Hu et al. parse the cell-autonomous and nonautonomous responses to a morphogen—in this case, Sonic hedgehog (Shh)—during muscle formation in the developing limb in the mouse and chick model systems. While Shh affects the limb muscular pattern non-cell-autonomously, its role is cell-autonomous in activating the myogenic program in the ventral limb, promoting slow muscle differentiation, and regulating directional muscle migration in the distal limb. The role of Net1 expression during muscle migration is also explored. See also the related study by Anderson et al. (in this issue).
Keywords: Shh, Smo, limb muscles, Net1, cell migration
Abstract
Muscle progenitor cells migrate from the lateral somites into the developing vertebrate limb, where they undergo patterning and differentiation in response to local signals. Sonic hedgehog (Shh) is a secreted molecule made in the posterior limb bud that affects patterning and development of multiple tissues, including skeletal muscles. However, the cell-autonomous and non-cell-autonomous functions of Shh during limb muscle formation have remained unclear. We found that Shh affects the pattern of limb musculature non-cell-autonomously, acting through adjacent nonmuscle mesenchyme. However, Shh plays a cell-autonomous role in maintaining cell survival in the dermomyotome and initiating early activation of the myogenic program in the ventral limb. At later stages, Shh promotes slow muscle differentiation cell-autonomously. In addition, Shh signaling is required cell-autonomously to regulate directional muscle cell migration in the distal limb. We identify neuroepithelial cell transforming gene 1 (Net1) as a downstream target and effector of Shh signaling in that context.
Developmentally, the vertebrate limb is a mosaic structure wherein some cell types, such as the skeletal and connective tissues, arise from the lateral plate-derived limb mesenchyme (Dhouailly and Kieny 1972; Chevallier et al. 1977), while others, such as the muscles and some endothelial cells, are derived from the somites (Christ et al. 1977; Kardon et al. 2002; Huang et al. 2003; Bryson-Richardson and Currie 2008; Hutcheson et al. 2009). In spite of their disparate origins, once in the limb bud, the progenitors of those tissues respond to the same signaling environment that patterns the limb and orchestrates its morphogenesis. For example, Sonic hedgehog (Shh) is a key signaling molecule expressed in the zone of polarizing activity (ZPA) in the posterior of the vertebrate limb bud from embryonic day 9.75 (E9.75) through E12.5 (Echelard et al. 1993; Riddle et al. 1993; Kruger et al. 2001). Shh protein has also been detected in the limb ectoderm (Bouldin et al. 2010). Functionally, Shh activity is both necessary and sufficient to establish the anterior–posterior (AP) pattern of the limb. Ectopic expression of the Shh at the anterior margin of the chick limb bud results in a mirror-image duplication of all of the tissues of the limb (Riddle et al. 1993), while removal of Shh in mice results in a loss of posterior pattern and the development of a single anterior digit (Chiang et al. 2001). However, within the limb bud, which tissues are directly affected by Shh signaling and which tissues are indirectly patterned have not been previously examined.
Shh appears to play multiple sequential roles in regulating the process of myogenesis. The myogenic precursor cells that populate the limb bud originate in the somites (Chevallier et al. 1977; Christ et al. 1977). Shh, produced in the midline by the notochord and the floor plate of the neural tube, is required to activate expression of myogenic determination genes such as Myf5 and MyoD in the epaxial portion of the somite (Münsterberg et al. 1995; Borycki et al. 1999; Gustafsson et al. 2002; McDermott et al. 2005), which gives rise to the deep back muscles. The hypaxial muscle precursors of the dermomyotome are not affected at this stage (Borycki et al. 1999).
The hypaxial cells, including the future limb muscle progenitors, express the paired domain transcription factor Pax3, which is initially expressed throughout the presomitic mesoderm but later becomes restricted to the dermomyotome (Goulding et al. 1991; Williams and Ordahl 1994). At E9.5 in mice or Hamburger and Hamilton stage 17 (HH17) in chicks, myogenic cells start to delaminate from the ventrolateral lip of the dermomyotome and migrate into the forelimb bud to where the dorsal and ventral muscle masses will form initially (Tajbakhsh and Buckingham 2000; Francis-West et al. 2003; Otto et al. 2006). The same process occurs slightly later in the hindlimb. During migration, muscle progenitor cells continue to proliferate and express Pax3 until arriving in the limb bud and differentiating to become myoblasts, concomitant with the expression of Myf5 and MyoD (Birchmeier and Brohmann 2000; Pownall et al. 2002).
This myogenic differentiation is integrated with the process of pattern formation such that as the muscle cells differentiate and begin to form muscle bundles, they do so in the correct location and orientation (Kardon 1998; Kardon et al. 2003; Li et al. 2010). The AP organization of the muscles is established in response to Shh activity. When Shh is applied to the anterior chick limb bud, anterior muscles are transformed into muscles with posterior identity, in concert with other tissues (Duprez et al. 1999). This is likely an indirect response to Shh, as the patterning of the limb muscles appears to be controlled by the prepattern of the muscle connective tissue (Kardon et al. 2003; Hasson et al. 2010). However, in addition to patterning changes, ectopic Shh also results in an expansion of the Pax3-expressing muscle precursor population, leading to muscle hypertrophy (Duprez et al. 1998). Furthermore, in the complete loss of Shh activity in mice, there is a total loss of limb musculature, with the exception of a small portion of the dorsal muscle adjacent to the humerus (Kruger et al. 2001). Explant cultures suggest that this may be due to a requirement for Shh to maintain the expression of Myf5 and MyoD (Kruger et al. 2001).
Shh has also been shown to regulate terminal differentiation of limb muscles. As the muscles differentiate within the limb, they express different compositions of myosin heavy chain (MyHC) isoforms, which are determinants of myofiber types—fatigue-enduring oxidative slow muscle fibers or force-generating glycolytic fast muscle fibers (Gunning and Hardeman 1991; Schiaffino and Reggiani 1996; Wigmore and Evans 2002). For instance, the soleus is enriched with slow fibers, while the tibialis anterior (TA) is largely composed of fast fibers (Agbulut et al. 2003). These differences enable each muscle to adapt to different physiological and functional demands. It has been reported that excessive Shh promotes the formation of slow fibers, while loss of Shh signaling reduces slow muscle fiber formation, due to precocious differentiation (Cann et al. 1999; Bren-Mattison and Olwin 2002; Li et al. 2004).
To attempt to distinguish whether these various effects of Shh on limb muscle development are direct or indirect, we took a genetic approach in mice. Smoothened (Smo) encodes a seven-pass transmembrane protein that acts downstream from the Shh receptor Patched and is required for Shh signaling (Alcedo et al. 1996; van den Heuvel and Ingham 1996; Zhang et al. 2001). While Smo is required for all hedgehog signaling, Shh is the only member of the Hh family expressed during the early steps of limb development (Echelard et al. 1993; Yang et al. 1998). Thus, by removing Smo tissue specifically from the muscle cell precursors or from the surrounding lateral plate-derived mesenchyme, we are able to dissect the cell-autonomous and non-cell-autonomous functions of Shh signaling during limb muscle development. We found that Shh acts non-cell-autonomously to pattern limb musculature through lateral plate-derived tissues. However, Hh signaling is required cell-autonomously to maintain cell survival in the dermomyotome, initiate prompt and robust early Myf5 and MyoD expression in the ventral limb muscle mass, and, at a later stage, promote slow muscle fiber formation in the limb. In addition, Shh signaling is essential for the maintenance of neuroepithelial cell transforming gene 1 (Net1) expression in the myogenic precursors, which in turn regulates directional muscle cell migration in the distal limb.
Results
Shh signaling patterns limb muscles non-cell-autonomously
In order to define the cell-autonomous and non-cell-autonomous functions of Shh signaling during limb muscle development, we removed the ability to respond to Shh signaling specifically from the somite or lateral plate-derived limb mesenchyme by using a conditional allele of Smoothened (Smofl) (Long et al. 2001) in conjunction with Pax3Cre (Engleka et al. 2005) or Prx1Cre (Logan et al. 2002), which encode Cre-recombinases that are expressed in these tissues, respectively. In some crosses, the Smofl allele was combined with a null allele of the gene Smodel. Smodel/+ did not show any phenotype. We first examined the cell-autonomous role of Shh signaling during the AP patterning of the forelimb muscles by creating either Pax3Cre; Smofl/fl or Pax3Cre; Smofl/del embryos [collectively referred to as Pax3Cre; SmoCKO (conditional knockout) when giving the same phenotype]. At E12.5, MyoD whole-mount in situ hybridization revealed a loss of MyoD expression in the cervical somites (Supplemental Fig. 1B). In regions where MyoD was expressed, the mutant myotomes were shorter than those of the wild-type Pax3Cre; Smofl/+ siblings (Supplemental Fig. 1A,B). They had additionally lost parts of the epaxial dermomyotome (Supplemental Fig. 1C,D), a phenotype that was also seen in the Shh−/− mutants (Borycki et al. 1999), presumably due to an inability to respond to the midline Shh. However, AP patterning in the mutant proximal limb muscles was unaffected, although the MyoD-expressing domain appeared to be reduced in size at E12.5 (Fig. 1C). This decrease in MyoD expression did not affect the eventual muscle mass and patterning, as supported by immunostaining at E16.5 using an antibody against smooth muscle actin (SMA) (Fig. 1D,F), which is expressed in the skeletal muscles at this stage (Supplemental Fig. 2A–B″). In these mutants, Pax3-Cre recombinase effectively led to recombination in the somites and all limb muscles but not in the lateral plate-derived tissues, hence enabling removal of Hh signaling specifically in the myogenic cells (Table 1; Supplemental Fig. 2C–D″,G,H). These data show that Shh does not pattern the proximal limb muscles cell-autonomously. While no proximal patterning changes were observed, we did note a complete loss of muscle tissue in the distal-most limb, a phenotype analyzed below.
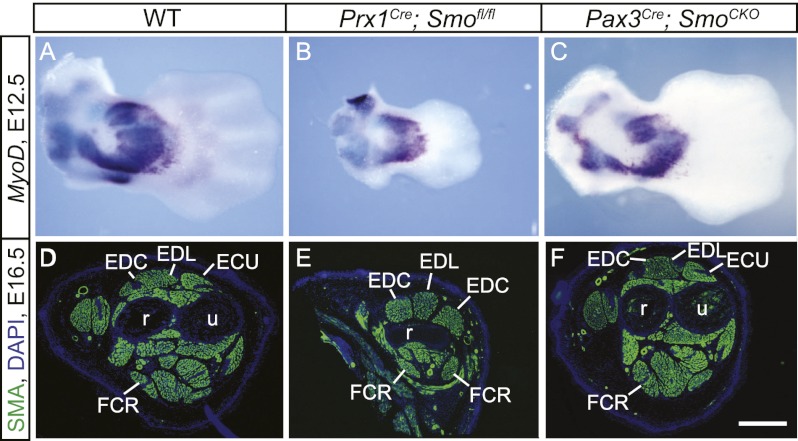
Figure 1.
Shh signaling patterns limb muscle non-cell-autonomously. (A–C) MyoD expression of mouse forelimbs was analyzed by whole-mount in situ hybridization at E12.5 (dorsal views). (D–F) The expression of SMA was detected by immunostaining at E16.5 (transverse sections). While the forelimb muscles of wild-type (WT) embryos were correctly patterned along the AP axis (A,D), AP patterning was lost in the mutant forelimb muscles, where Shh activity was removed in the nonmuscle limb mesenchyme (B,E). (C,F) Cell-autonomous removal of Shh activity in the muscles did not affect AP patterning. (ECU) Extensor carpi ulnaris; (EDC) extensor digitorum communis; (FCR) flexor carpi radialis; (r) radius; (u) ulna. Bar in F: A–C, 480 μm; D–F, 400 μm.
Table 1.
Microarray results
To verify that the patterning of the limb muscles by Shh is an indirect, nonautonomous effect, we specifically removed the ability of the nonmuscle cells of the limb to respond to Shh, while leaving Shh signal transduction intact in the myogenic populations. To this end, we generated Prx1Cre; Smofl/fl embryos where Prx1Cre is only active in the lateral plate-derived tissues (Supplemental Fig. 2E–F″). Prx1Cre; Smofl/fl embryos exhibited a Shh mutant-like limb phenotype with a much shortened limb and reduced posterior skeletal elements. Limb muscles in this mutant appear symmetrical, with an anatomy most consistent with a double anterior pattern along the AP axis (Fig. 1B,E). Therefore, these data do indeed confirm the idea that Shh patterns limb muscles along the AP axis through lateral plate-derived mesenchyme in a non-cell-autonomous manner, consistent with previous work indicating that the pattern of the musculature is established by the muscle connective tissue (Kardon et al. 2003; Hasson et al. 2010; Li et al. 2010).
