Abstract
When total splenectomy is inevitable, heterotopic splenic autotransplantation seems to be the only alternative to maintain the functions of the spleen. The present study was carried out to analyse the critical mass of splenic autotransplant (SAT) for the development of phagocytic activity in rats. Wistar rats were submitted to total splenectomy (TS) alone or in combination with slices of SAT ranging from an average rate of 21·9% (one slice) to 100% (five slices) of the total splenic mass implanted into the greater omentum. Sixteen weeks after the beginning of the experiment, the animals were inoculated intravenously with a suspension of Escherichia coli labelled with Tc-99m. After 20 min, the rats were killed and the liver, lung and spleen or SAT, as well as blood samples were removed to determine the percentage of labelled bacteria uptake in these tissues. As the percentage of the total splenic mass contained in the SAT increased, the bacteria remaining in the blood decreased. From the implant of 26% up to the implant of the total splenic mass (100%) there was no difference in the bacteria remaining in the blood between the healthy animals of the control group and those submitted to TS combined with SAT. This finding shows that the critical mass needed for the development of phagocytic activity of macrophages in splenic autotransplants in adult rats is 26% of the total splenic mass.
Keywords: bacteria phagocytosis, critical mass, spleen, splenic autotransplant, total splenectomy
Introduction
As the largest lymphoid organ in the human body, the spleen plays a very important immune function, clearing bacteria from the bloodstream and providing the early production of antibodies in response to various antigenic particles. Its role in the immune system is highlighted by the number and different types of immune cells found in the organ temporarily or permanently, and by its peculiar anatomical and histological structure, which favours, among others, the functions of opsonization, phagocytosis and destruction of foreign antigens [1–6].
The response of immunoglobulins (Ig) to antigens – mainly IgM – occurs early in the spleen. During infections, the sequestration of bacteria induces the synthesis of antibodies in subjects with a preserved spleen. After total splenectomy (TS), the levels of IgG and IgA vary widely and may be normal or elevated, but most of the available data suggest a reduction of IgM [3,7–10]. Furthermore, in patients undergoing TS, the levels of CD4+ T lymphocytes (helper), the proportion of CD4+/T CD8+ T lymphocytes (helper/suppressor) and the competence of T lymphocytes themselves, in general, is reduced, which may be due to the minimization of the mitogenic responsive capacity of these cells [11].
In addition to its direct action in defence against infection, the spleen also appears to act as a mediator in the regulation of certain cell subpopulations in other organs, such as lung [12–14], liver [15,16] and bowel [16]. In asplenic patients, the portion of the non-splenic mononuclear phagocytic system (MPS) makes up for the absence of the organ, but the antibody formation is less efficient, lacking the ability to produce antibodies by IgG, given that IgM is reduced [17].
In comparison to the liver, the spleen has a higher ability to clear microorganisms per gram of tissue [18]. The reason for this greater ability of the spleen is unknown. Although it is believed that there may be some difference in the macrophages of these two organs, the diversity in their histological architecture, responsible for the slower passage of blood through the spleen – allowing more intense and prolonged contact between antigens and phagocytes – probably plays an important role in this difference of phagocytic activity [3,7,19,20]. The spleen is the only organ in the MPS with this versatility, and several studies suggest that the heterotopic autotransplant of splenic tissue can recover this particular functional activity [3,21–32].
Clinical and experimental investigations have shown that, after a certain period, autotransplanted splenic tissue presents morphological regeneration and leads to the recovery of some functions of the spleen [3,6,8,10,12,21–32]. However, it is of fundamental importance to determine the amount of splenic tissue to be autotransplanted – critical mass – for the occurrence of functional regeneration. The objective of the present study was to determine the amount of splenic tissue to be autotransplanted in rats for the effective development of phagocytic activity.
Materials and methods
The study was approved by the Ethics Committee on Animal Research of the Biology Institute Roberto Alcantara Gomes, Rio de Janeiro State University, Brazil. All procedures followed current guidelines for animal experimentation rigorously [33].
Animals
Sixty-three male Wistar rats weighing 215·5–234·2 g were allocated randomly to seven groups: 1: sham-operated; 2: total splenectomy alone (TS); and 3–7: TS combined with splenic autotransplantation (SAT) of one to five slices containing an average rate of 21·9–100% of splenic tissue, respectively.
The animals were housed in appropriate cages, five to a cage at most, under conditions of controlled temperature and humidity, on a 12-h light/12-h dark photoperiod, and allowed free access to water and standard laboratory chow over a period of 16 weeks.
Surgical procedures
After a 12-h fast, the animals were anaesthetized with 80 mg/kg ketamine and 12 mg/kg xylazine, given intramuscularly, followed by extensive trichotomy of the abdominal wall. Anti-sepsis of the abdominal wall was carried out with a solution of polyvinylpyrrolidone–iodine containing 1% of active iodine. The entire procedure was performed under sterile conditions.
In all animals, the procedure was started with a supraumbilical midline incision, approximately 3 cm in length, and laparorrhaphy was carried out with continuous sutures on two planes (peritoneal–aponeurotic plane and skin) with polyglycolic acid 3-0.
In group 1 animals (control), the spleen was mobilized to the surgical field and returned subsequently to its usual place.
In the animals in groups 2–7, the spleen was mobilized to the surgical field and the splenic and splenogastric vessels were ligated with chromic catgut 3-0, followed by removal of the organ and weighing on a precision scale.
