Abstract
Distinguishing invasive high-grade urothelial carcinoma (UC) from other carcinomas occurring in the genitourinary tract may be difficult. The differential diagnosis includes high-grade prostatic adenocarcinoma, spread from an anal squamous cell carcinoma (SCC), or spread from a uterine cervical SCC. In terms of metastatic UC, the most common problem is differentiating spread of UC to the lung versus a primary pulmonary SCC. Immunohistochemistry (IHC) for GATA binding protein 3 (GATA3), thrombomodulin (THROMBO), and Uroplakin III was performed on a tissue microarray (TMA) containing 35 cases of invasive high-grade UC. GATA3 IHC was also performed on TMAs containing 38 high-grade (Gleason score 8) prostatic adenocarcinomas, representative tissue sections from 15 invasive anal SCCs, representative tissue sections from 19 invasive cervical SCCs, and TMAs with 12 invasive cervical carcinomas of the cervix [SCC (n=10), SCC with neuroendocrine features (n=1), adenosquamous carcinoma (n=1)]. Additionally, GATA3 IHC was performed on representative tissue sections from 15 pulmonary UC metastases and a TMA with 25 SCCs of the lung and 5 pulmonary non-small cell carcinomas with squamous features. GATA3, THROMBO, and Uroplakin III were positive in 28 (80%), 22 (63%), and 21 (60%) cases of high-grade UC, respectively. All GATA3 positive staining was non-focal, 25 (89%) cases demonstrated moderate-strong staining, and 3 (11%) cases demonstrated weak staining. Of the 7 cases that failed to express GATA3, 5 were positive for THROMBO and/or Uroplakin III, while 2 cases were negative for all 3 markers. None of 38 high-grade prostatic adenocarcinomas were positive for GATA3. Weak GATA3 staining was present in occasional basal cells of benign prostate glands, in a few benign atrophic glands, and in urothelial metaplasia. Of the 15 cases of anal SCCs, 2 (7%) cases showed focal weak staining and 1 (3%) case showed focal moderate staining. Weak staining was also rarely observed in the benign anal squamous epithelium. Of the 31 uterine cervical carcinomas, 6 (19%) showed weak GATA3 staining (3 non-focal, 3 focal) and 2 (6%) demonstrated focal moderate staining. Twelve (80%) of the metastatic UC to the lung were positive for GATA3 with 11 cases showing diffuse moderate or strong staining and 1 case showing focal moderate staining. None of the pulmonary SCC or non-small cell carcinomas with squamous features were GATA3 positive. GATA3 IHC is a sensitive marker for UC and positive staining in UC is typically non-focal and moderate or strong in intensity. GATA3 is also highly specific in excluding high-grade prostate adenocarcinoma. Although some cervical and anal SCCs can be GATA3 positive, unlike in UC, staining is more commonly focal and weak. GATA3 is also a useful maker when diagnosing metastatic UC to the lung.
INTRODUCTION
GATA binding protein 3 (GATA3) is a zinc finger transcription factor with a diverse range of biologic roles. GATA3 contributes to early T cell development, is required for normal mammary gland development, and decreased expression has been proposed as a poor prognostic indicator in breast cancer.(1,2,10,19) Haploinsufficiency of GATA3 results in Barakat syndrome, which clinically manifests as hypoparathyroidism, sensorineural deafness, and renal dysplasia.(2,19) Recent studies have identified GATA3 immunohistochemistry (IHC) as a sensitive marker for urothelial carcinoma (UC), ductal breast carcinoma, and transitional proliferations of the gynecological tract.(4,6)
Distinguishing invasive high-grade UC from other carcinomas occurring in the genitourinary tract may be difficult. Depending on gender, the differential diagnosis includes high-grade prostatic adenocarcinoma, spread from a squamous cell carcinoma (SCC) of the uterine cervix, or spread from an anal SCC. In terms of metastatic UC, the most common problem is differentiating spread of UC to the lung versus a primary pulmonary SCC. The current study evaluates the sensitivity of GATA3 IHC for high-grade UC and compares it to the sensitivities of thrombomodulin (THROMBO) and Uroplakin III. The specificity of GATA3 IHC for high-grade UC and the utility of GATA3 IHC in assessing metastatic UC to the lung are also examined.