Hh signaling promotes slow muscle fiber formation cell-autonomously
In addition to its early effect on muscle patterning, memory of Shh exposure could also have an effect on later muscle differentiation. Moreover, the related protein Indian hedgehog (Ihh), produced in the developing cartilage elements, could also influence later muscle development (Bren-Mattison et al. 2011). As Smo is required for both Shh and Ihh activity, its removal will block all Hh signaling in the developing limb. To determine whether Hh signaling is required non-cell-autonomously for the regulation of terminal muscle differentiation at later stages, we used Tcf4-Cre recombinase (encoded by Tcf4GFPCre+neo) to remove Smo from muscle connective tissue, which arises from the lateral plate-derived limb mesenchyme and has previously been reported to regulate muscle fiber type (Mathew et al. 2011). However, Tcf4GFPCre+neo ; Smofl/fl mutant embryos appeared to be normal in both connective tissue and muscle patterning and differentiation (Supplemental Fig. 1E,F; data not shown). Slow muscle fiber formation is a hallmark of limb muscle terminal differentiation and can be detected by using an antibody against myosin heavy chain I (MyHCI). To study whether terminal differentiation is affected, we therefore quantified the proportion of slow muscle fiber in the soleus and the extensor digitorum longus (EDL) of the hindlimb, which contains a higher proportion of slow muscle fibers than the forelimb and has been traditionally used to examine this aspect of muscle differentiation. However, no changes were observed (Fig. 2D,H).
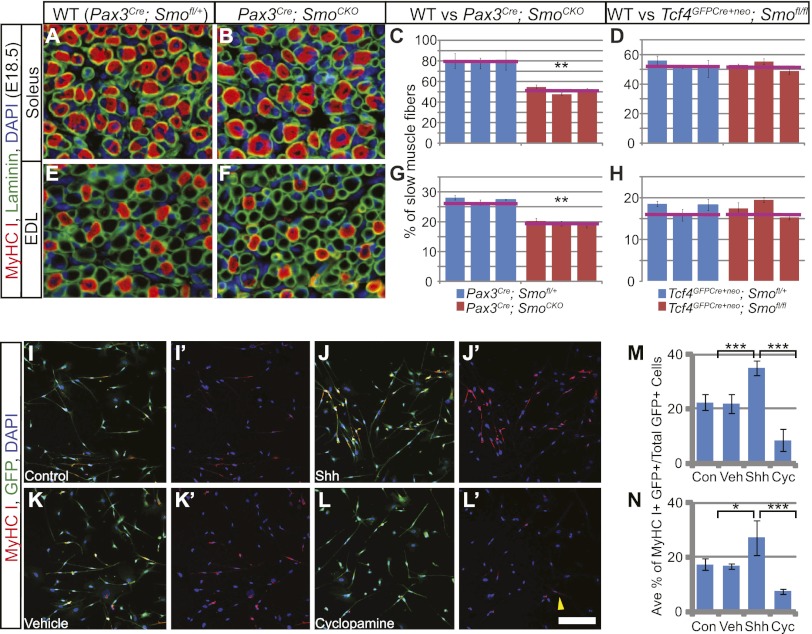
Figure 2.
Hh signaling promotes slow muscle fiber formation cell-autonomously. (A,B,E,F) Frozen sections from the hindlimb soleus (A,B) and EDL (E,F) were immunostained for laminin to mark all muscle fibers and type I myosin to label slow muscle fibers at E18.5. (C,D,G,H) The proportion of slow muscle fibers in the soleus (C,D) and EDL (G,H) was quantified as previously described (Hutcheson et al. 2009). Slow muscle fibers were found to be decreased in limbs with cell-autonomous removal of Hh signaling (C,G) but unchanged when Hh signaling was abrogated in muscle connective tissues (D,H). The red lines mark the average value from all three individuals of the same genotype. (I–L′) After 7 d of differentiation, hindlimb myogenic cells cultured in the presence of Shh protein exhibited more MyHCI-positive slow myofibers than the control cells (shown in I–J′). (L,L′) However, in the presence of cyclopamine, few MyHCI signals were detected, with occasional faintly stained cells (yellow arrowhead). (K,K′) Vehicle control had no effect on the cells. (M,N) When quantified, GFP-positive myogenic cells from both the hindlimb (M) and forelimb (N) showed a significant increase in the proportion of MyHCI slow myofibers when cultured with Shh protein and decreased slow muscle fibers in the presence of cyclopamine. Histograms are expressed as means and standard error of the mean (SEM). (Con) Control; (Veh) vehicle control; (Cyc) cyclopamine. Bar in L′: A,B,E,F, 20 μm; I–L′, 200 μm.
In contrast, when Hh responsiveness was removed directly from limb muscles in the Pax3Cre; SmoCKO mutant embryos, there was a significant decrease in the percentage of slow muscle fiber in the soleus and EDL compared with the wild type at E18.5 (Fig. 2A–C,E–G). This indicates that Hh signaling promotes slow muscle fiber formation in the limb in a cell-autonomous fashion, a mechanism that is analogous to the adaxial slow muscle fiber formation in the zebrafish (Baxendale et al. 2004; Feng et al. 2006). To directly assess whether Shh signaling is capable of regulating differentiation of myogenic limb bud cells, we next performed an in vitro differentiation assay. To isolate myogenic cells, we generated Pax3Cre; RC∷rePe embryos where RC∷rePe is a Cre-responsive GFP reporter at the Rosa26 locus (Ray et al. 2011), permitting isolation of all Pax3 descendants by fluorescence-activated cell sorting (FACS). Myogenic cells isolated from E13.5 forelimbs or hindlimbs were subsequently cultured and differentiated for 7 d in the presence or absence of Shh protein. Addition of Shh significantly increased the proportion of MyHCI-positive myofibers in the culture (Fig. 2I–J′,M,N). In contrast, the addition of the Smo inhibitor cyclopamine drastically decreased the percentage of slow myofibers when compared with either the vehicle control or cells cultured with Shh protein (Fig. 2K–N). Thus, Shh signaling is capable of inducing slow muscle fiber formation in culture, consistent with our in vivo findings.
Shh signaling is required cell-autonomously for initiation of the myogenic program in the early ventral limb
As Shh signaling has been implicated in regulating Myf5 expression through Gli2/3 in the somites (Borycki et al. 1999; McDermott et al. 2005), we reasoned that it might similarly promote the myogenic program in a cell-autonomous fashion during limb myogenesis. To study this early aspect of Shh function, we first performed section in situ hybridization to look at the expression of several myogenic differentiation markers between E10.5 and E11.25 in both wild-type and mutant embryos where Smo was removed by Pax3Cre. While Pax3 and Six1/4 expression was comparable in wild-type and mutant embryos (Fig. 3A,B; Supplemental Fig. 3A–D), cell-autonomous removal of Smo resulted in down-regulation of Myf5 expression at E10.5 in the forelimb ventral muscle mass and at E10.75 in the hindlimb ventral muscle mass (Fig. 3C,D; Supplemental Fig. 4C,D). However, its expression recovered by E10.75 and E11 in the forelimb and hindlimb, respectively, at which point the level of Myf5 expression became indistinguishable from wild type at the resolution of in situ hybridization. (Fig. 3I,J; Supplemental Fig. 4I,J). Thus, Shh signaling is required for the timely initiation of Myf5 expression in the early ventral limb muscle but not for ultimately establishing or maintaining its expression. Similar results were obtained by using Pax3 and Myf5 antibodies (Fig. 3E,F,K,L; Supplemental Fig. 4E,F,K,L).
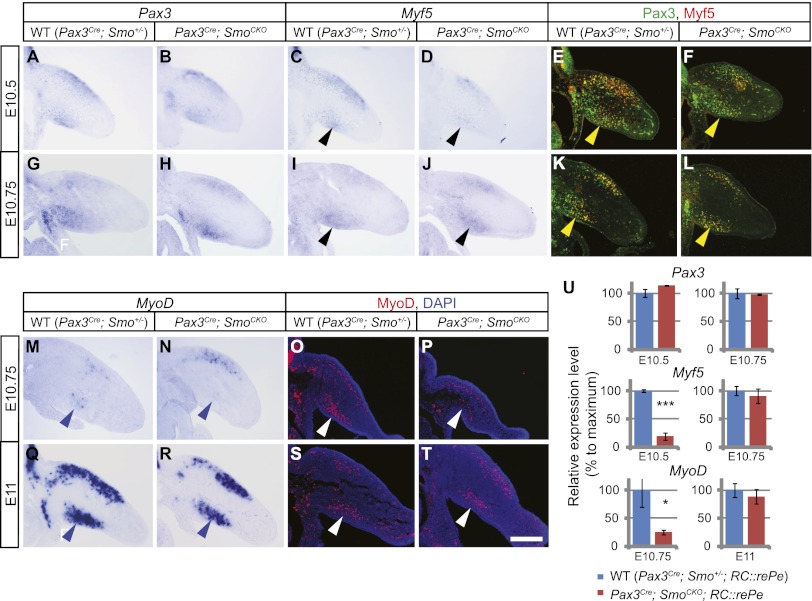
Figure 3.
The initiation of the myogenic program is delayed in the Pax3Cre; SmoCKO mutant forelimb ventral muscle cells. (A–D) When Hh signaling is removed cell-autonomously from muscle progenitor cells, section in situ hybridization showed that at E10.5, the initiation of Myf5 expression was delayed in the Pax3Cre; SmoCKO mutant forelimb ventral muscle cells (black arrowheads in C,D). (A,B) However, Pax3 expression was unaffected. (E,F) A similar observation (yellow arrowhead) was made by using antibodies against Myf5 (red) and Pax3 (green). (G–L) At E10.75, however, Myf5 expression had recovered (black and yellow arrowheads). (M–T) Like Myf5, the initiation of MyoD expression was delayed in the Pax3Cre; SmoCKO mutant forelimb ventral muscle cells at E10.75, as assessed by in situ hybridization (M,N, blue arrowheads) and immunostaining (O,P, white arrowheads). (Q–T) However, by E11, the expression of both MyoD transcripts and MyoD protein was restored. (U) A Cre-responsive GFP reporter (RC∷rePe) was used to generate GFP-positive muscle cells for FACS sorting. Myogenic cells from the ventral forelimb were isolated and analyzed by qPCR, which further demonstrated that the initiation of Myf5 and MyoD expression was delayed in the Pax3Cre; SmoCKO mutant ventral limb muscle but recovered to almost wild-type levels at a later stage. Expression levels were normalized to GAPDH expression. Histograms are expressed as means and standard error of the mean (SEM) (n = 3 for each genotype). (***) P < 0.001; (*) P < 0.05. Bar in T: A–T, 200 μm.
To further confirm this delay in Myf5 initiation, we next performed quantitative PCR (qPCR) analysis to quantify Pax3, Six1/4, and Myf5 expression in FACS-sorted muscle progenitor cells. GFP-positive myogenic cells were isolated from both Pax3Cre; SmoCKO; RC∷rePe mutant and wild-type ventral limbs across different stages. Measurement of RNA expression in these cells confirmed our observation of delayed Myf5 initiation (Fig. 3U; Supplemental Figs. 3E, S4Q).
This prompted us to further examine MyoD expression in order to address whether there was a general delay in the onset of the myogenic differentiation program. Similar to Myf5, the onset of MyoD expression in the ventral limb muscle was initiated 6 h later in the Pax3Cre; SmoCKO mutants than in their wild-type counterparts (Fig. 3M–U; Supplemental Fig. 4M–Q), indicating that Shh signaling is required cell-autonomously to promote the early initiation of the myogenic program in the ventral limb. However, as dorsal muscle cell differentiation was largely unaffected and the expression of both Myf5 and MyoD in the ventral muscle mass was ultimately restored, a Shh-independent mechanism must be in place to initiate muscle differentiation in these cells.