In the animals in groups 3–7, after TS the spleen was weighed and cut transversely into five segments, each about 2 mm thick. In these animals, the splenic sections were implanted into the greater omentum using continuous 4-0 polyglycolic acid sutures. Only one slice of splenic tissue was implanted in group 3 and two, three, four, and five slices were implanted in groups 4–7, respectively. Stitches were introduced alternately into the omentum and the slices to permit interposition of omental tissue between the splenic slices.
The animals were followed-up daily for 16 weeks.
Inoculation of labelled bacteria
A suspension of Escherichia coli AB1157 containing a bacterial concentration corresponding to 108 colony-forming units (CFU) was used.
E. coli strain AB1157 was labelled with 99mtechnetium (99mTc) in the form of sodium pertechnetate (Tc04Na), as described previously [34,35].
Sixteen weeks after the beginning of the experiment, the animals were anaesthetized with 80 mg/kg ketamine and 12 mg/kg xylazine given intramuscularly, submitted to cervical and abdominal trichotomy and disinfected with polyvinylpyrrolidone–iodine containing 1% active iodine. Rats were inoculated with 99mTc-labelled E. coli through direct transverse cervicotomy by dissecting the internal jugular vein. Twenty minutes after inoculation, the animals were killed with an anaesthetic overdose and submitted to midline thoracolaparotomy. The caudal vena cava was cut, causing intra-abdominal bleeding which, in turn, resulted in the formation of a blood clot. The liver, lung and spleen or SAT, as well as the blood clot, were removed, weighed and placed into appropriate tubes for radioactive counting with a gamma scintillation counter.
Calculation of labelled bacterial uptake
A standard dose containing the same volume and the same activity of the 99mTc-labelled E. coli suspension inoculated into the animals was used for calculation. The standard count was considered to be 100% of the radioactivity inoculated into the animals. The distribution of captured bacteria was normalized, considering that uptake in the liver, lung and spleen or SAT, plus blood (bacteria remaining in the bloodstream), was 100%. Percentage of bacterial uptake was calculated for each sample using the formula (cpm = counts per minute):
Taking into consideration the mass of the liver, lung, spleen or SAT and blood clot, percentage of uptake per g tissue was calculated using the formula:
Percentage of uptake in each organ (total mass) was calculated using the formula:
Calculation of circulating blood volume
Blood (1·0 ml) was collected from the caudal vena cava during the laparotomies for organ removal from five rats of each group, placed into test tubes and left to stand for 24 h. The tubes were centrifuged to separate serum and blood clots completely. The clots were weighed, with each millilitre of blood resulting, on average, in a 0·49 g clot. A rat weighing 250 g contains on average 16 ml of blood and this corresponds to approximately 7·85 g of clot material [36]. Based on this clot mass, percentage uptake in whole blood was estimated for each animal.
Histopathological study
Splenic implants were removed and placed into a solution containing buffered 10% formalin. The tissue was processed with increasing concentrations of alcohol and xylol, embedded in paraffin and cut into 4-µm-thick slices. Slides prepared from these slices were stained with haematoxylin and eosin (H&E) and Gram stain, and analysed by light microscopy.
Statistical analysis
Statistical analysis was carried out to compare the level of regeneration of the autotransplanted splenic mass of animals in groups 3–7. The analysis also aimed to compare the behaviour of the groups regarding the uptake of bacteria labelled with Tc-99m by the liver, lung, and spleen or SAT, and the bacteria remaining in the bloodstream. Specifically, for the study of uptake by the spleen or SAT, splenectomized animals were excluded from the analysis.
The groups of animals were compared by t-test, accounting for adjustment to equal and different variances, and by the non-parametric Kruskal–Wallis test. Additionally, multiple linear regression was used to identify the independent effect of belonging to different groups of animals on the bacteria remaining in the blood, as well as the effect of different ranges of percentage of implanted splenic mass on the same dependent variable. In all cases, the models included all the animals.
As a complement, using linear regression, we also explored the difference between groups and the role of bacteria remaining in the blood in explaining differences in the levels of bacterial uptake in liver, lung and spleen or SAT, with the last variable being used to observe the existence of compensation between organs and blood in bacterial uptake.
The analyses were performed using the sas statistical package, version 9·2 (2008), with the level of significance set at 5%.
Results
Animal's evolution
All animals in groups 1 (control), 2 (total splenectomy alone), 3 (SAT with one slice) and 4 (SAT with two slices) progressed satisfactorily throughout the experiment (16 weeks). One animal in each of the other groups (5, 6 and 7, SAT with three, four and five slices, respectively) died 16 weeks after the beginning of the experiment at the time of anaesthesia for intravenous inoculation of labelled bacteria. In these groups, analyses were performed with eight animals.
Except for the deaths reported above, the remaining animals showed good progress during the 16 weeks of the experiment, with normal activity, and none of them presented complications in the postoperative period.
Regarding the weight of the animals, there was no difference between groups at the beginning or at the end of the experiment.
Regeneration of autotransplanted splenic tissue
There was regeneration of SAT in all animals in groups 3–7, including the three who died 16 weeks after the beginning of the experiment. Although they were excluded from the analyses they were autopsied, showing a favourable outcome of this aspect.
Table 1 shows a descriptive analysis of the regeneration of implanted splenic masses in rats submitted to SAT. The mean percentage of implanted splenic mass ranged from 21·9% (in the group implanted with one splenic slice) to 100·0% (in the group implanted with five splenic slices).
Table 1.