MATERIAL AND METHODS
Tissue microarrays (TMAs) containing 35 invasive high-grade UCs (Fig. 1A), 38 high-grade prostatic adenocarcinomas [Gleason score 8 (n=2), Gleason score 9 (n=18), Gleason score 10 (n=18)] (Fig. 1B), 12 cases of invasive uterine cervical carcinomas [SCC (n =10), SCC with neuroendocrine features (n=1), adenosquamous carcinoma (n=1)], and 30 cases of primary lung carcinomas [SCC (n=25), non-small cell carcinoma with squamous features (n=5)] were retrieved. Of the high-grade prostatic adenocarcinomas, Gleason pattern 4 was represented by poorly formed glands. None of the high-grade prostatic adenocarcinomas with Gleason pattern 4 featured cribriform architecture. Of the UCs examined, none showed squamous differentiation. Representative tissue sections from 15 cases of invasive anal SCC, 19 cases of invasive uterine cervical SCC, and 15 cases of pulmonary metastases of UC were also obtained.
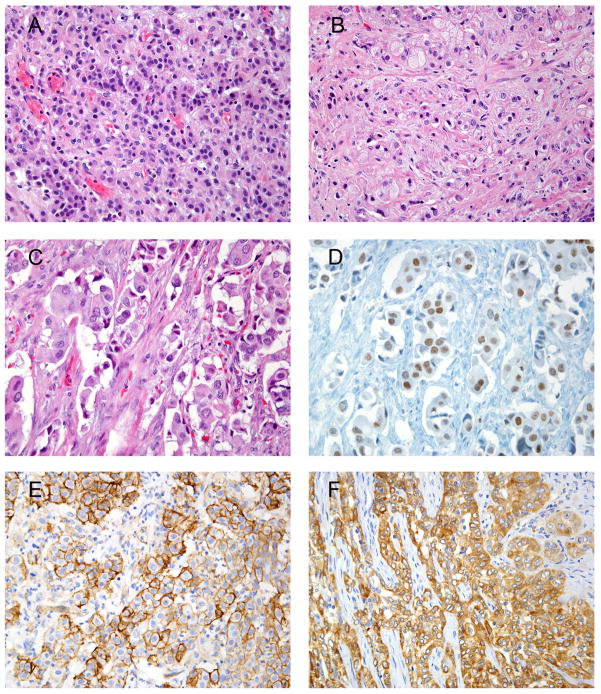
Figure 1.
A) Infiltrating poorly differentiated UC.
B) Gleason score 10 prostatic adenocarcinoma.
C) Invasive poorly differentiated UC.
D) GATA3with both moderate and strong nuclear staining (same case shown in 1C).
E) UC showing diffuse and strong staining with THROMBO.
F) UC showing diffuse and strong staining with Uroplakin III.
IHC was performed on 4 μm sections taken from formalin fixed paraffin embedded tissue blocks and TMAs. GATA3 IHC was performed on a Benchmark XT automated slide strainer (Ventana Medical Systems, Inc. Tucson, AZ). The sections were deparaffinized and subjected to antigen retrieval with Cell Conditioning Solution (high pH CC1 standard) for 60 minutes. The sections were then incubated for 44 minutes with mouse monoclonal anti-GATA3 antibodies (1:100 dilution, clone L50-823, Biocare Medical, Concord, CA) followed by an amplification step. The reaction was developed with biotin free, polymer detection (Ultra-view, Ventana Medical Systems, Inc. Tucson, AZ) as per manufacturer’s instructions.
THROMBO IHC was performed on a Bond-Leica automated slide stainer (Leica Microsystems, Inc., Bannocburn, IL). The sections were deparaffinized and subjected to heat induced antigen retrieval with high pH EDTA buffer for 20 minutes. The sections were then equilibrated with neutralizing buffer (Leica wash buffer) and incubated for 15 minutes with mouse monoclonal anti-THROMBO antibody (1:100 dilution, clone 1009, Leica Microsystems, Inc, Bannocburn, IL). Post primary antibody was applied for 8 minutes followed by biotin free polymer reagent (Leica Microsystems, Inc., Bannocburn, IL) for an additional 8 minutes. The reaction was visualized using substrate 3,3'-Diamino-benzidine hydrochloride.
