Abstract
Dendritic cells (DC) are mediators of the adaptive immune response responsible for antigen presentation to naive T cells in secondary lymph organs. Human immunodeficiency virus (HIV-1) has been reported to inhibit the maturation of DC, but a clear link between maturation and function has not been elucidated. To understand further the effects of HIV-1 on DC maturation and function, we expanded upon previous investigations and assessed the effects of HIV-1 infection on the expression of surface molecules, carbohydrate endocytosis, antigen presentation and lipopolysaccharide (LPS) responsiveness over the course of maturation. In vitro infection with HIV-1 resulted in an increase in the expression of DC-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) as well as decreases in maturation-induced CCR7 and major histocompatibility complex (MHC)-II expression. Retention of endocytosis that normally occurs with DC maturation as well as inhibition of antigen presentation to CD8+ T cells was also observed. Mitogen-activated protein kinase (MAPK) responsiveness to LPS as measured by phosphorylation of p38, c-Jun N-terminal kinase (JNK) and extracellular-regulated kinase (ERK)1/2 was not affected by HIV-1 infection. In summary, in-vitro HIV-1 impairs DC maturation, as defined by cell surface protein expression, with selective alterations in mature DC function. Understanding the mechanisms of DC dysfunction in HIV infection will provide further insight into HIV immune pathogenesis.
Keywords: antigen presentation; antigen processing; dendritic cells (myeloid, plasmacytoid, monocyte-derived); human immunodeficiency virus (AIDS, HIV-1, HIV-2); phenotype/cell markers
Introduction
Dendritic cells (DC) are critical mediators of the interaction between the adaptive and innate immune systems and are responsible for the presentation of antigens and co-stimulatory molecules to naive T cells in the secondary lymph organs [1]. When not presenting antigens in the secondary lymph organs, DC are located throughout the body in tissues in an immature form, where they constantly ‘sample’ their environment for pathogens through pattern recognition receptors [2]. During normal maturation, DC change from antigen capture cells to antigen-presenting cells [3]. Maturation is characterized by a decrease in phagocytic and pinocytic activities [3] and decreases in the expression of cell surface molecules associated with those functions, including mannose receptors, CD14 and C-type lectin receptors such as DC-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) [4–6]. These changes are accompanied by concomitant increases in the expression of surface molecules that facilitate antigen presentation and adaptive immune system activation such as CD80, CD86, CD40, major histocompatibility complex (MHC)-I and MHC-II [7–11]. Additionally, expression of the immunoregulatory surface molecule CD83 increases when DC mature and this is accompanied by decreases in the expression of the chemokine receptor CCR5 and increases in CCR7 expression [12–14].
Another important component of DC physiology involved in maturation is signal transduction by the mitogen-activated protein kinase (MAPK) pathways [15]. MAPKs are highly conserved signal transduction pathways important in the function and differentiation [16]. In the case of DC, three specific pathways have been identified as important components of normal DC physiology.
Stimulation of the p38 MAPK has been observed to be critical for normal maturation and function of DC [17]. Specifically, p38 activation has been implicated in the regulation of the surface expression of CD80, CD86, CD40, CCR7 and MHC-II molecules as well as cytoskeletal rearrangement, endocytosis, cytokine secretion and response [18–25]. Stimulation of the c-Jun N-terminal kinase (JNK) pathway has been found to be important in CD80 and CD86 expression as well as expression of CD83, MHC-II, Toll-like receptor (TLR) function, cytokine secretion and response and T cell stimulation [26–31]. Activation of the extracellular-regulated kinase (ERK) MAPK pathway has been observed contribute to TLR function and cytokine production and responsiveness [32–34].
During most viral infections, mature DC are responsible for the presentation of viral antigens to naive T cells within secondary lymphoid organs, resulting in the generation of an antigen-specific adaptive immune response and clearance of the virus [35]. However, this is not the case with human immunodeficiency virus (HIV-1) infection [36]. During infection with HIV-1, the virus is not cleared and a chronic systemic infection develops characterized by immune dysfunction, CD4+ T cell depletion, systemic inflammation and opportunistic infections [37–40].
How the virus evades immune system elimination is not completely understood. It has been suggested that initial HIV-1 interactions with DC may actually enhance viral spread to naive T cells in secondary lymphoid tissue. Rather than process and present critical viral antigens to induce a virus-specific adaptive immune response, there have been reports suggesting that DC enhance HIV-1 dissemination during infection via the transfer of intact cell surface and endosomal viral particles to naive T cells in the secondary lymphoid organs [41,42].
HIV-1 itself does not appear to stimulate the maturation of DC but, rather, may induce DC dysfunction, inhibit maturation and reduce DC numbers in vivo[43–46], although there are reports that suggest otherwise [47–54]. In fact, a number of HIV-1-derived peptides have also been observed to induce maturation of DC [55–57].
To describe more comprehensively the effects of HIV-1 on DC, we expanded upon previous studies of the influence of HIV-1 on DC maturation and function. In addition to investigating the effects of HIV-1 infection on the expression of surface molecules pertinent to DC maturation, we studied simultaneously the effects of HIV-1 on DC function, including endocytosis, antigen presentation and cell signalling, in response to bacterial lipopolysaccharide (LPS).
Materials and methods
Preparation of monocytes and DC
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood from healthy donors using Ficoll Paque™ PREMIUM (GE-Healthcare Bio-Sciences, Mississauga, ON, Canada) density separation by centrifugation. Monocytes were isolated from PBMCs with anti-CD14-coated microbeads (Miltenyi Biotec, Mississauga, ON, Canada) and maintained in complete media (RPMI-1640 medium containing L-glutamine, 100 µg/ml streptomycin and 100 U/ml penicillin; Invitrogen, Burlington, ON, Canada) at 1 × 106 cells/ml.
Generation of DC
Monocytes were differentiated into immature monocyte-derived DC (iMDDC), as described previously [58]. Isolated monocytes were incubated in complete media supplemented with 500 U/ml recombinant human interleukin (rhIL)-4 and 1000 U/ml recombinant human granulocyte–macrophage colony-stimulating factor (rhGM-CSF) (R&D Systems, Burlington, ON, Canada) at 1 × 106 cells/ml at 37°C and 5% CO2 for 24 h.
To induce maturation, iMDDCs in complete media at a density of 1 × 106 cells/ml were incubated with 1000 U/ml tumour necrosis factor (TNF)-α, 10 ng/ml IL-1β, 10 ng/ml IL-6 and 1 µM prostaglandin E2 (PGE2) (R&D Systems) for 48 h at 37°C and 5% CO2[58].
Flow cytometric analysis of monocytes and MDDCs
Monocytes and MDDCs were incubated with saturating concentrations of fluorescein isothiocyanate (FITC)-conjugated anti-CD14, DC-SIGN, CD80, CD86, CCR5, CCR7, MHC-I or MHC-II antibodies, phycoerythrin (PE)-conjugated anti-MHC-I antibodies or isotype controls in 5-ml polypropylene round-bottomed tubes (Becton Dickinson and Company, Franklin Lakes, NJ, USA). Surface expression was measured using a Coulter Epics Altra flow cytometer (Beckman-Coulter Canada Inc., Mississauga, ON, Canada) and analysed with FCS Express 2·00 software (De Novo Software, Los Angeles, CA, USA).
HIV-1 infection of MDDCs
Immature MDDCs were incubated with live dual tropic HIV-1CS204 (a gift from Dr Francisco Diaz-Mitoma at the Children's Hospital of Eastern Ontario, Ottawa, ON, Canada) [multiplicity of infection (MOI)] of 1 for 24 h at 37°C and 5% CO2. After 24 h, MDDCs were incubated with 20 µl of HIV-1CS204 or an equivalent volume of mock solution for 24 h, washed and suspended in complete media supplemented with rhIL-4 (500 U/ml) and of rhGM-CSF (1000 U/ml) in 12-well tissue culture plates at a density of 1 × 106 cells/ml at 37°C and 5% CO2. HIV-1 infection was evaluated 3 days post-infection using Alu-nested polymerase chain reaction (PCR) detection and a commercially available p24 antigen enzyme-linked immunosorbent assay (ELISA) kit (National Cancer Institute, Frederick, MD, USA).