Descriptive analysis of the regeneration of implanted splenic mass in the groups of rats submitted to splenic autotransplant
| Total splenic mass (g) | % implanted splenic mass | % recovered/implanted mass | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Group | n | Mean | SD | Median | Mean | SD | Median | Mean | SD | Median |
| SAT – 1 | 9 | 0·6934 | 0·0945 | 0·6819 | 21·9 | 4·0 | 22·1 | 32·6 | 14·9 | 27·5 |
| SAT – 2 | 9 | 0·6569 | 0·0775 | 0·6824 | 31·9 | 4·8 | 30·3 | 42·1 | 6·2 | 40·6 |
| SAT – 3 | 8 | 0·7000 | 0·1066 | 0·6896 | 60·6 | 6·4 | 61·4 | 48·1 | 13·8 | 45·1 |
| SAT – 4 | 8 | 0·6127 | 0·0554 | 0·6136 | 75·4 | 6·2 | 76·0 | 56·7 | 14·1 | 53·0 |
| SAT – 5 | 8 | 0·7756 | 0·0813 | 0·7685 | 100·0 | 0·0 | 100·0 | 57·9 | 7·3 | 56·5 |
SAT: splenic autotransplant; 1: one splenic slice; 2: two splenic slices; 3: three splenic slices; 4: four splenic slices; 5: five splenic slices; SD: standard deviation.
Morphological analysis of regenerated SAT
At the abdominal reintervention performed on animals 16 weeks after the beginning of the experiment, the regenerated splenic implants presented a bright violet colour, with mild and scarce whitish stippling. Most of the recovered segments presented anatomical conformation resembling the slices that were implanted and, most notably, when they were merged with others they led to the appearance of a portion of a normal spleen. On palpation, their consistency was elastic, also resembling normal splenic tissue.
At microscopic examination by H&E staining, the morphological aspect found was similar in all groups of animals (3–7), with the presence of well-defined red and white pulps, with moderate architectural disarrangement. A reduction of white pulp was observed, but with the presence of lymphoid follicles. A decrease of the marginal zone was also observed, with no germinal centres being detected. In the splenic parenchyma, there were numerous macrophages containing intracytoplasmic haemosiderin pigments. With regard to the blood vessels, their walls were preserved without evidence of thrombosis or vasculitis.
Under ×1000 magnification, Fig. 1 illustrates the microscopic morphology of a splenic macrophage of regenerated autotransplanted tissue. Using H&E staining, numerous bacterial clumps were identified in the cytoplasm, and using Gram staining (Brown & Brenn's method) there were found to be clumps of Gram-negative bacteria around the macrophage nucleus.
Fig. 1.
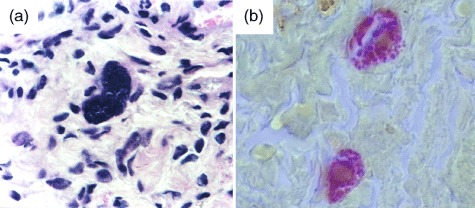
(a) Microscopic morphology of a splenic macrophage of a regenerated splenic autotransplant, with bacterial clumps in the cytoplasm (haematoxylin and eosin staining); (b) microscopic morphology of Gram-negative bacterial clumps around the nucleus of a macrophage (Gram stain) (×1000). Bar: 100 µm.
Uptake of labelled E. coli with Tc-99m
Table 2 presents the descriptive statistics of bacterial uptake by the liver, lung, spleen/autotransplant and blood in the various groups of animals, highlighting the results of the Kruskal–Wallis test which indicated the rejection of the hypothesis of equality of groups regarding uptake by the liver, lung and blood, but not the rejection of the same hypothesis (excluding the group submitted to total splenectomy alone) in relation to bacterial uptake by the spleen/autotransplant.
Table 2.
Descriptive statistics of bacterial uptake by the liver, lung, spleen/autotransplant and blood in the groups of rats in the study
| Liver | Lung | Spleen/autotransplant | Blood | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | n | Mean | SD | Median | Mean | SD | Median | Mean | SD | Median | Mean | SD | Median |
| Control | 9 | 71·5 | 4·0 | 70·4 | 21·1 | 2·7 | 21·6 | 4·8 | 1·4 | 4·3 | 2·5 | 0·9 | 2·5 |
| TS | 9 | 75·3 | 2·4 | 74·5 | 18·0 | 2·4 | 17·9 | – | – | – | 6·7 | 2·0 | 6·6 |
| SAT–1 | 9 | 68·3 | 2·9 | 67·6 | 23·4 | 2·2 | 23·4 | 3·8 | 1·2 | 3·7 | 4·4 | 1·1 | 4·1 |
| SAT–2 | 9 | 70·7 | 2·6 | 70·3 | 21·2 | 2·9 | 21·2 | 4·4 | 0·6 | 4·7 | 3·7 | 0·9 | 3·6 |
| SAT–3 | 8 | 71·3 | 2·8 | 70·9 | 21·7 | 2·6 | 21·2 | 4·4 | 1·5 | 4·2 | 2·7 | 0·9 | 2·7 |
| SAT–4 | 8 | 70·9 | 2·3 | 71·1 | 21·7 | 2·0 | 21·8 | 4·8 | 1·2 | 4·6 | 2·6 | 0·9 | 2·3 |
| SAT–5 | 8 | 72·0 | 3·8 | 71·4 | 20·8 | 3·3 | 21·1 | 4·7 | 1·4 | 4·3 | 2·5 | 0·9 | 2·3 |
| Kruskal–Wallis test (P) | 0·0061 | 0·0147 | 0·6438 | <0·0001 | |||||||||
TS: total splenectomy; SAT: splenic autotransplant; 1: one splenic slice; 2: two splenic slices; 3: three splenic slices; 4: four splenic slices; 5: five splenic slices; SD: standard deviation.
