Abstract
Circulating growth hormone (GH) levels rise in response to nutrient deprivation and fall in states of nutrient excess. Since GH regulates carbohydrate, lipid and protein metabolism, defining the mechanisms by which changes in metabolism alters GH secretion will aid in our understanding of the cause, progression and treatment of metabolic diseases. This review will summarize what is currently known regarding the impact of systemic metabolic signals on GH-axis function. In addition, ongoing studies using the Cre/loxP system to generate mouse models with selective somatotrope resistance to metabolic signals, will be discussed, where these models will serve to enhance our understanding of the specific role the somatotrope plays in sensing the metabolic environment and adjusting GH output in metabolic extremes.
Keywords: growth hormone, somatotrope, fasting, obesity
Introduction
This review will briefly summarize the impact of nutrient deficiency and nutrient excess on circulating GH and insulin-like growth factor I (IGF-I) in humans and other mammals. The mechanisms by which these changes are thought to occur will be discussed, with a particular emphasis on studies exploring the direct effects of systemic signals on somatotrope function. This review is in part an extension of a previous review1, which examined how GH is regulated under metabolic extremes and how changes in GH modify metabolic function. For an overview of what is known regarding metabolic regulation of somatotropes in non-mammalian species, refer to Gahete et al2.
Effects of nutrient deficiency on GH/IGH-I
In humans, circulating GH levels are elevated in response to fasting, diabetes type I and anorexia nervosa3–5. Circulating GH levels have also been shown to be elevated in response to fasting, caloric restriction or diabetes type I in a variety of animal models including; pigs, dogs, sheep, goats, cows, rabbits, guinea pigs, and mice as reviewed in Luque et al6; with one exception, the male rat, where fasting and diabetes type I suppresses GH secretion7–9.
Despite an increase in GH levels in the majority of species tested, short term fasting is characterized by a decrease in “free” bioavailable IGF-I, attributed in part to a rise in circulating IGF-I binding protein 1 (IGFBP-1)10. More prolonged fasting leads to a reduction of hepatic IGF-I production attributed to a decrease in hepatic sensitivity to GH actions, where GH binding is reduced11 and GH-mediated phosphorylation of the transcription factor, signal transducer and activator of transcription 5b (STAT5b), is blunted12,13. Recent data indicates that the fasting-induced reduction in GH binding may be mediated by a cell surface protein, leptin receptor overlapping transcript (LEPROT), which is induced in response to fasting and is thought to promote internalization and degradation of GH receptor (GHR)11. In addition, fasting increases hepatic expression of fibroblast growth factor 21 (FGF21) in animal models and humans14–16, where FGF21 can directly decrease STAT5b phosphorylation14. The fasting induced rise in GH and fall in IGF-I are considered part of the natural adaptive response to nutritional stress3. It is believed that the anabolic effects of GH may protect lean muscle mass, while its anti-lipogenic, pro-lipolytic and anti-insulin actions, in the context of low insulin and IGF-I, contribute to the shift in peripheral energy utilization from carbohydrate to fatty acid oxidation, thereby maintaining circulating glucose concentrations for central energy consumption.
Fasting-induced changes in hypothalamic and pituitary expression
In humans, it has been hypothesized that the fasting-induced rise in GH may reflect an increase in hypothalamic GH releasing hormone (GHRH) input and a decrease in somatostatin (SST) tone, as well as an increase in pituitary sensitivity to GHRH and ghrelin and a decrease in the inhibitory effects of SST4. These hypotheses are supported by animal studies. In food-restricted sheep, hypothalamic expression of GHRH is increased, and SST is decreased, where the later is reflected by a decrease in SST in the portal vasculature system17. Hypothalamic GHRH mRNA levels also increase in the mouse following short-term fasting (12h and 24h)6, which is associated with a reciprocal shift in the pituitary receptor expression profile (increased GHRH-R and ghrelin receptor [GHS-R] and decreased SST receptor isoform/variants [sst2, sst3 and sst5] mRNA levels6,18,19 where all of these changes would favor GH release, as illustrated in Fig. 1. Similar changes in pituitary receptor expression have been reported in the fasted and diabetic type I male rat9,20–23. These receptor changes may be functional in that the GH response to exogenous administration of GHRH and GHS-R agonists is augmented in fasted rats and dogs21,24,25. However, it should be noted, short-term fasting was recently shown to severely blunt the GH response to ghrelin in sheep26. These species dependent differences may be, in part, related to species dependent time course in homologous regulation of these receptors, where both ghrelin and GHRH can acutely downregulate the expression of their own receptors, but augment receptor expression after prolonged exposure27–30.
Figure 1.
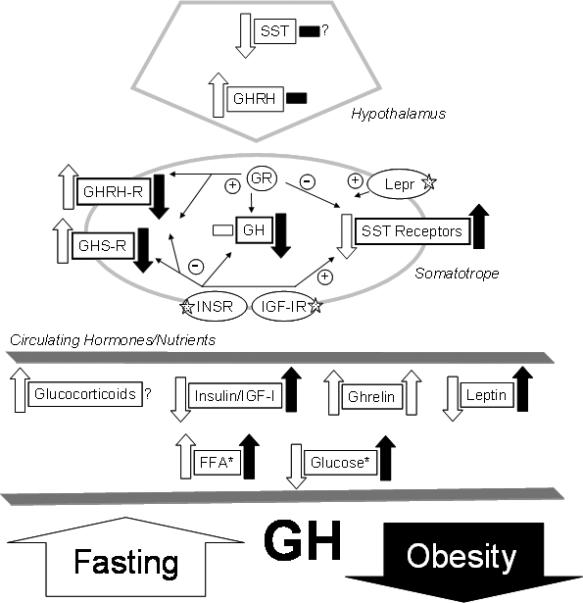
The impact of metabolic extremes on GH-axis function. The directional impact of metabolic extremes on hypothalamic and somatotrope gene expression important in GH-axis function, as well as circulating factors important in these changes are illustrated by block arrows (up or down) and horizontal blocks (no change). Open (white) blocks represent the impact of fasting, while solid (black) blocks indicate the impact of obesity. Fine arrows connect receptor activation to positive or negative regulation of genes within the somatotrope, as determined by experiments conducted in primary pituitary cell cultures. Question marks (?) denote signals or endpoints in which conflicting or incomplete data has been generated. Five-point stars (✰) indicates receptor genes which have been selectively inactivated using the Cre/loxP system. *, Both free fatty acids (FFA) and glucose negatively impact somatotrope function, but the mechanism of action remains to be clarified. Abbreviations – somatostatin (SST), GH releasing hormone (GHRH), GHRH receptor (GHRH-R), leptin receptor (Lepr), insulin receptor (INSR), insulin-like growth factor I (IGF-I), IGF-I receptor (IGF-IR). This figure represents a modification of a figure published in a previous review (1).
Potential mechanisms
IGF-I and insulin
Receptors for both IGF-I and insulin are found within the hypothalamus of the rat31–33 and intraventricular infusion of insulin or IGF-I can decrease GHRH, while increasing SST34,35. Therefore the fasting-induced fall in insulin and IGF-I could initiate a reciprocal shift in hypothalamic GHRH and SST neuronal activity. However, in rats the fasting induced fall in IGF-I and insulin is associated with a decrease in GH pulse release, therefore it is unlikely that these actions are critical for fasting-induced GH changes in this species.
Independent of the central actions of IGF-I and insulin, it has long been recognized that both can directly inhibit somatotrope function by suppressing GH release and synthesis36–40. In addition, IGF-I and insulin suppress GHRH-R and GHS-R37–40 and augment Sst2 expression (unpublished data) in primary pituitary cultures of rats, mice and baboons. The fact that the inhibitory actions of IGF-I on somatotrope function can be mimicked by insulin, at doses not predicted to bind and activate the IGF-I receptor, coupled with the observation that mouse and baboon pituitaries express receptors for both insulin and IGF-I at comparable levels39,40, supports the hypothesis that the fasting-mediated increase in GH release may be explained, in part, by a decrease in IGF-I and insulin inhibitory tone at the level of the pituitary.