Alu-nested PCR detection of HIV-1 DNA
Viral infection was confirmed by Alu-nested PCR amplification adapted from previous work [59]. The first-round PCR cycle conditions consisted of a denaturation step (7 min at 94°C) and 12 cycles of amplification (94°C for 1 min, 59°C for 1 min and 72°C for 1 min) using Taq PCR Mastermix (Qiagen, Mississauga, ON, Canada) with two outward-facing Alu primers (300 nM) and an HIV-1 long terminal repeat (LTR)-specific primer (300 nM). During the second round of PCR, 1/10 of the first-round PCR products were amplified using a lambda-specific primer (Lambda T) (300 nM) and an LTR primer (AA55M) (300 nM). Second-round PCR cycle conditions consisted of a denaturation step (7 min at 94°C) and 30 amplification cycles (94°C for 1 min, 59°C for 1 min and 72°C for 1 min) in Taq PCR Mastermix using an Eppendorf Mastercycler ep 543X instrument (Eppendorf, Mississauga, Canada).
Primers
The primers used were as follows: L-M667 – ATGCCACGTAAGCGAAACTCTGGCTAACTAGGGAACCCACTG; Alu 1 – TCCCAGCTACTGGGGAGGCTGAGG; Alu 2 – GCCTCCCAAAGTGCTGGGATTACAG; Lambda T – ATGCCACGTAAGCGAAACT; and AA55M – GCTAGAGATTTTCCACACTGACTAA.
Dextran endocytosis assay
A total of 250 000 MDDCs differentiated and infected as described above were incubated in 5 ml polypropylene round-bottomed tubes with 1 mg of FITC-conjugated dextran (Sigma-Aldrich, Milwaukee, WI, USA) in the dark for 1 h on ice or at 37°C and 5% CO2. Cells were then washed in phosphate-buffered saline (PBS) and subjected to flow cytometric analysis using FCS Express 2·00 software.
Preparation of cell lysates and immunoblot analysis
Changes in the phosphorylation of the ERK, JNK and p38 proteins in response to LPS after HIV-1 infection were measured using immunoblot analysis, as described previously [60]. HIV-1-infected or -uninfected MDDCs were centrifuged, incubated in the presence or absence of 2 µg/µl LPS (Escherichia coli, 0111:B4; Sigma-Aldrich) for 1 h at 37°C and 5% CO2. Cells were then collected by centrifugation, washed, and then lysed on ice using 250 µl lysis buffer [0·05 M HEPES, 0·15 M NaCl, 10% glycerol, 1% Triton-X-100, 7·5 × 10−4 M MgCl2, 0·1 M NaF and 0·001 M ethylene glycol tetraacetic acid (EGTA) (pH 7·7)] (Fisher Scientific Canada Limited, Ottawa, ON, Canada). Samples were boiled with ×4 treatment buffer [8% sodium dodecyl sulphate (SDS), 10% 2-mercaptoethanol, 30% glycerol, 0·008% bromophenol blue, 0·25 M Tris HCl] for 10 min, and 40 µg of total protein of each lysate was added to each well of an 8% SDS polyacrylamide gel and subjected to electrophoresis. Next, proteins were transferred electrophoretically to nitrocellulose sheets (Protran®, Bioscience, Schleicher and Schuell, Mandel, ON, Canada) via semidry electrophoretic transfer (Biorad Labratories Inc., Burlington, ON, Canada) and blocked with Amersham™ ECL Advance Blocking agent (GE-Healthcare Bio-Sciences).
The membranes were incubated at 4°C with the primary phosphorylated anti-p38, JNK/stress-activated protein kinase (SAPK) or ERK1/2 and β-actin antibodies (9215S, 9251S, 99101S and 4967; Cell Signaling Technologies, New England Biolabs Limited, Toronto, ON, Canada) at a titre of 1:500 in Amersham™ ECL Advance Blocking agent in ×1 Tris-buffered saline (TBS) (Fisher Scientific Canada Limited) plus Tween 20 (Fisher Scientific Canada Limited) (TBST) for 24 h. The membranes were washed and incubated with secondary antibodies bound covalently to horseradish peroxidase (HRP) (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a titre of 1:1000 in Amersham™ ECL advance blocking agent in TBST at 4°C for 24 h. The membranes were washed and signals were detected using Supersignal HRP substrate (Pierce Chemical Company, Brockville, ON, Canada) and AlphaEase FC 6·0·0 software (Cell Biosciences, Santa Clara, CA, USA).
MDDC co-culture
MDDCs, differentiated and infected as above, were pulsed for 3 h with 3 µg/ml of a CEF peptide pool containing 23 human leucocyte antigen (HLA)-ABC-restricted T cell epitopes from human cytomegalovirus, Epstein–Barr and influenza viruses (CEF) (Anaspec Inc., Fremont, CA, USA).
The negative fraction obtained from the monocyte isolation (to serve as the pool of autologous T cells) was suspended at 1 × 107 cells/ml in 5 mM CellTrace™ carboxyfluorescein succinimidyl ester (CFSE) 10 min at 37°C and 5% CO2 in 15 ml polypropylene conical tubes in the dark. The cells were then washed, incubated for 5 min on ice, pelleted by centrifugation and suspended at 1 × 106 cells/ml in complete media.
A total of 250 000 CFSE-labelled autologous cells from the negative fraction and 25 000 DC from each condition were co-cultured together in the dark for 7 days at 37°C and 5% CO2 with a negative control culture containing colchicine (100 ng/ml) (Sigma-Aldrich, Milwaukee, WI, USA). Co-cultures were then transferred to 5-ml polypropylene round-bottomed tubes and stained with PE-conjugated anti-CD8 antibodies (R&D Systems). CD8+ T cell proliferation was measured by flow cytometric analysis (CFSE dilution). Only those cultures that proliferated in response to the CEF antigen pool beyond the level of media controls were included in the analysis (six of 12).
Statistical analyses
Data were analysed using paired t-tests or the Wilcoxon rank-sum test when appropriate for identification of statistically significant differences (P ≤ 0·05 was considered significant) between experimental groups using Sigma Plot 8·0 (Systat Software Inc., Chicago, IL, USA).
Results
Monocyte and DC phenotype
Monocytes isolated from PBMCs of healthy donors using CD14+ magnetic bead isolation expressed high surface levels of CD14, CD40 and MHC I and low levels of surface DC-SIGN/CD209, CD83, CD80, CD86 and MHC II (Fig. 1a), consistent with the published literature [3,61]. Immature MDDCs differentiated from monocytes using GM-CSF and IL-4 expressed low surface levels of CD14 and high levels of DC-SIGN (Fig. 2). Immature MDDC also expressed higher levels of surface CD83, CD80, CD86, CD40, MHC-I and MHC-II (Fig. 1b). Finally, after incubation of the iMDDC with the maturation-inducing cytokine cocktail consisting of TNF-α, IL-1β, IL-6 and PGE2 for 48 h, mMDDC were observed to express high levels of CD83, CD80, CD86, CD40, CCR7 and MHC-I and MHC-II, with a low level of DC-SIGN expression and negligible CD14 expression (Fig. 1c). Therefore, monocytes, iMDDCs and mMDDCs all expressed surface molecules consistent with that reported in the literature [58].
Fig. 1.