Focusing specifically on the bacteria remaining in the blood, we also emphasize the high average percentage (6·7%) of bacteria in the blood of rats submitted to TS and its reduction in the groups submitted to SAT, reaching levels comparable to those of the control group.
Table 3 shows the lack of difference between the groups of animals submitted to SAT of at least three slices and the control group, as well as the contrast with the group submitted to TS, with percentages of bacteria in the blood ranging between 3·8 and 9·9%, and mean values much higher than found in the control group. The groups submitted to SAT with one or two slices also differed from the control group, with higher mean levels of bacteria remaining in the blood, although lower than in the TS group.
Table 3.
Comparison of the remaining bacteria in the blood of the animals submitted to total splenectomy alone or combined with splenic autotransplant with the control group
| Group | 95% CI | P |
|---|---|---|
| Control group (reference) | 1·85; 3·20 | |
| TS | 5·18; 8·23 | <0·0001 |
| SAT – 1 | 3·62; 5·27 | 0·0007 |
| SAT – 2 | 2·96; 4·38 | 0·0154 |
| SAT – 3 | 1·91; 3·40 | 0·7650 |
| SAT – 4 | 1·90; 3·32 | 0·8408 |
| SAT – 5 | 1·70; 3·23 | 0·8961 |
TS: total splenectomy; SAT: splenic autotransplant; 1: one splenic slice; 2: two splenic slices; 3: three splenic slices; 4: four splenic slices; 5: five splenic slices; CI: confidence interval.
Table 4 shows linear regression models explaining the variation of the bacteria remaining in the blood considering the different groups of animals and the range of implanted splenic mass. The results confirm the different behaviour, in relation to the control group, of the groups submitted to TS without SAT and SAT up to two slices, with a gradient favourable to the autotransplant groups. They also confirm the absence of difference between groups of SAT with at least three slices and the control group. Additionally, they exhibit consistent behaviour of the groups of animals submitted to TS alone or combined with SAT up to about 26% of the total splenic mass, with a decrease in remaining bacteria, but significantly higher than that found in the control group. The groups with SAT of at least 26% of the splenic mass appeared not to differ significantly from the control group.
Table 4.
Linear regression models: explanatory factors of the variation in blood remaining bacteria (%) of the animals with regard to the number of implanted splenic slices and to the percentage of implanted splenic mass (control group as reference)
| Model regarding implanted splenic slices | Model regarding percentage of implanted splenic mass | |||||
|---|---|---|---|---|---|---|
| Variable | Coef. | SE | P > |t| | Coef. | SE | P > |t| |
| Intercept | 2·52 | 0·38 | <0·0001 | 2·52 | 0·37 | <0·0001 |
| TS | 4·18 | 0·54 | <0·0001 | 4·18 | 0·52 | <0·0001 |
| SAT – 1 | 1·92 | 0·54 | 0·0008 | |||
| SAT – 2 | 1·15 | 0·54 | 0·0389 | |||
| SAT – 3 | 0·13 | 0·56 | 0·8161 | |||
| SAT – 4 | 0·09 | 0·56 | 0·8784 | |||
| SAT – 5 | −0·06 | 0·56 | 0·9181 | |||
| SAT, 10 ├ 20% SM | 2·99 | 0·74 | 0·0002 | |||
| SAT, 20 ├ 22% SM | 2·78 | 1·17 | 0·0212 | |||
| SAT, 22 ├ 26% SM | 1·44 | 0·62 | 0·0237 | |||
| SAT, 26 ├ 28% SM | 1·48 | 0·87 | 0·0938 | |||
| SAT, 28 ├ 30% SM | 0·92 | 0·87 | 0·2935 | |||
| SAT, 30 ├┤ 100% SM | 0·18 | 0·42 | 0·6743 | |||
| R2 | 0·647 | 0·680 | ||||
Coef.: coefficient; SE: standard error; TS: total splenectomy; SAT: splenic autotransplant; 1: one splenic slice; 2: two splenic slices; 3: three splenic slices; 4: four splenic slices; 5: five splenic slices; SM: splenic mass; ├, indication of the inclusion of the lower interval limit and of the exclusion of the higher interval limit.
Table 5 presents linear regression models explaining the variation in the percentage of bacterial uptake by the liver, lung and spleen/autotransplant, considering the groups of animals and the remaining bacteria in the blood.
Table 5.
Linear regression models: explanatory factors of the variation in the percentage of bacterial uptake in the liver, lung and spleen/autotransplant of the animals
| % bacterial uptake in the liver | % bacterial uptake in the lung | % bacterial uptake in the spleen/autotransplant | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Coef. | SE | P > |t| | Coef. | SE | P > |t| | Coef. | SE | P > |t| |
| Intercept | 73·97 | 0·96 | <0·0001 | 21·29 | 0·39 | <0·0001 | 5·31 | 0·48 | <0·0001 |
| TS | 8·28 | 1·44 | <0·0001 | −3·32 | 0·93 | 0·0008 | |||
| % of bacterial uptake in the blood | −1·03 | 0·28 | 0·0006 | −0·27 | 0·15 | 0·0716 | |||
| SAT – 1 | 2·08 | 0·93 | 0·0293 | ||||||
| R2 | 0·369 | 0·270 | 0·065 | ||||||
TS: total splenectomy; SAT – 1: autotransplant of one splenic slice; SE: standard error.
There was a significantly higher level of bacteria uptake by the liver in the group submitted to TS compared to the others, which showed similar uptake. There still was some compensation between the liver and blood, with an increase to 1·00% of bacteria remaining in the blood being associated, on average, with a 1·03% reduction of bacterial uptake by the liver.
