH2-like bronchoalveolar T-lymphocyte population in atopic asthma. N Engl J Med. 1992;326:298–304. doi: 10.1056/NEJM199201303260504. [DOI] [PubMed] [Google Scholar]
- 5.Cohn L, Homer RJ, Marinov A, Rankin J, Bottomly K. Induction of airway mucus production by T helper 2 (Th2) cells: a critical role for interleukin 4 in cell recruitment but not mucus production. J Exp Med. 1997;186:1737–47. doi: 10.1084/jem.186.10.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werenskiold AK, Hoffmann S, Klemenz R. Induction of a mitogen responsive gene after expression of the Ha-ras oncogene in NIH 3T3 fibroblasts. Mol Cell Biol. 1989;9:5207–14. doi: 10.1128/mcb.9.11.5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Trajkovic V, Sweet MJ, Xu D. T1/ST2 – an IL-1 receptor-like modulator of immune responses. Cytokine Growth Factor Rev. 2004;15:87–95. doi: 10.1016/j.cytogfr.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Iwahana H, Yanagisawa K, Ito-Kosaka A, et al. Different promoter usage and multiple transcription initiation sites of the interleukin-1 receptor-related human ST2 gene in UT-7 and TM12 cells. Eur J Biochem. 1999;264:397–406. doi: 10.1046/j.1432-1327.1999.00615.x. [DOI] [PubMed] [Google Scholar]
- 9.Tago K, Noda T, Hayakawa M, et al. Tissue distribution and subcellular localization of a variant form of the human ST2 gene product, ST2V. Biochem Biophys Res Commun. 2001;285:1377–83. doi: 10.1006/bbrc.2001.5306. [DOI] [PubMed] [Google Scholar]
- 10.Oboki K, Ohno T, Kajiwara N, Saito H, Nakae S. IL-33 and IL-33 receptors in host defense and diseases. Allergol Int. 2010;59:143–60. doi: 10.2332/allergolint.10-RAI-0186. [DOI] [PubMed] [Google Scholar]
- 11.Sweet MJ, Leung BP, Kang D, et al. A novel pathway regulating lipopolysaccharide-induced shock by ST2/T1 via inhibition of Toll-like receptor 4 expression. J Immunol. 2001;166:6633–9. doi: 10.4049/jimmunol.166.11.6633. [DOI] [PubMed] [Google Scholar]
- 12.Brunner M, Krenn C, Roth G, et al. Increased levels of soluble ST2 protein and IgG1 production in patients with sepsis and trauma. Intens Care Med. 2004;30:1468–73. doi: 10.1007/s00134-004-2184-x. [DOI] [PubMed] [Google Scholar]
- 13.Shimpo M, Morrow DA, Weinberg EO, et al. Serum levels of the interleukin-1 receptor family member ST2 predict mortality and clinical outcome in acute myocardial infarction. Circulation. 2004;109:2186–90. doi: 10.1161/01.CIR.0000127958.21003.5A. [DOI] [PubMed] [Google Scholar]
- 14.Leung BP, Xu D, Culshaw S, McInnes IB, Liew FY. A novel therapy of murine collagen-induced arthritis with soluble T1/ST2. J Immunol. 2004;173:145–50. doi: 10.4049/jimmunol.173.1.145. [DOI] [PubMed] [Google Scholar]
- 15.Fagundes CT, Amaral FA, Souza AL, et al. ST2, an IL-1R family member, attenuates inflammation and lethality after intestinal ischemia and reperfusion. J Leukoc Biol. 2007;81:492–9. doi: 10.1189/jlb.0606422. [DOI] [PubMed] [Google Scholar]
- 16.Yin H, Huang BJ, Yang H, et al. Pretreatment with soluble ST2 reduces warm hepatic ischemia/reperfusion injury. Biochem Biophys Res Commun. 2006;351:940–6. doi: 10.1016/j.bbrc.2006.10.166. [DOI] [PubMed] [Google Scholar]
- 17.Ramaprakash H, Shibata T, Duffy KE, et al. Targeting ST2L potentiates CpG-mediated therapeutic effects in a chronic fungal asthma model. Am J Pathol. 2011;179:104–15. doi: 10.1016/j.ajpath.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuda A, Okayama Y, Terai N, et al. The role of interleukin-33 in chronic allergic conjunctivitis. Invest Ophthalmol Vis Sci. 2009;50:4646–52. doi: 10.1167/iovs.08-3365. [DOI] [PubMed] [Google Scholar]
- 19.Walzl G, Matthews S, Kendall S, et al. Inhibition of T1/ST2 during respiratory syncytial virus infection prevents T helper cell type 2 (Th2)- but not Th1-driven immunopathology. J Exp Med. 2001;193:785–92. doi: 10.1084/jem.193.7.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin H, Li XY, Jin XB, et al. IL-33 prolongs murine cardiac allograft survival through induction of TH2-type immune deviation. Transplantation. 2010;89:1189–97. doi: 10.1097/TP.0b013e3181d720af. [DOI] [PubMed] [Google Scholar]
