Abstract
Allergy is one of the most common diseases with constantly increasing incidence. The identification of prognostic markers pointing to increased risk of allergy development is of importance. Cord blood represents a suitable source of cells for searching for such prognostic markers. In our previous work, we described the increased reactivity of cord blood cells of newborns of allergic mothers in comparison to newborns of healthy mothers, which raised the question of whether or not this was due to the impaired function of regulatory T cells (Tregs) in high-risk children. Therefore, the proportion and functional properties of Tregs in cord blood of children of healthy and allergic mothers were estimated by flow cytometry. The proportion of Tregs[CD4+CD25highCD127lowforkhead box protein 3 (FoxP3+)] in cord blood of children of allergic mothers tends to be higher while, in contrast, the median of fluorescence intensity of FoxP3 was increased significantly in the healthy group. Intracellular presence of regulatory cytokines interleukin (IL)-10 and transforming growth factor (TGF)-beta was also higher in Tregs of children of healthy mothers. Although we detected an increased proportion of Tregs in cord blood of children of allergic mothers, the functional indicators (intracellular presence of regulatory cytokines IL-10 and TGF-beta, median of fluorescence intensity of FoxP3) of those Tregs were lower in comparison to the healthy group. We can conclude that impaired function of Tregs in cord blood of children of allergic mothers could be compensated partially by their increased number. Insufficient function of Tregs could facilitate allergen sensitization in high-risk individuals after subsequent allergen encounter.
Keywords: allergy, cord blood, FoxP3, regulatory cytokines, Tregs
Introduction
Allergy is one of the most common medical disorders with a constantly increasing incidence. One of the theories explaining such a tremendous increment of allergies is the hygiene hypothesis, which postulates that lower exposure to microbes, especially in developed countries, alters the development of the immune system, thus promoting allergy development in predisposed infants. There is a down-regulatory bias to T helper type 2 (Th2) immune responses in the prenatal period preventing undesirable interactions with antigenically different maternal constituents [1]. The establishment of a new immunological balance proceeds post-natally after encountering the external environment. Prevalent Th2 responses support allergy development; Th1 and Th17 responses are important for anti-infection defence, but their exaggeration facilitates autoimmune reactions [2]. Therefore, very precise regulation preventing aberrant immune responses is important after birth. Regulatory T cells (Tregs) play an irreplaceable role in this fine tuning and limit pathological reactions, including allergy-associated Th2 responses.
There is a strong need to find early prognostic markers indicating increased risk of allergization. The finding of such a prognostic marker would make possible the introduction of preventive measures reducing allergy development, or at least lowering its clinical severity. Many authors have already tried to find some indications of future allergy development in cord blood. The responsiveness of cord blood cells of high- and low-risk children to allergens was followed [3,4], and polyclonal G+/G– bacteria stimulation [5–8] was tested. The proportion of both Th1 and Th2 cytokines in cord blood of high- and low-risk infants was tested [9–12]. Other researchers considered immunoglobulin (Ig)E levels in cord blood sera as a possible prognostic marker [13,14]. None of these measures have been found to be reliable prognostic indicators. It is possible to conclude with Prescott that the only reliable marker in allergy risk evaluation is the allergy status of the mother [5].
Several studies have tried to correlate the proportion of Tregs with the clinical symptoms of allergy in adults; unfortunately, results have as yet been contradictory [15–20]. Flow cytometry is usually used in these studies. Conflicting results may reflect variation in gating strategies used, different Tregs markers tested and also different ethnic groups examined. All these inconsistencies, together with the low number of individuals included in some studies [21,22], have led to ambiguous conclusions. Studies concerning cord blood Tregs and allergy are somewhat limited [22,23]. Based on the available studies, we postulate that some functional insufficiency of Tregs could contribute to allergy development. We tested this hypothesis by analysing and comparing Tregs in cord blood of high-risk newborns (children of allergic mothers) and low-risk newborns (children of healthy mothers). Using flow cytometry, we compared the proportion of Tregs (percentage of Tregs in the CD4+ population) and their functional properties [median of fluorescence intensity (MFI) of forkhead box protein 3 (FoxP3), interleukin (IL)-10 and transforming growth factor (TGF)-beta].
Materials and methods
Subjects
Healthy and allergic mothers with normal pregnancy and children delivered vaginally at full term in the Institute for the Care of Mother and Child in Prague, Czech Republic were included into the study. The diagnosis of allergy in mothers was based on the clinical manifestation of allergy persisting for longer than 24 months (allergy against respiratory and food allergens manifested by various individual combinations of hay fever, conjunctivitis, bronchitis, asthma, eczema and other allergic manifestations), monitoring by an allergist, positive skin prick tests or positive specific IgE antibodies and anti-allergic treatment before pregnancy. The study was approved by the Ethical Committee of the Institute for the Care of Mother and Child (Prague, Czech Republic) and was carried out with the written informed consent of the mothers.