Uroplakin III IHC was performed on sections that were deparaffinized and subjected to heat induced antigen retrieval with DAKO Target Retrieval Solution (DAKO North America Co, Carpintera, CA) for 20 minutes. The sections were then incubated for 20 minutes with monoclonal mouse anti-Uroplakin antibodies (1:00 dilution, clone 12A3, DAKO North America Co, Carpintera, CA). A biotin free polymer reagent (DAKO North America Co, Carpintera, CA) was then applied for 20 minutes. The reaction was visualized using substrate 3,3'-Diamino-benzidine hydrochloride.
Following antigen retrieval, primary antibody incubation, and visualization, all slides were counterstained with hematoxylin, dehydrated and cover slipped. Only moderate to strong staining was considered as positive for THROMBO and Uroplakin III. Nuclear staining for GATA3 was graded as weak, moderate, or strong, and focal ( 20% of cells) or non-focal (>20% of cells).
RESULTS
Immunohistochemical results for UC TMA are shown in Table 1. GATA3, THROMBO, and Uroplakin III were positive in 28 (80%), 22 (63%), and 21 (60%) cases of invasive high-grade UCs, respectively (Fig. 1C-F). Of the GATA3 positive cases, 25 (89%) demonstrated moderate-strong staining and 3 (11%) had weak staining. All positive staining was non-focal. Combining 2 antibodies, Uroplakin III and THROMBO had a sensitivity of 77.5%, THROMBO and GATA3 had a sensitivity of 92.5%, and Uroplakin III and GATA3 had a sensitivity of 95.0%. When using all three markers, a sensitivity of 95% was achieved. Six of the 8 of the UCs that were negative for THROMBO and Uroplakin III, showed moderate to strong staining with GATA3. Conversely, of the 7 GATA3 negative, 5 cases had staining for either THROMBO or Uroplakin III. There were 2 cases of invasive high-grade UC that were negative with all 3 stains.
TABLE 1.
Sensitivity of “Urothelial Markers” in High Grade Urothelial Carcinoma
| GATA3 | 28/35 (80%) | |
| Weak | 3 (11%) | |
| Moderate-Strong | 25 (89%) | |
| Thrombomodulin | 22/35 (63%) | |
| Uroplakin III | 21/35 (60%) |
None of the 38 high-grade prostatic adenocarcinomas were GATA3 positive. In the prostate, weak GATA3 staining was rarely encountered in basal cells of benign glands, benign atrophic glands, and glands with urothelial metaplasia. There was focal weak and focal moderate GATA3 staining in 2 (13%) and 1 (7%) of the 15 anal SCCs, respectively. Weak staining was also rarely observed in the benign anal squamous epithelium. Of the 31 uterine cervical carcinomas, 6 (19%) showed weak GATA3 staining (3 non-focal, 3 focal) and 2 (6%) demonstrated focal moderate staining. Twelve (80%) of the 15 pulmonary UC metastases were positive for GATA3 with 11 showing diffuse moderate or strong staining and 1 showing focal moderate staining. None of the 25 pulmonary SCC or 5 non-small cell carcinomas with squamous features was GATA3 positive.
DISCUSSION
Invasive high-grade UC can be difficult to differentiate from other high-grade carcinomas as the morphology of high-grade UC is not always specific. Most commonly, high-grade prostatic adenocarcinoma must be excluded. This scenario is frequently encountered in transurethral resections of large tumors involving the bladder neck, where clinically it is virtually impossible to distinguish between a prostate or bladder primary. Certain morphologic features are diagnostically helpful. High-grade prostate adenocarcinoma is composed of atypical but uniform cells with prominent nucleoli typically growing in sheets, cords, and/or as individual cells. UC is composed of atypical pleomorphic cells that tend to form nests. Cribriform architecture is characteristic of prostate adenocarcinoma and not a feature of UC. Subtle ill-defined cribriform architecture may be a clue to the prostatic origin of the tumor. Confounding factors include gland-like lumina and true glandular differentiation in UC mimicking cribriform architecture. A minority of high-grade prostatic adenocarcinomas may have nest formation similar to UC. Additionally, prostate adenocarcinoma may uncommonly demonstrate prominent pleomorphism that at the extreme has been termed “pleomorphic giant cell adenocarcinoma of the prostate.”(16)
High-grade UC may also demonstrate a squamoid appearance and thus morphologically overlap with invasive SCC. Therefore, excluding spread from an anal primary or a uterine cervical primary in female patients is necessary. While clinical history in these situations is of great value, that information is not always readily accessible or may be inaccurate. Additionally, the possibility of UC arising in the setting of an anal or cervical SCC must be considered. Given the morphologic overlap of high-grade UC with high-grade prostatic adenocarcinoma and SCC, IHC should routinely be performed in the assessment of a high-grade carcinoma in genitourinary tract when the primary site is not certain.