Regarding the uptake of bacteria by the lung, the level was significantly lower in rats submitted to TS, and greater with SAT of only one slice compared to all others. In the case of the lung, no association was identified with the bacteria remaining in the blood.
The bacterial uptake by the spleen/autotransplant, in turn, was not statistically different between the groups with SAT and the control group. The analysis excluded the group submitted to TS and was not associated statistically (α = 0·05) with the level of remaining bacteria in the bloodstream. However, a borderline association in the latter case (P = 0·0716) should not be overlooked, and may also suggest some compensation between spleen and blood.
Discussion
Previously, we showed the morphological regeneration and phagocytic activity of macrophages of SAT corresponding to the total splenic mass on the greater omentum of young and adult Wistar rats of both sexes after 16 weeks. By intravenous inoculation of E. coli, we presented the existence of a large number of macrophages containing aggregates of Gram-negative bacteria engulfed in its cytoplasm, leading to the conclusion that SAT provides protection against bacteraemia caused by this bacteria [23,24].
In this work, we used an experimental model with autotransplant of one (average of 21·9% of the total splenic mass) to five (total splenic mass) thin splenic slices sutured to the greater omentum, intercalating omental tissue between them, to allow better vascularization. There was a direct correlation with the mean percentage of regenerated splenic mass (splenic mass recovered after 16 weeks) and implanted splenic mass.
In humans, approximately 50% of all lymphocytes migrate daily throughout the body, passing through the spleen [20]. As the vasculature of the spleen and the greater omentum comes from the same region, the route of lymphocyte migration of omental implants, in contrast to other sites, can be related more to the path of splenic migration [37]. This observation shows that the new splenic capsule depends on the bed of the implant and is not constituted only in a product of the autotransplanted splenic tissue [30,37–40], and is ratified by the fact that after splenic trauma, when spontaneous splenosis occurs and the incidence of overwhelming post-splenectomy infection (OPSI) is known to be lower, the omentum is involved in virtually all cases. This suggests that the omental implant can become more efficient than in other sites. This may be due to its abundant blood supply, with the passage of a large number of inflammatory cells, growth factors and cytokines, possibly by the maintenance of venous drainage to the portal system, similar to what occurs with the spleen in situ[3,26,29,30].
Several authors suggest that after 16 weeks SAT is indistinguishable from a normal spleen, and that its functional regeneration is already present at this time [23,24,30]. After 16 weeks from the beginning of the experiment, it was found that all implants presented splenic regeneration, with the presence of red and white pulp, lymphoid follicles and marginal zone. However, it is known that even in the presence of morphological regeneration, functional recovery may not occur.
Corazza et al. [41] were pioneers in warning that the mere presence of tissue derived from the spleen was not enough to ensure the satisfactory return of its function. These authors showed that the presence of 20–30 cm3 of residual splenic tissue would be the ‘inflexion point’ beyond which the function of the spleen shows some degree of return [41]. Previously, in 1980, using Sprague–Dawley rats with variable splenic mass (25–300 mg) due to graduated partial splenectomy and subjected to intravenous injection of a suspension of Streptococcus pneumoniae type III, Van Wyck et al. [42], indicated that it would require more than one-third of spleen mass to confer resistance to such animals, which they called ‘critical mass of spleen’. Iinuma et al. [30] reported a success rate of 100% in the implantation of autogen fragments of the spleen in the greater omentum of 6-week-old rats. Sixteen weeks after SAT, these authors carried out intravenous inoculation of a suspension of S. pneumoniae type 6 and concluded that at least 50% of the original splenic mass would be necessary to confer protection against sepsis. However, in the medical literature no study has attempted specifically and conclusively to use the minimum amount of splenic tissue for the regeneration of phagocytic capacity of SAT macrophages.
The study of bacterial phagocytosis between different organs of the mononuclear phagocytic system (MPS) constitutes an experimental model that appears suitable for checking the effectiveness of clearance by splenic macrophages, being possibly more appropriate than the study of the clearance of colloidal substances and providing the safest evidence regarding the phagocytic activity of such organs.
We used the wild strain of E. coli AB1157 labelled with Tc-99m due to extensive knowledge of these bacteria, as they participate 12% of all cases of OPSI [43,44].
Liver, lung and spleen or SAT contain more than 95% of the total amount of macrophages of the MPS [25,27,45]. As the measurement of bacterial uptake in other organs of SMF (e.g. Peyer's patches, bone marrow and lymph nodes) is not routinely feasible, we carried out the measurement of uptake in these main organs, plus the bacteria remaining in the blood.
Using H&E staining we identified a large number of bacteria arranged in the cytoplasm of regenerated SAT macrophages, confirming its recovery of activity, followed by the use of the Gram staining method, which showed that these bacteria were Gram-negative bacteria (E. coli) and were arranged around the nucleus of numerous macrophages, arranged diffusely in the splenic parenchyma, confirming regeneration of the phagocytic function of SAT. The presence of haemosiderin pigment within macrophages, distributed diffusely throughout the splenic parenchyma, may be due to the phagocytosis of blood components from the haemorrhagic process during the initial phase of necrosis of the implant or to the phagocytosis of senescent erythrocytes in the bloodstream.