A total of 153 maternal–child pairs were included in our study. Newborns were divided into two groups according to their mothers' allergy status: 77 children of healthy mothers (non-allergic) and 76 children of allergic mothers. Detailed description of the different types of allergy mothers involved in our study is summarized in Table 1.
Table 1.
Detailed characteristics of mothers participating in this study
| Allergy status | No. | Median age | Min | Max | Pollen | Mites | Dust | Cat dander | Food | Metal | Medicaments | Insects | Eczema | Others | 10–90 percentile IgE (r) IU/ml | 10–90 percentile IgE (f) IU/ml |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Allergic mothers | 76 | 31 | 24 | 37 | 27 | 9 | 14 | 2 | 8 | 2 | 35 | 6 | 7 | 18 | 0·21–5·59 | 0·11–2·49 |
| Non-allergic mothers | 77 | 32 | 20 | 39 | 0·20–1·36 | 0·13–0·67 |
r: Specific IgE to a mixture of respiratory allergens; f: specific immunoglobulin (Ig)E to a mixture of food allergens.
Cord blood sampling
Typically, 10–20 ml of cord blood of children was collected in sterile heparinized tubes for cell analysis (Tregs). A questionnaire inquiring about the allergy status of the mother was completed during the stay at the Institute for the Care of Mother and Child.
Tregs ratio, FoxP3 staining
The proportion of Tregs was estimated in cord blood samples immediately after delivery. The whole cord blood was stained for Treg cell surface markers using the following antibodies: CD4 fluorescein isothiocyanate (FITC), cat. no. 555346, CD25 phycoerythrin-cyanin 7 (PE-Cy7), cat. no. 557741 and CD127 Alexa 647, cat. no. 558598, all from Becton Dickinson (Franklin Lakes, NJ, USA). Lysis of erythrocytes was achieved by 12-min incubation with 3 ml of red blood cell (RBC) lysis buffer (cat. no. 00–4333) included in the human regulatory T cell whole blood staining kit (cat. no. 88–8996-40; eBioscience, San Diego, CA, USA). After centrifugation, followed by decantation of supernatant and washing (using 2 ml of flow staining buffer, cat. no. 00–4222, also included in the human regulatory T cell whole blood staining kit), cells were permeabilized/fixed by incubation with 1 ml FoxP3 lysed whole blood (LWB) fixation/permeabilization working solution at 4°C for 1 h in the dark. After washing with 2 ml of flow staining buffer, cord blood samples were stained using the ‘gold standard’ marker for identifying Tregs with anti-human FoxP3 PE antibody, cat. no. 12-4776-41A (clone PCH101), also included in the human regulatory T cell whole blood staining kit (cat. no. 88–8996-40; eBioscience), for 30 min. After washing with 2 ml of flow staining buffer, the pellet was resuspended in 100 µl of flow staining buffer (no fixative added). Samples were examined immediately in order to prevent loss of fluorescence. The lymphocyte gate was set based on forward-scatter (FCS) and side-scatter (SSC) characteristics with doublets exclusion (FCS-A × FCS-H), then CD4+ population was gated in the lymphocyte gate. Approximately 500 000 total events per sample were acquired for proper statistical evaluation of Treg functional parameters. Tregs were analysed in the CD4 gate as an intercept of three subpopulations of CD4+ lymphocytes using CD25, CD127 and FoxP3 markers (CD25 × CD127, CD25 × FoxP3, CD127 × FoxP3). Detailed gating strategy for estimation of the Treg ratio is shown in Fig. 1. Results are expressed as Treg ratio and MFI.
Fig. 1.
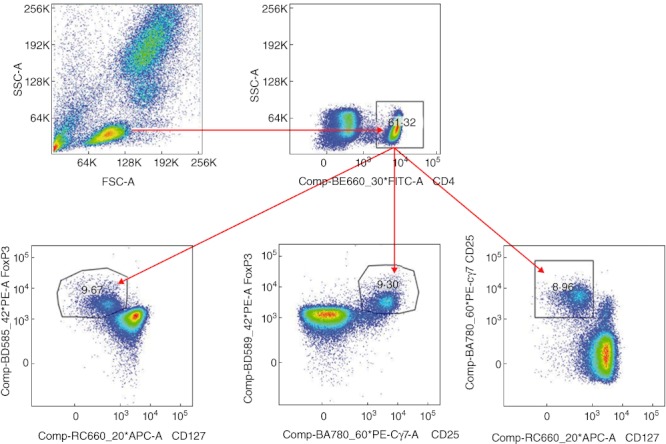
Gating strategy of proportion of regulatory T cells (Tregs) in cord blood of children of healthy and allergic mothers. Expression of the proportion of Tregs in CD4+ lymphocyte gate was considered as an intercept of three gates based on the combination of staining cell surface markers (CD4, CD25, CD127) and intracellular staining of transcription factor forkhead box protein 3 (FoxP3).