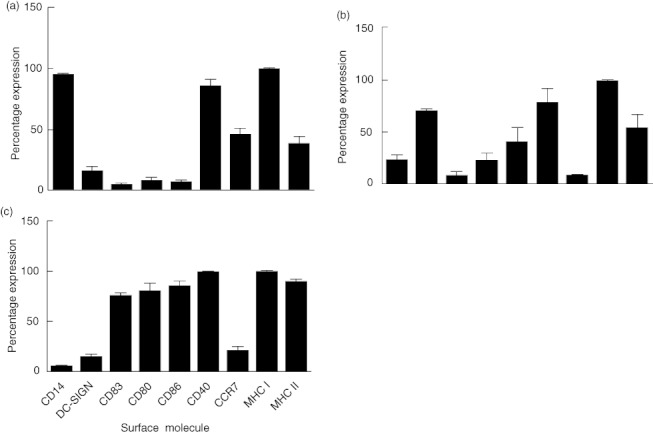
Cell surface phenotype of monocytes, immature monocyte-derived dendritic cells (iMDDC) and mature MDDC (mMDDC). Monocytes isolated from peripheral blood mononuclear cells (PBMCs) from healthy donors were differentiated into iMDDCs with interleukin (IL)-4 and granulocyte–macrophage colony-stimulating factor (GM-CSF) and then matured into mMDDCs with tumour necrosis factor (TNF)-α, IL-1β, IL-6 and prostaglandin E2 (PGE2). Expression of CD14, dendritic cell (DC)-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN), CD83, CD80, CD86, CD40, CCR7, major histocompatibility complex (MHC)-I and MHC-II as measured by flow cytometry on (a) monocytes, (b) iMDDCs and (c) mMDDC were observed at expected levels. Results are expressed as means ± standard deviation of percentage of total cells expressing each surface molecule (n = 3).
Fig. 2.
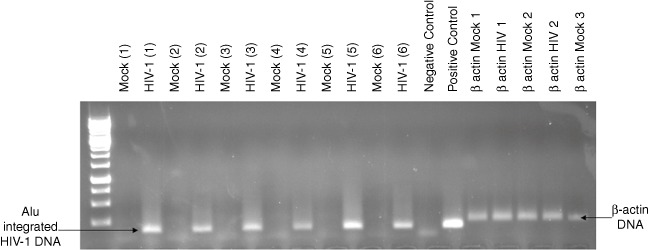
Monocyte-derived dendritic cells (MDDC) are infected by human immunodeficiency virus (HIV)-1. HIV-1 DNA was detected by nested polymerase chain reaction (PCR) using two outward-facing Alu primers and an HIV-1 LTR-specific primer during the first round of replication. First-round PCR products were then amplified using a Lambda T and a long terminal repeat (LTR) primer during a second round of replication. Water was used as a negative control and DNA isolated from HIVCS204-infected peripheral blood mononuclear cells (PBMCs) served as a positive control for detection of integrated HIV-1 DNA.
HIV infection of DCs: evidence of integrated HIV-1 DNA
After a 24-h incubation with HIV-1 and 48 h of culture, HIV-1 DNA was detected consistently in HIV-1-infected cultures (Fig. 2). There was no detectable HIV-1 DNA in the mock-infected cultures over the same period of time (Fig. 4).
Fig. 4.
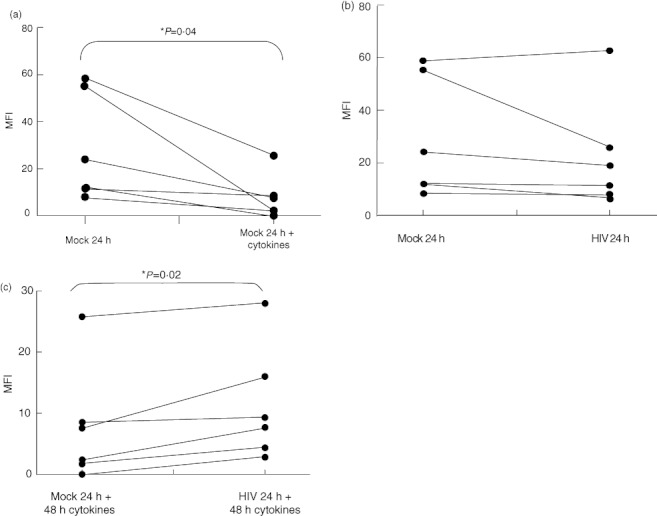
Human immunodeficiency virus (HIV)-1 infection blunts the maturation-induced down-regulation of endocytosis. Immature monocyte-derived dendritic cells (iMMDC) were infected with HIV-1CS204 and incubated with or without tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and prostaglandin E2 (PGE2). Cells were then incubated with saturating concentrations of fluorescein isothiocyanate (FITC)-conjugated dextran. (a) Endocytosis of FITC-conjugated dextran was reduced in mature MDDC compared to (b) iMDDC. (b) HIV-1CS204 infection of iMDDCs was not associated with any change in FITC-conjugated dextran endocytosis compared to mock controls. (c) HIV-1CS204 infection of iMDDCs was associated with blunted down-regulation of endocytosis after the induction of maturation. Results are expressed as means ± standard deviation. *P < 0·05 by paired t-test (n = 6).
HIV infection inhibits maturation of MDDC
Phenotypic analysis
HIV-1 has been observed to have a variety of effects on dendritic cell maturation both ex vivo and in vitro. While HIV-1 has been reported to induce DC maturation [47,62], there is considerably more evidence to suggest that HIV-1 does not induce maturation [44,63–67]. Because one measure of DC maturation is the surface expression of distinct surface molecules, we first determined if HIV-1 infection influences the cell surface phenotype of MDDC during the course of maturation.
After incubation with HIV-1 for 24 h and 48 h of culture, there was no change in the expression of CD80, CD86, CD83, CD40, CCR7, MHC-I or MHC-II, indicating that HIV-1 itself was not capable of inducing DC maturation. There was, however, an increase in DC-SIGN expression following HIV-1 infection (Fig. 3a).
Fig. 3.
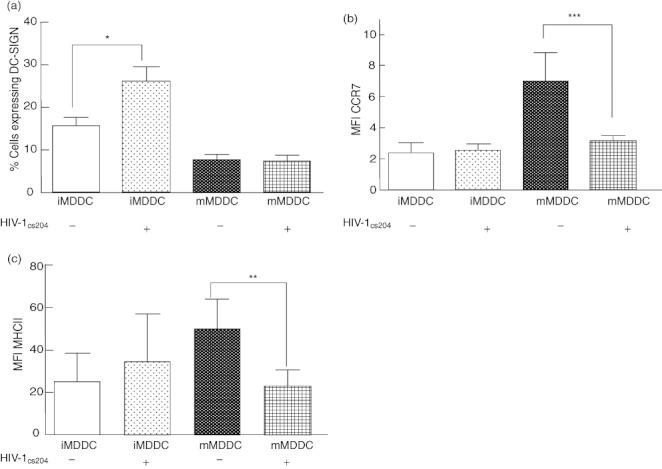
Human immunodeficiency virus (HIV)-1 infection causes an increase in dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN) expression on immature monocyte-derived dendritic cells (iMDDC) and decreases in CCR7 and major histocompatibility complex (MHC)-II expression on mature MDDC (mMDDC). Immature MDDC were incubated with a live dual tropic HIV-1CS204 (multiplicity of infection = 1) and incubated with or without tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6, and prostaglandin E2 (PGE2) to induce maturation. (a) iMDDCs infected with HIV-1CS204 had increased surface expression of DC-SIGN as measured by flow cytometry. (b) Expression of CCR7 on mMDDC was found to be reduced on HIV-1CS204-infected cells. A similar pattern was observed when analysed as the proportion of cells expressing CCR7 (data not shown). (c) Surface expression of MHC-II on mMDDCs was also found to be lower after HIV-1CS204 infection. Results are expressed as means ± standard deviation. *P = 0·02; **P = 0·04; ***P = 0·02 by paired t-test (n = 6).
After iMDDC were infected with HIV-1 and then stimulated to mature, they expressed lower levels of CCR7 and MHC-II than that observed in uninfected cells (Fig. 3b,c), suggesting that HIV-1 inhibits the full maturation of iMDDCs.
Functional analysis
Analyses were conducted as follows.
1. Endocytosis: while a phenotypic analysis of MDDC can be used to partially identify the maturation status of an MDDC, determining the effects of HIV-1 on the functional character of MDDC over the course of maturation is required to elucidate a comprehensive picture of the effects of HIV-1 on MDDC maturation.
One critical function of DC is the uptake of antigen from the periphery for processing and presentation in lymphoid organs [3]. After endocytosing antigens, immature DC undergo maturation and move from the anatomic periphery to secondary lymphoid organs where their role becomes that of antigen presentation and not uptake [3,68]. As a measure of endocytic activity, and therefore the maturation state of MDDC, the effect of HIV-1 on dextran uptake was evaluated.