When excluding a high-grade prostatic adenocarcinoma, our routine immunohistochemical panel includes the following prostatic markers: prostate specific antigen (PSA), prostate specific membrane antigen (PSMA), P501S (Prostein), and NKX3.1. We have previously compared PSA staining in a group of poorly differentiated prostatic adenocarcinomas with “poor” PSA staining to PSMA and P501S and NKX 3.1.(3) Completely negative staining was seen in 15% (PSA), 12% (PSMA), 17% (P501S) and 5% (NKX 3.1) of the cases. Five per cent of the cases were negative for all four markers combined. Therefore, the lack of immunoreactivity to prostate specific markers in a poorly differentiated tumor within the prostate or bladder, especially if present in limited amount, does not exclude the diagnosis of a poorly differentiated prostatic adenocarcinoma.
Prior to the current study, our panel of immunohistochemical markers for UC was THROMBO, p63, and high molecular weight cytokeratin (HMWCK). The reported sensitivities of THROMBO, p63, and HMWCK for UC are 61%–91%, 83%–87%, and 90%, respectively.(3,6,13,15,17) These stains are variably reliable when excluding a high-grade prostatic adenocarcinoma, however, there is significant immunohistochemical overlap between UCs and SCCs. Positive THROMBO IHC has been observed in 5% of high-grade prostatic adenocarcinomas and is a common finding in SCCs from various organ systems including the uterine cervix. (3,13) While positive p63 IHC has not been reported in high-grade prostatic adenocarcinoma, p63 is positive in SCCs from a variety of sites including the uterine cervix and anus.(7,14,18) When differentiating UC and high-grade prostate adenocarcinoma, HMWCK is the least specific marker as it is expressed in a small percentage of cells in almost 10% of high-grade prostatic adenocarcinomas.(3,12) HMWCK is also present in a large proportion of SCCs.(7) As a consequence, HMWCK was not included in this study.
Uroplakin III is considered the most specific marker for urothelial differentiation, but it has not received popularity due to lack of uniform expression in UCs. The reported sensitivity is 31%–60% in primary invasive UC and 53% in metastatic UCs.(8,9) In a recent paper, Gaisa et al. identified significantly less sensitivity (22%) in invasive high-grade UCs.(5) Others have also noticed a loss of Uroplakin III expression with increase in grade and stage of UC.(11,15)
We have shown that GATA3 IHC is a sensitive marker for high-grade UC. Eighty percent of the cases of UC examined were GATA3 positive. All positive cases demonstrated non-focal staining and most showed moderate to strong staining intensity. The sensitivity of GATA3 IHC for UC exceeded that of THROMBO and Uroplakin III. GATA3 is also highly specific when differentiating high-grade UC from high-grade prostatic adenocarcinoma. None of the 38 high-grade prostatic adenocarcinomas were GATA3 positive. Sensitivity was maintained in cases of metastatic UC. Eighty percent of pulmonary UC metastases were GATA3 positive, and none of the pulmonary SCCs or non-small cell carcinomas with squamous features were GATA3 positive. We have also shown that in general moderate to strong GATA3 immunohistochemical staining can be used to exclude spread from an anal or uterine cervical SCC. Weak GATA3 IHC can be seen in a minority of cervical and anal SCCs, but tended to be more commonly focal. Focal moderate staining was only seen in 6% and 7% of uterine cervical and anal SCCs, respectively. Therefore, when distinguishing high-grade UC from uterine cervical or anal SCC, weak and focal moderate staining must be interpreted with caution. Typically (71% of cases), high-grade UCs will demonstrate non-focal moderate to strong staining. In difficult cases, in situ hybridization studies for high risk human papilloma viruses may also be of additional value.