Ghrelin
Another factor thought to regulate the GH-axis in response to fasting, at both the central and pituitary level, is the acylated form of the gastro-intestinal peptide, ghrelin, which has been reported to be elevated in nutrient deficient states (for review see41–43). And indeed, central infusion of ghrelin or GHS-R agonists augment GH release44. This effect, unlike the well characterized stimulatory effect of ghrelin on food intake, does not require vagal afferents, at least in humans45 and may be due to a direct stimulatory effect of ghrelin on hypothalamic GHRH neurons, as observed in mice with green fluorescent protein-expressing GHRH neurons46. At the level of the pituitary, ghrelin is less effective than GHRH in releasing GH in primary pituitary cell cultures prepared from rats47,48. However, ghrelin is as effective as GHRH in releasing GH in primary pituitary cell cultures from mice (unpublished data), pigs48 and baboons [Papio anubis]30, acting through intracellular pathways distinct from GHRH30,48. In vivo, ghrelin is important in regulating basal GH secretion, based on a study showing mice with an inactivating mutation in GHS-R have reduced GH and IGF-I levels associated with a decrease in pituitary expression of GH, however these changes only modestly alters growth49. Ghrelin also contributes to the rise in circulating GH in response nutrient deprivation, based on a recent observation that mice lacking ghrelin O-acylatransferease (GOAT), the enzyme that converts des-acyl ghrelin to acylated ghrelin, show a blunted GH response to caloric restriction with reduced glucose levels, compared to GOAT intact mice50. In the same study, circulating GH and glucose levels could be recovered in GOAT knockout mice by infusion of acyl ghrelin. It should be noted that hypoglycemia was not evident until fat mass was less than 2%50. Therefore the authors speculate that ghrelin-mediated GH release may be critical in maintaining fasting glucose levels only in severe catabolic states such as anorexia nervosa, consistent with the observations that circulating acyl ghrelin and GH show no association in lean or obese humans in response to short-term fasting where only modest weight loss is observed51,52.
It is clear that the primary source of circulating ghrelin is the gastrointestinal tract, however ghrelin is also produced within the pituitary6,53–56 and in neurons within the hypothalamus6,55–57. In fact, fasting enhances pituitary expression of ghrelin, as well as increasing hypothalamic and pituitary expression of GHS-R6,56. Therefore it is possible that local changes in ghrelin synthesis and sensitivity may play a significant role in promoting GH release in response to fasting. In addition, the hypothalamus and pituitary express GOAT, and transcript levels in both tissues are increased in the fasted mouse56, favoring the possibility that local conversion of des-acyl to acyl ghrelin could take place to promote GH secretion.
Other signals
The acute rise in glucocorticoids, in response to fasting, may also directly contribute to changes in somatotrope function because glucocorticoids can increase GH, GHRH-R and GHS-R mRNA levels in primary pituitary cell cultures from rats and baboons, while having a predominately inhibitory effect on SST receptor expression40,58–61. In vivo, adrenalectomy dramatically blunts the fasting-induced rise in pituitary GHRH-R expression, but does not alter the fasting-induced increase in GHS-R expression, suggesting factors other than glucocorticoids are involved in this response62.
Effects of nutrient excess (obesity) on GH/IGF-1
In humans, circulating GH levels are negatively correlated with body mass index63,64. Obese patients also show a blunted or absent response to all known GH stimuli65–71, where in some cases these changes can be difficult to differentiate from organic GH deficiency (GHD)71. Significant weight loss (by exercise/diet or gastric bypass), results in the recovery of circulating GH concentrations72,73,74. Therefore, the metabolic alterations associated with weight gain are thought to be the precipitating events leading to suppression of the GH-axis.
The mechanisms by which obesity leads to a decline in GH output are poorly understood. Multiple theories, based on clinical and animal studies, provide evidence implicating defects in hypothalamic input (suppressed GHRH and enhanced SST) and/or defects in somatotrope function, where both central and pituitary changes may be mediated by changes in circulating FFA, glucocorticoids, ghrelin, IGF-I or insulin, as previously reviewed63,64. All of these factors may ultimately contribute to obesity-associated GH-deficiency, depending on the experimental model or severity of the condition. However, examination of the GH-axis of the ob/ob and diet-induced (high-fat fed) obese mouse revealed obesity can be associated with a defect in somatotrope function (i.e. decreased expression of GH, GHRH-R and GHS-R), independent of changes in hypothalamic GHRH and SST expression75. Although we cannot rule out the possibility that GHRH and SST release may be modified, independent of changes in their gene expression, these results serve to minimize the role of a GHRH/SST imbalance in precipitating somatotrope defects in the obese state. These findings are consistent with an earlier observation showing normal male rats, which become obese after feeding a cafeteria-style diet, had normal hypothalamic GHRH and SST expression, but were insensitive to GHRH challenge76. Also, the fact that 8-day treatment with GHRH alone, or in combination with arginine (to suppress endogenous SST release), failed to restore the GH response of obese patients77, supports the theory that obesity-associated defects in somatotrope function may be directly mediated by systemic signals acting directly to inhibit somatotrope function, as illustrated in Fig. 1.
Potential mechanisms
Insulin and IGF-I
In obesity, low GH levels are paradoxically associated with normal levels of total IGF-I but bioavailable IGF-I is elevated, attributed to a direct inhibitory effect of insulin on IGFBP1 production by the liver10,78. Therefore, it is hypothesized that in the obese state, more IGF-I is available to directly inhibit somatotrope function, leading to a reduction in GH synthesis and release. And indeed, the inhibitory impact of IGF-I on GHRH-stimulated GH release was reported to be preserved in obese patients79.
In addition to the direct inhibitory effect of IGF-I on somatotrope function, obesity-associated hyperinsulinemia might also directly contribute to somatotrope dysfunction since several reports have shown a negative correlation between circulating insulin levels and GH65,74,75,80. In order for insulin to contribute to somatotrope dysfunction in the obese state, the pituitary must remain responsive to insulin, despite systemic insulin resistance. This appears to be the case in diet-induced obese mice, where the pituitaries remain responsive to the acute in vivo actions of insulin, as assessed by phosphorylation of Akt, despite systemic (skeletal muscle and fat) insulin resistance75.
Ghrelin
Ghrelin also appears to play a role in releasing GH under fed conditions, where acyl ghrelin levels were shown to be positively correlated with GH pulse release in healthy subjects when supplied regular meals51. In obesity, total ghrelin levels (des-acyl plus acyl)) are reduced63,75,81,82. However, more recent studies indicate that acyl ghrelin is selectively elevated in obesity83,84. Therefore circulating ghrelin is unlikely to be a key player in obesity-associated GH suppression, but is hypothesized to directly contribute to hyperphagia and fat accumulation85.
Adipokines
The most studied of the adipokines, in relationship to GH output, is leptin where circulating levels increase in obesity and decrease in response to fasting. Curiously, leptin has been shown to stimulate GH release in vivo and this action has been associated with increased GHRH and/or suppressed SST output depending on the species and nutritional status studied, as previously discussed86,87. Leptin receptors are also located on pituitary somatotropes, and leptin can directly stimulate GH release from primary pituitary cell cultures with species- and dose-dependent effects differences on GH, GHRH-R and GHS-R expression86–89. Therefore, the rise and fall of leptin in nutrient extremes are unlikely to explain changes in GH production under these conditions, however leptin may play a role in informing the somatotrope when nutrients are replete, as discussed in the last section of this review. Two other adipokines, resistin and adiponectin have also been shown to acutely stimulate GH release from primary pituitary cell cultures90,91, however circulating levels of these adipokines are oppositely controlled in fasting and obesity, where resistin levels follow those of leptin, but adiponectin levels are elevated in fasting and suppressed in obesity. Therefore, further studies are required to appreciate the intricacies of adipose regulation of somatotrope function.