As expected, maturation of uninfected iMDDC resulted in a decrease in FITC–dextran uptake (Fig. 4a). While HIV infection had no impact on the ability of iMDDC to take up dextran (Fig. 4b), HIV-1 infection was associated with blunted down-regulation of endocytosis by iMDDC (Fig. 4c). HIV-1 infection therefore appeared to inhibit maturation reflected by the fact that HIV-1 infected DC partially retain their endocytic function.
2. Antigen presentation: a primary function of DC is the presentation of antigens to naive T cells in peripheral lymphoid tissue [3]. The effect of HIV-1 infection on the ability of MDDC to present antigen to autologous CD8+ T cells was determined by incubating HIV-1-infected MDDC with autologous PBMC in the presence of a CEF peptide pool, as described previously [69].
After culturing CEF peptide-pulsed iMDDC with autologous PBMCs for 7 days, CD8+ T cells proliferated as expected (Fig. 5). When iMDDC were infected with HIV-1, however, CD8+ T cell proliferation in response to the CEF peptide pool was not observed (Fig. 5), suggesting that HIV-1 infection of DC prevented or interfered with antigen presentation.
Fig. 5.
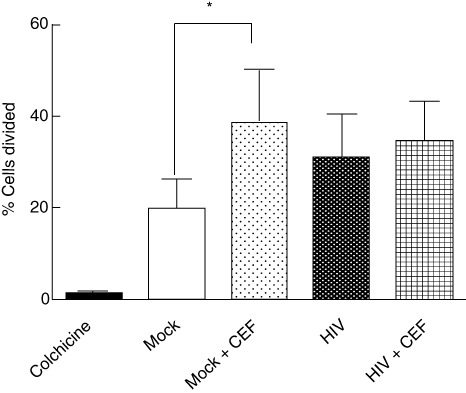
Human immunodeficiency virus (HIV)-1 infection of monocyte-derived dendritic cells (MDDC) inhibits antigen presentation to autologous CD8+ T cells. HIV-1CS204-infected immature MDDC (iMDDC) were pulsed with a cytomegalovirus, Epstein–Barr and influenza viruses (CEF) peptide pool and then co-cultured with autologous 5 mM CellTrace™ carboxyfluorescein succinimidyl ester (CFSE)-stained peripheral blood mononuclear cells (PBMC) at a ratio of 1:10 for 7 days. While preincubation of uninfected iMDDC induced the proliferation of autologous monocyte depleted PBMC, HIV-1CS204 infection of iMDDCs impaired the presentation of a CEF peptide pool as measured by CFSE dilution. Results are expressed as means ± standard deviation. *P = 0·02 by paired t-test (n = 6).
3. MAP kinase analysis: the p38, JNK and ERK MAPKs have been found to be integral for the maturation of DC [70–72] so, to characterize further the effect of HIV-1 on DC maturation, MAPK activation in response to LPS was evaluated.
When iMDDC were infected with HIV-1, they exhibited similar patterns of LPS-induced phosphorylation of p38, JNK and ERK (Fig. 6a–c) to that observed in uninfected cells. Similarly, the patterns of MAPK phosphorylation observed after LPS stimulation of mMDDC were not affected by HIV-1-infection (Fig. 6d–f).
Fig. 6.
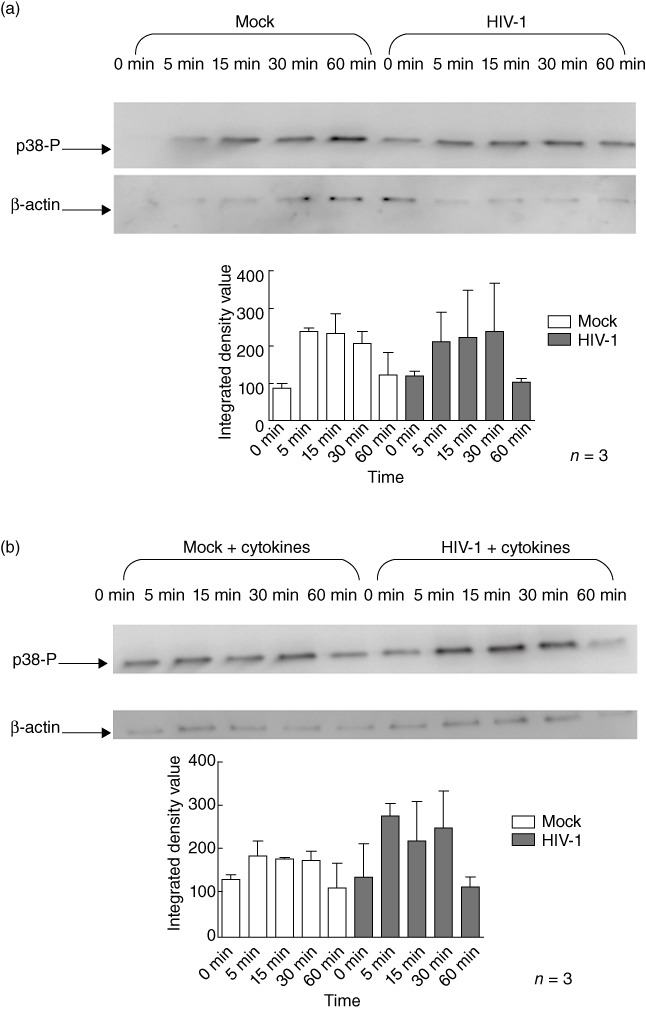
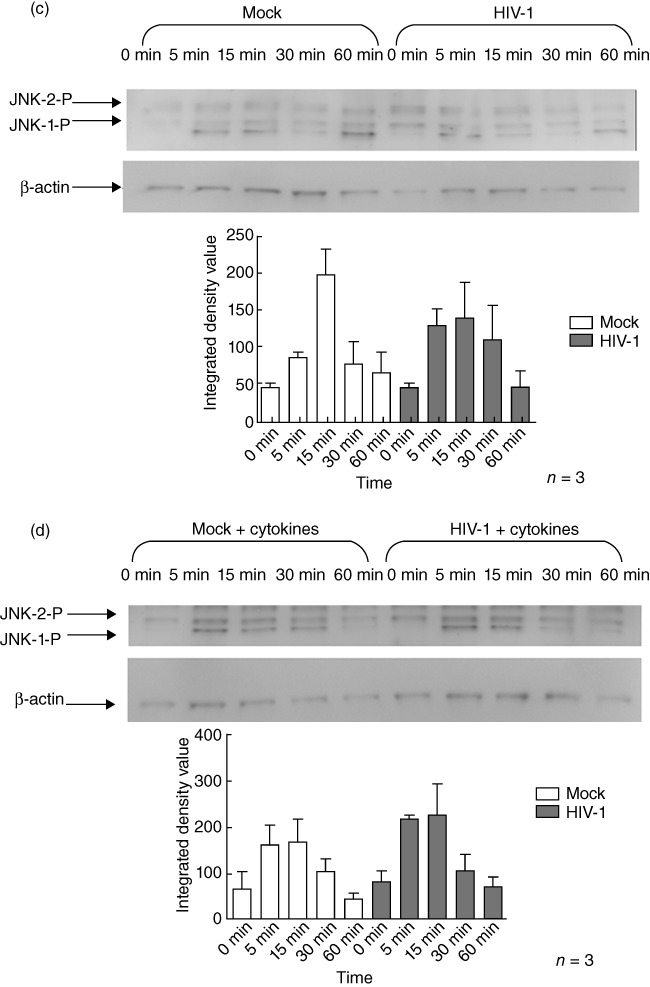
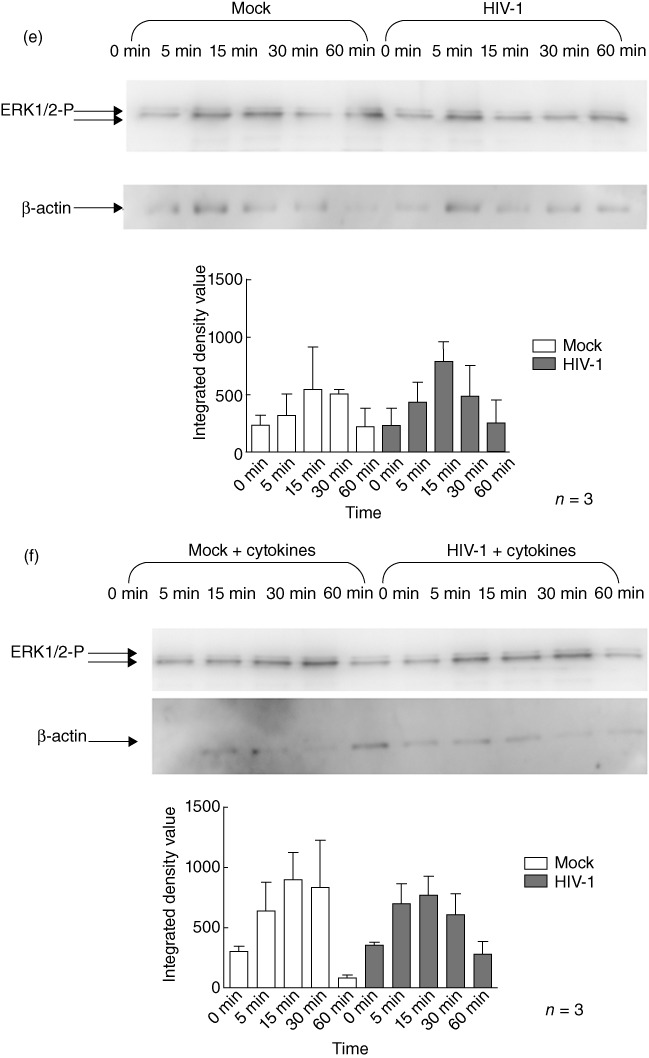
Human immunodeficiency virus (HIV)-1 infection does not alter lipopolysaccharide (LPS)-induced mitogen-activated protein kinase (MAPK) phosphorylation in monocyte-derived dendritic cells (MDDC). HIV-1-infected or uninfected immature monocyte-derived DC (iMDDC) were incubated with or without tumour necrosis factor (TNF)-α, interleukin (IL)-1β, IL-6 and prostaglandin E2 (PGE2) and then stimulated with LPS for 1 h. Changes in the phosphorylation of the extracellular-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38 proteins in response to LPS after HIV-1 infection were measured using immunoblot analysis. HIV-1CS204 infection did not affect LPS-induced phosphorylation of p38 [a: iMDDC; b: mature monocyte-derived DC (mMDDC); JNK, c: iMDDC; d: mMDDC] or ERK1/2 (e: iMDDC; f: mMDDC). Densitometry analysis was performed on three individual experiments and results are expressed as means ± standard deviation.
Discussion
Mature DCs are primarily responsible for the presentation of foreign antigens to T cells in secondary lymphoid tissues. Most viral infections stimulate immature DCs to mature through infection or by activation of TLRs. In either case, after maturation, DCs present viral antigens to T cells within the secondary lymph organs and initiate an adaptive immune response that results in clearance of the infection. During HIV-1 infection, however, the virus evades immune clearance and chronic, persistent infection results.
Integrative, productive HIV-1 infection of DC occurs at low levels compared to that of T cells [73]. Proposed explanations for the observed low infectivity of DC by HIV-1 include level of DC maturation [74], low levels of HIV-1 receptor and co-receptor expression [75], the characteristic ability of DC to degrade attached virions [76] and intrinsic host resistance factors that prevent productive HIV-1 infection [77]. Despite this, HIV-1 infection of DC has been observed with a number of effects on their maturation and function [78].