IL-10 and TGF-beta in Tregs
Regulatory cytokines were detected in non-stimulated cord blood cells. After red blood cell lysis and cell surface staining of CD4, CD25, CD127 (using the antibodies indicated above), intracellular staining of cytokines IL-10 (IL-10 PE, cat. no. 506804; BioLegend, San Diego, CA, USA) and TGF-beta [anti-human latency associated peptide (LAP) TGF-beta1 peridinin chlorophyll (PerCP)-Cy5·5, cat. no. 341803; BioLegend] was performed using fixation buffer, cat. no. 420801 and permeabilization wash buffer, cat. no. 421002 (both BioLegend) exactly according to the manufacturer's recommendations. For proper statistical evaluation, at least 100 000 total events were acquired per sample.
Data analysis and statistics
Flow cytometry data were acquired on a BD fluorescence activated cell sorter (FACS) Canto II instrument using BD FACS Diva version 6·1.2. software (Becton Dickinson). FlowJo 7·2.2. (TreeStar, Ashland, OR, USA) was used for data evaluation. Differences between groups were compared using the unpaired Student's t-test for data normally distributed (Treg ratio, MFI of FoxP3); otherwise the non-parametric Mann–Whitney test was used (comparing proportion of IL-10+ Tregs and TGF-beta+Tregs). Statistical and graphical analysis was performed in GrapPad Prism (GraphPad Software, La Jolla, CA, USA). Statistical significance was set at P ≤ 0·05.
Results
The immunological characteristics of Tregs in cord blood of high-risk children (children of allergic mothers) and low-risk children (children of healthy mothers) were compared. The proportion of Tregs was evaluated. To elucidate possible differences in functional properties of Tregs, MFI of FoxP3 and intracellular regulatory cytokines IL-10 and TGF-beta were tested. Differences in Treg proportions and their functional properties were found between the groups.
Treg ratio
Using our gating strategy (Fig. 1) and antibodies against CD4, CD25, CD127 and FoxP3, we did not find significant differences in the proportion of Tregs in the cord blood of children of healthy and allergic mothers, although the trend towards an increased number of Tregs in the CD4+ lymphocyte population from the allergic group was obvious (P = 0·07) (Fig. 2a). A significantly increased proportion of Tregs in cord blood of children of allergic mothers was observed when Tregs were considered only as CD4+CD25+ cells (P = 0·0117) (Fig. 2b). Different gating strategies together with using different Treg markers may account for variation among the results of different research groups.
Fig. 2.
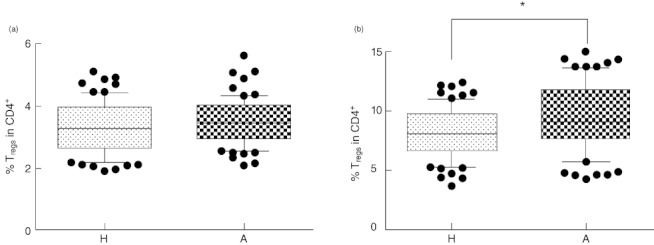
Proportion of regulatory T cells (Tregs) in cord blood. (a) Four-colour flow cytometry analysis (intercept of CD4+CD25highCD127low and CD4+CD25high forkhead box protein 3 (FoxP3+) and CD4+CD127lowFoxP3+. (b) Two-colour flow cytometry analysis (CD4+CD25+). H: proportion of Tregs in CD4+ lymphocytes in cord blood of children of healthy mothers (mean of 77 tested cord blood samples). A: proportion of Tregs in CD4+ lymphocytes in cord blood of children of allergic mothers (mean of 76 tested cord blood samples). *P ≤ 0·05.
MFI of FoxP3
Transcription factor FoxP3 is considered to be a master marker for identifying Tregs[24] (as CD25 can be expressed on other activated CD4+ T lymphocytes and CD127 is present on various cell types). The values of MFI of FoxP3 in cord blood of children of allergic mothers followed an opposite trend to the proportion of Tregs. A significantly higher MFI of FoxP3 (P = 0·0159) in cord blood Tregs of children of healthy mothers was detected in comparison to children of allergic mothers (Fig. 3).
Fig. 3.