Circulating nutrients
In the obese human80 and mouse75 GH output is negatively correlated with glucose, as well as insulin. Certainly, acute hyperglycemia leads to suppression of GH via central activation of SST neuronal activity92 and high glucose can suppress GHRH-stimulated GH release in primary pituitary cell cultures93,94. Therefore, it is possible that chronic hyperglycemia, associated with obesity/diabetes type II may signal through similar pathways to reduce GH output. FFA can also block basal GH release and suppress GH, GHRH-R and GHS-R expression in primary pituitary cell cultures from baboons and other species as reviewed in Luque et al40. An inhibitory effect of FFA on GH output in vivo is supported by the observation that oral administration of acipimox, to block lipolysis and lower circulating FFA, enhanced GHRH-stimulated GH release in obese patients65. However, based on the fact that in obese patients, acipimox treatment or a euglycemic insulin clamp, lowered FFA and glucose levels to that observed in similarly treated lean controls, but did not normalize insulin levels or the GH response to GHRH65, the authors concluded that hyperinsulinemia is a major determinant of GH suppression in the obese state. It should also be noted that circulating FFA increase in response to fasting, in part due to the lipolytic effect of GH95, and therefore are unlikely to play a dominant inhibitory role on GH release.
New tools
Although many hormones and nutrients can directly regulate somatotrope function and may in fact mimic the effect of metabolic extremes on GH output, as reviewed above, this does not prove that these direct actions are critical for the changes in GH output observed in vivo (i.e. exclude the contribution of altered hypothalamic signals). In order to circumvent this problem, our laboratory has developed and validated a mouse model that allows for somatotrope-specific gene modification by the Cre/loxP system96. Specifically, 310 bp 5' of the initiation codon of the rat GH gene (rGHp) was used to drive somatotrope-specific, Cre recombinase expression. This promoter was selected based on previous reports showing it was effective in targeting transgene expression to the somatotrope in other genetically engineered mouse models97,98. Since their development, the rGHpCre transgenic mice have been backcrossed and maintained in a C57Bl/6 background, where this strain has been favored in studies of hormonal regulation of metabolic function. To date, we have used the rGHpCre mice to generate somatotrope-specific knockouts of the IGF-I, insulin and leptin receptors and the data accumulated thus far is summarized below.
Somatotrope-specific knockout of IGF-I and insulin receptors
The use of the Cre/loxP system to explore the differential effects of IGF-I and insulin on somatotrope function is particularly relevant given the fact that 1) circulating concentrations of IGF-I and insulin track together in metabolic extremes, 2) pituitaries express comparable levels of insulin receptors (INSR) and IGF-I receptors (IGF-IR) and 3) insulin and IGF-I at high concentrations can activate each others receptors and stimulate common intracellular signaling pathways. Therefore, rGHpCre mice were crossbred to mice carrying a loxP-modified IgfIr allele (IgfIrfl/fl, developed by Jens Bruning, Univ. of Mainz99) and/or mice carrying a loxP-modified Insr allele (Insrfl/fl, developed by Dr CR Kahn, Joslin100), generating somatotrope-specific knockouts of IgfIr (IgfIrrGHpCre), Insr (InsrrGHpCre) or both receptors (IgfIr/InsrrGHpCre). Preliminary findings of the phenotype of these mouse models were recently reported at the 2010 Endocrine Society meeting101 and the ICN2010102, where a full characterization of the GH- and metabolic-axis of the IgfIrrGHpCre mice has been previously reported103. Knockout of either the IgfIr or Insr alone did not alter pituitary morphology or cell composition, while IgfIr/InsrrGHpCre pituitaries were smaller than homozygous floxed, Cre negative controls, which appears to be due to cell size, not cell number. IgfIrrGHpCre and InsrrGHpCre mice showed modest elevations in GH, that were most pronounced in IgfIrrGHpCre mice. Body weight of IgfIrrGHpCre and InsrrGHpCre mice did not differ from controls up to 3 months, however IgfIrrGHpCre mice had reduced fat mass101,103. Knocking out both receptors (IgfIr/InsrrGHpCre) had an additive stimulatory effect on GH that was sufficient to clearly elevate IGF-I and increase growth101. The additive effect of knocking out both receptors on GH/IGF-I production indicates IGF-I and insulin signal through separate systems to inhibit somatotrope function, which was confirmed in vitro by knocking out the IGF-I and insulin receptors in primary pituitary cell cultures of IgfIrfl/fl or Insrfl/fl, using a Cre recombinase adenoviral vector101. The fact that somatotrope-specific loss of both IgfIr and Insr, in vivo, elevated GH levels indicates negative feedback to hypothalamus is not sufficient to compensate, suggesting the somatotrope serves as the primary sensor of circulating IGF-I and insulin. Therefore, studies are ongoing to determine if somatotrope-specific loss of these receptors modifies fasting and obesity-induced changes in GH output.
Somatotrope-specific knockout of the leptin receptor (Lepr)
The rGHpCre transgenic mice have also been crossbred to mice carrying a loxP-modified Lepr allele (Leprfl/fl, developed by Dr. Streamson C. Chua, Albert Einstein College of Medicine104) generating mice with somatotrope specific knockout of the Lepr87. Consistent with the stimulatory effect of leptin on GH release observed in primary pituitary cell cultures of a number of mammalian species86,88,89, including baboons (unpublished data), the loss of the Lepr was associated with a significant decrease in circulating GH levels and a reduction in the proportion of GH immunopositive cells. However the total pituitary cell number and gross pituitary morhphology was not altered, suggesting leptin regulates GH secretion in the adult, but not somatotrope development. Interestingly, the decline in GH did not impact early growth curves but did result in an increase in body weight later in life due to excess fat accumulation, with no significant change in lean mass, consistent with the impact of adult onset GH deficiency on body composition. These observations provide further evidence that the somatotrope plays an important role as a metabolic sensor, where hypothalamic signals are unable to compensate to normalize GH output. These results also indicate that leptin plays a role in informing the somatotrope of nutrient excess, where leptin-mediated maintainance of GH release could serve to keep excess fat accumulation in check.
Future directions
As loxP-modified models become available, the specific roles that systemic hormones and nutrients play in directly regulating somatotrope function and subsequent GH output, in the context of metabolic extremes, will be revealed. A similar strategy can be applied to understand the relative contribution of hypothalamic neurons (such as GHRH and SST) at sensing systemic metabolic changes and altering GH secretion, however this will require the development of mouse models with neuron-specific Cre-recombinase expression. This basic strategy can be refined to include models of inducible, tissue-specific Cre-recombinase expression, which will allow for temporal regulation of gene modification105,106 and avoid the potential confounding effects of early gene modification on cell-specific development. Since GH has profound effects on carbohydrate, lipid and protein metabolism107, use of the Cre/loxP system will greatly expand our understanding of the mechanisms by which changes in metabolic function alters GH output and will in turn aid in the understanding and treatment of metabolic diseases.
Acknowledgments
Grants: The original work conducted by our laboratory and discussed throughout this review was supported by the “Programa Ramon y Cajal del Ministerio de Educación y Ciencia (RYC-2007-00186) and the BFU2008-01136/BFI grant (to RML), Spain” (to RML), FI06/00804 (to JCC), R03 HD059066, 1R01HD059056 and core facilities funded by NCRR P20 RR020146 and P30 NS047546 (to GVC), and grants from the University of Illinois at Chicago Campus Research Board, Veterans Affairs Merit Award and R01DK030677 (to RDK).