Initial investigations into the effects of HIV-1 on DC maturation and function revealed that DC from HIV-1-infected individuals had impaired ability to stimulate autologous T cell recall and proliferation [79,80]. Their ability to induce a mixed leucocyte reaction in co-culture was also compromised [79,80]. More recent examination of the effects of HIV-1 on DC have included additional analyses of the effects of HIV-1 on their maturation that support these initial investigations. Granelli-Piperno et al. found that HIV-1 infection of DC did not induce their maturation as measured by CD83, MHC-II and DC-lysosomal-associated membrane protein (LAMP) surface expression, but rather inhibited cytokine-induced maturation of DC [42]. While confirming previous reports that HIV-1 impairs the ability of DC to stimulate allogeneic T cells, they also observed an increase in IL-10 secretion from HIV-1-infected DC co-cultures that may contribute to the observed inhibition of T cell stimulation by HIV-1-infected DC [42].
While the majority of evidence suggests that the effect of HIV-1 on DC is one of inhibition of maturation and induction of DC dysfunction, other groups have reported contrasting results. In 2006, Harman et al. published findings detailing increases in myeloid DC maturation measured through increases in both co-stimulatory molecule mRNA and surface expression [47]. While their results describing increases in co-stimulatory molecule expression were contrary to the findings of the group led by Granelli-Piperno, they were in agreement with the partial DC activation observed in ex-vivo studies [81–84].
Other reports that describe HIV-1 induced maturation of DCs focus on highly virus-sensitive plasmacytoid DC which have immunologically and anatomically distinct characteristics from those of myeloid lineage [48–54]. The activation of pDC by HIV-1 has also been reported to induce the maturation of bystander DC of myeloid origin [49]. However, in this case it is not a direct effect of HIV-1.
In the present study, our initial investigations focused on the effects of HIV-1 infection on DC maturation as evaluated by cell surface molecule expression. Consistent with previous reports that described HIV-1-induced inhibition of DC maturation [44,63–67], we also found that HIV-1 inhibited the expression of several cell surface molecules associated with a mature phenotype. Specifically, it was observed that up-regulation of CCR7 and MHC-II was inhibited by HIV-1. The observed inhibition of MHC-II expression in the presence of sustained co-stimulatory molecule expression after incubation with maturation-inducing cytokines also complements previous ex-vivo observations in which DC expressing only select maturation markers were found to accumulate abnormally in the lymphoid tissues of HIV-1 infected individuals [81–84]. This lower MHC-II molecule expression could result in impaired DC-mediated presentation of exogenous antigens in both the periphery and in secondary lymphoid organs.
The significance of blunted CCR7 up-regulation is unknown, but may contribute to HIV-1 pathogenesis. While reduced CCR7 expression may not facilitate the dissemination of HIV-1 to naive T cells in secondary lymphoid tissue, it could delay the development of an effective adaptive immune response. Specifically, impaired expression of CCR7 by activated DC in an inflammatory cytokine-rich environment would allow for the maintenance of partially activated HIV-1-infected DC in the anatomical periphery in the presence of virus-susceptible resident effector T cells and potentially increase HIV-1 infectivity [3].
To complement the characterization of the effects of HIV-1 on cell surface molecule expression, we also investigated several functional aspects of mature DC. Maturation of DC is associated with decreases in endocytic activity [3,68], which was confirmed in our experimental system (Fig. 4a). When DC were infected with HIV-1, this inhibition of endocytosis was blunted (Fig. 4c), demonstrating that HIV-1 infection inhibits functions associated with mature DC in addition to its effects on surface marker expression.
To define further the effects of HIV-1 on the functional aspects of mature DC stimulated to undergo maturation, we evaluated antigen presentation as measured by autologous T cell proliferation. To do so, HIV-1-infected (or -uninfected) DC were pulsed with a peptide pool derived from CEF and then co-cultured with CFSE-stained autologous T cells. Consistent with published reports [79,80], we found that HIV-1 infection of DC inhibited autologous T cells proliferation. This impaired T cell proliferation occurred despite the fact that HIV-1 had no effect on MHC-I expression (data not shown). This indicates that the degree of MHC-I expression does not appear to be a factor in the observed HIV-1 effects on T cell proliferation.
Because a critical aspect of immature DC physiology concerns appropriate MAPK responses to pathogenic stimulation that trigger the maturation of DC [3], we next investigated whether HIV-1 had any effect on LPS-induced MAPK signalling. Interestingly, we found that HIV-1 infection had no effect on the p38, JNK or ERK MAPK signalling pathways in immature DC or in-vitro matured DC. This was consistent with our phenotypic observations that HIV-1 did not affect CD14 expression on DC (data not shown), which is necessary for TLR-4 recognition of bacterial LPS [3].