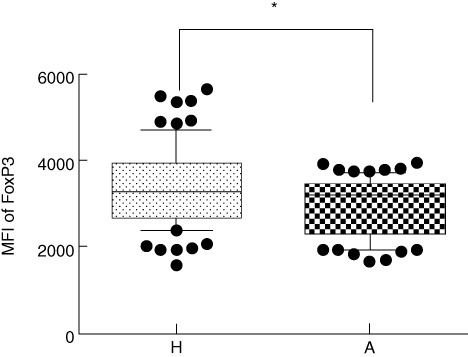
Median of fluorescence intensity (MFI) of forkhead box protein 3 (FoxP3) in CD4+CD25highCD127low regulatory T cells (Tregs). H: MFI of FoxP3 in Tregs from cord blood of children of healthy mothers (mean of 77 tested cord blood samples). A: MFI of FoxP3 in Tregs from cord blood of children of allergic mothers (mean of 76 tested cord blood samples). *P ≤ 0·05.
Intracellular regulatory cytokines IL-10 and TGF-beta
To evaluate the possible differences in functional characteristics of Tregs, the presence of regulatory cytokines IL-10 and TGF-beta was estimated by intracellular staining. A significantly higher number of IL-10+ Tregs in cord blood of children of healthy mothers was detected in comparison to children of allergic mothers (P = 0·0012) (Fig. 4). Similarly, a significantly higher proportion of TGF-beta+ Tregs in cord blood of children of healthy mothers is documented in Fig. 5 (P = 0·0174).
Fig. 4.
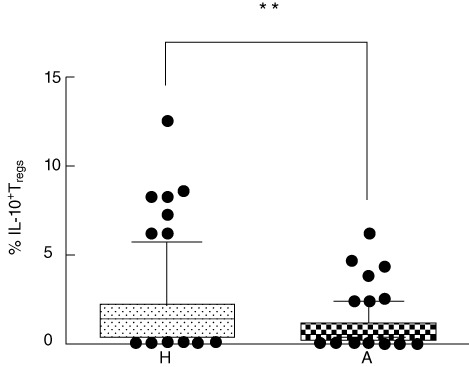
Intracellular presence of IL-10 in regulatory T cells (Tregs). Percentage of interleukin (IL)-10-positive Tregs (CD4+CD25highCD127low). H: percentage of IL-10+ Tregs in cord blood of children of healthy mothers (mean of 77 tested cord blood samples). A: percentage of IL-10+ Tregs in cord blood of children of allergic mothers (mean of 76 tested cord blood samples). **P ≤ 0·001.
Fig. 5.
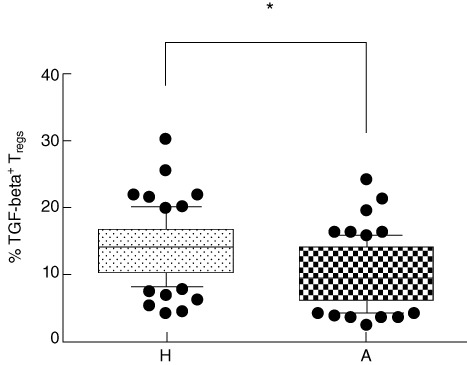
Intracellular presence of transforming growth factor (TGF)-beta in regulatory T cells (Tregs). Percentage of TGF-beta-positive Tregs (CD4+CD25highCD127low). H: percentage of TGF-beta+ Tregs in cord blood of children of healthy mothers (mean of 77 tested cord blood samples). A: percentage of TGF-beta+ Tregs in cord blood of children of allergic mothers (mean of 76 tested cord blood samples). *P ≤ 0·05.
Discussion
The importance of Tregs in immune regulations consists mainly in their role in induction of peripheral tolerance against autoantigens and harmless food and environmental antigens [25]. An insufficiency of Tregs can result in autoimmunity and allergy development [26–29]. We followed the status of newborn Tregs as a possible prognostic marker for future allergy manifestation. It is possible to assume that changes of immune regulation in allergy-prone infants can be evident prior to development of the clinical signs of allergy.
We found differences in immune characteristics of Tregs in the cord blood of children of allergic mothers in comparison to children of healthy mothers. Tregs were assessed on the basis of their cell surface markers (CD4, CD25high and CD127low), typical transcription factor FoxP3 and intracellular regulatory cytokines IL-10 and TGF-beta. The lower presence of regulatory cytokines, together with decreased MFI of FoxP3 in Tregs in cord blood of children of allergic mothers, points to the lower functional efficiency of these cells [23,30]. Impaired function of Tregs in the cord blood of children of allergic mothers could be compensated partially by an increased number of Tregs in comparison with the healthy group.