References
- 1.LUQUE RM, LIN Q, KINEMAN RD. Understanding the interrelationship between metabolism and the GH-Axis. In: Clemmons DR, Attanasio AF, editors. Hypothalamic-Pituitary Disease and Obesity, 11th International HypoCCS Meeting; Bristol, UK. BioScientifica Ltd; 2009. pp. 33–56. [Google Scholar]
- 2.GAHETE MD, DURAN-PRADO M, LUQUE RM, MARTINEZ-FUENTES AJ, QUINTERO A, GUTIERREZ-PASCUAL E, CORDOBA-CHACON J, MALAGON MM, GRACIA-NAVARRO F, CASTANO JP. Understanding the multifactorial control of growth hormone release by somatotropes. Ann N Y Acad Sci. 2009;1163:137–153. doi: 10.1111/j.1749-6632.2008.03660.x. [DOI] [PubMed] [Google Scholar]
- 3.NORRELUND H. The metabolic role of growth hormone in humans with particular reference to fasting Growth Horm. IGF Res. 2005;15:95–122. doi: 10.1016/j.ghir.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 4.SCACCHI M, IDA PINCELLI A, CAVAGNINI F. Nutritional status in the neuroendocrine control of growth hormone secretion: the model of anorexia nervosa Front. Neuroendocrinol. 2003;24:200–224. doi: 10.1016/s0091-3022(03)00014-1. [DOI] [PubMed] [Google Scholar]
- 5.MERCADO M, BAUMANN G. Characteristics of the somatotropic axis in insulin dependent diabetes mellitus Arch. Med. Res. 1995;26:101–109. [PubMed] [Google Scholar]
- 6.LUQUE RM, PARK S, KINEMAN RD. Severity of the catabolic condition differentially modulates hypothalamic expression of growth hormone-releasing hormone (GHRH) in the fasted mouse; potential role of neuropeptide Y (NPY) and corticotropin-releasing hormone (CRH) Endocrinology. 2007;148:300–309. doi: 10.1210/en.2006-0592. [DOI] [PubMed] [Google Scholar]
- 7.TANNENBAUM GS, MARTIN JB, COLLE E. Ultradian growth hormone rhythm in the rat: effects of feeding, hyperglycemia, and insulin-induced hypoglycemia. Endocrinology. 1976;99:720–727. doi: 10.1210/endo-99-3-720. [DOI] [PubMed] [Google Scholar]
- 8.GIUSTINA A, WEHRENBERG B. Growth hormone neuroregulation in diabetes mellitus. TEM. 1994;5:73–78. doi: 10.1016/1043-2760(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 9.KIM E, SOHN S, LEE M, JUNG J, KINEMAN RD, PARK S. Differential responses of the growth hormone axis in two rat models of streptozotocin-induced insulinopenic diabetes. J. Endocrinol. 2006;188:263–270. doi: 10.1677/joe.1.06501. [DOI] [PubMed] [Google Scholar]
- 10.FRYSTYK J. Free insulin-like growth factors-measurements and relationships to growth hormone secretion and glucose homeostasis Growth Horm. IGF Res. 2004;14:337–375. doi: 10.1016/j.ghir.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 11.TOUVIER T, CONTE-AURIOL F, BRIAND O, CUDEJKO C, PAUMELLE R, CARON S, BAUGE E, ROUILLE Y, SALLES JP, STAELS B, BAILLEUL B. LEPROT and LEPROTL1 cooperatively decrease hepatic growth hormone action in mice. J. Clin. Invest. 2009;119:3830–3838. doi: 10.1172/JCI34997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.BEAULOYE V, WILLEMS B, DE CONINCK V, FRANK SJ, EDERY M, THISSEN J-P. Impairment of liver GH receptor signaling by fasting. Endocrinology. 2002;143:792–800. doi: 10.1210/endo.143.3.8692. [DOI] [PubMed] [Google Scholar]
- 13.GEVERS EF, HANNAH MJ, WATERS MJ, ROBINSON ICAF. Regulation of rapid signal transducer and activator of transcription-5 phosphorylation in the resting cells of the growth plate and in the liver by growth hormone and feeding. Endocrinology. 2009;150:3627–3636. doi: 10.1210/en.2008-0985. [DOI] [PubMed] [Google Scholar]
- 14.KLIEWER SA, MANGELSDORF DJ. Fibroblast growth factor 21: from pharmacology to physiology. Am J Clin Nutr. 2010;91:254S–257. doi: 10.3945/ajcn.2009.28449B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.GALMAN C, LUNDASEN T, KHARITONENKOV A, BINA HA, ERIKSSON M, HAFSTROM I, DAHLIN M, AMARK P, ANGELIN B, RUDLING M. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPAR alpha activation in man. Cell Metabolism. 2008;8:169–174. doi: 10.1016/j.cmet.2008.06.014. [DOI] [PubMed] [Google Scholar]
- 16.FAZELI PK, MISRA M, GOLDSTEIN M, MILLER KK, KLIBANSKI A. Fibroblast growth factor-21 may mediate growth hormone resistance in anorexia nervosa. J. Clin. Endocrinol. Metab. 2010;95:369–374. doi: 10.1210/jc.2009-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.HENRY BA, RAO A, TILBROOK AJ, CLARKE IJ. Chronic food-restriction alters the expression of somatostatin and growth hormone-releasing hormone in the ovariectomized ewe. J. Endocrinol. 2001;170:R1–R5. doi: 10.1677/joe.0.170r001. [DOI] [PubMed] [Google Scholar]
- 18.CORDOBA-CHACON J, GAHETE MD, CASTANO JP, KINEMAN RD, LUQUE RM. AJP - Endocrinology and Metabolism. 2010. Somatostatin and its receptors contribute, in a tissue-specific manner, to the geneder-dependent, metabolic (fed/fasting) control of growth hormone axis in mice. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CORDOBA-CHACON J, GAHETE MD, DURAN-PRADO M, POZO-SALAS AI, MALAGON MM, GRACIA-NAVARRO F, KINEMAN RD, LUQUE RM, CASTANO JP. Identification and characterization of new functional truncated variants of somatostatin receptor subtype 5 in rodents. Cellular and Molecular Life Sciences. 2010;67:1147–1163. doi: 10.1007/s00018-009-0240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.PARK S, SOHN S, KINEMAN RD. Fasting-induced changes in the hypothalamic-pituitary-GH axis in the absence of GH expression: lessons from the spontaneous dwarf rat. J. Endocrinol. 2004;180:369–378. doi: 10.1677/joe.0.1800369. [DOI] [PubMed] [Google Scholar]
- 21.SUGIHARA H, EMOTO N, SHIBASAKI T, MINAMI S, WAKABAYASHI I. Increased pituitary growth hormone-releasing factor (GRF) receptor messenger ribonucleic acid expression in food-deprived rats. Brain Res. 1996;742:355–358. doi: 10.1016/s0006-8993(96)01100-6. [DOI] [PubMed] [Google Scholar]
- 22.BRUNO JF, XU Y, SONG J, BERELOWITZ M. Pituitary and hypothalamic somatostatin receptor subtype messenger ribonucleic acid expression in the food-deprived and diabetic rat. Endocrinology. 1994;135:1787–1792. doi: 10.1210/endo.135.5.7956902. [DOI] [PubMed] [Google Scholar]
- 23.KIM MS, YOON CY, PARK KH, SHIN CS, PARK KS, KIM SY, CHO BY, LEE HK. Changes in ghrelin and ghrelin receptor expression according to feeding status. Neuroreport. 2003;14:1317–1320. doi: 10.1097/01.wnr.0000078703.79393.d2. [DOI] [PubMed] [Google Scholar]