Shh signaling is required cell-autonomously for distal limb muscle formation
As noted above, in our analysis of muscle patterning, we observed a loss of muscle in the distal limb bud of Pax3Cre; SmoCKO mutant limbs (Fig. 4; Supplemental Fig. 5). Indeed, even after MyoD expression was restored in the ventral muscle mass at E11, it remained absent in the distal forelimb at E11.5 (Fig. 4A,B). In principle, this could have represented a continuation of the failure in myogenic differentiation in this domain; however, the absence of distal Pax3 in situ staining at the same developmental stage suggests that the muscle progenitor cells themselves were absent in the mutant autopod (Fig. 4E,F). To confirm this interpretation, we again took advantage of the RC∷rePe allele to perform recombinase-based fate mapping to definitively establish that no somite-derived cells were present in the distal limb. At E11.5, Pax3Cre; SmoCKO; RC∷rePe mutants showed a clear truncation in the distal end of the forelimb ventral muscle mass (Fig. 4G–J). As a consequence of this absence of myogenic cells in the distal limb bud, there is a complete loss of muscles in the forelimb autopod, as indicated by SMA and laminin immunostaining at E16.5 (Fig. 4K,L; Supplemental Fig. 6B′,E′). Similarly, although less affected, the hindlimb autopod exhibited reduced MyoD expression at E12.5, which corresponded to a partial muscle loss in the feet at E16.5 (Supplemental Fig. 5A–D).
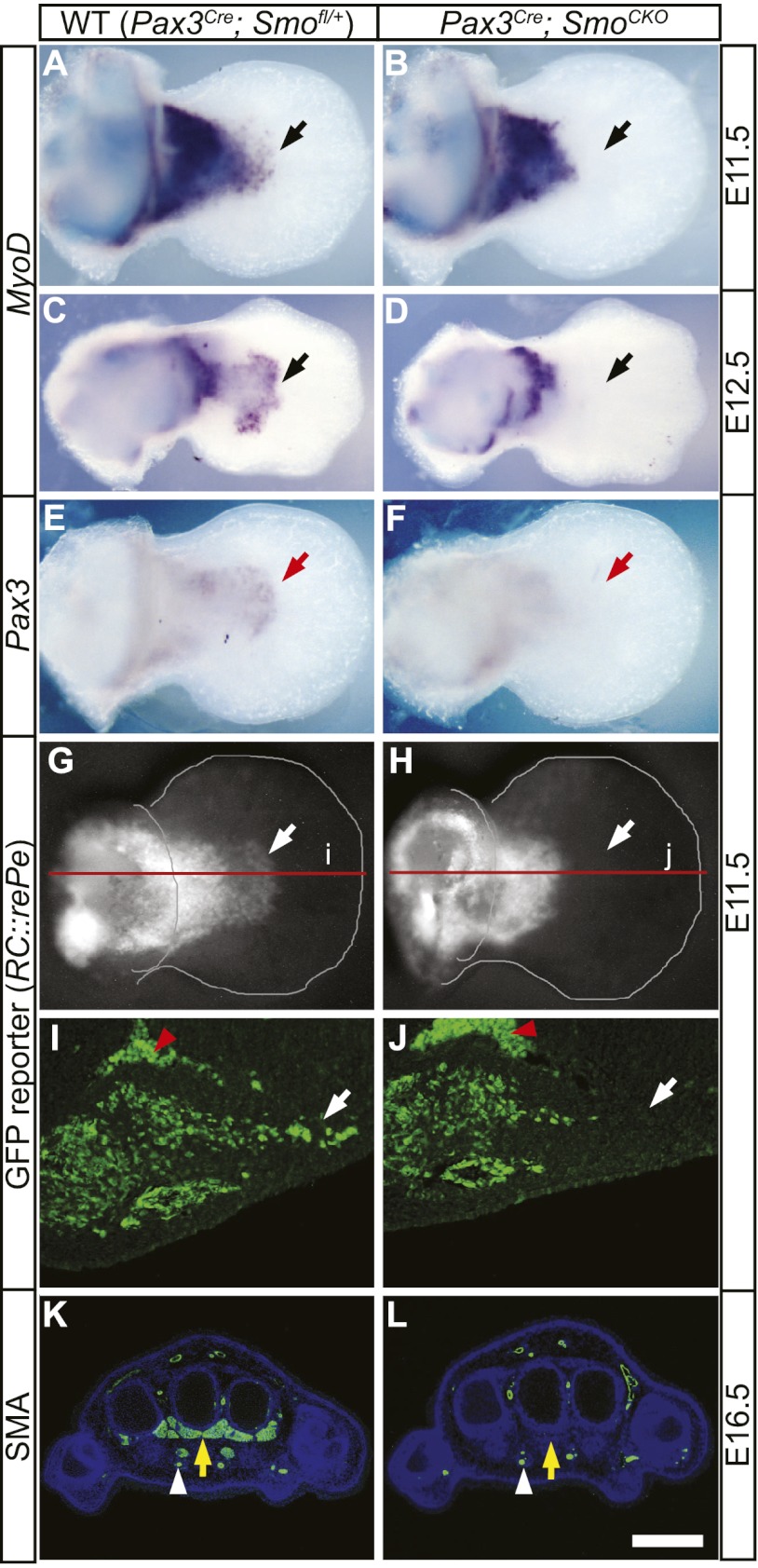
Figure 4.
Shh signaling is required cell-autonomously for distal limb muscle formation. (A,C) The presence of MyoD-expressing myogenic cells in the ventral autopod (black arrows) was evident in the wild-type (WT) forelimbs at E11.5 (A) and E12.5 (C). (B,D) However, these distal cells were lost in mutant limbs (black arrows), where Hh activity was removed in muscle progenitors. (E,F) Pax3 expression was similarly lost in the ventral autopod of Pax3Cre; SmoCKO mutants (F, red arrow) when compared with the wild-type forelimb (E) at E11.5. (G–J) The loss of distal limb muscles was confirmed by using a Cre-responsive GFP reporter (RC∷rePe) at E11.5, which marked all Pax3 descendants, including those in the wild-type distal limb (white arrows in G,I). (H,J) However, the autopod Pax3 descendants was lost in the Pax3Cre; SmoCKO; RC∷rePe mutant limb (white arrows). I and J are sections through the red lines labeled i and j in G and H, respectively. Red arrowheads mark autofluorescent blood cells. (K,L) Using an antibody against SMA, we noted that the early loss of distal myogenic cells led to a complete absence of limb muscles in the Pax3Cre; SmoCKO mutant autopod (L, yellow arrow) at E16.5, while they were formed normally in the wild-type distal limb (K, yellow arrow). Endothelial cells (white arrowheads), also positive for SMA, remain unaffected in the mutant autopod. Bar in L: A,B,E–H, 320 μm; C,D 500 μm; I,J, 80 μm; K,L, 400 μm.
The loss of distal muscles was also seen in the fate-mapping of descendants of Pax3-expressing cells. At E16.5, GFP-positive Pax3 muscle descendants were present in the stylopod and zeugopod but absent in the autopod of the mutant embryos (Supplemental Fig. 6). Interestingly, GFP-positive endothelial cells that are derived from the somites were unaffected and still present in the autopod blood vessels of Smo-deficient animals (Supplemental Fig. 6E). Together, these results indicate that Hh signaling is autonomously required for the formation of distal limb muscles, although dispensable for the more proximal muscle tissues and the vasculature.
Loss of distal limb muscles is not due to increased cell death in the dermomyotome
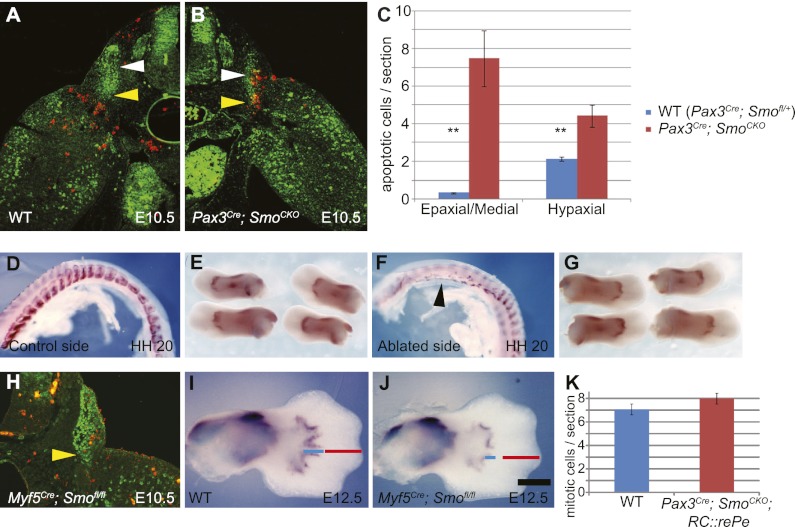
One potential mechanism that could result in the loss of distal limb muscle is increased cell death in muscle progenitor cells. TUNEL staining and Pax3/anti-cleaved caspase-3 coimmunostaining were performed in order to observe such changes. We found that at E10.25, there was increased apoptosis in the dorsal dermomyotome (data not shown), which could partially explain the dermomyotomal truncation that we observed earlier. At E10.5, cell death also increased at the ventral lateral lip of the dermomyotome, which gives rise to the limb muscles (Fig. 5A–C). However, no increased apoptosis was observed in muscle cells that have migrated into the limb bud, suggesting that Hh signaling is not required for the cell survival once muscle progenitor cells have entered the limb (Fig. 5B; Supplemental Fig. 7B). By E11.5, all abnormal apoptosis was resolved, and the amount of cell death in Pax3Cre; SmoCKO was comparable with the wild type (Supplemental Fig. 7A,B).
Figure 5.
Loss of distal limb muscles is not due to increased cell death in the dermomyotome caused by removal of cell-autonomous Shh activity. (A,B) Sections of E10.5 wild-type (WT) (A) and Pax3Cre; SmoCKO mutant (B) embryos were analyzed for apoptosis, marked by an antibody against cleaved caspase-3 (red), in muscle progenitor cells, labeled by Pax3 antibody (green). Apoptotic cells were counted in the epaxial/medial and hypaxial dermomyotome (white and yellow arrowheads, respectively). (C) An increase in apoptosis was observed in both domains of the dermomyotome in the Pax3Cre; SmoCKO mutants. (D–G) In the chick system, myogenic cells were marked by Myf5 in situ hybridization. Early removal of the lateral somite at HH20 (F, black arrowhead) did not affect the formation of distal limb muscles in the ventral wing (G), which were comparable with the control left side (D,E). (H) In mice, when Hh activity was removed by using Myf5Cre, no apparent apoptosis (marked by red anti-cleave caspase-3 antibody) was observed in the Pax3-labeled (green) hypaxial dermomyotome (yellow arrowhead). (I,J) However, MyoD expression revealed that the distal myocytes in the Myf5Cre; SmoCKO mutant limb (J) did not extend as distally as those in the wild-type limb (I) (cf. blue and red lines). (K) Finally, the numbers of proliferating myocytes were quantified by using an antibody against phospho-histone-H3 on limb sections from Pax3Cre; SmoCKO; RC∷rePe mutants and wild-type siblings at E11.5. No significant difference was detected. Histograms are expressed as means and standard error of the mean (SEM) (n = 3 for each genotype). (**) P < 0.01. Bar in J: A,B, 300 μm; D–G, 1 mm; H, 200 μm; I,J, 440 μm.
In order to address whether this initial loss of muscle progenitors in the ventral–lateral dermomyotome could be the cause for the autopod muscle loss, we turned to the chick system, where embryos can be easily manipulated by surgery. We ablated the lateral somites of HH16 or HH20 chick embryos and let them continue to develop in the incubator. The removal of the somites was confirmed by the loss of Myf5 in situ staining. However, upon further development, the lateral somites eventually grew back, mimicking the recovery of abnormal cell death in E11.5 mouse mutants. Interestingly, no autopod muscle loss was observed (Fig. 5D–G; data not shown). In another experiment, we inserted a metal barrier between the somites and the limb in HH20 chick embryos to delay the entry of muscle cells into the limb bud. Without removal of the barrier, ventral limb muscles failed to form (Supplemental Fig. 7H). However, if the barrier was removed either 24 or 48 h post-insertion, all limb muscles were formed properly (Supplemental Fig. 7I,J), confirming that an initial delay in the entry of the muscle cells into the limb bud does not affect the eventual formation of the distal muscles.