We documented an increased proportion of CD4+CD25highCD127lowFoxP3+ Tregs in children of allergic mothers. As indicated by Steinborn [23], FoxP3 is an important marker of regulatory cells reflecting their suppressor potency. When Tregs were detected only as CD4+CD25+ cells, their number was still higher. It is necessary to keep in mind that the above phenotype is characteristic not only for Tregs, but also for various subpopulations of activated T cells [31]. An increased proportion of the CD4+CD25+ subpopulation in cord blood of children of allergic mothers is in concordance with our previous observation of increased proliferation activity of both in-vitro-stimulated and non-stimulated cord blood cells of newborns of allergic mothers [32]. Discrimination between regulatory and activated T cells could be conducted on the basis of a recently described inverse correlation between CD127 and FoxP3 expression [33,34].
Regulatory cytokines IL-10 and TGF-beta are important effectors of Tregs[2,35,36]. Increased secretion of IL-10 (detected by ELISA) correlated with increased Tregs markers after stimulation of cord blood cells of children of healthy mothers, as reported by Schaub [37]. To the best of our knowledge, we are the first to report on the differences in the presence of intracellular IL-10 and TGF-beta between Tregs of children of healthy and allergic mothers. A lower proportion of Tregs producing IL-10 and TGF-beta in cord blood of children of allergic mothers (Figs 4 and 5) can signal a decreased predisposition to limiting the aberrant immune reaction to allergens in future, and can partially explain the increased proliferation activity of cord blood lymphocytes of children of allergic mothers mentioned above.
Tregs are a very heterogeneous population of cells and many methodological problems arise in the course of their study. Different gating strategies used for quantification of Tregs (CD4+CD25+[38], CD4+CD25high[30], CD4+CD25highCD127low[22], CD4+CD25highFoxP3+[39], CD4+CD25highCD127lowFoxP3+[40] or the gating we chose, based on the intercept of three different gates on CD4 subpopulation (as indicated in Fig. 1), can give quite different results leading to controversial conclusions. Furthermore, using different clones of FoxP3 antibodies could lead to different values of Treg ratio [41,42]. Using different clones of FoxP3 antibodies allows the detection of different Treg subpopulations. In our early experimental setting, we used two antibody clones (PCH101, eBioscience; and 259D/C7, Becton Dickinson) with appropriate buffers. A higher proportion of FoxP3+ Tregs was detected using PCH101, which was then used for further experiments. However, it is necessary to realize that the number of Tregs alone is not decisive for effective suppression function [43]. Functional analyses of Tregs are probably more informative. Further, it is necessary to keep in mind that not all lymphocytes exerting suppressor function express FoxP3 [44]. Another obstacle can be caused by cell isolation. Many studies analyse Tregs in peripheral blood after Ficoll-Paque separation. We compared the detection of Tregs in whole blood and in the population of isolated cord blood mononuclear cells (CBMC) – the results were similar, but the analyses obtained with the whole blood were more convincing and consistent and less time-consuming (data not shown).
We acknowledge some limitations of our study, namely the heterogeneity of mothers' allergies, but differentiation of the children into subgroups according to different kinds of maternal allergy decreased the power of statistical analyses. Individual types of maternal allergies are listed in Table 1.
Tregs are thought to play an important role in immune regulations even during intrauterine life [7]. Increased numbers of Tregs in this period can be partially responsible for decreased neonatal immune responses. The function of Tregs is critical in the early postnatal period, when the tuning of the immature immune system takes place. The impairment of Tregs could be the underlying mechanism contributing to heightened allergy development in predisposed children.
Our proof of decreased functionality of Tregs in cord blood of children of allergic mothers is in full agreement with the work of Prescott [22], who tested the immune function of neonatal CD4+CD25+CD127 low/– Tregs. However, both Prescott [22] and Schaub [30] did not find significant differences in transcription factor FoxP3 between high- and low-risk infants, whereas other studies pointed to decreased function of Tregs based on the lower presence of FoxP3 (MFI) [23]. This could be explained either by low numbers of individuals included [22] or by different methods used for the quantification of FoxP3. Quantitiative PCR (qPCR) was often used for the detection of FoxP3 gene expression [22,30]. Conversely, we exploited flow cytometry for FoxP3 protein detection. Schaub [30] suggests that the mRNA level of FoxP3 in Tregs is not regulated differently in dependence on maternal atopy. Nevertheless, the same group observed quantitatively and qualitatively increased Tregs in the cord blood of children of farming mothers whose children were postulated to be low-risk individuals for allergy development [7].