The evidence that asplenia is associated with lower phagocytic capacity is confirmed by the higher bacteria reminiscent in the blood of animals submitted to TS. Similarly, splenectomized animals presented a higher amount of bacteria remaining in the blood than the animals in groups 3–7, in which SAT of different percentages of splenic mass were performed. In these groups of animals, the higher the percentage of splenic tissue that was autotransplanted (one to five splenic slices), the lower were the residual bacteria remaining in the bloodstream. Thus, the experimental model used seems adequate for the study of the regeneration of the phagocytic activity of SAT macrophages considering that, when all the splenic mass (five slices) was implanted, the animals achieved the same percentage of bacteria remaining in the blood as found in the animals with spleen in situ (2·5%).
The best phagocytic function of MPS organs and the decrease of bacteria remaining in the blood (confirming the presence of a more appropriate clearing function) was achieved by animals submitted to SAT with higher percentages of total splenic mass compared to splenectomized animals (absence of splenic mass) or to those with a smaller mass percentage. In agreement with the literature, this finding suggests that SAT, starting from a certain percentage of the total splenic mass (critical mass), preserves the phagocytic function of the spleen [41,42].
The liver is the largest organ of the MPS and, as might be expected, presented the highest bacterial uptake. Among all groups of animals, the one showing increased uptake in the liver was the group submitted to TS (mean 75·3%), but this was also the group with the highest percentage of bacteria remaining in the blood (mean 6·7%), showing that, although the liver attempted to compensate for the absence of the spleen, it could not perform this action fully. Control animals or those with SAT of two or more spleen slices showed no difference with regard to bacterial uptake by the liver. Furthermore, we observed a negative association between such uptake and the bacteria remaining in the bloodstream, suggesting compensation between uptake in the liver and the bacteria remaining in the blood.
Conversely, the lowest rate of pulmonary uptake occurred in the group of animals submitted to TS (18·0%) and, similar to hepatic uptake, there was no difference between animals of the control group or with implantation of two or more splenic slices. Shennib et al. [12] and Müftüoglu et al. [14] found a decrease in phagocytic activity against pneumococci by lung macrophages in splenectomized rats. However, when there were splenic fragments in the abdominal cavity this decrease did not occur [12]. Similarly, Petroianu et al. [46] showed increased pulmonary uptake in adult rats inoculated intravenously with E. coli after partial splenectomy. Such findings may suggest that, with less functioning splenic mass, either by the splenic remnant in situ (partial or subtotal splenectomy) or by a regenerated autotransplant, alveolar macrophages experience some change in their phagocytic activity in trying to compensate for a possible decline in the splenic phagocytic index.
The healthy control animals showed a mean 4·8% bacterial uptake by the spleen, while group 3 rats (SAT of one slice, with an average of 21·9% of the total splenic mass) showed a mean uptake of 3·8% by SAT. However, from SAT of two or more slices (31·9–100% of the total splenic mass) there was no difference in the bacterial uptake of the spleen or SAT, confirming the functional regeneration of the organ.
Regarding the bacteria remaining in the blood, animals in the groups with at least three slices of SAT showed no difference from the control group, in contrast to the results observed in the group submitted to TS. Even with lower levels than the group submitted to TS, the groups with one (21·9% mean autotransplanted mass) or two slices (31·9% mean autotransplanted mass) of SAT also showed differences from the control group, with higher mean levels of bacteria remaining in the blood.
This experimental model shows that the bacteria remaining in the blood decrease with the increasing percentage of splenic mass contained in SAT. Thus, although the percentage of bacteria remaining in the blood is decreasing, with the autotransplant of up to 22·0% of the splenic mass there is no difference from animals submitted to TS. On the basis of the present data, explorations of the cut-off for the percentage of splenic mass to be autotransplanted in order to achieve a performance similar to that observed in normal animals with an intact spleen in situ indicated a value of 26·0%. These explorations were based on various categorizations according to the range of the percentages of implanted splenic mass, with autotransplants starting from 22·0% of the total spleen mass achieving levels of remaining bacteria in the blood similar to those detected in the control group. However, the pattern of variability could not be sustained statistically except when starting from a value of 26·0%.
Thus, with the implantation of 26·0–100% of the splenic mass the model used becomes fully consistent, considering that there is no difference in bacteria remaining in the blood between the healthy control animals and those submitted to SAT. This observation shows that the critical mass needed to achieve the development of phagocytic activity in macrophages of adult rats is 26·0% of the total splenic mass. Although this finding cannot be extrapolated to direct clinical application, it is plausible to state that a critical mass also may exist in humans, from which we would observe the occurrence of increased resistance to OPSI.
This experimental model should be applied to a larger number of animals, as well as to younger (after weaning) and older rats, in order to verify the phagocytic function of MPS organs in extreme age groups. Similarly, other animal species, as well as the inoculation of other bacteria, need to be tested to elucidate more clearly the critical mass of splenic autotransplant for the development of phagocytic activity.
Acknowledgments
This work was supported partially by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro – FAPERJ.
Disclosure
None.