- 24.TANNENBAUM GS, PAINSON JC, LENGYEL AMJ, BRAZEAU P. Paradoxical enhancement of pituitary growth hormone (GH) responsiveness to GH-releasing factor in the face of high somatostatin tone. Endocrinology. 1989;124:1380–1388. doi: 10.1210/endo-124-3-1380. [DOI] [PubMed] [Google Scholar]
- 25.RIGAMONTI AE, MARAZZI N, CELLA G, CATTANEO L, MULLER EE. Growth hormone responses to growth hormone-releasing hormone and hexarelin in fed and fasted dogs: effect of somatostatin infusion or pretreatment with pirenzepine. J. Endocrinol. 1998;156:341–348. doi: 10.1677/joe.0.1560341. [DOI] [PubMed] [Google Scholar]
- 26.TAKAHASHI H, KUROSE Y, SUZUKI Y, KOJIMA M, YAMAGUCHI T, YOSHIDA Y, OGINO M, HODATE K, AZUMA Y, SUGINO T, KOJIMA M, KANGAWA K, HASEGAWA Y, KOBAYASHI S. Ghrelin differentially modulates the GH secretory response to GHRH between the fed and fasted states in sheep. Domestic Animal Endocrinology. 2009;37:55–60. doi: 10.1016/j.domaniend.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 27.ALEPPO G, MOSKAL SF, 2, DEGRANDIS PA, KINEMAN RD, FROHMAN LA. Homologous down-regulation of growth hormone-releasing hormone receptor messenger ribonucleic acid levels. Endocrinology. 1997;138:1058–1065. doi: 10.1210/endo.138.3.5029. [DOI] [PubMed] [Google Scholar]
- 28.KINEMAN RD, KAMEGAI J, FROHMAN LA. Growth hormone- releasing hormone (GHRH) and the growth hormone secretagogue (GHS), L692,585, differentially modulate rat pituitary GHS receptor (GHS-R) and GHRH receptor (GHRH-R) mRNA levels. Endocrinology. 1999;140:3581–3586. doi: 10.1210/endo.140.8.6918. [DOI] [PubMed] [Google Scholar]
- 29.LUQUE RM, KINEMAN RD, PARK S, PENG XD, GRACIA-NAVARRO F, CASTANO JP, MALAGON MM. Homologous and heterologous regulation of pituitary receptors for ghrelin and growth hormone-releasing hormone. Endocrinology. 2004;145:3182–3189. doi: 10.1210/en.2003-1626. [DOI] [PubMed] [Google Scholar]
- 30.KINEMAN RD, LUQUE RM. Evidence that ghrelin is as potent as growth hormone (GH)-releasing hormone (GHRH) in releasing GH from primary pituitary cell cultures of a nonhuman primate (Papio anubis), acting through intracellular signaling pathways distinct from GHRH. Endocrinology. 2007;148:4440–4449. doi: 10.1210/en.2007-0441. [DOI] [PubMed] [Google Scholar]
- 31.UNGER J, MCNEILL TH, MOXLEY RT, 3, WHITE M, MOSS A, LIVINGSTON JN. Distribution of insulin receptor-like immunoreactivity in the rat forebrain. Neuroscience. 1989;31:143–157. doi: 10.1016/0306-4522(89)90036-5. [DOI] [PubMed] [Google Scholar]
- 32.LESNIAK MA, HILL JM, KIESS W, ROJESKI M, PERT CB, ROTH J. Receptors for insulin-like growth factors I and II: autoradiographic localization in rat brain and comparison to receptors for insulin. Endocrinology. 1988;123:2089–2099. doi: 10.1210/endo-123-4-2089. [DOI] [PubMed] [Google Scholar]
- 33.MARKS JL, PORTE D, Jr., BASKIN DG. Localization of type I insulin-like growth factor receptor messenger RNA in the adult rat brain by in situ hybridization. Mol. Endocrinol. 1991;5:1158–1168. doi: 10.1210/mend-5-8-1158. [DOI] [PubMed] [Google Scholar]
- 34.ITOH M. Immunoreactive somatostatin in the hypothalamus and other regions of the rat brain: effects of insulin, glucose, alpha- or beta-blocker and L-dopa Endocrinol. Jpn. 1979;26:41–58. doi: 10.1507/endocrj1954.26.41. [DOI] [PubMed] [Google Scholar]
- 35.SATO M, FROHMAN LA. Differential effects of central and peripheral administration of growth hormone (GH) and insulin-like growth factor on hypothalamic GH-releasing hormone (GRH) and somatostatin gene expression in GH-deficient dwarf rats. Endocrinology. 1993;133:793–799. doi: 10.1210/endo.133.2.8102097. [DOI] [PubMed] [Google Scholar]
- 36.MELMED S, YAMASHITA S, YAMASAKI H, FAGIN J, NAMBA H, YAMAMOTO H, WEBER M, MORITA S, WEBSTER J, PRAGER D. IGF-I receptor signalling: lessons from the somatotroph. Rec. Prog. Horm. Res. 1996;51:189–215. [PubMed] [Google Scholar]
- 37.SUGIHARA H, EMOTO N, TAMURA H, KAMEGAI J, SHIBASAKI T, MINAMI S, WAKABAYASHI I. Effect of insulin-like growth factor-I on growth hormone-releasing factor receptor expression in primary rat anterior pituitary cell culture. Neurosci. Lett. 1999;276:87–90. doi: 10.1016/s0304-3940(99)00801-0. [DOI] [PubMed] [Google Scholar]
- 38.KAMEGAI J, TAMURA H, SHIMIZU T, ISHII S, SUGIHARA H, OIKAWA S. Insulin-like growth factor-I regulates ghrelin receptor (growth hormone secretagogue receptor) expression in the rat pituitary. Regul. Pept. 2005;127:203–206. doi: 10.1016/j.regpep.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 39.LUQUE RM, GAHETE MD, KINEMAN RD. Endocrine Society Meetings. San Diego, CA: 2005. Comparison of the effects of insulin and IGF-I on expression of growth hormone (GH), GH-releasing hormone receptors (GHRH-R) and ghrelin receptors (GHS-R), in primary pituitary cell cultures of mice and baboons (papio anubis) [abstract] pp. P2–11. [Google Scholar]
- 40.LUQUE RM, GAHETE MD, VALENTINE RJ, KINEMAN RD. Examination of the direct effects of metabolic factors on somatotrope function in a non-human primate model, Papio anubis. J Mol Endocrinol. 2006;37:25–38. doi: 10.1677/jme.1.02042. [DOI] [PubMed] [Google Scholar]
- 41.KOJIMA M, KANGAWA K. Ghrelin: structure and function. Physiol. Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 42.VAN DER LELY AJ, TSCHOP M, HEIMAN ML, GHIGO E. Biological, physiological, pathophysiological, and pharmacological aspects of ghrelin. Endocr Rev. 2004;25:426–457. doi: 10.1210/er.2002-0029. [DOI] [PubMed] [Google Scholar]
- 43.HASSOUNA R, ZIZZARI P, TOLLE V. The ghrelin/obstatin balance in the physiological and pathological control of growth hormone secretion, body composition and food intake. J.Neuroendocrinol. 2010;22:793–804. doi: 10.1111/j.1365-2826.2010.02019.x. [DOI] [PubMed] [Google Scholar]
- 44.DATE Y, MURAKAMI N, KOJIMA M, KUROIWA T, MATSUKURA S, KANGAWA K, NAKAZATO M. Central effects of a novel acylated peptide, ghrelin on growth release in rats. Biochem. Biophys. Res. Commun. 2000;275:480. doi: 10.1006/bbrc.2000.3342. [DOI] [PubMed] [Google Scholar]
- 45.LE ROUX CW, NEARY NM, HALSEY TJ, SMALL CJ, MARTINEZ-ISLA AM, GHATEI MA, THEODOROU NA, BLOOM SR. Ghrelin Does Not Stimulate Food Intake in Patients with Surgical Procedures Involving Vagotomy. Journal of Clinical Endocrinology Metabolism. 2005;90:4521–4524. doi: 10.1210/jc.2004-2537. [DOI] [PubMed] [Google Scholar]