Despite some conflicting reports, it is generally accepted that HIV-1 inhibits DC maturation. This is based largely on the effects of HIV-1 on the expression of cell surface markers associated with the state of DC maturation. Within the present comprehensive set of experiments, not only have we confirmed that HIV-1 alters cell surface marker expression consistent with the inhibition of maturation, but for the first time have clearly linked these changes with a number of aspects of DC function (endocytosis, antigen presentation). The fact that HIV-1 interferes with important aspects of DC function has implications in both HIV-1 pathogenesis as it relates to the immunological control of HIV replication, and in the immunodeficiency and risk of opportunistic infections associated with HIV disease.
Acknowledgments
This work was supported by a Canadian Institutes of Health grant to JBA (grant no. HOP-98830). J.B.A. is supported by a Career Scientist Award from the Ontario HIV Treatment Network.
Disclosure
None of the authors has conflicts of interest to declare, or any relevant financial interest, in any company or institution that might benefit from this publication.
References
- 1.Fearon DT, Locksley RM. The instructive role of innate immunity in the acquired immune response. Science. 1996;272:50–3. doi: 10.1126/science.272.5258.50. [DOI] [PubMed] [Google Scholar]
- 2.Lambrecht BN, Salomon B, Klatzmann D, Pauwels RA. Dendritic cells are required for the development of chronic eosinophilic airway inflammation in response to inhaled antigen in sensitized mice. J Immunol. 1998;160:4090–7. [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 4.Kato M, Neil TK, Fearnley DB, McLellan AD, Vuckovic S, Hart DN. Expression of multilectin receptors and comparative FITC–dextran uptake by human dendritic cells. Int Immunol. 2000;12:1511–9. doi: 10.1093/intimm/12.11.1511. [DOI] [PubMed] [Google Scholar]
- 5.Zhou LJ, Tedder TF. CD14+ blood monocytes can differentiate into functionally mature CD83+ dendritic cells. Proc Natl Acad Sci USA. 1996;93:2588–92. doi: 10.1073/pnas.93.6.2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Nunez RT, Louafi F, Friedmann PS, Sanchez-Elsner T. MicroRNA-155 modulates the pathogen binding ability of dendritic cells (DCs) by down-regulation of DC-specific intercellular adhesion molecule-3 grabbing non-integrin (DC-SIGN) J Biol Chem. 2009;284:16334–42. doi: 10.1074/jbc.M109.011601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verhasselt V, Buelens C, Willems F, De Groote D, Haeffner-Cavaillon N, Goldman M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: evidence for a soluble CD14-dependent pathway. J Immunol. 1997;158:2919–25. [PubMed] [Google Scholar]
- 8.Kanto T, Hayashi N, Takehara T, et al. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584–91. [PubMed] [Google Scholar]
- 9.Lechmann M, Berchtold S, Steinkasserer A, Hauber J. CD83 on dendritic cells: more than just a marker for maturation. Trends Immunol. 2002;23:273–5. doi: 10.1016/s1471-4906(02)02214-7. [DOI] [PubMed] [Google Scholar]
- 10.Sallusto F, Schaerli P, Loetscher P, et al. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol. 1998;28:2760–9. doi: 10.1002/(SICI)1521-4141(199809)28:09<2760::AID-IMMU2760>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 11.Seger R, Krebs EG. The MAPK signaling cascade. FASEB J. 1995;9:726–35. [PubMed] [Google Scholar]
- 12.Ahn NG. The MAP kinase cascade. Discovery of a new signal transduction pathway. Mol Cell Biochem. 1993;127–128:201–9. doi: 10.1007/BF01076771. [DOI] [PubMed] [Google Scholar]
- 13.Arrighi JF, Rebsamen M, Rousset F, Kindler V, Hauser C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J Immunol. 2001;166:3837–45. doi: 10.4049/jimmunol.166.6.3837. [DOI] [PubMed] [Google Scholar]
- 14.Boislève F, Kerdine-Römer S, Rougier-Larzat N, Pallardy M. Nickel and DNCB induce CCR7 expression on human dendritic cells through different signalling pathways: role of TNF-alpha and MAPK. J Invest Dermatol. 2004;123:494–502. doi: 10.1111/j.0022-202X.2004.23229.x. [DOI] [PubMed] [Google Scholar]
- 15.West MA, Wallin RP, Matthews SP, et al. Enhanced dendritic cell antigen capture via Toll-like receptor-induced actin remodeling. Science. 2004;305:1153–7. doi: 10.1126/science.1099153. [DOI] [PubMed] [Google Scholar]
- 16.Yu Q, Kovacs C, Yue FY, Ostrowski MA. The role of the p38 mitogen-activated protein kinase, extracellular signal-regulated kinase, and phosphoinositide-3-OH kinase signal transduction pathways in CD40 ligand-induced dendritic cell activation and expansion of virus-specific CD8+ T cell memory responses. J Immunol. 2004;172:6047–56. doi: 10.4049/jimmunol.172.10.6047. [DOI] [PubMed] [Google Scholar]
- 17.Jackson AM, Mulcahy LA, Porte J, et al. Role of mitogen-activated protein kinase and PI3K pathways in the regulation of IL-12-family cytokines in dendritic cells and the generation of TH-responses. Eur Cytokine Netw. 2010;21:319–28. doi: 10.1684/ecn.2010.0219. [DOI] [PubMed] [Google Scholar]
- 18.Mitchell D, Olive C. Regulation of Toll-like receptor-induced chemokine production in murine dendritic cells by mitogen-activated protein kinases. Mol Immunol. 2010;47:2065–73. doi: 10.1016/j.molimm.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 19.Wu WL, Ho LJ, Chang DM, Chen CH, Lai JH. Triggering of DC migration by dengue virus stimulation of COX-2-dependent signaling cascades in vitro highlights the significance of these cascades beyond inflammation. Eur J Immunol. 2009;39:3413–22. doi: 10.1002/eji.200939306. [DOI] [PubMed] [Google Scholar]
- 20.Mäkelä SM, Österlund P, Julkunen I. TLR ligands induce synergistic interferon-β and interferon-λ1 gene expression in human monocyte-derived dendritic cells. Mol Immunol. 2011;48:505–15. doi: 10.1016/j.molimm.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Fedele G, Spensieri F, Palazzo R, et al. Bordetella pertussis commits human dendritic cells to promote a Th1/Th17 response through the activity of adenylate cyclase toxin and MAPK-pathways. PLoS ONE. 2010;5:e8734. doi: 10.1371/journal.pone.0008734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakahara T, Uchi H, Urabe K, Chen Q, Furue M, Moroi Y. Role of c-Jun N-terminal kinase on lipopolysaccharide induced maturation of human monocyte-derived dendritic cells. Int Immunol. 2004;16:1701–9. doi: 10.1093/intimm/dxh171. [DOI] [PubMed] [Google Scholar]
- 23.Sato K, Nagayama H, Tadokoro K, Juji T, Takahashi TA. Extracellular signal-regulated kinase, stress-activated protein kinase/c-Jun N-terminal kinase, and p38mapk are involved in IL-10-mediated selective repression of TNF-alpha-induced activation and maturation of human peripheral blood monocyte-derived dendritic cells. J Immunol. 1999;162:3865–72. [PubMed] [Google Scholar]
- 24.Iijima N, Yanagawa Y, Clingan JM, Onoé K. CCR7-mediated c-Jun N-terminal kinase activation regulates cell migration in mature dendritic cells. Int Immunol. 2005;17:1201–12. doi: 10.1093/intimm/dxh297. [DOI] [PubMed] [Google Scholar]
- 25.Häcker H, Mischak H, Miethke T, et al. CpG-DNA-specific activation of antigen-presenting cells requires stress kinase activity and is preceded by non-specific endocytosis and endosomal maturation. EMBO J. 1998;17:6230–40. doi: 10.1093/emboj/17.21.6230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu Y, Yvashkiv LB. Costimulation of chemokine receptor signaling by matrix metalloproteinase-9 mediates enhanced migration of IFN-alpha dendritic cells. J Immunol. 2006;176:6022–33. doi: 10.4049/jimmunol.176.10.6022. [DOI] [PubMed] [Google Scholar]