Prior to this study, Higgins et al. examined GATA3 IHC in 321 UCs of the bladder and observed that 67% were GATA3 positive with most exhibiting intense non-focal staining.(6) Two hundred and eight of the UCs were invasive at least into the lamina propria and 113 were noninvasive. Higgins et al. also studied 257 prostate adenocarcinomas from radical prostatectomy specimens [Gleason score 3+3 (n=46); 3+4 (n=136); 4+3 (n=48); 4+4 (n=2); 3+2 (n=1); and 4+ 5 (n=1)]. None of the cases were GATA3 positive. The authors also studied a small group uterine cervical SCCs (n=6) and found no GATA3 staining.
The current study and that of Higgins et al. differ in several respects. Each study obtained a GATA3 antibody from separate sources (Biocare Medical versus Santa Cruz Biotechnology). This difference could account for the higher rate of GATA3 positivity in UC observed in the current study (80% versus 67%). It could also be attributed to the relatively large numbers of low grade and non-invasive UCs studied by Higgins et al. In contrast, the current study only examined high-grade UCs with muscularis propria invasion. The groups of prostate cancers examined in each study also differ. Only 3 of 257 prostate cancers in the Higgins study were Gleason score 8 or 9, and it is not stated whether cases with Gleason pattern 4 featured cribriform architecture. However, all of the cases in the current study were Gleason score 8 or higher, with all but 2 having a Gleason score of 9–10 and none having cribriform architecture. The high-grade cases lacking cribriform architecture in our study more closely reflect the situation where one would apply IHC for the differential diagnosis of UC and prostate cancer. Other differences in assessing GATA3 IHC between the current study and that of Higgins et al. included the examination of anal SCCS, a larger cohort of uterine cervical SCCs, and the evaluation of metastatic UC to the lung, and primary lung SCCs.
In conclusion, GATA3 IHC is a sensitive marker for UC and positive staining in UC is typically non-focal and moderate or strong in intensity. GATA3 is also highly specific in excluding high-grade prostate adenocarcinoma. Although some cervical and anal SCCs can be GATA3 positive, unlike in UC, staining is more commonly focal and weak. GATA3 is also a useful maker when diagnosing metastatic UC to the lung.
Footnotes
Disclosures: The authors have no conflicts of interest or relevant funding to disclose.
References
- 1.Asselin-Labat ML, Sutherland KD, Barker H, et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 2.Chou J, Provot S, Werb Z. GATA3 in development and cancer differentiation: cells GATA have it! J Cell Physiol. 2010;222:42–49. doi: 10.1002/jcp.21943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chuang AY, DeMarzo AM, Veltri RW, et al. Immunohistochemical differentiation of high-grade prostate carcinoma from urothelial carcinoma. Am J Surg Pathol. 2007;31:1246–1255. doi: 10.1097/PAS.0b013e31802f5d33. [DOI] [PubMed] [Google Scholar]
- 4.Esheba GE, Longacre TA, Atkins KA, et al. Expression of the urothelial differentiation markers GATA3 and placental S100 (S100P) in female genital tract transitional cell proliferations. Am J Surg Pathol. 2009;33:347–353. doi: 10.1097/PAS.0b013e3181908e24. [DOI] [PubMed] [Google Scholar]
- 5.Gaisa NT, Braunschweig T, Reimer N, et al. Different immunohistochemical and ultrastructural phenotypes of squamous differentiation in bladder cancer. Virchows Arch. 2011;458:301–312. doi: 10.1007/s00428-010-1017-2. [DOI] [PubMed] [Google Scholar]