Finally, in an attempt to bypass the initial cell death in mice, we generated Myf5Cre; Smofl/fl; and MyoDCre; Smofl/fl embryos. Both Myf5Cre and MyoDCre are expressed later than Pax3Cre (Chen et al. 2005; Gensch et al. 2008). Removal of Smo using either of these Cre drivers results in a normal appearance of the hypaxial dermomyotome, with no evidence of ectopic or increased cell death in the ventral lateral lip (Fig. 5H). In both cases, where Hh signaling was abrogated later than in the Pax3Cre; SmoCKO mutants, muscles did form to some extent in the autopod but did not extend as distally as the wild-type siblings at E12.5 or E13.5 (Fig. 5I,J; Supplemental Fig. 7E,F), hence giving a milder phenotype that is nonetheless consistent with what we observed in the Pax3Cre; SmoCKO mutants. Together, these results suggest that it is unlikely that the cell death that we detected in the dermomyotome is the main cause of the distal limb muscle loss in the cell-autonomous mutants.
If increased apoptosis did not play an etiological role in the loss of distal limb muscles, we had to consider the converse explanation that the loss could be due to a decrease in proliferation. This was particularly important to check, as Shh has been shown to act as a mitogen and can affect muscle proliferation in other contexts (Duprez et al. 1998; Bren-Mattison and Olwin 2002). Therefore, we examined muscle cell proliferation in the limb by performing anti-phospho-histone H3 immunostaining at E11. However, no changes in cell proliferation were detected in the limb ventral muscle mass, and it is therefore not likely that a change in proliferation underlies distal muscle loss in the Pax3Cre; SmoCKO mutants (Fig. 5K).
Hh signaling is required for limb muscle cell migration
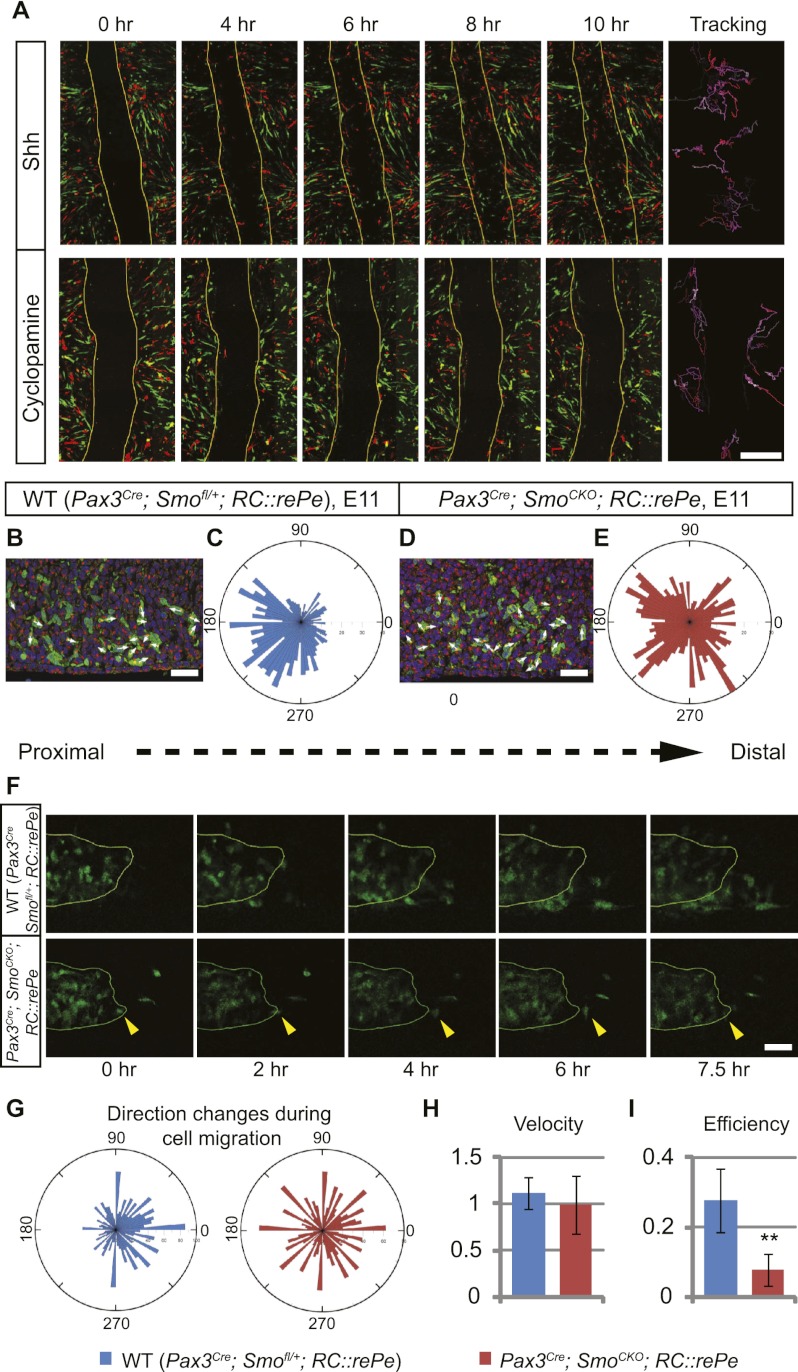
Another possible explanation for the loss of distal muscles in the absence of Hh signaling could be that Hh acts cell-autonomously to maintain proper cell migration, allowing muscle progenitor cells to continue their distal migration into the autopod. In order to test whether Hh signaling is required for muscle cell migration, we used in vitro “scratch assays.” This assay examines the ability of confluent cells to migrate back into a stripe on a plate, where cells have been removed by creating a scratch. We cultured FACS-sorted GFP- or DsRed-positive myogenic cells that had been harvested from chick embryos following electroporation of pCAG-GFP or -DsRed in the somites. The use of chick embryos allowed us to obtain a large amount of muscle cells, and the inclusion of both GFP and DsRed signals permitted us to easily track cells after imaging. When FACS-sorted primary muscle progenitors were cultured in the presence of Shh protein, they quickly responded to the scratch and migrated over the scratch to close the wound (n = 3 of 3) (Fig. 6A; Supplemental Movie 1). However, in the presence of cyclopamine, Hh signaling was inhibited and the cells failed to migrate to the scratched region (n = 3 of 3) (Fig. 6A; Supplemental Movie 2). Interestingly, cells that had been treated with cyclopamine did migrate but appeared to be moving randomly, rather than directionally toward the gap. Similar results were obtained using FACS-sorted mouse myogenic cells (Supplemental Movies 3, 4) This therefore suggests a model wherein exposure to Shh is necessary for myoblasts to respond to other directional cues, such as the signals that are generated by cells wounded at the scratch. This raised the possibility that Hh signaling in the limb bud might be similarly required for myogenic cells to respond to directional migratory cues in the distal limb.
Figure 6.
Cell-autonomous Hh activity is required for the migration of distal limb muscle cells. (A) Scratch assays were performed to test the requirement of Shh for cell migration. Confluent chick primary muscle cells were labeled with either GFP or DsRed and cultured in the presence of Shh or cyclopamine. Time-lapse images showed that in the presence of Shh, cells began to move into the stripe at the fourth hour and closed the gap by the eighth hour. When Shh signaling was blocked by cyclopamine, cells continued to move but failed to close the gap. Cell movement was tracked, with the beginning of the migration coded white and the subsequent movements coded with increasing red intensity. (B,D) Sections of E11 mouse ventral limbs (distal to the right) were analyzed for the position of the Golgi complex (marked by GM130 antibody, red) relative to the nucleus (DAPI, blue) in Pax3 descendants (GFP antibody, green). White arrows indicate the directions of cell movements. (C,E) The relative position of the Golgi to the nucleus was measured as the angle between the Golgi/nucleus alignment and the proximal–distal axis. The numbers of Golgi complexes at a particular angle were quantified and binned at a 5°-interval, as represented on the rosette graph. (C) In the wild type (WT), the majority of the Golgi complexes were positioned in the proximal half, indicating distal movements (n = 1157 cells from three individuals). (E) In contrast to this, Golgi complexes of muscle progenitor cells that had lost Hh activity were more randomly distributed (n = 1043 cells from three individuals). This difference is statistically significant, with a χ2 value of 136.312; P < 0.00001. (F) Time-lapse images of E11 limb tissue slices showed that GFP-positive Pax3 descendants in the wild-type embryos migrated distally, while Pax3Cre; SmoCKO; RC∷rePe mutant myogenic cells did not. In a few cases where cells did migrate distally, they often retracted in the end (yellow arrowheads). (G) Cells were tracked, and the angle of turning between each frame was quantified, showing that wild-type cells (n = 22) tended to turn toward the distal end of the limb, while mutant cells (n = 19) turn randomly. This difference is statistically significant, with a χ2 value of 166; P < 0.00001. (H,I) The velocity and efficiency (i.e., ratio between actual displacement and total distance traveled) of these cells were also quantified, showing that while Hh signaling does not affect muscle cell speed, it modulates its migration efficiency. Histograms are expressed as means and standard error of the mean (SEM). (**) P < 0.01. Bars: A, 500 μm; B,D, 30 μm; F, 40 μm.
To test the plausibility of this model, we examined migrating behavior of myoblasts in vivo. We first analyzed the relative position of the Golgi complex to the nucleus in the muscle progenitor cells from E11 mouse ventral forelimbs, which had a more pronounced distal muscle loss phenotype than the hindlimbs, thereby permitting robust identification of subtle differences. The location of the Golgi apparatus has been found to be at the trailing end of a directionally migrating cell in a three-dimensional (3D) environment and can therefore be used as an indicator of the direction of cell migration (Serrador et al. 1999; Pouthas et al. 2008; Petrie et al. 2009). In GFP-labeled myoblasts within the wild-type limb buds, the Golgi complex was usually found proximal to the nucleus, indicating that muscle progenitors tended to move toward the distal end of the limb bud (Fig. 6B,C). However, the position of the Golgi complex relative to the nucleus was more randomly distributed in the Pax3Cre; SmoCKO; RC∷rePe mutants, pointing to more randomized cell movements in these embryos (Fig. 6D,E). Thus, in the absence of Shh activity, the migration of limb muscle progenitors becomes less oriented.
Finally, to directly visualize cell migration in vivo, we performed time-lapse imaging on forelimb tissue slices that were prepared from E11 Pax3Cre; SmoCKO; RC∷rePe mutants and wild-type siblings. In the wild-type limb bud, the GFP-positive muscle cells in the ventral muscle mass were observed to migrate distally over a period of 7.5 h (n = 7 of 9) (Fig. 6F; Supplemental Movie 5). However, such distal movement was rarely detected in Pax3Cre; SmoCKO; RC∷rePe mutants (n = 10 of 10) (Fig. 6F; Supplemental Movie 6). When cells did initially appear to move distally, they often subsequently wandered back to their original position and made little overall progress toward the distal limb bud (Fig. 6F, yellow arrowheads; Supplemental Movie 4). By tracking individual cells, we were also able to acquire their X and Y coordinates, which were used to calculate the angle of cell turning between each frame. Such analysis revealed that wild-type cells tended to turn distal-ward, in contrast to mutant cells, which moved more randomly (Fig. 6G). The velocity and efficiency (directness of the path traversed) of these cells were also calculated, showing that even though both wild-type and mutant cells moved at a comparable speed, wild-type cells were consistently more efficient in reaching their final destination (Fig. 6H,I). Therefore, in agreement with our previous results, Shh activity is required for distally oriented muscle migration.