- 27.Rescigno M, Martino M, Sutherland CL, Gold MR, Ricciardi-Castagnoli P. Dendritic cell survival and maturation are regulated by different signaling pathways. J Exp Med. 1998;188:2175–80. doi: 10.1084/jem.188.11.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.An H, Yu Y, Zhang M, et al. Involvement of ERK, p38 and NF-kappaB signal transduction in regulation of TLR2, TLR4 and TLR9 gene expression induced by lipopolysaccharide in mouse dendritic cells. Immunology. 2002;106:38–45. doi: 10.1046/j.1365-2567.2002.01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caparrós E, Munoz P, Sierra-Filardi E, et al. DC-SIGN ligation on dendritic cells results in ERK and PI3K activation and modulates cytokine production. Blood. 2006;107:3950–8. doi: 10.1182/blood-2005-03-1252. [DOI] [PubMed] [Google Scholar]
- 30.Yanagawa Y, Iijima N, Iwabuchi K, Onoé K. Activation of extracellular signal-related kinase by TNF-alpha controls the maturation and function of murine dendritic cells. J Leukoc Biol. 2002;71:125–32. [PubMed] [Google Scholar]
- 31.Puig-Kröger A, Relloso M, Fernández-Capetillo O, et al. Extracellular signal-regulated protein kinase signaling pathway negatively regulates the phenotypic and functional maturation of monocyte-derived human dendritic cells. Blood. 2001;98:2175–82. doi: 10.1182/blood.v98.7.2175. [DOI] [PubMed] [Google Scholar]
- 32.Cui GY, Diao HY. Recognition of HBV antigens and HBV DNA by dendritic cells. Hepatobiliary Pancreat Dis Int. 2010;9:584–92. [PubMed] [Google Scholar]
- 33.Altfeld M, Fadda L, Frleta D, Bhardwaj N. DCs and NK cells: critical effectors in the immune response to HIV-1. Nat Rev Immunol. 2011;11:176–86. doi: 10.1038/nri2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haseltine WA. Replication and pathogenesis of the AIDS virus. J Acquir Immune Defic Syndr. 1988;1:217–40. [PubMed] [Google Scholar]
- 35.Bedoui S, Gebhardt T. Interaction between dendritic cells and T cells during peripheral virus infections: a role for antigen presentation beyond lymphoid organs? Curr Opin Immunol. 2011;23:124–30. doi: 10.1016/j.coi.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg ES, Billingsley JM, Caliendo AM, et al. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–50. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 37.Price P, Mathiot N, Krueger R, Stone S, Keane NM, French MA. Immune dysfunction and immune restoration disease in HIV patients given highly active antiretroviral therapy. J Clin Virol. 2001;22:279–87. doi: 10.1016/s1386-6532(01)00200-1. [DOI] [PubMed] [Google Scholar]
- 38.Douek DC, Brenchley JM, Betts MR, et al. HIV preferentially infects HIV-specific CD4+ T cells. Nature. 2002;417:95–8. doi: 10.1038/417095a. [DOI] [PubMed] [Google Scholar]
- 39.Appay V, Sauce D. Immune activation and inflammation in HIV-1 infection: causes and consequences. J Pathol. 2008;214:231–41. doi: 10.1002/path.2276. [DOI] [PubMed] [Google Scholar]
- 40.Mofenson LM, Oleske J, Serchuck L, Van Dyke R, Wilfert C. Treating opportunistic infections among HIV-exposed and infected children: recommendations from CDC, the National Institutes of Health, and the Infectious Diseases Society of America. Clin Infect Dis. 2005;40(Suppl. 1):S1–84. doi: 10.1086/427295. [DOI] [PubMed] [Google Scholar]
- 41.Izquierdo-Useros N, Naranjo-Gómez M, Erkizia I, et al. HIV and mature dendritic cells: Trojan exosomes riding the Trojan horse? PLoS Pathog. 2010;6:e1000740. doi: 10.1371/journal.ppat.1000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wiley RD, Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proc Natl Acad Sci USA. 2006;103:738–43. doi: 10.1073/pnas.0507995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smed-Sörensen A, Loré K, Walther-Jallow L, Andersson J, Spetz AL. HIV-1-infected dendritic cells up-regulate cell surface markers but fail to produce IL-12 p70 in response to CD40 ligand stimulation. Blood. 2004;104:2810–7. doi: 10.1182/blood-2003-07-2314. [DOI] [PubMed] [Google Scholar]
- 44.Granelli-Piperno A, Golebiowska A, Trumpfheller C, Siegal FP, Steinman RM. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proc Natl Acad Sci USA. 2004;101:7669–74. doi: 10.1073/pnas.0402431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Donaghy H, Gazzard B, Gotch F, Patterson S. Dysfunction and infection of freshly isolated blood myeloid and plasmacytoid dendritic cells in patients infected with HIV-1. Blood. 2003;101:4505–11. doi: 10.1182/blood-2002-10-3189. [DOI] [PubMed] [Google Scholar]
- 46.Hsieh SM, Pan SC, Hung CC, Chen MY, Chang SC. Differential impact of late-stage HIV-1 infection on in vitro and in vivo maturation of myeloid dendritic cells. J Acquir Immune Defic Syndr. 2003;33:413–9. doi: 10.1097/00126334-200308010-00001. [DOI] [PubMed] [Google Scholar]
- 47.Harman AN, Wilkinson J, Bye CR, et al. HIV induces maturation of monocyte-derived dendritic cells and Langerhans cells. J Immunol. 2006;177:7103–13. doi: 10.4049/jimmunol.177.10.7103. [DOI] [PubMed] [Google Scholar]
- 48.Yonezawa A, Morita R, Takaori-Kondo A, et al. Natural alpha interferon-producing cells respond to human immunodeficiency virus type 1 with alpha interferon production and maturation into dendritic cells. J Virol. 2003;77:3777–84. doi: 10.1128/JVI.77.6.3777-3784.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fonteneau JF, Larsson M, Beignon AS, et al. Human immunodeficiency virus type 1 activates plasmacytoid dendritic cells and concomitantly induces the bystander maturation of myeloid dendritic cells. J Virol. 2004;78:5223–32. doi: 10.1128/JVI.78.10.5223-5232.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smed-Sörensen A, Loré K, Vasudevan J, et al. Differential susceptibility to human immunodeficiency virus type 1 infection of myeloid and plasmacytoid dendritic cells. J Virol. 2005;79:8861–9. doi: 10.1128/JVI.79.14.8861-8869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beignon AS, McKenna K, Skoberne M, et al. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor–viral RNA interactions. J Clin Invest. 2005;115:3265–75. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schmidt B, Ashlock BM, Foster H, Fujimura SH, Levy JA. HIV-infected cells are major inducers of plasmacytoid dendritic cell interferon production, maturation, and migration. Virology. 2005;343:256–66. doi: 10.1016/j.virol.2005.09.059. [DOI] [PubMed] [Google Scholar]
- 53.Manches O, Munn D, Fallahi A, et al. HIV-activated human plasmacytoid DCs induce Tregs through an indoleamine 2,3-dioxygenase-dependent mechanism. J Clin Invest. 2008;118:3431–9. doi: 10.1172/JCI34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rossetti M, Gregori S, Hauben E, et al. HIV-1-derived lentiviral vectors directly activate plasmacytoid dendritic cells, which in turn induce the maturation of myeloid dendritic cells. Hum Gene Ther. 2011;22:177–88. doi: 10.1089/hum.2010.085. [DOI] [PubMed] [Google Scholar]