- 6.Higgins JP, Kaygusuz G, Wang L, et al. Placental S100 (S100P) and GATA3: markers for transitional epithelium and urothelial carcinoma discovered by complementary DNA microarray. Am J Surg Pathol. 2007;31:673–680. doi: 10.1097/01.pas.0000213438.01278.5f. [DOI] [PubMed] [Google Scholar]
- 7.Kaufmann O, Fietze E, Mengs J, et al. Value of p63 and cytokeratin 5/6 as immunohistochemical markers for the differential diagnosis of poorly differentiated and undifferentiated carcinomas. Am J Clin Pathol. 2001;116:823–830. doi: 10.1309/21TW-2NDG-JRK4-PFJX. [DOI] [PubMed] [Google Scholar]
- 8.Kaufmann O, Volmerig J, Dietel M. Uroplakin III is a highly specific and moderately sensitive immunohistochemical marker for primary and metastatic urothelial carcinomas. Am J Clin Pathol. 2000;113:683–687. doi: 10.1309/PYQC-17CB-063T-Q07J. [DOI] [PubMed] [Google Scholar]
- 9.Koga F, Kawakami S, Fujii Y, et al. Impaired p63 expression associates with poor prognosis and uroplakin III expression in invasive urothelial carcinoma of the bladder. Clin Cancer Res. 2003;9:5501–5507. [PubMed] [Google Scholar]
- 10.Kouros-Mehr H, Kim JW, Bechis SK, et al. GATA-3 and the regulation of the mammary luminal cell fate. Curr Opin Cell Biol. 2008;20:164–170. doi: 10.1016/j.ceb.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Matsumoto K, Satoh T, Irie A, et al. Loss expression of uroplakin III is associated with clinicopathologic features of aggressive bladder cancer. Urology. 2008;72:444–449. doi: 10.1016/j.urology.2007.11.128. [DOI] [PubMed] [Google Scholar]
- 12.Oliai BR, Kahane H, Epstein JI. Can basal cells be seen in adenocarcinoma of the prostate?: an immunohistochemical study using high molecular weight cytokeratin (clone 34betaE12) antibody. Am J Surg Pathol. 2002;26:1151–1160. doi: 10.1097/00000478-200209000-00005. [DOI] [PubMed] [Google Scholar]
- 13.Ordonez NG. Thrombomodulin expression in transitional cell carcinoma. Am J Clin Pathol. 1998;110:385–390. doi: 10.1093/ajcp/110.3.385. [DOI] [PubMed] [Google Scholar]
- 14.Owens SR, Greenson JK. Immunohistochemical staining for p63 is useful in the diagnosis of anal squamous cell carcinomas. Am J Surg Pathol. 2007;31:285–290. doi: 10.1097/01.pas.0000213362.10756.d3. [DOI] [PubMed] [Google Scholar]
- 15.Parker DC, Folpe AL, Bell J, et al. Potential utility of uroplakin III, thrombomodulin, high molecular weight cytokeratin, and cytokeratin 20 in noninvasive, invasive, and metastatic urothelial (transitional cell) carcinomas. Am J Surg Pathol. 2003;27:1–10. doi: 10.1097/00000478-200301000-00001. [DOI] [PubMed] [Google Scholar]
- 16.Parwani AV, Herawi M, Epstein JI. Pleomorphic giant cell adenocarcinoma of the prostate: report of 6 cases. Am J Surg Pathol. 2006;30:1254–1259. doi: 10.1097/01.pas.0000209841.77595.4b. [DOI] [PubMed] [Google Scholar]
- 17.Varma M, Morgan M, Amin MB, et al. High molecular weight cytokeratin antibody (clone 34betaE12): a sensitive marker for differentiation of high-grade invasive urothelial carcinoma from prostate cancer. Histopathology. 2003;42:167–172. doi: 10.1046/j.1365-2559.2003.01560.x. [DOI] [PubMed] [Google Scholar]
- 18.Wang TY, Chen BF, Yang YC, et al. Histologic and immunophenotypic classification of cervical carcinomas by expression of the p53 homologue p63: a study of 250 cases. Hum Pathol. 2001;32:479–486. doi: 10.1053/hupa.2001.24324. [DOI] [PubMed] [Google Scholar]
- 19.Zheng R, Blobel GA. GATA Transcription Factors and Cancer. Genes Cancer. 2010;1:1178–1188. doi: 10.1177/1947601911404223. [DOI] [PMC free article] [PubMed] [Google Scholar]