- 21.Yin H, Li XY, Yuan BH, et al. Adenovirus-mediated overexpression of soluble ST2 provides a protective effect on lipopolysaccharide-induced acute lung injury in mice. Clin Exp Immunol. 2011;164:248–55. doi: 10.1111/j.1365-2249.2011.04326.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu X, Li M, Wu Y, Zhou Y, Zeng L, Huang T. Anti-IL-33 antibody treatment inhibits airway inflammation in a murine model of allergic asthma. Biochem Biophys Res Commun. 2009;386:181–5. doi: 10.1016/j.bbrc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 23.Kearley J, Buckland KF, Mathie SA, Lloyd CM. Resolution of allergic inflammation and airway hyperreactivity is dependent upon disruption of the T1/ST2-IL-33 pathway. Am J Respir Crit Care Med. 2009;179:772–81. doi: 10.1164/rccm.200805-666OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu B, Aoyama K, Kusumoto M, et al. Lack of lymphoid chemokines CCL19 and CCL21 enhances allergic airway inflammation in mice. Int Immunol. 2007;19:775–84. doi: 10.1093/intimm/dxm046. [DOI] [PubMed] [Google Scholar]
- 25.Gajewska BU, Swirski FK, Alvarez D, et al. Temporal–spatial analysis of the immune response in a murine model of ovalbumin-induced airways inflammation. Am J Respir Cell Mol Biol. 2001;25:326–34. doi: 10.1165/ajrcmb.25.3.4482. [DOI] [PubMed] [Google Scholar]
- 26.Burrows B, Martinez FD, Halonen M, Barbee RA, Cline MG. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–7. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 27.Giri JG, Wells J, Dower SK, et al. Elevated levels of shed type II IL-1 receptor in sepsis. Potential role for type II receptor in regulation of IL-1 responses. J Immunol. 1994;153:5802–9. [PubMed] [Google Scholar]
- 28.Sato TA, Widmer MB, Finkelman FD, et al. Recombinant soluble murine IL-4 receptor can inhibit or enhance IgE responses in vivo. J Immunol. 1993;150:2717–23. [PubMed] [Google Scholar]
- 29.Xu W, Presnell SR, Parrish-Novak J, et al. A soluble class II cytokine receptor, IL-22RA2, is a naturally occurring IL-22 antagonist. Proc Natl Acad Sci USA. 2001;98:9511–6. doi: 10.1073/pnas.171303198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurst SM, Wilkinson TS, McLoughlin RM, et al. Il-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity. 2001;14:705–14. doi: 10.1016/s1074-7613(01)00151-0. [DOI] [PubMed] [Google Scholar]
- 31.Levine SJ. Molecular mechanisms of soluble cytokine receptor generation. J Biol Chem. 2008;283:14177–81. doi: 10.1074/jbc.R700052200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmitz J, Owyang A, Oldham E, et al. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2- associated cytokines. Immunity. 2005;23:479–90. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 33.Hayakawa H, Hayakawa M, Kume A, Tominaga SI. Soluble ST2 blocks IL-33 signaling in allergic airway inflammation. J Biol Chem. 2007;36:26369–80. doi: 10.1074/jbc.M704916200. [DOI] [PubMed] [Google Scholar]
- 34.Oshikawa K, Kuroiwa K, Tago K, et al. Elevated soluble ST2 protein levels in sera of patients with asthma with an acute exacerbation. Am J Respir Crit Care Med. 2001;164:277–81. doi: 10.1164/ajrccm.164.2.2008120. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki M, Suzuki S, Yamamoto N, et al. Immune responses against replication-deficient adenovirus inhibit ovalbumin-specific allergic reactions in mice. Hum Gene Ther. 2000;11:827–38. doi: 10.1089/10430340050015446. [DOI] [PubMed] [Google Scholar]
- 36.Wang CC, Fu CL, Yang YH, et al. Adenovirus expressing interleukin-1 receptor antagonist alleviates allergic airway inflammation in a murine model of asthma. Gene Ther. 2006;13:1414–21. doi: 10.1038/sj.gt.3302798. [DOI] [PubMed] [Google Scholar]
- 37.Behera AK, Kumar M, Lockey RF, Mohapatra SS. Adenovirus mediated interferon gamma gene therapy for allergic asthma: involvement of interleukin 12 and STAT4 signaling. Hum Gene Ther. 2002;13:1697–709. doi: 10.1089/104303402760293547. [DOI] [PubMed] [Google Scholar]
- 38.Coyle AJ, Lloyd C, Tian J, et al. Crucial role of the interleukin 1 receptor family member T1/ST2 in T helper cell type 2-mediated lung mucosal immune responses. J Exp Med. 1999;190:895–902. doi: 10.1084/jem.190.7.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kurowska-Stolarska M, Stolarski B, Kewin P, et al. IL-33 amplifies the polarization of alternatively activated macrophages that contribute to airway inflammation. J Immunol. 2009;183:6469–77. doi: 10.4049/jimmunol.0901575. [DOI] [PubMed] [Google Scholar]