References
- 1.Altamura M, Caradonna L, Amati L, Pellegrino NM, Urgesi G, Miniello S. Splenectomy and sepsis: the role of the spleen in the immune-mediated bacterial clearance. Immunopharmacol Immunotoxicol. 2001;23:153–61. doi: 10.1081/iph-100103856. [DOI] [PubMed] [Google Scholar]
- 2.Lynch AM, Kapila R. Overwhelming postsplenectomy infection. Infect Dis North Am. 1996;10:693–707. doi: 10.1016/s0891-5520(05)70322-6. [DOI] [PubMed] [Google Scholar]
- 3.Timens W, Leemans R. Splenic autotransplantation and the immune system. Adequate testing required for evaluation of effect. Ann Surg. 1992;215:256–60. doi: 10.1097/00000658-199203000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saba TM, Cho E. Reticuloendothelial systemic response to operative trauma as influenced by cryoprecipitate or cold-insoluble globulin therapy. J Reticuloendothel Soc. 1979;26:171–86. [PubMed] [Google Scholar]
- 5.Marques RG, Petroianu A, Oliveira MB, Bernardo-Filho M. Evaluation of possible failure of the mononuclear phagocyte system after total splenectomy in rats. Braz Arch Biol Technol. 2004;47:199–204. [Google Scholar]
- 6.Marques RG, Petroianu A, Oliveira MB, Bernardo-Filho M. [Relevance of splenic tissue preservation to bactéria phagcytosis] Acta Cir Bras. 2002;17:388–93. [Google Scholar]
- 7.Hazlewood M, Kumararatne DS. The spleen? Who needs it anyway? Clin Exp Immunol. 1992;89:327–9. doi: 10.1111/j.1365-2249.1992.tb06956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scher KS, Scott-Conner C, Jones CW, Wroczynski AF. Methods of splenic preservation and their effect on clearance of pneumococcal bacteremia. Ann Surg. 1985;202:595–9. doi: 10.1097/00000658-198511000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaimoff C, Douer D, Pick IA, Pinkhas J. Serum immunogloblulin changes after accidental splenectomy in adults. Am J Surg. 1978;136:332–3. doi: 10.1016/0002-9610(78)90287-8. [DOI] [PubMed] [Google Scholar]
- 10.Fernandes BF, Rezende AB, Alves CCS, et al. Splenic autotransplantation restores IL-17 production and antibody response in Streptococcus penumoniae in splenectomized mice. Transpl Immunol. 2010;22:195–7. doi: 10.1016/j.trim.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Pachter HL, Grau J. The current status of splenic preservation. Adv Surg. 2000;34:137–74. [PubMed] [Google Scholar]
- 12.Shennib H, Chiu RCJ, Mulder DS. The effects of splenectomy and splenic implantation on alveolar macrophage function. J Trauma. 1983;23:7–12. doi: 10.1097/00005373-198301000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Hebert JC. Pulmonary antipneumococcal defenses after hemisplenectomy. J Trauma. 1989;29:1217–20. doi: 10.1097/00005373-198909000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Müftüoglu TM, Köksal N, Ozkutlu D. Evaluation of phagocytic function of macrophages in rats after partial splenectomy. J Am Coll Surg. 2000;191:668–71. doi: 10.1016/s1072-7515(00)00739-0. [DOI] [PubMed] [Google Scholar]
- 15.Billiar TR, West MA, Hyland BJ, Simmons RL. Splenectomy alters Kupffer cell response to endotoxin. Arch Surg. 1988;123:327–32. doi: 10.1001/archsurg.1988.01400270061009. [DOI] [PubMed] [Google Scholar]
- 16.Spaeth G, Specian RD, Berg RD, Deitch EA. Splenectomy influences endotoxin-induced bacterial translocation. J Trauma. 1990;30:1267–72. doi: 10.1097/00005373-199010000-00012. [DOI] [PubMed] [Google Scholar]
- 17.Krivit W, Giebink GS, Leonard A. Overwhelming postsplenectomy infection. Surg Clin North Am. 1979;59:223–33. doi: 10.1016/s0039-6109(16)41782-2. [DOI] [PubMed] [Google Scholar]
- 18.Cheslyn-Curtis S, Aldridge MC, Giglin JEJ, Dye J, Chadwick SJD, Dudley HAF. Effect of splenectomy on Gram-negative bacterial clearance in the presence and absence of sepsis. Br J Surg. 1988;75:177–80. doi: 10.1002/bjs.1800750231. [DOI] [PubMed] [Google Scholar]
- 19.Bohnsack JF, Brown EJ. The role of the spleen in resistance to infection. Annu Rev Med. 1986;37:49–59. doi: 10.1146/annurev.me.37.020186.000405. [DOI] [PubMed] [Google Scholar]
- 20.Pabst R. The spleen in lymphocyte migration. Immunol Today. 1988;9:43–5. doi: 10.1016/0167-5699(88)91258-3. [DOI] [PubMed] [Google Scholar]
- 21.Resende V, Petroianu A. [Late functional study of human spleen autotransplantation after severe splenic injuries] Rev Col Bras Cir. 2001;28:165–70. [Google Scholar]
- 22.Pabst R. Regeneration of autotransplanted splenic fragments: basic immunological and clinical relevance. Clin Exp Immunol. 1999;117:423–4. doi: 10.1046/j.1365-2249.1999.00979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marques RG, Petroianu A, Coelho JM, Portela MC. Regeneration of splenic autotransplants. Ann Hematol. 2002;81:622–6. doi: 10.1007/s00277-002-0564-2. [DOI] [PubMed] [Google Scholar]
- 24.Marques RG, Petroianu A, Oliveira MB, Bernardo-Filho M, Boasquevisque E, Portela MC. Bacterial clearance after total splenectomy and splenic autotransplantation. Appl Radiat Isot. 2002;57:767–71. doi: 10.1016/s0969-8043(02)00135-5. [DOI] [PubMed] [Google Scholar]