- 55.Fantuzzi L, Purificato C, Donato K, Belardelli F, Gessani S. Human immunodeficiency virus type 1 gp120 induces abnormal maturation and functional alterations of dendritic cells: a novel mechanism for AIDS pathogenesis. J Virol. 2004;78:9763–72. doi: 10.1128/JVI.78.18.9763-9772.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Quaranta MG, Tritarelli E, Giordani L, Viora M. HIV-1 Nef induces dendritic cell differentiation: a possible mechanism of uninfected CD4(+) T cell activation. Exp Cell Res. 2002;275:243–54. doi: 10.1006/excr.2002.5497. [DOI] [PubMed] [Google Scholar]
- 57.Fanales-Belasio E, Moretti S, Nappi F, et al. Native HIV-1 Tat protein targets monocyte-derived dendritic cells and enhances their maturation, function, and antigen-specific T cell responses. J Immunol. 2002;168:197–206. doi: 10.4049/jimmunol.168.1.197. [DOI] [PubMed] [Google Scholar]
- 58.Dauer M, Obermaier B, Herten J, et al. Mature dendritic cells derived from human monocytes within 48 h: a novel strategy for dendritic cell differentiation from blood precursors. J Immunol. 2003;170:4069–76. doi: 10.4049/jimmunol.170.8.4069. [DOI] [PubMed] [Google Scholar]
- 59.Yamamoto N, Tanaka C, Wu Y, et al. Analysis of human immunodeficiency virus type 1 integration by using a specific, sensitive and quantitative assay based on real-time polymerase chain reaction. Virus Genes. 2006;32:105–13. doi: 10.1007/s11262-005-5851-2. [DOI] [PubMed] [Google Scholar]
- 60.Gallagher S, Winston SE, Fuller SA, Hurrell JG. Immunoblotting and immunodetection. Curr Protoc Immunol. 2008;83 doi: 10.1002/0471142735.im0810s26. 8.10.1–28. November; Unit 8·10. [DOI] [PubMed] [Google Scholar]
- 61.Svajger U, Anderluh M, Jeras M, Obermajer N. C-type lectin DC-SIGN: an adhesion, signalling and antigen-uptake molecule that guides dendritic cells in immunity. Cell Signal. 2010;22:1397–405. doi: 10.1016/j.cellsig.2010.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sabado RL, O'Brien M, Subedi A, et al. Evidence of dysregulation of dendritic cells in primary HIV infection. Blood. 2010;116:3839–52. doi: 10.1182/blood-2010-03-273763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Masso M. DC-SIGN points the way to a novel mechanism for HIV-1 transmission. MedGenMed. 2003;5:2. Review. PMID: 14603101. [PubMed] [Google Scholar]
- 64.Lekkerkerker AN, van Kooyk Y, Geijtenbeek TB. Viral piracy: HIV-1 targets dendritic cells for transmission. Curr HIV Res. 2006;4:169–76. doi: 10.2174/157016206776055020. [DOI] [PubMed] [Google Scholar]
- 65.Hanley TM, Blay Puryear W, Gummuluru S, Viglianti GA. PPARgamma and LXR signaling inhibit dendritic cell-mediated HIV-1 capture and trans-infection. PLoS Pathog. 2010;6:e1000981. doi: 10.1371/journal.ppat.1000981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Manel N, Hogstad B, Wang Y, Levy DE, Unutmaz D, Littman DR. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature. 2010;467:214–7. doi: 10.1038/nature09337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhang R, Zhang S, Li M, Chen C, Yao Q. Incorporation of CD40 ligand into SHIV virus-like particles (VLP) enhances SHIV-VLP-induced dendritic cell activation and boosts immune responses against HIV. Vaccine. 2010;28:5114–27. doi: 10.1016/j.vaccine.2010.03.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zanoni I, Granucci F. Regulation of antigen uptake, migration, and lifespan of dendritic cell by Toll-like receptors. J Mol Med (Berl) 2010;88:873–80. doi: 10.1007/s00109-010-0638-x. [DOI] [PubMed] [Google Scholar]
- 69.O'Connor AM, Crawley AM, Angel JB. Interleukin-7 enhances memory CD8(+) T-cell recall responses in health but its activity is impaired in human immunodeficiency virus infection. Immunology. 2010;131:525–36. doi: 10.1111/j.1365-2567.2010.03325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ardeshna KM, Pizzey AR, Devereux S, Khwaja A. The PI3 kinase, p38 SAP kinase, and NF-kappaB signal transduction pathways are involved in the survival and maturation of lipopolysaccharide-stimulated human monocyte-derived dendritic cells. Blood. 2000;96:1039–46. [PubMed] [Google Scholar]
- 71.Stepnik M, Arkusz J. Molecular events associated with dendritic cells activation by contact sensitizers. Int J Occup Med Environ Health. 2003;16:191–9. [PubMed] [Google Scholar]
- 72.Iijima N, Yanagawa Y, Onoé K. Role of early- or late-phase activation of p38 mitogen-activated protein kinase induced by tumour necrosis factor-alpha or 2,4-dinitrochlorobenzene during maturation of murine dendritic cells. Immunology. 2003;110:322–8. doi: 10.1046/j.1365-2567.2003.01746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.McIlroy D, Autran B, Cheynier R, et al. Infection frequency of dendritic cells and CD4+ T lymphocytes in spleens of human immunodeficiency virus-positive patients. J Virol. 1995;69:4737–45. doi: 10.1128/jvi.69.8.4737-4745.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bakri Y, Schiffer C, Zennou V, et al. The maturation of dendritic cells results in postintegration inhibition of HIV-1 replication. J Immunol. 2001;166:3780–8. doi: 10.4049/jimmunol.166.6.3780. [DOI] [PubMed] [Google Scholar]
- 75.Granelli-Piperno A, Moser B, Pope M, et al. Efficient interaction of HIV-1 with purified dendritic cells via multiple chemokine coreceptors. J Exp Med. 1996;184:2433–8. doi: 10.1084/jem.184.6.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Turville SG, Santos JJ, Frank I, et al. Immunodeficiency virus uptake, turnover, and 2-phase transfer in human dendritic cells. Blood. 2004;103:2170–9. doi: 10.1182/blood-2003-09-3129. [DOI] [PubMed] [Google Scholar]
- 77.Pion M, Granelli-Piperno A, Mangeat B, et al. APOBEC3G/3F mediates intrinsic resistance of monocyte-derived dendritic cells to HIV-1 infection. J Exp Med. 2006;203:2887–93. doi: 10.1084/jem.20061519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu L, KewalRamani VN. Dendritic-cell interactions with HIV: infection and viral dissemination. Nat Rev Immunol. 2006;6:859–68. doi: 10.1038/nri1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Knight SC, Patterson S, Macatonia SE. Stimulatory and suppressive effects of infection of dendritic cells with HIV-1. Immunol Lett. 1991;30:213–8. doi: 10.1016/0165-2478(91)90028-9. [DOI] [PubMed] [Google Scholar]
- 80.Roberts M, Gompels M, Pinching AJ, Knight SC. Dendritic cells from HIV-1 infected individuals show reduced capacity to stimulate autologous T-cell proliferation. Immunol Lett. 1994;43:39–43. doi: 10.1016/0165-2478(94)00147-2. [DOI] [PubMed] [Google Scholar]
- 81.Loré K, Sönnerborg A, Broström C, et al. Accumulation of DC-SIGN+CD40+ dendritic cells with reduced CD80 and CD86 expression in lymphoid tissue during acute HIV-1 infection. AIDS. 2002;16:683–92. doi: 10.1097/00002030-200203290-00003. [DOI] [PubMed] [Google Scholar]
- 82.Dillon SM, Robertson KB, Pan SC, et al. Plasmacytoid and myeloid dendritic cells with a partial activation phenotype accumulate in lymphoid tissue during asymptomatic chronic HIV-1 infection. J Acquir Immune Defic Syndr. 2008;48:1–12. doi: 10.1097/QAI.0b013e3181664b60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Knight SC, Patterson S. Bone marrow-derived dendritic cells, infection with human immunodeficiency virus, and immunopathology. Annu Rev Immunol. 1997;15:593–615. doi: 10.1146/annurev.immunol.15.1.593. [DOI] [PubMed] [Google Scholar]
- 84.Desai S, Chaparro A, Liu H, et al. Impaired CCR7 expression on plasmacytoid dendritic cells of HIV-infected children and adolescents with immunologic and virologic failure. J Acquir Immune Defic Syndr. 2007;45:501–7. doi: 10.1097/QAI.0b013e3180654811. [DOI] [PubMed] [Google Scholar]