- 46.OSTERSTOCK G, ESCOBAR P, MITUTSOVA V, GOUTY-COLOMER LA, FONTANAUD P, MOLINO F, FEHRENTZ JA, CARMIGNAC D, MARTINEZ J, GUERINEAU NC, ROBINSON ICAF, MOLLARD P, M+¬RY PF. Ghrelin stimulation of growth hormone-releasing hormone neurons Is direct in the arcuate nucleus. PLoS ONE. 2010;5:e9159. doi: 10.1371/journal.pone.0009159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.YAMAZAKI M, NAKAMURA K, KOBAYASHI H, MATSUBARA M, HAYASHI Y, KANGAWA K, SAKAI T. Regulational effect of ghrelin on growth hormone secretion from perifused rat anterior pituitary cells. J Neuroendocrinol. 2002;14:156–162. doi: 10.1046/j.0007-1331.2001.00757.x. [DOI] [PubMed] [Google Scholar]
- 48.MALAGON MM, LUQUE RM, RUIZ-GUERRERO E, RODRIGUEZ-PACHECO F, GARCIA-NAVARRO S, C.F.F., GRACIA-NAVARRO F, CASTANO JP. Intracellular signaling mechanisms mediating ghrelin-stimulated growth hormone release in somatotropes. Endocrinology. 2003;144:5372–5380. doi: 10.1210/en.2003-0723. [DOI] [PubMed] [Google Scholar]
- 49.YANG H, DIXIT VD, PATEL K, VANDANMAGSAR B, COLLINS G, SUN YX, SMITH RG, TAUB DD. Reduction in hypophyseal growth hormone and prolactin expression due to deficiency in ghrelin receptor signaling is associated with Pit-1 suppression: Relevance to the immune system. Brain Behavior and Immunity. 2008;22:1138–1145. doi: 10.1016/j.bbi.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ZHAO TJ, LIANG GS, LI RL, XIE XF, SLEEMAN MW, MURPHY AJ, VALENZUELA DM, YANCOPOULOS GD, GOLDSTEIN JL, BROWN MS. Ghrelin O-acyltransferase (GOAT) is essential for growth hormone-mediated survival of calorie-restricted mice. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7467–7472. doi: 10.1073/pnas.1002271107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.NASS R, FARHY L, LIU J, PRUDOM C, JOHNSON ML, VELDHUIS P, PEZZOLI SS, OLIVERI MC, GAYLINN BD, GEYSEN HM, THORNER MO. Evidence for Acyl-ghrelin modulation of growth hormone release in the fed state. J Clin Endocrinol Metab. 2008;93:1988–1994. doi: 10.1210/jc.2007-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.ESPELUND U, HANSEN TK, HOJLUND K, BECK-NIELSEN H, CLAUSEN JT, HANSEN BS, ORSKOV H, JORGENSEN JOL, FRYSTYK J. Fasting unmasks a strong inverse association between ghrelin and cortisol in serum: studies in obese and normal-weight subjects. Journal of Clinical Endocrinology Metabolism. 2005;90:741–746. doi: 10.1210/jc.2004-0604. [DOI] [PubMed] [Google Scholar]
- 53.KAMEGAI J, TAMURA H, SHIMIZU T, ISHII S, SUGIHARA H, OIKAWA S. Regulation of the ghrelin gene: growth hormone-releasing hormone upregulates ghrelin mRNA in the pituitary. Endocrinology. 2001;142:4154–4157. doi: 10.1210/endo.142.9.8492. [DOI] [PubMed] [Google Scholar]
- 54.KAMEGAI J, TAMURA A, SHIMIZU T, ISHII S, TATSUGUCHI A, SUGIHARA H, OIKAWA S, KINEMAN RD. The role of pituitary ghrelin in growth hormone (GH) secretion: GH-releasing hormone-dependent regulation of pituitary ghrelin gene expression and peptide content. Endocrinology. 2004;145:3731–3738. doi: 10.1210/en.2003-1424. [DOI] [PubMed] [Google Scholar]
- 55.KEDZAI A, OBARA-MOSCYNSKA M, CHMIELNICKA-KOPAXZYK M. Assessment of ghrelin, GHS-R, GH, and neurohormones in human fetal pituitary glands and central nervous system: and immunohistochemical study. Folia Histochem.Cytobiol. 2009;47:505–510. doi: 10.2478/v10042-009-0106-z. [DOI] [PubMed] [Google Scholar]
- 56.GAHETE MD, CORDOBA-CHACON J, SALVATORI R, CASTANO JP, KINEMAN RD, LUQUE RM. Metabolic regulation of ghrelin O-acyl transferase (GOAT) expression in the mouse hypothalamus, pituitary, and stomach. Mol. Cell. Endocrinol. 2010;317:154–160. doi: 10.1016/j.mce.2009.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.SATO T, FUKUE Y, TERANISHI H, YOSHIDA Y, KOJIMA M. Molecular forms of hypothalamic ghrelin and its regulation by fasting and 2-deoxy-D-glucose administration. Endocrinology. 2005;146:2510–2516. doi: 10.1210/en.2005-0174. [DOI] [PubMed] [Google Scholar]
- 58.TAMAKI M, SATO M, MATSUBARA S, WADA Y, TAKAHARA J. Dexamethasone increases growth hormone (GH)-releasing hormone (GRH) receptor mRNA levels in cultured rat anterior pituitary cells. J. Neuroendocrinol. 1996;8:475–480. doi: 10.1046/j.1365-2826.1996.04779.x. [DOI] [PubMed] [Google Scholar]
- 59.MILLER TL, MAYO KE. Glucocorticoids regulate pituitary growth hormone-releasing hormone receptor messenger ribonucleic acid expression. Endocrinology. 1997;138:2458–2465. doi: 10.1210/endo.138.6.5184. [DOI] [PubMed] [Google Scholar]
- 60.TAMURA H, KAMEGAI J, SUGIHARA H, KINEMAN RD, FROHMAN LA, WAKABAYASHI I. Glucocorticoids regulate pituitary growth hormone secretagogue receptor gene expression. J. Neuroendocrinol. 2000;12:481–485. doi: 10.1046/j.1365-2826.2000.00446.x. [DOI] [PubMed] [Google Scholar]
- 61.PARK S, KAMEGAI J, KINEMAN RD. Role of glucocorticoids in the regulation of pituitary somatostatin receptor subtype (sst1-sst5) mRNA levels: evidence for direct and somatostatin-mediated effects. Neuroendocrinology. 2003;78:163–175. doi: 10.1159/000072798. [DOI] [PubMed] [Google Scholar]
- 62.KIM E, SANGHEE S, CHUNG H, PARK S. Role of glucocorticoids in fasting-induced changes in hypothalamic and pituitary components of the growth hormone (GH)-axis. Korean J.Physiol.Pharmacol. 2008;12:217–233. doi: 10.4196/kjpp.2008.12.5.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.SCACCHI M, PINCELLI AI, CAVAGNINI F. Growth hormone in obesity. Int. J. Obes. Relat. Metab. Disord. 1999;23:260–271. doi: 10.1038/sj.ijo.0800807. [DOI] [PubMed] [Google Scholar]
- 64.MACCARIO M, GROTTOLI S, PROCOPIO M, OLEANDRI SE, ROSSETTO R, GAUNA C, ARVAT E, GHIGO E. The GH/IGF-I axis in obesity: influence of neuro-endocrine and metabolic factors. Int. J. Obes. Relat. Metab. Disord. 2000;24:S96–S99. doi: 10.1038/sj.ijo.0801289. [DOI] [PubMed] [Google Scholar]
- 65.LANZI R, LUZI L, CAUMO A, ANDREOTTI AC, MANZONI MF, MALIGHETTI ME, SERENI LP, PONTIROLI AE. Elevated insulin levels contribute to the reduced growth hormone (GH) response to GH-releasing hormone in obese subjects. Metabolism. 1999;48:1152–1156. doi: 10.1016/s0026-0495(99)90130-0. [DOI] [PubMed] [Google Scholar]