It is believed that lower exposure to non-pathogenic microbes together with reduced regulatory T function early in life could lead to Th1/Th2 imbalance, increasing the risk of allergy development [3]. The relationship between immune function of cord blood Tregs and allergy development requires further detailed studies. Our next intention is to follow the possible future allergy status of children participating in this study and to correlate this with the proportion and functional characteristics of Tregs in their cord blood. It could allow us to assess the value of Treg characteristics as prognostic markers of allergy development. It would be of importance to include other recently described markers characterizing various subsets of Tregs in further studies (e.g. transcription factor Helios differentiating between nTregs and iTregs). Until now, no work has been focused on the presence of Helios in cord blood Tregs. Studies [7,43] suggest using the Treg-specific demethylated region (TSDR), which seems to be a good qualitative marker indicative of functional activity. In fact, Hinz et al. [45] described a decreased proportion of Tregs characterized by TSDR in cord blood of children who develop allergy. Further studies characterizing Tregs by currently described Treg markers are needed to assess the suitability of these markers to serve as prognostic indicators of functional impairment of Tregs.
In conclusion, our study points to the decreased immunological capacity of Tregs in the cord blood of children of allergic mothers in comparison to healthy mothers. Insufficient Treg function can facilitate allergy induction in predisposed children. Long-term monitoring of children at risk is necessary for assessing the significance of the prognostic value of Treg insufficiency at birth for future allergy development.
Acknowledgments
We thank Professor Robert L. Owen for the language correction of the manuscript. This work was supported by grants of the Ministry of Education, Youth and Sports of the Czech Republic MSM0021620806, Grant Agency of Charles University GAUK259911, Charles University project SVV-2012–264506, Charles University research program PRVOUK P25/LF1/2.
References
- 1.Aluvihare VR, Kallikourdis M, Betz AG. Tolerance, suppression and the fetal allograft. J Mol Med (Berl) 2005;83:88–96. doi: 10.1007/s00109-004-0608-2. [DOI] [PubMed] [Google Scholar]
- 2.Jutel M, Akdis CA. T-cell subset regulation in atopy. Curr Allergy Asthma Rep. 2011;11:139–45. doi: 10.1007/s11882-011-0178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Joerink M, Oortveld MA, Stenius F, Rindsjo E, Alm J, Scheynius A. Lifestyle and parental allergen sensitization are reflected in the intrauterine environment at gene expression level. Allergy. 2010;65:1282–9. doi: 10.1111/j.1398-9995.2010.02328.x. [DOI] [PubMed] [Google Scholar]
- 4.Rindsjo E, Joerink M, Johansson C, Bremme K, Malmstrom V, Scheynius A. Maternal allergic disease does not affect the phenotype of T and B cells or the immune response to allergens in neonates. Allergy. 2010;65:822–30. doi: 10.1111/j.1398-9995.2009.02266.x. [DOI] [PubMed] [Google Scholar]
- 5.Prescott SL, King B, Strong TL, Holt PG. The value of perinatal immune responses in predicting allergic disease at 6 years of age. Allergy. 2003;58:1187–94. doi: 10.1034/j.1398-9995.2003.00263.x. [DOI] [PubMed] [Google Scholar]
- 6.Lappalainen M, Roponen M, Pekkanen J, Huttunen K, Hirvonen MR. Maturation of cytokine-producing capacity from birth to 1 yr of age. Pediatr Allergy Immunol. 2009;20:714–25. doi: 10.1111/j.1399-3038.2009.00865.x. [DOI] [PubMed] [Google Scholar]
- 7.Schaub B, Liu J, Hoppler S, et al. Maternal farm exposure modulates neonatal immune mechanisms through regulatory T cells. J Allergy Clin Immunol. 2009;123:774–82. doi: 10.1016/j.jaci.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 8.Gold DR, Bloomberg GR, Cruikshank WW, et al. Parental characteristics, somatic fetal growth, and season of birth influence innate and adaptive cord blood cytokine responses. J Allergy Clin Immunol. 2009;124:1078–87. doi: 10.1016/j.jaci.2009.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung EK, Miller RL, Wilson MT, McGeady SJ, Culhane JF. Antenatal risk factors, cytokines and the development of atopic disease in early childhood. Arch Dis Child Fetal Neonatal Ed. 2007;92:F68–F73. doi: 10.1136/adc.2006.106492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hrdy J, Zanvit P, Novotna O, Kocourkova I, Zizka J, Prokesova L. Cytokine expression in cord blood cells of children of healthy and allergic mothers. Folia Microbiol (Praha) 2010;55:515–9. doi: 10.1007/s12223-010-0085-7. [DOI] [PubMed] [Google Scholar]