Net1 acts downstream from Hh signaling to promote continued cell migration
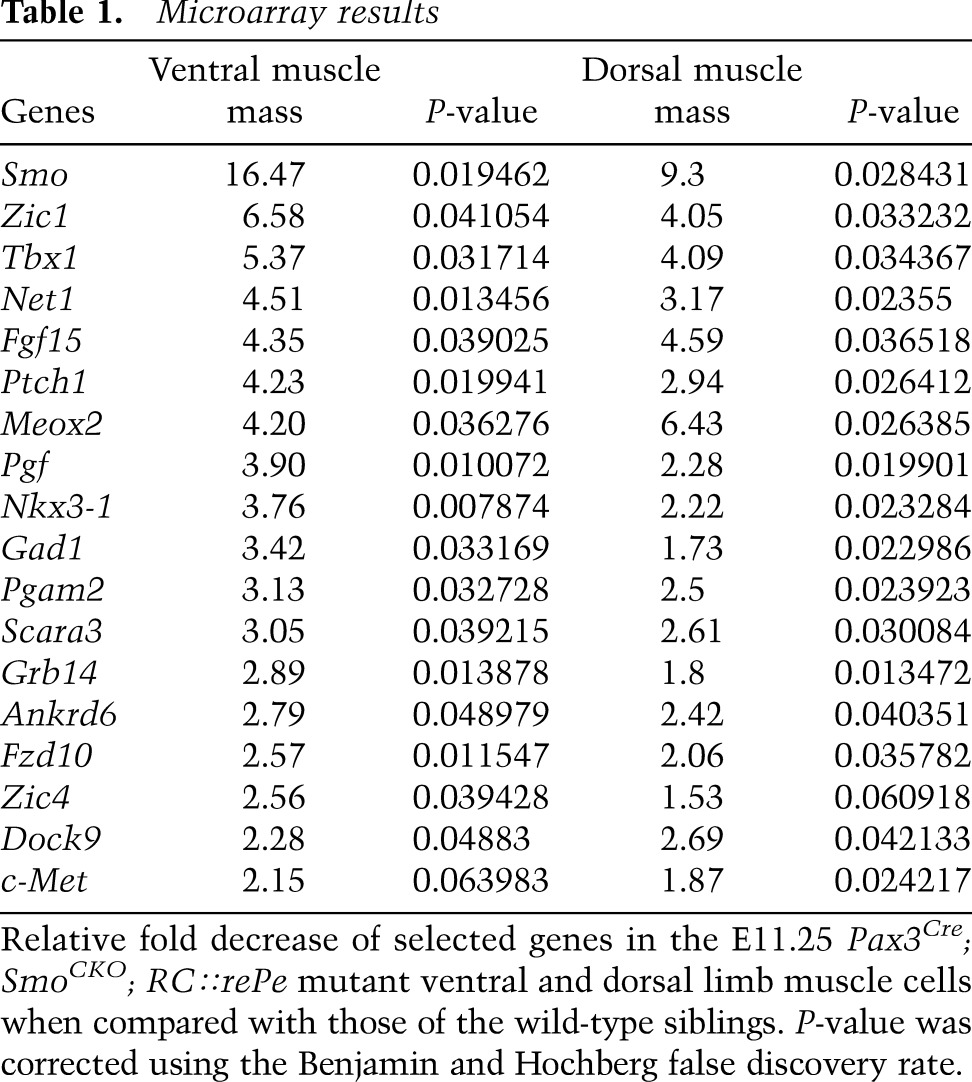
In order to identify potential targets of Hh signaling in the muscle progenitor cells, we FACS-sorted GFP-positive dorsal and ventral muscle cells from E11.25 Pax3Cre; SmoCKO; RC∷rePe mutant embryos and wild-type Pax3Cre; Smofl/+; RC∷rePe siblings and extracted RNA for microarray analysis. As expected, both Smo itself and the Hh signaling-responsive gene Ptch1 were down-regulated in the mutants (Table 1). However, we did not detect any change in the expression level of Pax3 or any of the myogenic regulatory factors (Myf5, MyoD, and Myogenin) at this stage, consistent with our earlier observation that these genes were restored to the wild-type level post-E11.
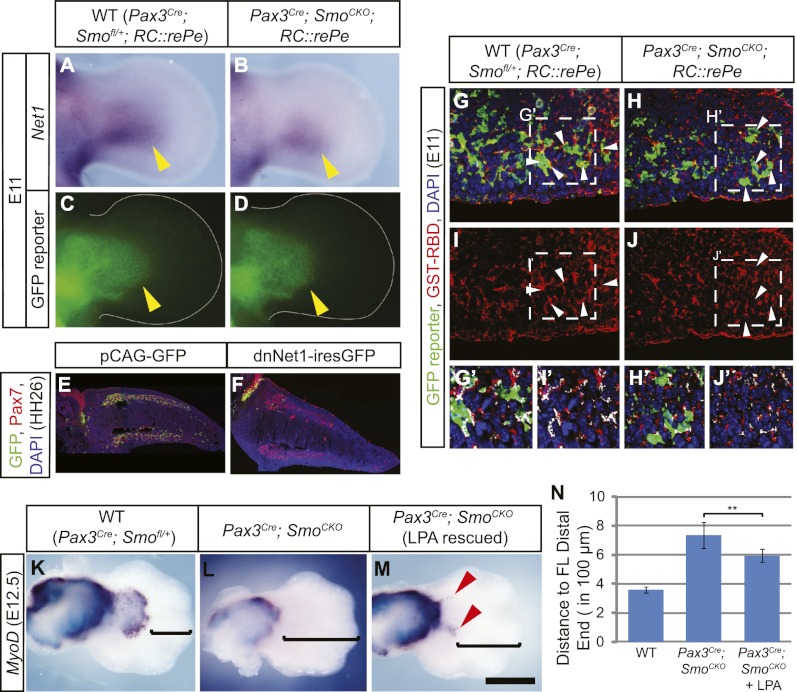
Interestingly, one of the genes that was significantly down-regulated in Smo-deficient myoblasts relative to the wild type was Net1. Net1 encodes a guanine nucleotide exchange factor (GEF) that specifically activates the small GTPase RhoA (Alberts and Treisman 1998). Because RhoA plays an important role during cell migration, it raised the possibility that Hh signaling regulates myogenic cell migration in the limb bud through Net1. Net1 showed a 4.5-fold decrease in the relative expression level in the Pax3Cre; SmoCKO; RC∷rePe mutant muscles. This down-regulation of Net1 in muscle cells was validated by whole-mount in situ hybridization in E11 embryo limbs (Fig. 7A–D; Supplemental Fig. 8A–J). Interestingly, its expression appeared to be normal at E10.5, indicating that Hh signaling is not required for Net1 initiation, in spite of being needed for its maintenance from E10.5 onward.
Figure 7.
Net1 acts downstream from Hh signaling to promote cell migration. (A,B) Net1 in situ hybridization showed that Net1 expression was down-regulated in the Pax3Cre; SmoCKO; RC∷rePe mutant forelimb (B, yellow arrowhead) at E11 when compared with the wild-type (WT) limb bud (A). (C,D) This down-regulation was not due to a loss of muscle progenitors in the limb, marked by the Pax3Cre-responsive GFP reporter. (E,F) In the chick system, when the lateral somites at the hindlimb level were electroporated with pCAG-GFP at HH18, GFP-positive cells (green) were found throughout the Pax7-positive dorsal and ventral muscle masses (red) at HH26 (shown in E). (F) However, when two dominant-negative forms of Net1 were coelectroporated into the lateral somites, cells failed to migrate into the limb, demonstrating that functional Net1 activity is required for muscle cell migration. (G–J) Active RhoA, as detected by GST-RBD, was reduced in the Pax3Cre; SmoCKO; RC∷rePe mutant forelimb ventral muscle cells, marked by the Cre-responsive GFP reporter, at E11 (white arrowheads). (G′–J′) Blow-ups of the squares in G–J show that there are less overlapping green and red signals (white patches) in the mutant myogenic cells. (K–M) The loss of distal forelimb muscles in the Pax3Cre; SmoCKO mutants was partially rescued (red arrowheads) by serial injection of the RhoA activator LPA. (N) This rescue can be quantified as the distance from the distal-most MyoD staining to the tip of the hand plate (as indicated by the brackets in K–M). Histograms are expressed as means and standard error of the mean (SEM) (n = 6 for each genotype). (**) P < 0.01. Bar in M: A–D, 315 μm; E,F, 350 μm; G–J, 60 μm; G′–J′, 45 μm; K–M, 500 μm.
In order to test the function of Net1 during muscle migration, we again took advantage of the chick system, where Net1 is similarly expressed in the limb muscles (data not shown). We electroporated HH18 chick embryo somites at the hindlimb level (somites 26–33) with GFP (pCAG-GFP) or two previously described dominant-negative forms of Net1 (Cag-dnNet1-iresGFP) carrying a deletion in either the Dbl homology (DH) or pleckstrin homology (PH) domain (Guerra et al. 2008). When somitic cells were electroporated with just pCAG-GFP, they continued to migrate and populated the entire muscle mass (n = 7 of 7) (Fig. 7E). However, coelectroporation of both dominant-negative Net1 constructs severely inhibited muscle migration and even prevented muscle progenitors from entering the limb bud in many cases (n = 18 of 25 affected, nine embryos had no GFP in the limb) (Fig. 7F), demonstrating that Net1 is indeed required for muscle cell migration.
These results are consistent with the view that, in the normal limb bud, Hh signaling is required to maintain Net1 expression in the migrating myoblasts. In the absence of Hh signaling, Net1 is down-regulated, which could be predicted to render reduced RhoA activity. Indeed, by using GST-RBD to label active RhoA and an antibody to detect GST, it became apparent that in the mutant ventral limb, there was diminished RhoA activity in the distal muscle cells (Fig. 7G–J′), which likely led to inefficient cell migration at E11 and the loss of distal muscles at E11.5. This model would therefore suggest that it might be possible to rescue the Smo-deficient phenotype by restoring RhoA activity. We injected lysophosphotidic acid (LPA), a RhoA activator, into pregnant female mice starting at E9.5 and every 0.5 d afterward until E11.5. This treatment was able to partially rescue the loss of distal muscle in Pax3Cre; SmoCKO mutants at E12.5 (n = 5 of 6) (Fig. 7K–N; Supplemental Fig. 9). While the rescue was incomplete, we also noted that Dock9, a GEF for another small GTPase, CDC42, was additionally down-regulated in the Pax3Cre; SmoCKO mutants (Table 1; Supplemental Fig. 8K–N), hinting at a common theme of migratory regulation by the Hh signaling.
Discussion
By conditionally removing Smo activity, and hence the ability to respond to Hh signaling, from various cell populations in the limb, we demonstrated that the function of Hh during limb muscle development is multifaceted and that Hh signaling acts both cell-autonomously and non-cell-autonomously in directing limb muscle formation. A parallel study by Anderson et al. (2012) also reached similar conclusions.
We observed that when Hh signaling is disrupted in the lateral plate-derived limb mesenchyme, normal muscle AP patterning is lost and the resultant muscle bundles are more symmetrical. The nonmuscle mesenchyme has long been considered as the main source for muscle patterning, and it has been established that the muscle connective tissue sets up a prepattern for correct muscle patterning to take place (Christ et al. 1977; Jacob and Christ 1980; Kardon et al. 2003; Hasson et al. 2010). Our results further confirm this notion and show that Shh does not pattern limb muscles directly but through the lateral plate-derived cells.
Hh signaling also promotes slow muscle fiber myogenesis in a cell-autonomous fashion. This finding is consistent with a previous study demonstrating that muscle cells are responsive to Hh signaling after they have migrated into the limb between E10.5 and E11.5 (Ahn and Joyner 2004). Whether these cells are responding to Shh or Ihh remains unclear. While Ihh from the developing long bones could be responsible for this, we cannot rule out the possibility that at an earlier stage, Shh sets up cellular memory of exposure in a population of muscle cells that are destined to form the slow fibers. When fiber type becomes specified in myogenic cells has been controversial. For example, at least in birds, it has been shown that the first muscle progenitor cells to enter the limb contribute to the proximal slow muscles and that the fast muscles are formed by later-migrating populations (Van Swearingen and Lance-Jones 1995). However, other studies have suggested that cell fate is not predetermined in the somites (Kardon et al. 2002; Rees et al. 2003).
Regardless of the timing of slow muscle determination, the cell-autonomous requirement for Hh signaling that we observed in our study is similar to that seen in the formation of zebrafish adaxial slow muscle fibers (Baxendale et al. 2004; Feng et al. 2006). However, contrary to the near complete loss of slow fibers in zebrafish smo mutants (Barresi et al. 2000), there is only ∼20% reduction in the Pax3Cre; SmoCKO mutants, suggesting that additional mechanisms are in place for specifying the slow fiber myogenesis in the mammalian limbs. Additional regulation of this process is likely provided by the muscle connective tissue, which is known to affect slow fiber myogenesis via the WNT/β-catenin pathway (Anakwe et al. 2003; Mathew et al. 2011).