- 66.MACCARIO M, AIMARETTI G, GROTTOLI S, GAUNA C, TASSONE F, CORNELI G, ROSSETTO R, WU Z, STRASBURGER CJ, GHIGO E. Effects of 36 hour fasting on GH/IGF-I axis and metabolic parameters in patients with simple obesity. Comparison with normal subjects and hypopiutitary patients with severe GH deficiency. Int. J. Obes. Relat. Metab. Disord. 2001;25:1233–1239. doi: 10.1038/sj.ijo.0801671. [DOI] [PubMed] [Google Scholar]
- 67.BONERT VS, ELASHOFF JD, BARNETT P, MELMED S. Body mass index determines evoked growth hormone (GH) responsiveness in normal healthy male subjects: Diagnostic caveat for adult GH deficiency. J Clin Endocrinol Metab. 2004;89:3397–3401. doi: 10.1210/jc.2003-032213. [DOI] [PubMed] [Google Scholar]
- 68.HAIJIMA SV, VAN DAM PS, DE VRIES WR, MAITIMU-SMEELE I, DIEGUEZ C, CASANUEVA FF, KOPPESCHAAR HP. The GHRH/GHRP-6 test for the diagnosis of GH deficiency in elderly or severely obese men. Eur. J. Endocrinol. 2005;152:575–580. doi: 10.1530/eje.1.01887. [DOI] [PubMed] [Google Scholar]
- 69.QU X-D, GAW GONZALO IT, AL SYED MY, COHAN P, CHRISTENSON PD, SWERDLOFF RS, KELLY DF, WANG C. Influence of body mass index and gender on growth hormone (GH) responses to GH-releasing hormone plus arginine and insulin tolerance tests. J Clin Endocrinol Metab. 2004;90:1563–1569. doi: 10.1210/jc.2004-1450. [DOI] [PubMed] [Google Scholar]
- 70.CORDIDO F, ALVAREZ-CASTRO P, ISIDRO ML, CASANUEVA FF, DIEGUEZ C. Comparison between insulin tolerance test, growth hormone (GH)-releasing hormone (GHRH), GHRH plus acipimox and GHRH plus GH-releasing peptide-6 for the diagnosis of adult GH deficiency in normal subjects, obese and hypopituitary patients. Eur. J. Endocrinol. 2003;149:117–122. doi: 10.1530/eje.0.1490117. [DOI] [PubMed] [Google Scholar]
- 71.KAUSHAL K, SHALET SM. Defining growth hormone status in adults with hypopituitarism. Horm. Res. 2007;68:185–194. doi: 10.1159/000101286. [DOI] [PubMed] [Google Scholar]
- 72.VAHL N, JORGENSEN JO, SKJAERBAEK C, VELDHUIS JD, ORSKOV H, CHRISTIANSEN JS. Abdominal adiposity rather than age and sex predicts mass and regularity of GH secretion in healthy adults. Am. J. Physiol. 1997;272:E1108–E1116. doi: 10.1152/ajpendo.1997.272.6.E1108. [DOI] [PubMed] [Google Scholar]
- 73.PIJL H, LANGENDONK JG, BURGGRAAF J, FROLICH M, COHEN AF, VELDHUIS JD, MEINDERS AE. Altered neuroregulation of GH secretion in viscerally obese premenopausal women. J Clin Endocrinol Metab. 2001;86:5509–5515. doi: 10.1210/jcem.86.11.8061. [DOI] [PubMed] [Google Scholar]
- 74.DE MARINIS L, BIANCHI A, MANCINI A, GENTILELLA R, PERRELLI M, GIAMPIETRO A, PORCELLI T, TILARO L, FUSCO A, VALLE D, TACCHINO RM. Growth hormone secretion and leptin in morbid obesity before and after biliopancreatic diversion: relationships with insulin and body composition. Endocrinology. 2004;89:174–180. doi: 10.1210/jc.2002-021308. [DOI] [PubMed] [Google Scholar]
- 75.LUQUE RM, KINEMAN RD. Impact of obesity on the growth hormone axis: evidence for a direct inhibitory effect of hyperinsulinemia on pituitary function. Endocrinology. 2006;147:2754–2763. doi: 10.1210/en.2005-1549. [DOI] [PubMed] [Google Scholar]
- 76.CATTANEO L, DE GENNARO-COLONNA V, ZOLI M, MULLER E, COCCHI D. Characterization of the hypothalamo-pituitary-IGF-I axis in rats made obese by overfeeding. J. Endocrinol. 1996;148:347–353. doi: 10.1677/joe.0.1480347. [DOI] [PubMed] [Google Scholar]
- 77.GHIGO E, PROCOPIO M, MACCARIO M, BELLONE J, ARVAT E, CAMPANA S, BOGHEN MF, CAMANNI F. Repetitive GHRH administration fails to increase the response to GHRH in obese subjects: Evidence for a somatotrope defect in obesity? Horm. Metab. Res. 1993;25:305–308. doi: 10.1055/s-2007-1002105. [DOI] [PubMed] [Google Scholar]
- 78.YEAGLEY D, GUO S, UNTERMAN T, QUINN PG. Gene- and activation-specific mechanisms for insulin inhibition of basal and glucocorticoid-induced insulin-like growth factor binding protein-1 and phosphoenolpyruvate carboxykinase transcription. Roles of forkhead and insulin response sequences. J. Biol. Chem. 2001;276:33705–33710. doi: 10.1074/jbc.M101215200. [DOI] [PubMed] [Google Scholar]
- 79.MACCARIO M, TASSONE F, GIANOTTI L, LANFRANCO F, GROTTOLI S, ARVAT E, MULLER EE, GHIGO E. Effects of recombinant human Insulin-like growth factor I administration on the growth hormone (GH) response to GH-releasing hormone in obesity. Journal of Clinical Endocrinology Metabolism. 2001;86:167–171. doi: 10.1210/jcem.86.1.7110. [DOI] [PubMed] [Google Scholar]
- 80.CARMICHAEL JD, DANOFF A, MILANI D, ROUBENOFF R, LESSER ML, LIVOTE E, REITZ RE, FERRIS S, KLEINBERG DL. GH peak response to GHRH-arginine: relationship to insulin resistance and other cardiovascular risk factors in a population of adults aged 50–90. Clinical Endocrinology. 2006;65:169–177. doi: 10.1111/j.1365-2265.2006.02569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.TSCHOP M, WEYER C, TATARANNI PA, DEVANARAYAN V, RAVUSSIN E, HEIMAN ML. Circulating Ghrelin levels are decreased in human obesity. Diabetes. 2001;50:707–709. doi: 10.2337/diabetes.50.4.707. [DOI] [PubMed] [Google Scholar]
- 82.SHIIYA T, NAKAZATO M, MIZUTA M, DATE Y, MONDAL S, TANAKA M, NOZOE S, HOSODA H, KANGAWA K, MATSUKURA S. Plasma ghrelin levels in lean and obese humans and the effect of glucose on ghrelin secretion. J. Clin. Endocrinol. Metab. 2002;87:240–244. doi: 10.1210/jcem.87.1.8129. [DOI] [PubMed] [Google Scholar]
- 83.ST PIERRE DH, KARELIS AD, CODERRE L, MALITA F, FONTAINE J, MIGNAULT D, BROCHU M, BASTARD JP, CIANFLONE K, DOUCET E, IMBEAULT P, RABASA-LHORET R. Association of Acylated and Nonacylated Ghrelin with Insulin Sensitivity in Overweight and Obese Postmenopausal Women. J. Clin. Endocrinol. Metab. 2007;92:264–269. doi: 10.1210/jc.2006-1603. [DOI] [PubMed] [Google Scholar]
- 84.BARAZZONI R, ZANETTI M, FERREIRA C, VINCI P, PIRULLI A, MUCCI M, DORE F, FONDA M, CIOCCHI B, CATTIN L, GUARNIERI G. Relationships between Desacylated and Acylated Ghrelin and Insulin Sensitivity in the Metabolic Syndrome. Journal of Clinical Endocrinology Metabolism. 2007;92:3935–3940. doi: 10.1210/jc.2006-2527. [DOI] [PubMed] [Google Scholar]
- 85.WIEDMER P, NOGUEIRAS R, BROGLIO F, D'ALESSIO D, TSCHÖP MH. Ghrelin, obesity and diabetes. Nat.Clin. 2007;3:705–712. doi: 10.1038/ncpendmet0625. [DOI] [PubMed] [Google Scholar]