- 25.Malangoni MA, Dawes LG, Droege EA, Rao SA, Collier BD, Almagro UA. Splenic phagocytic function after partial splenectomy and splenic autotransplantation. Arch Surg. 1985;120:275–8. doi: 10.1001/archsurg.1985.01390270015003. [DOI] [PubMed] [Google Scholar]
- 26.Miko I, Brath E, Nemeth N, et al. Spleen autotransplantation. Morphological and functional follow-up after spleen autotransplantation in mice: a research summary. Microsurgery. 2007;27:312–6. doi: 10.1002/micr.20362. [DOI] [PubMed] [Google Scholar]
- 27.Malangoni MA, Evers BM, Peyton JC, Wellhausen SR. Reticuloendothelial clearance and splenic mononuclear cell populations after resection and autotransplantation. Am J Surg. 1988;155:298–302. doi: 10.1016/s0002-9610(88)80720-7. [DOI] [PubMed] [Google Scholar]
- 28.Pabst R, Kamran D. Autotransplantation of splenic tissue. J Pediatr Surg. 1986;21:120–4. doi: 10.1016/s0022-3468(86)80062-8. [DOI] [PubMed] [Google Scholar]
- 29.Weber T, Hanisch E, Baum RP, Seufert RM. Late results of heterotopic autotransplantation of splenic tissue into the greater omentum. World J Surg. 1998;22:883–9. doi: 10.1007/s002689900487. [DOI] [PubMed] [Google Scholar]
- 30.Iinuma H, Okinaga K, Sato S, Tomioka M, Matsumoto K. Optimal site and amount of splenic tissue for autotransplantation. J Surg Res. 1992;53:109–16. doi: 10.1016/0022-4804(92)90021-q. [DOI] [PubMed] [Google Scholar]
- 31.Leemans R, Harms G, Rijkers GT, Timens W. Spleen autotransplantation provides restoration of functional splenic lymphoid compartments and improves the humoral immune response to pneumococcal polysaccharide vaccine. Clin Exp Immunol. 1999;117:596–604. doi: 10.1046/j.1365-2249.1999.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pabst R, Reilmann H. Regeneration of heterotopically transplanted autologous splenic tissue. Cell Tissue Res. 1980;209:137–43. doi: 10.1007/BF00219930. [DOI] [PubMed] [Google Scholar]
- 33.Marques RG, Morales MM, Petroianu A. Brazilian law for scientific use of animals. Acta Cir Bras. 2009;24:69–74. doi: 10.1590/s0102-86502009000100015. [DOI] [PubMed] [Google Scholar]
- 34.Bernardo-Filho M, Pereira JAA, Boasquevisque EM. Labeling of Klebsiella pneumoniae with technetium-99m – a preliminary communication. Rev Microbiol. 1986;17:188–93. [Google Scholar]
- 35.Diniz SOF, Resende BM, Nunan EA, Simal CJR, Cardoso VN. 99mTechnetium labelled Escherichia coli. Appl Radiat Isot. 1999;51:33–6. doi: 10.1016/s0969-8043(98)00185-7. [DOI] [PubMed] [Google Scholar]
- 36.Diehl KH, Hull R, Morton D, et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appl Toxicol. 2001;21:15–23. doi: 10.1002/jat.727. [DOI] [PubMed] [Google Scholar]
- 37.Pabst R, Westermann J. The role of the spleen in lymphocyte migration. Scanning Microsc. 1991;5:1075–9. [PubMed] [Google Scholar]
- 38.Thalhamer J, Leitner W, Kuraz ME, et al. Immunoarchitecture and specific functions of splenic autotransplants at different implantation sites. Eur Surg Res. 1992;24:22–36. doi: 10.1159/000129185. [DOI] [PubMed] [Google Scholar]
- 39.Patel JM, Williams JS, Naim JO, Hinshaw JR. The effect of site and technique of splenic tissue reimplantation on pneumococcal clearance from the blood. J Pediatr Surg. 1986;21:877–80. doi: 10.1016/s0022-3468(86)80012-4. [DOI] [PubMed] [Google Scholar]
- 40.Sasaki K, Kiuchi Y, Sato Y, Yamamori S. Morphological analysis of neovascularization at early stages of rat splenic autografts in comparison with tumor angiogenesis. Cell Tissue Res. 1991;265:503–10. doi: 10.1007/BF00340873. [DOI] [PubMed] [Google Scholar]
- 41.Corazza GR, Tarozzi C, Vaira D, Frisoni M, Gasbarrini G. Return of splenic function after splenectomy: how much tissue is needed? BMJ. 1984;289:861–4. doi: 10.1136/bmj.289.6449.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Van Wyck DB, Witte MH, Witte CL, Thies AC., Jr Critical splenic mass for survival from experimental pneumococcemia. J Surg Res. 1980;28:14–7. doi: 10.1016/0022-4804(80)90076-1. [DOI] [PubMed] [Google Scholar]
- 43.Singer DB. Postsplenectomy sepsis. Perspect Pediatr Pathol. 1973;1:285–311. [PubMed] [Google Scholar]
- 44.Hansen K, Singer DB. Asplenic–hyposplenic overwhelming sepsis: postsplenectomy sepsis revisited. Pediatr Dev Pathol. 2001;4:105–21. doi: 10.1007/s100240010145. [DOI] [PubMed] [Google Scholar]
- 45.Kaplan JE, Scovill WA, Bernard H, Saba TM, Gray V. Reticuloendothelial phagocytic responses to bacterial challenge after traumatic shock. Circ Shock. 1977;4:1–12. [PubMed] [Google Scholar]
- 46.Petroianu A, da Silva RG, Nascimento-Cardoso V, Alberti LR, da Silva MG. Effect of spleen surgeries on Escherichia coli distribution on the mononuclear phagocytic system. Int J Surg. 2010;8:48–51. doi: 10.1016/j.ijsu.2009.10.006. [DOI] [PubMed] [Google Scholar]