We did not see any significant changes in proliferation of the limb myogenic cells in the absence of cell-autonomous Hh signaling. We also did not see any increase in myogenic cell death within the limb bud. Similar results were obtained in the parallel study by Anderson et al. (2012). However, we did detect cell death in both the epaxial and hypaxial dermomyotome in the Pax3Cre; SmoCKO mutants. Our finding that dermomyotomal cell survival depends on cell-autonomous Hh signaling is intriguing as, while Shh has been shown to be critical for cell survival in the ventral somite, it is considered to be dispensable for the developing dermomyotome (Borycki et al. 1999). This discrepancy could be due to the stage and the somite level of the embryo at the time of apoptosis detection, as apoptosis occurs transiently at E10.5 and is more apparent in the more mature somites at the forelimb level than in those at the hindlimb level. It is also possible that Ihh from the endoderm maintains the survival of dermomyotomal cells in the previously analyzed Shh mutants, while the removal of Smo alleles in our study blocks all Hh activity. Indeed, at least at E8.5, the Hh response gene Ptch1 is still expressed in the somites of Shh-null embryos. Moreover, in general, Smo mutants phenocopy Shh−/−; Ihh−/− double mutants, which have more severe phenotypes than the Shh−/− single mutant (Zhang et al. 2001).
The Hh signaling pathway in the limb has been previously shown to drive myogenic proliferation (Duprez et al. 1998), maintain cell survival (Kruger et al. 2001), and regulate the size of the muscle progenitor pool in the limb (and hence muscle mass within the limb) by controlling myoblast proliferation and differentiation during primary myogenesis and maintaining cell survival over secondary myogenesis (Bren-Mattison and Olwin 2002; Bren-Mattison et al. 2011). However, it was unclear whether these processes were direct effects of cell-autonomous Shh function. By removing Smo specifically from muscle progenitor cells, both this work and that of the accompanying study by Anderson et al. (2012) demonstrated a cell-autonomous requirement for Shh to initiate the myogenic program in the early ventral muscle mass. In this context, Shh appears to function similar to its role in the somites to directly regulate the expression of Myf5 (Borycki et al. 1999; McDermott et al. 2005). However, as the expression of Myf5 and MyoD in the ventral muscle cells recovered and the dorsal muscle mass was largely unaffected, additional mechanisms must be in place to compensate for the loss of Shh signaling in order to initiate the myogenic differentiation program. Moreover, as noted above, neither cell proliferation nor survival was affected in the absence of cell-autonomous Shh activity; these effects of loss of Shh observed in previous studies are therefore most likely due to non-cell-autonomous Shh function mediated by other tissue types.
Both our work and that of Anderson et al. (2012) further identified a specific loss of distal muscles when Shh responsiveness was conditionally removed from the myogenic cells. This could be the result of the loss of early Myf5 and MyoD expression. In this scenario, proper Shh-induced myogenic differentiation may be required at the beginning of limb myogenesis to specify a subpopulation that will eventually become the distal limb muscle. However, our study strongly suggests that there is also a cell-autonomous requirement for Hh signaling to maintain proper muscle cell migration within the limb. These explanations are certainly not mutually exclusive, and it is additionally plausible that a prompt onset of myogenic differentiation is required to maintain Net1 expression for Hh-dependent muscle migration.
Shh has previously been reported to regulate cell movement in various contexts, such as medial migration of the endothelial progenitors in zebrafish, the navigation of axons in the neural tube, the distribution of oligodendrocytes along the developing optic nerves, and the migration of adult neural precursor cells to the olfactory bulb (Charron et al. 2003; Gering and Patient 2005; Merchán et al. 2007; Angot et al. 2008; Yam et al. 2009). In these cases, however, Shh acts as a chemoattractant, which is unlikely to be the governing mechanism by which Hh signaling regulates limb muscle migration because Shh is expressed in the posterior limb bud and muscle cells migrate distally.
In cell culture studies, Shh has been shown to be required for cell motility by activating Rho GTPases through a “noncanonical” or Gli-independent pathway (Renault et al. 2010; Sasaki et al. 2010; Polizio et al. 2011). In these scenarios, signaling activation is relayed from Smo to the G proteins or Tiam1, a GEF for Rac1 (Sasaki et al. 2010; Polizio et al. 2011). While we cannot rule out these possibilities, our results strongly indicate that in the limb myogenic cells, Hh signaling is required cell-autonomously to maintain the expression of Net1, which is in turn required for adequate RhoA activity and cell migration.
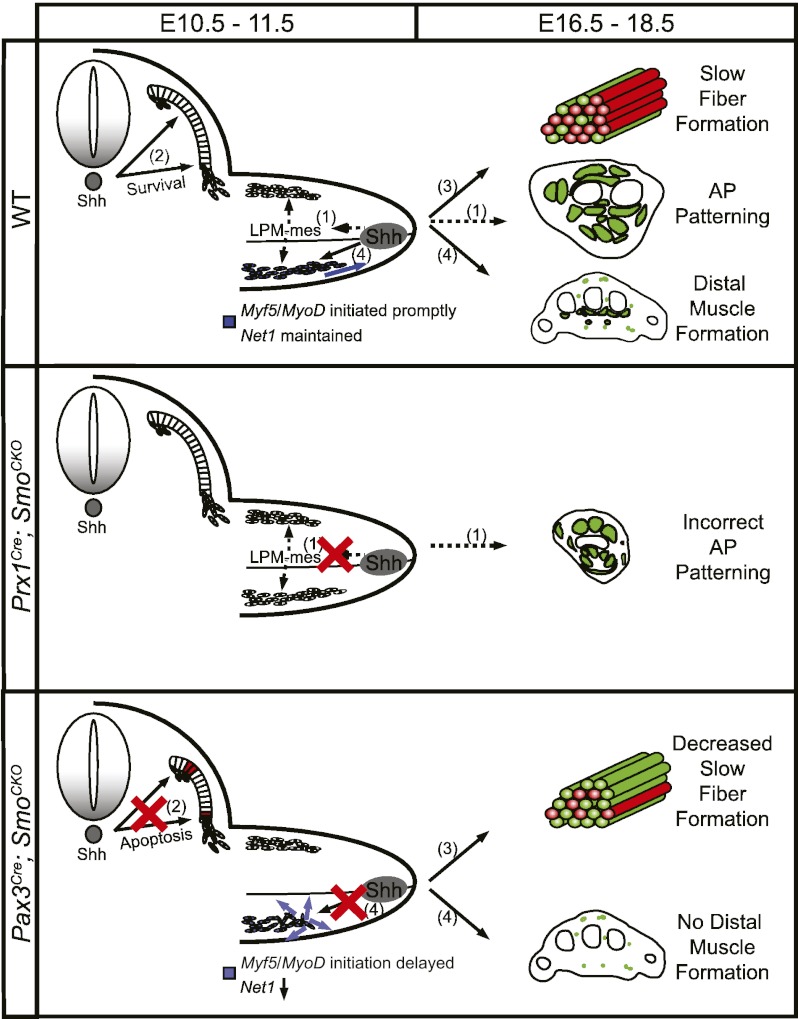
From our study, a model for the regulation of limb muscle development by the Hh signaling pathway emerges (Fig. 8). As limb muscle progenitor cells are formed at the ventral lateral lip of the dermomyotome, Shh from the midline is transiently required cell-autonomously at E10.5 to promote their survival. Subsequently, Shh from the ZPA patterns the lateral plate-derived limb mesenchyme along the AP axis, which in turn patterns the muscle progenitor cells that have migrated into the limb bud. At the same time, Shh promotes the early phase of myogenic differentiation and maintains Net1 expression cell-autonomously in the migrating muscle cells. Net1 is the GEF for RhoA, and its up-regulation by Shh signaling is permissively required for continued muscle cell migration toward the distal limb bud between E10.5 and E11.5. Consequently, in the absence of Shh signaling in the muscle progenitor cells, Net1 is down-regulated, and directionally persistent migration is lost in these cells, resulting in the truncation of distal muscles. At a later stage, myocytes undergo terminal differentiation, and again Hh signaling is required cell-autonomously to promote slow muscle fiber formation, which is key to establishing optimally functional adult muscles.
Figure 8.
Schematic representation of the function of Shh during limb muscle development. In normal conditions, Shh patterns limb muscles non-cell-autonomously along the AP axis through the lateral plate-derived limb mesenchyme (1) such that when Hh activity is removed in the nonmuscle limb mesenchyme by Prx1-Cre recombinase AP patterning of the limb muscles is affected. Meanwhile, cell-autonomous function of Hh signaling is required to maintain cell survival in the dermomyotome (2), promote the formation of slow muscle fibers (3), initiate Myf5 and MyoD expression in a timely manner, and regulate directional migration of the distal myocytes by maintaining Net1 expression (4). Thus, in the absence of Hh activity in the muscle progenitor cells, there is increased apoptosis in the dermomyotome, decreased slow muscle fibers, and loss of distal muscles.
Last, the vertebrate limb has been thought to have evolved in two phases: The proximal structures are Shh-independent and more basal, and the distal structures are Shh-dependent and evolutionarily novel (Shubin and Alberch 1986; Ahlberg and Milner 1994; Sordino et al. 1995; Kardon 1998; Francis-West et al. 2003). In addition, while Shh expression persists until the beginning of digit formation in the tetrapods, it disappears at an earlier stage, prior to ray formation in the teleosts, which lack muscles in the fin ray region (López-Martínez et al. 1995; Sordino et al. 1995; Thorsen and Hale 2005). Therefore, despite the ongoing debate on the evolutionary origin of the autopod in the tetrapods (Holmgren 1933; Gregory and Raven 1941; Wagner and Chiu 2001; Shubin et al. 2006; Boisvert et al. 2008), it is tempting to propose that early tetrapods have co-opted an existing pathway (Shh signaling from ZPA) to extend the distribution of the limb muscles to the more distally located and structurally complicated autopod, a potentially neomorphic structure.
Materials and methods
Mouse genetics and manipulations
Smofl/fl, Smodel/+, Pax3Cre, Prx1Cre, Myf5Cre, MyoDCre, and Tcf4GFPCre+neo mice and RC∷rePe (Cre-responsive GFP reporter) were previously reported and genotyped as described (Long et al. 2001; Logan et al. 2002; Chen et al. 2005; Engleka et al. 2005; Gensch et al. 2008; Purcell et al. 2009; Mathew et al. 2011; Ray et al. 2011). Generation of mutant embryos and wild-type siblings are described in the text. Noon of the day of vaginal plug discovery was designated as E0.5. For the microarray analysis of gene expression level, dissociated ventral and dorsal E11.25 limb mesenchyme was FACS-sorted (Hematologic Neoplasia Flow Cytometry Facility at Dana-Farber Cancer Institute), and RNA was extracted using the RNeasy kit (Qiagen). Fifty-eight Pax3Cre; SmoCKO; RC∷rePe mutant forelimbs and 55 wild-type sibling forelimbs were pooled into three different samples for triplicate. Microarray was performed on the Illumina Mouse WG-6 Expression BeadChip by the Molecular Genetics Core Facility at Children's Hospital Boston (supported by NIH-P50-NS40828 and NIH-P30-HD18655). For qPCR analysis, RNA from FACS-sorted muscle cells at different stages (as indicated in the text) was reverse-transcribed to cDNA using SuperScript VILO cDNA Synthesis kit (Invitrogen). qPCR was performed using the Roche LightCycler with the following conditions: 2 min at 95°C and 40 cycles of amplification (5 sec at 95°C, 10 sec at 56°C, and 20 sec at 72°C). The primer sets used are listed in Supplemental Table 1. All measurements were normalized to GAPDH and β-actin. A water sample was used as the negative control and set as 0% for expression level. An in vitro differentiation assay was performed as previously described (Mathew et al. 2011). Rescue of the distal muscle loss in the SmoCKO; Pax3Cre mutant limbs was done by intraperitoneal injection of LPA (20 μg/g body weight) at E9.5. Serial injections were subsequently made every 12 h until E11.5.