- 11.Schaub B, Tantisira KG, Gibbons FK, et al. Fetal cord blood: aspects of heightened immune responses. J Clin Immunol. 2005;25:329–37. doi: 10.1007/s10875-005-4180-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silberer J, Ihorst G, Kopp MV. Cytokine levels in supernatants of whole blood and mononuclear cell cultures in adults and neonates reveal significant differences with respect to interleukin-13 and interferon-gamma. Pediatr Allergy Immunol. 2008;19:140–7. doi: 10.1111/j.1399-3038.2007.00605.x. [DOI] [PubMed] [Google Scholar]
- 13.Peters JL, Cohen S, Staudenmayer J, Hosen J, Platts-Mills TA, Wright RJ. Prenatal negative life events increases cord blood IgE: interactions with dust mite allergen and maternal atopy. Allergy. 2012;67:545–51. doi: 10.1111/j.1398-9995.2012.02791.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prokesova L, Novotna O, Janatkova I, et al. IgE against food and respiratory allergens in healthy and allergic mothers and their children. Folia Microbiol (Praha) 2008;53:67–72. doi: 10.1007/s12223-008-0010-5. [DOI] [PubMed] [Google Scholar]
- 15.Almeida AR, Zaragoza B, Freitas AA. Competition controls the rate of transition between the peripheral pools of CD4+ Int Immunol. 2006;18:1607–13. doi: 10.1093/intimm/dxl093. [DOI] [PubMed] [Google Scholar]
- 16.Kang SM, Tang Q, Bluestone JA. CD4+CD25+ regulatory T cells in transplantation: progress, challenges and prospects. Am J Transplant. 2007;7:1457–63. doi: 10.1111/j.1600-6143.2007.01829.x. [DOI] [PubMed] [Google Scholar]
- 17.Faria AM, Weiner HL. Oral tolerance. Immunol Rev. 2005;206:232–59. doi: 10.1111/j.0105-2896.2005.00280.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Benson MJ, Pino-Lagos K, Rosemblatt M, Noelle RJ. All-trans retinoic acid mediates enhanced T reg cell growth, differentiation, and gut homing in the face of high levels of co-stimulation. J Exp Med. 2007;204:1765–74. doi: 10.1084/jem.20070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ou LS, Goleva E, Hall C, Leung DY. T regulatory cells in atopic dermatitis and subversion of their activity by superantigens. J Allergy Clin Immunol. 2004;113:756–63. doi: 10.1016/j.jaci.2004.01.772. [DOI] [PubMed] [Google Scholar]
- 20.Taylor AL, Dunstan JA, Prescott SL. Probiotic supplementation for the first 6 months of life fails to reduce the risk of atopic dermatitis and increases the risk of allergen sensitization in high-risk children: a randomized controlled trial. J Allergy Clin Immunol. 2007;119:184–91. doi: 10.1016/j.jaci.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 21.Amoudruz P, Holmlund U, Malmstrom V, et al. Neonatal immune responses to microbial stimuli: is there an influence of maternal allergy? J Allergy Clin Immunol. 2005;115:1304–10. doi: 10.1016/j.jaci.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 22.Smith M, Tourigny MR, Noakes P, Thornton CA, Tulic MK, Prescott SL. Children with egg allergy have evidence of reduced neonatal CD4(+)CD25(+)CD127(lo/–) regulatory T cell function. J Allergy Clin Immunol. 2008;121:1460–6. doi: 10.1016/j.jaci.2008.03.025. 1466. [DOI] [PubMed] [Google Scholar]
- 23.Steinborn A, Engst M, Haensch GM, et al. Small for gestational age (SGA) neonates show reduced suppressive activity of their regulatory T cells. Clin Immunol. 2010;134:188–97. doi: 10.1016/j.clim.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto N, Oida T, Hirota K, et al. Foxp3-dependent and -independent molecules specific for CD25+CD4+ natural regulatory T cells revealed by DNA microarray analysis. Int Immunol. 2006;18:1197–209. doi: 10.1093/intimm/dxl060. [DOI] [PubMed] [Google Scholar]
- 25.Dimeloe S, Nanzer A, Ryanna K, Hawrylowicz C. Regulatory T cells, inflammation and the allergic response – the role of glucocorticoids and vitamin D. J Steroid Biochem Mol Biol. 2010;120:86–95. doi: 10.1016/j.jsbmb.2010.02.029. [DOI] [PubMed] [Google Scholar]
- 26.Curotto de Lafaille MA, Lafaille JJ. CD4(+) regulatory T cells in autoimmunity and allergy. Curr Opin Immunol. 2002;14:771–8. doi: 10.1016/s0952-7915(02)00408-9. [DOI] [PubMed] [Google Scholar]