- 86.LUQUE RM, HUANG ZH, SHAH B, MAZZONE T, KINEMAN RD. Effects of eptin replacement on hypothalamic-pituitary growth hormone axis function and circulating ghrelin levels in ob/ob mice. Am. J. Physiol. Endocrinol. Metab. 2007;292:E891–E899. doi: 10.1152/ajpendo.00258.2006. [DOI] [PubMed] [Google Scholar]
- 87.CHILDS GV, AKHTER N, HANEY A, SYED M, ODLE A, COZART M, BRODRICK Z, GADDY D, SUVA LJ, AKEL N, CARNE C, BENES H, CHARLESWORTH A, LUQUE RM, CHUA S, KINEMAN RD. The somatotrope as a metabolic sensor:deletion of leptin receptors causes obesity. Endocrinology. 2010 doi: 10.1210/en.2010-0498. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.SALERI R, GIUSTINA A, TAMANINI C, VALLE D, BURATTIN A, WEHRENBERG WB, BARATTA M. Leptin stimulates growth hormone secretion via a direct pituitary effect combined with a decreased somatostatin tone in a median eminence-pituitary perifusion study. Neuroendocrinology. 2004;79:221–228. doi: 10.1159/000078103. [DOI] [PubMed] [Google Scholar]
- 89.MIZUNO I, OKIMURA Y, TAKAHASHI Y, KAJI H, ABE H, CHIHARA K. Leptin stimulates basal and GHRH-induced GH release from cultured rat anterior pituitary cells in vitro. Kobe J. Med. Sci. 1999;45:221–227. [PubMed] [Google Scholar]
- 90.RODRIGUEZ-PACHECO F, MARTINEZ-FUENTES AJ, TOVAR S, PINILLA L, TENA-SEMPERE M, DIEGUEZ C, CASTANO JP, MALAGON MM. Regulation of pituitary cell function by adiponectin. Endocrinology. 2007;148:401–410. doi: 10.1210/en.2006-1019. [DOI] [PubMed] [Google Scholar]
- 91.RODRIGUEZ-PACHECO F, VAZQUEZ-MARTINEZ R, MARTINEZ-FUENTES AJ, PULIDO MR, GAHETE MD, VAUDRY H, GRACIA-NAVARRO F, DIEGUEZ C, CASTANO JP, MALAGON MM. Resistin regulates pituitary somatotrope cell function through the activation of multiple signaling pathways. Endocrinology. 2009;150:4643–4652. doi: 10.1210/en.2009-0116. [DOI] [PubMed] [Google Scholar]
- 92.PENALVA A, BURGUERA B, CASABIELL X, TRESGUERRES JAF, DIEGUEZ C, CASANUEVA FF. Activation of cholinergic neurotransmission by pyridostigmine reverses the inhibitory effect of hyperglycemia on growth hormone (GH) releasing hormone-induced GH secretion in man: Does acute hyperglycemia act through hypothalamic release of somatostatin. Neuroendocrinology. 1989;49:551–554. doi: 10.1159/000125166. [DOI] [PubMed] [Google Scholar]
- 93.RENIER G, SERRI O. Effects of acute and prolonged glucose excess on growth hormone release by cultured rat anterior pituitary cells. Neuroendocrinology. 1991;54:521–525. doi: 10.1159/000125947. [DOI] [PubMed] [Google Scholar]
- 94.BARB CR, KRAELING RR, RAMPACEK GB. Glucose and free fatty acid modulation of growth hormone and luteinizing hormone secretion by cultured porcine pituitary cells. J. Anim. Sci. 1995;73:1416–1423. doi: 10.2527/1995.7351416x. [DOI] [PubMed] [Google Scholar]
- 95.MOLLER L, NORRELUND H, JESSEN N, FLYVBJERG A, PEDERSEN SB, GAYLINN BD, LIU J, THORNER MO, MOLLER N, LUNDE-JORGENSEN JO. Impact of growth hormone receptor blockade on substrate metabolism during fasting in healthy subjects. Journal of Clinical Endocrinology Metabolism. 2009;94:4524–4532. doi: 10.1210/jc.2009-0381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.LUQUE RM, AMARGO G, ISHII S, LOBE C, FRANKS R, KIYOKAWA H, KINEMAN RD. Reporter expression, induced by a GH promoter-driven Cre recombinase (rGHp-Cre) transgene, questions the developmental relationship between somatotropes and lactotropes in the adult mouse pituitary gland. Endocrinology. 2007;148:1946–1953. doi: 10.1210/en.2006-1542. [DOI] [PubMed] [Google Scholar]
- 97.BEHRINGER RR, MATHEWS LS, PALMITER RD, BRINSTER RL. Dwarf mice produced by genetic ablation of growth hormone-expressing cells. Genes Dev. 1988;2:453–461. doi: 10.1101/gad.2.4.453. [DOI] [PubMed] [Google Scholar]
- 98.BURTON FH, HASEL KW, BLOOM FE, SUTCLIFFE JG. Pituitary hyperplasia and gigantism in mice caused by a cholera toxin transgene. Nature. 1991;350:74–77. doi: 10.1038/350074a0. [DOI] [PubMed] [Google Scholar]
- 99.KLOTING N, KOCH L, WUNDERLICH T, KERN M, RUSCHKE K, KRONE W, BRUNING JC, BLUHER M. Autocrine IGF-1 action in adipocytes controls systemic IGF-1 concentrations and growth. Diabetes. 2008;57:2074–2082. doi: 10.2337/db07-1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.BRUNING JC, MICHAEL MD, WINNAY JN, HAYASHI T, HORSCH D, ACCILI D, GOODYEAR LJ, KAHN CR. A muscle-specific insulin receptor knockout exhibits features of the metabolic syndrome of NIDDM without altering glucose tolerance. Molecular Cell. 1998;2:559–569. doi: 10.1016/s1097-2765(00)80155-0. [DOI] [PubMed] [Google Scholar]
- 101.LUQUE RM, CORDOBA-CHACON J, LIN Q, KAHN CR, KOCH L, BRUNING JC, KINEMAN RD. Use of the Cre-loxP system to dissect out the individual roles IGF-I receptors (IGFIR) and insulin receptors (INSR) play in mediating somatotrope function, in vitro and in vivo [abstract] Endocr. Rev. 2010;30(Suppl 1):S1954. [Google Scholar]
- 102.LUQUE RM, GAHETE MD, CORDOBA-CHACON J, LIN Q, CHILDS GV, KINEMAN RD. International Congress of Neuroendocrinology. Rouen France: 2010. The interrelationship between GH and metabolism: the somatotrope as a metabolic sensor [abstract] [Google Scholar]
- 103.ROMERO CJ, NG Y, LUQUE RM, KINEMAN RD, KOCH L, BRUNING JC, RADOVICK S. Targeted deletion of somatotroph insulin-like growth factor-I signaling in a cell-specific knockout mouse model. Mol. Endocrinol. 2010;24:1077–1089. doi: 10.1210/me.2009-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.BALTHASAR N, COPPARI R, MCMINN J, LIU SM, LEE CE, TANG V, KENNY CD, MCGOVERN RA, CHUA SC, Jr., LMQUIST JK, OWELL BB. Leptin receptor signaling in POMC neurons Is required for normal body weight homeostasis. Neuron. 2004;42:983–991. doi: 10.1016/j.neuron.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 105.BOCKAMP E, MARINGER M, SPANGENBERG C, FEES S, FRASER S, ESHKIND L, OESCH F, ZABEL B. Of mice and models: improved animal models for biomedical research. Physiol. Genomics. 2002;11:115–132. doi: 10.1152/physiolgenomics.00067.2002. [DOI] [PubMed] [Google Scholar]
- 106.MOROZOV A. Conditional gene expression and targeting in neuroscience research. Curr.Protoc.Neurosci. 2008;Chapter 4(Unit 4.31):1–10. doi: 10.1002/0471142301.ns0431s44. [DOI] [PubMed] [Google Scholar]
- 107.MOLLER N, JORGENSEN JOL. Effects of growth hormone on glucose, lipid, and protein metabolism in human subjects. Endocr.Rev. 2009;30:152–177. doi: 10.1210/er.2008-0027. [DOI] [PubMed] [Google Scholar]