Chick embryos, surgeries, and electroporation
Fertilized chick eggs from commercial sources (Charles River) were incubated at 38°C and staged according to Hamburger and Hamilton (1951). Lateral somite ablation was done by cutting the lateral 200 μm of somite 16–21 using a sharpened tungsten needle. The barrier experiment was performed by inserting a tantalum foil barrier between the developing limb bud and the somites at HH20. The barriers were either left in situ or removed 24 or 48 h after insertion. Embryos were either immediately processed for whole-mount in situ hybridization to check Myf5 expression in the somites or put back into the incubator to allow further development until HH26 or HH34 to assess limb muscle formation. Somite electroporation experiments were carried out as previously described (Scaal et al. 2004). pCAG-GFP was used as a positive control (Matsuda and Cepko 2004). Dominant-negative Net1 alleles carrying a deletion in the DH or PH domain were gifts from Alan Hall (Memorial Sloan-Kettering Cancer Center) and were subcloned into pCAGIG (Matsuda and Cepko 2004).
In situ hybridization and immunohistochemistry
For whole-mount in situ hybridization, samples were fixed in 4% PFA overnight at 4°C, and the hybridization was carried out as previously described (Riddle et al. 1993). DIG-labeled probes were detected with NBT/BCIP (Sigma) or BM Purple (Roche). Probes include mouse MyoD (Brent et al. 2003), mouse Pax3 (Goulding et al. 1991), chick Myf5 (Brent and Tabin 2004), mouse Net1 (RT–PCR product using primers 5′-TGGGAGCATCAAGGGTTACT-3′ and 5′-AATGAATGCAGAAGGCGAAC-3′), and mouse Dock9 (RT–PCR product using primers 5′-GGGACATGCTTTGTCATGTG-3′ and 5′-TCAGTGCTGCTTTGTCTGCT-3′). For antibody staining, all samples were fixed in 4% PFA for 4–6 h at 4°C (unless stated otherwise in Supplemental Table 2) and prepared for either frozen or paraffin sectioning. The primary antibodies used are tabulated in Supplemental Table 2. Secondary antibodies (Molecular Probes) used include Alexa Fluor 594 goat anti-rabbit IgG, Alexa Fluor 488 goat anti-rabbit IgG, Alexa Fluor 594 goat anti-mouse IgG, and Alexa Fluor 488 goat anti-chick IgG (all at 1:250 dilution for 1 h at room temperature). For active RhoA detection, sectioned samples were blocked in 0.05% BSA in HBS before GST-RBD (Cytoskeleton) application. GST-RBD was diluted in HBS at a concentration of 200 μg/mL and incubated with samples for 90 min at room temperature. Samples were fixed for 15 sec with cold acetone–formalin before antibody detection.
Scratch assay
Chick somites at the hindlimb level were electroporated with pCAG-GFP or pCAG-DsRed (Matsuda and Cepko 2004) at HH18 and allowed to develop at 38°C until HH25/26. In experiments where Hh signaling was to be abrogated, 1 μg of cyclopamine (Calbiochem) was delivered to the electroporated chick embryos by pipetting. Cyclopamine was prepared as previously described (Incardona et al. 1998). HH25/26 limb mesenchyme was subsequently incubated in 0.5% trypsin for 10 min at room temperature and dissociated by pipetting in DMEM/10% FBS. Dissociated cells were FACS-sorted for GFP- or DsRed-positive myogenic cells, which were then plated in a 96-well plate with a glass bottom (MatTek) coated with ECL (entactin-collagen IV-laminin; Millipore). The plated cells were incubated in DMEM/20% FBS/2.5 ng/mL bFGF plus either 20 nM Shh protein (Curis) or 50 μM cyclopamine for 24 h at 37°C with 5% CO2 to reach confluence, and a scratch wound was generated by a fine pipette tip. Cell migration was recorded by performing time-lapse microscopy.
Time-lapse microscopy and image analysis
Time-lapse imaging was performed as previously described (Gros et al. 2010). Myogenic cells at the scratch wound were imaged every 8 min for 12 h at 37°C and 5% CO2 at a wavelength of 910 nm to capture the migration of both GFP- and DsRed-positive myogenic cells. For live imaging of limb explants, E10.75–E11 mouse embryos were dissected in PBS and sectioned manually at a thickness of ∼200 μm using obsidian scalpels (Fine Science Tools). The tissue slices were cultured in 40% DMEM/60% rat serum (Harlan Laboratories)/1% low-melting agarose/0.5% glucose/2.5 mM HEPES (Gibco)/1% penicillin–streptomycin, supplemented with 20 nM of Shh protein (Curis). Images of ventral limb muscle mass were taken at 850 nm every 5 min for 12 h. Time-lapse movies were generated using NIH ImageJ, and cells were tracked using the manual tracking plug-in contributed by Fabrice Cordeli. Colocalization of active RhoA signals and muscle cells was done by using the ImageJ colocalization plug-in contributed by Pierre Bourdoncle.
Acknowledgments
We thank Jérôme Gros for technical advice with confocal live imaging and electroporation. We also thank Susan Dymecki, Jonathon Epstein, Gabrielle Kardon, and Andrew McMahon for mouse strains. B.D.H. was funded by the March of Dimes. This work was supported by NIH/NICHD grant R37HD032443 to C.J.T.
Footnotes
Supplemental material is available for this article.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.187385.112.
References
- Agbulut O, Noirez P, Beaumont F, Butler-Browne G 2003. Myosin heavy chain isoforms in postnatal muscle development of mice. Biol Cell 95: 399–406 [DOI] [PubMed] [Google Scholar]
- Ahlberg PE, Milner AR 1994. The origin and early diversification of tetrapods. Nature 368: 507–514 [Google Scholar]
- Ahn S, Joyner AL 2004. Dynamic changes in the response of cells to positive hedgehog signaling during mouse limb patterning. Cell 118: 505–516 [DOI] [PubMed] [Google Scholar]
- Alberts AS, Treisman R 1998. Activation of RhoA and SAPK/JNK signalling pathways by the RhoA-specific exchange factor mNET1. EMBO J 17: 4075–4085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcedo J, Ayzenzon M, Von Ohlen T, Noll M, Hooper JE 1996. The Drosophila smoothened gene encodes a seven-pass membrane protein, a putative receptor for the hedgehog signal. Cell 86: 221–232 [DOI] [PubMed] [Google Scholar]
- Anakwe K, Robson L, Hadley J, Buxton P, Church V, Allen S, Hartmann C, Harfe B, Nohno T, Brown AMC, et al. 2003. Wnt signalling regulates myogenic differentiation in the developing avian wing. Development 130: 3503–3514 [DOI] [PubMed] [Google Scholar]
- Anderson C, Williams VC, Moyon B, Daubas P, Tajbakhsh S, Buckingham ME, Shiroishi T, Hughes SM, Borycki A-G Sonic hedgehog acts cell-autonomously on muscle precursor cells to generate limb muscle diversity. (this issue). doi: 10.1101/gad.187807.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angot E, Loulier K, Nguyen-Ba-Charvet KT, Gadeau A-P, Ruat M, Traiffort E 2008. Chemoattractive activity of sonic hedgehog in the adult subventricular zone modulates the number of neural precursors reaching the olfactory bulb. Stem Cells 26: 2311–2320 [DOI] [PubMed] [Google Scholar]
- Barresi MJ, Stickney HL, Devoto SH 2000. The zebrafish slow-muscle-omitted gene product is required for Hedgehog signal transduction and the development of slow muscle identity. Development 127: 2189–2199 [DOI] [PubMed] [Google Scholar]
- Baxendale S, Davison C, Muxworthy C, Wolff C, Ingham Philip W, Roy S 2004. The B-cell maturation factor Blimp-1 specifies vertebrate slow-twitch muscle fiber identity in response to Hedgehog signaling. Nat Genet 36: 88–93 [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Brohmann H 2000. Genes that control the development of migrating muscle precursor cells. Curr Opin Cell Biol 12: 725–730 [DOI] [PubMed] [Google Scholar]
- Boisvert CA, Mark-Kurik E, Ahlberg PE 2008. The pectoral fin of Panderichthys and the origin of digits. Nature 456: 636–638 [DOI] [PubMed] [Google Scholar]
- Borycki AG, Brunk B, Tajbakhsh S, Buckingham M, Chiang C, Emerson CP Jr 1999. Sonic hedgehog controls epaxial muscle determination through Myf5 activation. Development 126: 4053–4063 [DOI] [PubMed] [Google Scholar]
- Bouldin CM, Gritli-Linde A, Ahn S, Harfe BD 2010. Shh pathway activation is present and required within the vertebrate limb bud apical ectodermal ridge for normal autopod patterning. Proc Natl Acad Sci 107: 5489–5494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bren-Mattison Y, Olwin BB 2002. Sonic hedgehog inhibits the terminal differentiation of limb myoblasts committed to the slow muscle lineage. Dev Biol 242: 130–148 [DOI] [PubMed] [Google Scholar]
- Bren-Mattison Y, Hausburg M, Olwin BB 2011. Growth of limb muscle is dependent on skeletal-derived Indian hedgehog. Dev Biol 356: 486–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent AE, Tabin CJ 2004. FGF acts directly on the somitic tendon progenitors through the Ets transcription factors Pea3 and Erm to regulate scleraxis expression. Development 131: 3885–3896 [DOI] [PubMed] [Google Scholar]
- Brent AE, Schweitzer R, Tabin CJ 2003. A somitic compartment of tendon progenitors. Cell 113: 235–248 [DOI] [PubMed] [Google Scholar]
- Bryson-Richardson RJ, Currie PD 2008. The genetics of vertebrate myogenesis. Nat Rev Genet 9: 632–646 [DOI] [PubMed] [Google Scholar]
- Cann GM, Lee JW, Stockdale FE 1999. Sonic hedgehog enhances somite cell viability and formation of primary slow muscle fibers in avian segmented mesoderm. Anat Embryol 200: 239–252 [DOI] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M 2003. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell 113: 11–23 [DOI] [PubMed] [Google Scholar]
- Chen JCJ, Mortimer J, Marley J, Goldhamer DJ 2005. MyoD-cre transgenic mice: A model for conditional mutagenesis and lineage tracing of skeletal muscle. Genesis 41: 116–121 [DOI] [PubMed] [Google Scholar]
- Chevallier A, Kieny M, Mauger A 1977. Limb–somite relationship: Origin of the limb musculature. J Embryol Exp Morphol 41: 245–258 [PubMed] [Google Scholar]
- Chiang C, Litingtung Y, Harris MP, Simandl BK, Li Y, Beachy PA, Fallon JF 2001. Manifestation of the limb prepattern: Limb development in the absence of sonic hedgehog function. Dev Biol 236: 421–435 [DOI] [PubMed] [Google Scholar]
- Christ B, Jacob HJ, Jacob M 1977. Experimental analysis of the origin of the wing musculature in avian embryos. Anat Embryol (Berl) 150: 171–186 [DOI] [PubMed] [Google Scholar]
- Dhouailly D, Kieny M 1972. The capacity of the flank somatic mesoderm of early bird embryos to participate in limb development. Dev Biol 28: 162–175 [DOI] [PubMed] [Google Scholar]
- Duprez D, Fournier-Thibault C, Le Douarin N 1998. Sonic Hedgehog induces proliferation of committed skeletal muscle cells in the chick limb. Development 125: 495–505 [DOI] [PubMed] [Google Scholar]
- Duprez D, Lapointe F, Edom-Vovard F, Kostakopoulou K, Robson L 1999. Sonic hedgehog (SHH) specifies muscle pattern at tissue and cellular chick level, in the chick limb bud. Mech Dev 82: 151–163 [DOI] [PubMed] [Google Scholar]
- Echelard Y, Epstein DJ, St-Jacques B, Shen L, Mohler J, McMahon JA, McMahon AP 1993. Sonic hedgehog, a member of a family of putative signaling molecules, is implicated in the regulation of CNS polarity. Cell 75: 1417–1430 [DOI] [PubMed] [Google Scholar]
- Engleka KA, Gitler AD, Zhang M, Zhou DD, High FA, Epstein JA 2005. Insertion of Cre into the Pax3 locus creates a new allele of Splotch and identifies unexpected Pax3 derivatives. Dev Biol 280: 396–406 [DOI] [PubMed] [Google Scholar]
- Feng X, Adiarte EG, Devoto SH 2006. Hedgehog acts directly on the zebrafish dermomyotome to promote myogenic differentiation. Dev Biol 300: 736–746 [DOI] [PubMed] [Google Scholar]