- 27.Bettini M, Vignali DA. Regulatory T cells and inhibitory cytokines in autoimmunity. Curr Opin Immunol. 2009;21:612–8. doi: 10.1016/j.coi.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryanna K, Stratigou V, Safinia N, Hawrylowicz C. Regulatory T cells in bronchial asthma. Allergy. 2009;64:335–47. doi: 10.1111/j.1398-9995.2009.01972.x. [DOI] [PubMed] [Google Scholar]
- 29.Lloyd CM, Hawrylowicz CM. Regulatory T cells in asthma. Immunity. 2009;31:438–49. doi: 10.1016/j.immuni.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schaub B, Liu J, Hoppler S, et al. Impairment of T-regulatory cells in cord blood of atopic mothers. J Allergy Clin Immunol. 2008;121:1491–9. doi: 10.1016/j.jaci.2008.04.010. 1499. [DOI] [PubMed] [Google Scholar]
- 31.Tulic MK, Andrews D, Crook ML, et al. Changes in thymic regulatory T-cell maturation from birth to puberty: differences in atopic children. J Allergy Clin Immunol. 2011;129:199–206. doi: 10.1016/j.jaci.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 32.Zizka J, Hrdy J, Lodinova-Zadnikova R, et al. Effect of breast milk of healthy and allergic mothers on in vitro stimulation of cord blood lymphocytes. Pediatr Allergy Immunol. 2007;18:486–94. doi: 10.1111/j.1399-3038.2007.00563.x. [DOI] [PubMed] [Google Scholar]
- 33.Seddiki N, Santner-Nanan B, Martinson J, et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu W, Putnam AL, Xu-Yu Z, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ Treg cells. J Exp Med. 2006;203:1701–11. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Taylor A, Verhagen J, Blaser K, Akdis M, Akdis CA. Mechanisms of immune suppression by interleukin-10 and transforming growth factor-beta: the role of T regulatory cells. Immunology. 2006;117:433–42. doi: 10.1111/j.1365-2567.2006.02321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 37.Schaub B, Campo M, He H, et al. Neonatal immune responses to TLR2 stimulation: influence of maternal atopy on Foxp3 and IL-10 expression. Respir Res. 2006;7:40–8. doi: 10.1186/1465-9921-7-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Amelsfort JM, Jacobs KM, Bijlsma JW, Lafeber FP, Taams LS. CD4(+)CD25(+) regulatory T cells in rheumatoid arthritis: differences in the presence, phenotype, and function between peripheral blood and synovial fluid. Arthritis Rheum. 2004;50:2775–85. doi: 10.1002/art.20499. [DOI] [PubMed] [Google Scholar]
- 39.Thunberg S, Gafvelin G, Nord M, et al. Allergen provocation increases TH2-cytokines and FOXP3 expression in the asthmatic lung. Allergy. 2010;65:311–8. doi: 10.1111/j.1398-9995.2009.02218.x. [DOI] [PubMed] [Google Scholar]
- 40.Segundo DS, Fernandez-Fresnedo G, Gago M, et al. Kidney transplant recipients show an increase in the ratio of T-cell effector memory/central memory as compared to nontransplant recipients on the waiting list. Transplant Proc. 2010;42:2877–9. doi: 10.1016/j.transproceed.2010.07.072. [DOI] [PubMed] [Google Scholar]
- 41.Law JP, Hirschkorn DF, Owen RE, Biswas HH, Norris PJ, Lanteri MC. The importance of Foxp3 antibody and fixation/permeabilization buffer combinations in identifying CD4+CD25+Foxp3+ regulatory T cells. Cytometry A. 2009;75:1040–50. doi: 10.1002/cyto.a.20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Presicce P, Moreno-Fernandez ME, Lages CS, Orsborn KI, Chougnet CA. Association of two clones allows for optimal detection of human FOXP3. Cytometry A. 2010;77:571–9. doi: 10.1002/cyto.a.20875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu J, Lluis A, Illi S, et al. T regulatory cells in cord blood – FOXP3 demethylation as reliable quantitative marker. PLoS ONE. 2010;5:e13267. doi: 10.1371/journal.pone.0013267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hall BM, Verma ND, Tran GT, Hodgkinson SJ. Distinct regulatory CD4+T cell subsets; differences between naive and antigen specific T regulatory cells. Curr Opin Immunol. 2011;23:641–7. doi: 10.1016/j.coi.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 45.Hinz D, Bauer M, Roder S, et al. Cord blood Tregs with stable FOXP3 expression are influenced by prenatal environment and associated with atopic dermatitis at the age of one year. Allergy. 2012;67:380–9. doi: 10.1111/j.1398-9995.2011.02767.x. [DOI] [PubMed] [Google Scholar]