- Francis-West PH, Antoni L, Anakwe K 2003. Regulation of myogenic differentiation in the developing limb bud. J Anat 202: 69–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gensch N, Borchardt T, Schneider A, Riethmacher D, Braun T 2008. Different autonomous myogenic cell populations revealed by ablation of Myf5-expressing cells during mouse embryogenesis. Development 135: 1597–1604 [DOI] [PubMed] [Google Scholar]
- Gering M, Patient R 2005. Hedgehog signaling is required for adult blood stem cell formation in zebrafish embryos. Dev Cell 8: 389–400 [DOI] [PubMed] [Google Scholar]
- Goulding MD, Chalepakis G, Deutsch U, Erselius JR, Gruss P 1991. Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. EMBO J 10: 1135–1147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory WK, Raven HC 1941. Studies on the origin and early evolution of paired fins and limbs. Ann N Y Acad Sci 42: 273–360 [Google Scholar]
- Gros J, Hu JH, Vinegoni C, Feruglio PF, Weissleder R, Tabin CJ 2010. WNT5A/JNK and FGF/MAPK pathways regulate the cellular events shaping the vertebrate limb bud. Curr Biol 20: 1993–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerra L, Carr HS, Richter-Dahlfors A, Masucci MG, Thelestam M, Frost JA, Frisan T 2008. A bacterial cytotoxin identifies the RhoA exchange factor Net1 as a key effector in the response to DNA damage. PLoS ONE 3: e2254 doi: 10.1371/journal.pone.0002254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning P, Hardeman E 1991. Multiple mechanisms regulate muscle fiber diversity. FASEB J 5: 3064–3070 [DOI] [PubMed] [Google Scholar]
- Gustafsson MK, Pan H, Pinney DF, Liu Y, Lewandowski A, Epstein Douglas J, Emerson CP Jr 2002. Myf5 is a direct target of long-range Shh signaling and Gli regulation for muscle specification. Genes Dev 16: 114–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger H, Hamilton V 1951. A series of normal stages in the development of the chick embryo. J Morphol 88: 49–92 [PubMed] [Google Scholar]
- Hasson P, DeLaurier A, Bennett M, Grigorieva E, Naiche LA, Papaioannou VE, Mohun TJ, Logan MPO 2010. Tbx4 and tbx5 acting in connective tissue are required for limb muscle and tendon patterning. Dev Cell 18: 148–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren N 1933. On the origin of the tetrapod limb. Acta Zoologica 14: 185–295 [Google Scholar]
- Huang R, Zhi Q, Christ B 2003. The relationship between limb muscle and endothelial cells migrating from single somite. Anat Embryol (Berl) 206: 283–289 [DOI] [PubMed] [Google Scholar]
- Hutcheson DA, Zhao J, Merrell A, Haldar M, Kardon G 2009. Embryonic and fetal limb myogenic cells are derived from developmentally distinct progenitors and have different requirements for β-catenin. Genes Dev 23: 997–1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona JP, Gaffield W, Kapur RP, Roelink H 1998. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development 125: 3553–3562 [DOI] [PubMed] [Google Scholar]
- Jacob HJ, Christ B 1980. On the formation of muscular pattern in the chick limb. In Teratology of the limbs (ed. HJ Merker et al.), pp. 89–97. Walter de Gruyter and Co., Berlin [Google Scholar]
- Kardon G 1998. Muscle and tendon morphogenesis in the avian hind limb. Development 125: 4019–4032 [DOI] [PubMed] [Google Scholar]
- Kardon G, Campbell JK, Tabin CJ 2002. Local extrinsic signals determine muscle and endothelial cell fate and patterning in the vertebrate limb. Dev Cell 3: 533–545 [DOI] [PubMed] [Google Scholar]
- Kardon G, Harfe BD, Tabin CJ 2003. A Tcf4-positive mesodermal population provides a prepattern for vertebrate limb muscle patterning. Dev Cell 5: 937–944 [DOI] [PubMed] [Google Scholar]
- Kruger M, Mennerich D, Fees S, Schafer R, Mundlos S, Braun T 2001. Sonic hedgehog is a survival factor for hypaxial muscles during mouse development. Development 128: 743–752 [DOI] [PubMed] [Google Scholar]
- Li X, Blagden CS, Bildsoe H, Bonnin MA, Duprez D, Hughes SM 2004. Hedgehog can drive terminal differentiation of amniote slow skeletal muscle. BMC Dev Biol 4: 9 doi: 10.1186/1471-213X-4-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Qiu Q, Watson SS, Schweitzer R, Johnson RL 2010. Uncoupling skeletal and connective tissue patterning: Conditional deletion in cartilage progenitors reveals cell-autonomous requirements for Lmx1b in dorsal-ventral limb patterning. Development 137: 1181–1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ 2002. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 33: 77–80 [DOI] [PubMed] [Google Scholar]
- Long F, Zhang XM, Karp S, Yang Y, McMahon AP 2001. Genetic manipulation of hedgehog signaling in the endochondral skeleton reveals a direct role in the regulation of chondrocyte proliferation. Development 128: 5099–5108 [DOI] [PubMed] [Google Scholar]
- López-Martínez A, Chang DT, Chiang C, Porter JA, Ros MA, Simandl BK, Beachy PA, Fallon JF 1995. Limb-patterning activity and restricted posterior localization of the amino-terminal product of Sonic hedgehog cleavage. Curr Biol 5: 791–796 [DOI] [PubMed] [Google Scholar]
- Mathew SJ, Hansen JM, Merrell AJ, Murphy MM, Lawson JA, Hutcheson DA, Hansen MS, Angus-Hill M, Kardon G 2011. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Development 138: 371–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T, Cepko CL 2004. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc Natl Acad Sci 101: 16–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott A, Gustafsson M, Elsam T, Hui C-C, Emerson CP Jr, Borycki A-G 2005. Gli2 and Gli3 have redundant and context-dependent function in skeletal muscle formation. Development 132: 345–357 [DOI] [PubMed] [Google Scholar]
- Merchán P, Bribián A, Sánchez-Camacho C, Lezameta M, Bovolenta P, de Castro F 2007. Sonic hedgehog promotes the migration and proliferation of optic nerve oligodendrocyte precursors. Mol Cell Neurosci 36: 355–368 [DOI] [PubMed] [Google Scholar]
- Münsterberg AE, Kitajewski J, Bumcrot DA, McMahon AP, Lassar AB 1995. Combinatorial signaling by Sonic hedgehog and Wnt family members induces myogenic bHLH gene expression in the somite. Genes Dev 9: 2911–2922 [DOI] [PubMed] [Google Scholar]
- Otto A, Schmidt C, Patel K 2006. Pax3 and Pax7 expression and regulation in the avian embryo. Anat Embryol 211: 293–310 [DOI] [PubMed] [Google Scholar]
- Petrie RJ, Doyle AD, Yamada KM 2009. Random versus directionally persistent cell migration. Nat Rev Mol Cell Biol 10: 538–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polizio AH, Chinchilla P, Chen X, Kim S, Manning DR, Riobo NA 2011. Heterotrimeric Gi proteins link Hedgehog signaling to activation of Rho small GTPases to promote fibroblast migration. J Biol Chem 286: 19589–19596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouthas F, Girard P, Lecaudey V, Ly TB, Gilmour D, Boulin C, Pepperkok R, Reynaud EG 2008. In migrating cells, the Golgi complex and the position of the centrosome depend on geometrical constraints of the substratum. J Cell Sci 121: 2406–2414 [DOI] [PubMed] [Google Scholar]
- Pownall ME, Gustafsson MK, Emerson CP Jr 2002. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Annu Rev Cell Dev Biol 18: 747–783 [DOI] [PubMed] [Google Scholar]
- Purcell P, Joo BW, Hu JK, Tran PV, Calicchio ML, O'Connell DJ, Maas RL, Tabin CJ 2009. Temporomandibular joint formation requires two distinct hedgehog-dependent steps. Proc Natl Acad Sci 106: 18297–18302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM 2011. Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science 333: 637–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees E, Young RD, Evans DJR 2003. Spatial and temporal contribution of somitic myoblasts to avian hind limb muscles. Dev Biol 253: 264–278 [DOI] [PubMed] [Google Scholar]
- Renault M-A, Roncalli J, Tongers J, Thorne T, Klyachko E, Misener S, Volpert OV, Mehta S, Burg A, Luedemann C, et al. 2010. Sonic hedgehog induces angiogenesis via Rho kinase-dependent signaling in endothelial cells. J Mol Cell Cardiol 49: 490–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riddle R, Johnson R, Laufer E, Tabin C 1993. Sonic hedgehog mediates the polarizing activity of the ZPA. Cell 75: 1401–1416 [DOI] [PubMed] [Google Scholar]
- Sasaki N, Kurisu J, Kengaku M 2010. Sonic hedgehog signaling regulates actin cytoskeleton via Tiam1–Rac1 cascade during spine formation. Mol Cell Neurosci 45: 335–344 [DOI] [PubMed] [Google Scholar]
- Scaal M, Gros J, Lesbros C, Marcelle C 2004. In ovo electroporation of avian somites. Dev Dyn 229: 643–650 [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C 1996. Molecular diversity of myofibrillar proteins: Gene regulation and functional significance. Physiol Rev 76: 371–423 [DOI] [PubMed] [Google Scholar]
- Serrador JM, Nieto M, Sánchez-Madrid F 1999. Cytoskeletal rearrangement during migration and activation of T lymphocytes. Trends Cell Biol 9: 228–233 [DOI] [PubMed] [Google Scholar]
- Shubin NH, Alberch P 1986. A morphogenetic approach to the origin and basic organization of the tetrapod limb. Evol Biol 20: 319–387 [Google Scholar]
- Shubin NH, Daeschler EB, Jenkins FA 2006. The pectoral fin of Tiktaalik roseae and the origin of the tetrapod limb. Nature 440: 764–771 [DOI] [PubMed] [Google Scholar]
- Sordino P, van der Hoeven F, Duboule D 1995. Hox gene expression in teleost fins and the origin of vertebrate digits. Nature 375: 678–681 [DOI] [PubMed] [Google Scholar]
- Tajbakhsh S, Buckingham M 2000. The birth of muscle progenitor cells in the mouse: Spatiotemporal considerations. Curr Top Dev Biol 48: 225–268 [DOI] [PubMed] [Google Scholar]
- Thorsen DH, Hale ME 2005. Development of zebrafish (Danio rerio) pectoral fin musculature. J Morphol 266: 241–255 [DOI] [PubMed] [Google Scholar]
- van den Heuvel M, Ingham PW 1996. smoothened encodes a receptor-like serpentine protein required for hedgehog signalling. Nature 382: 547–551 [DOI] [PubMed] [Google Scholar]
- Van Swearingen J, Lance-Jones C 1995. Slow and fast muscle fibers are preferentially derived from myoblasts migrating into the chick limb bud at different developmental times. Dev Biol 170: 321–337 [DOI] [PubMed] [Google Scholar]
- Wagner GP, Chiu CH 2001. The tetrapod limb: A hypothesis on its origin. J Exp Zool 291: 226–240 [DOI] [PubMed] [Google Scholar]
- Wigmore PM, Evans DJR 2002. Molecular and cellular mechanisms involved in the generation of fiber diversity during myogenesis. Int Rev Cytol 216: 175–232 [DOI] [PubMed] [Google Scholar]
- Williams BA, Ordahl CP 1994. Pax-3 expression in segmental mesoderm marks early stages in myogenic cell specification. Development 120: 785–796 [DOI] [PubMed] [Google Scholar]
- Yam PT, Langlois SD, Morin S, Charron F 2009. Sonic hedgehog guides axons through a noncanonical, Src-family-kinase-dependent signaling pathway. Neuron 62: 349–362 [DOI] [PubMed] [Google Scholar]
- Yang Y, Guillot P, Boyd Y, Lyon MF, McMahon AP 1998. Evidence that preaxial polydactyly in the Doublefoot mutant is due to ectopic Indian Hedgehog signaling. Development 125: 3123–3132 [DOI] [PubMed] [Google Scholar]
- Zhang XM, Ramalho-Santos M, McMahon AP 2001. Smoothened mutants reveal redundant roles for Shh and Ihh signaling including regulation of L/R symmetry by the mouse node. Cell 106: 781–792 [PubMed] [Google Scholar]